Characterization of the CYP3A4 Enzyme Inhibition Potential of Selected Flavonoids
Abstract
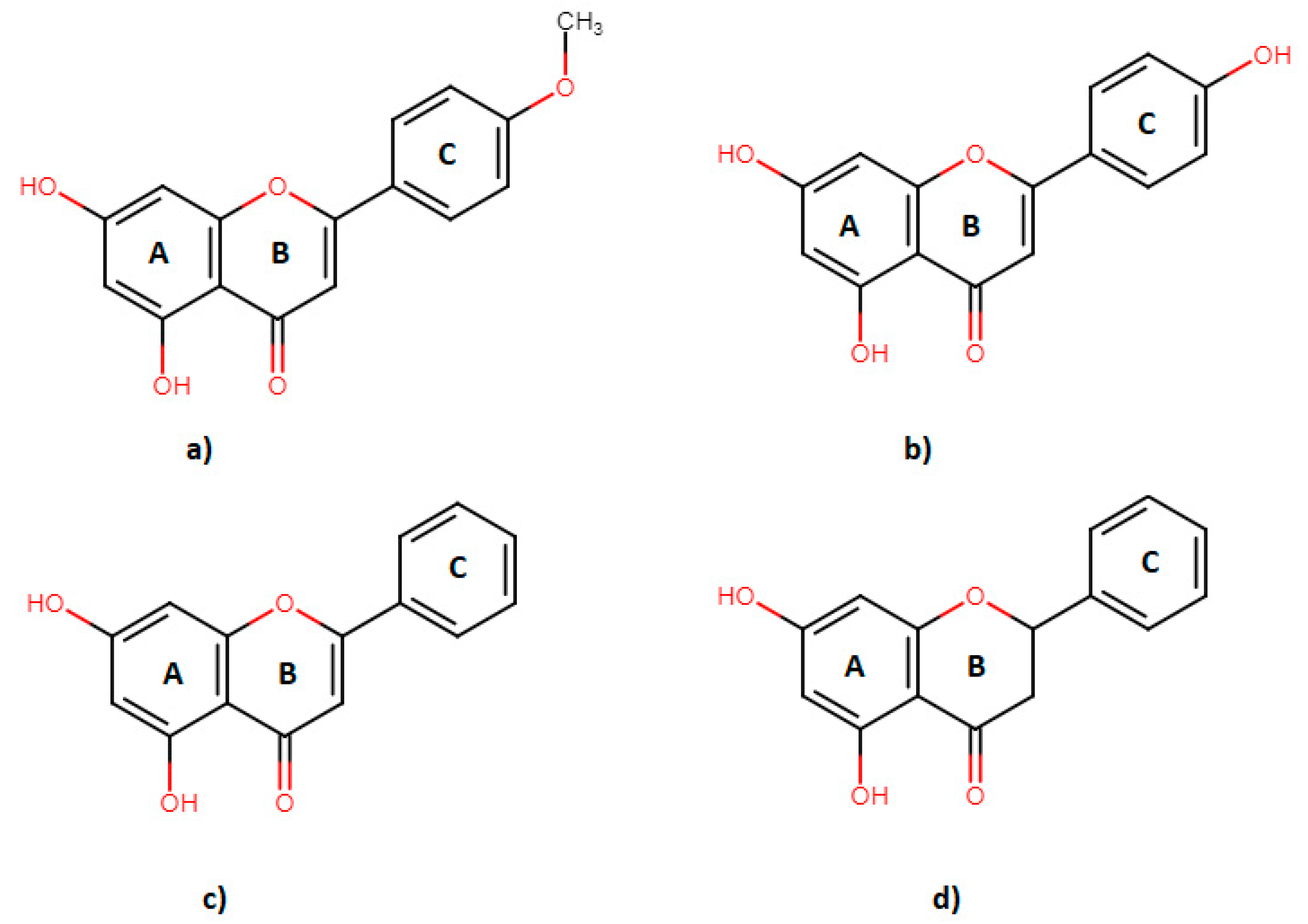
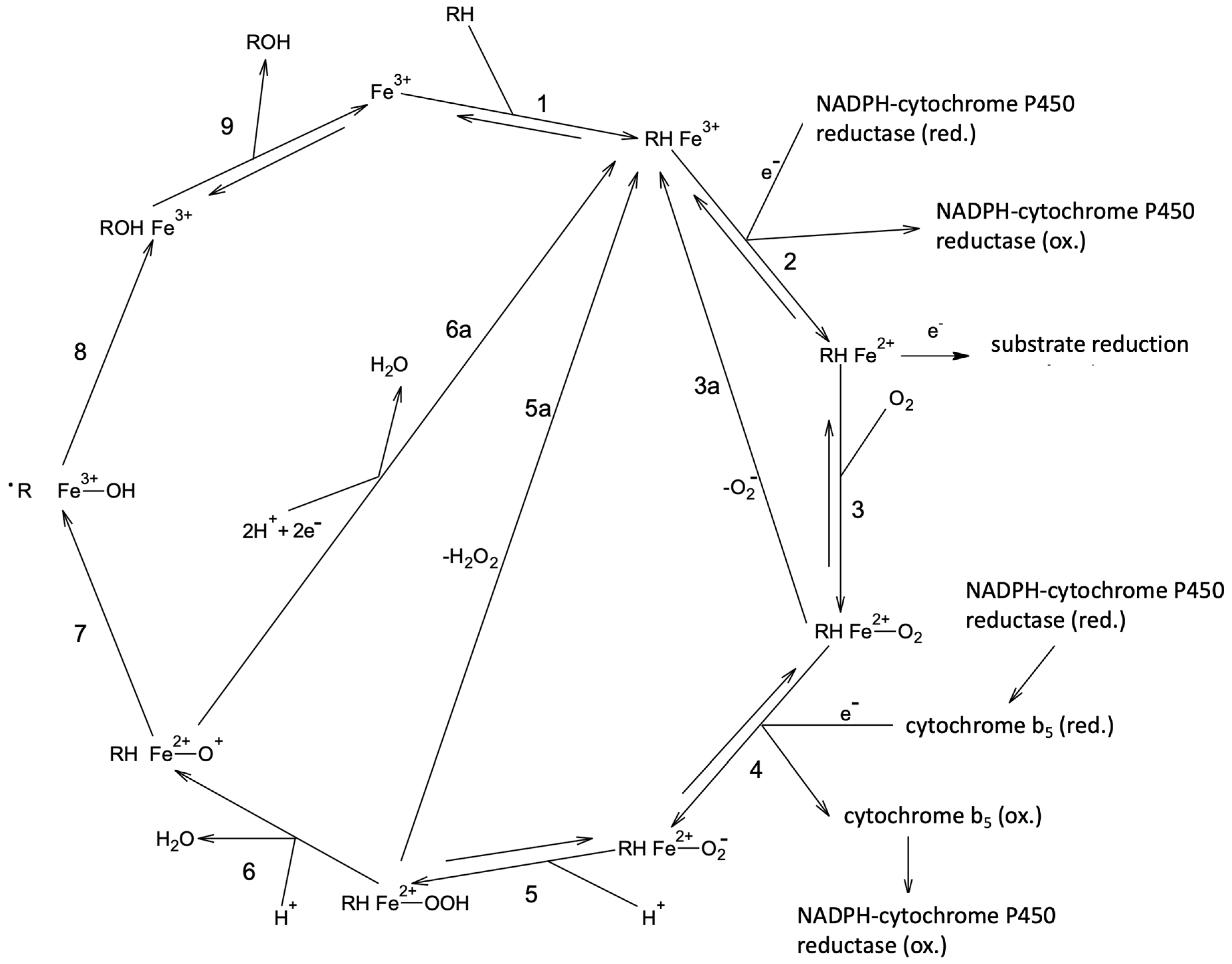
1. Introduction
2. Results
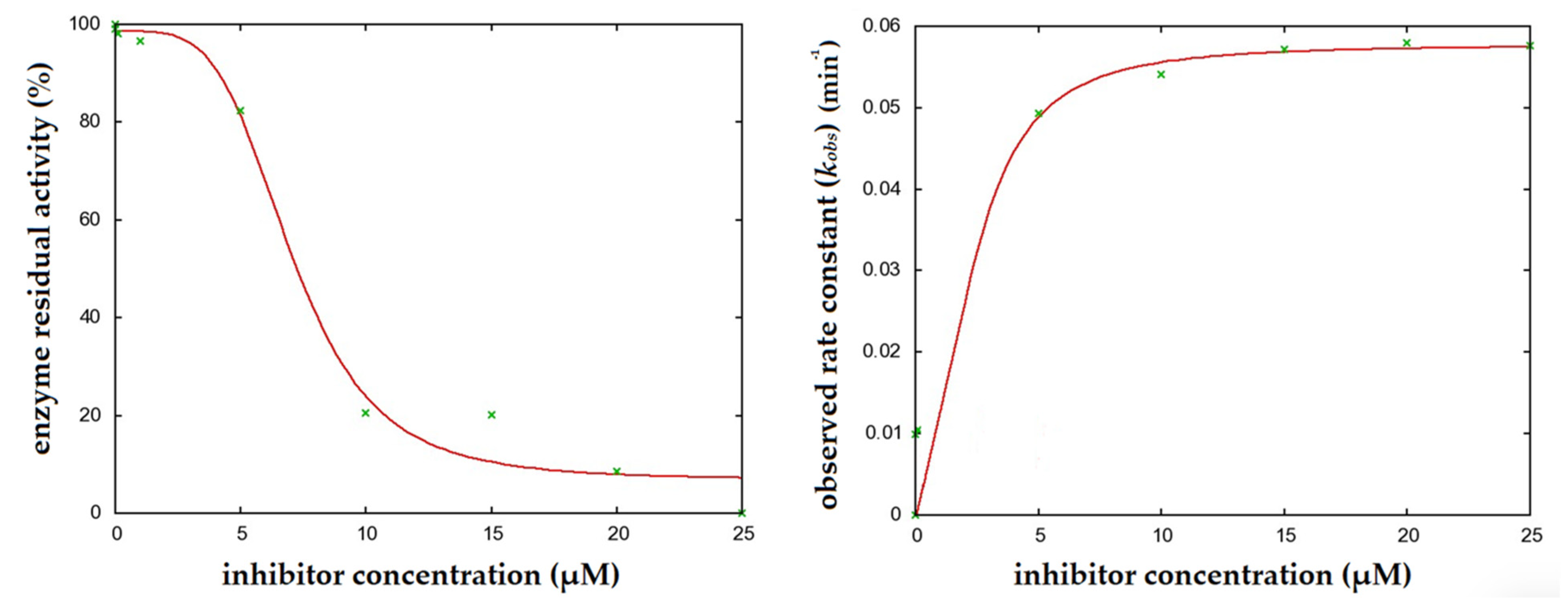
2.1. Enzyme Kinetics
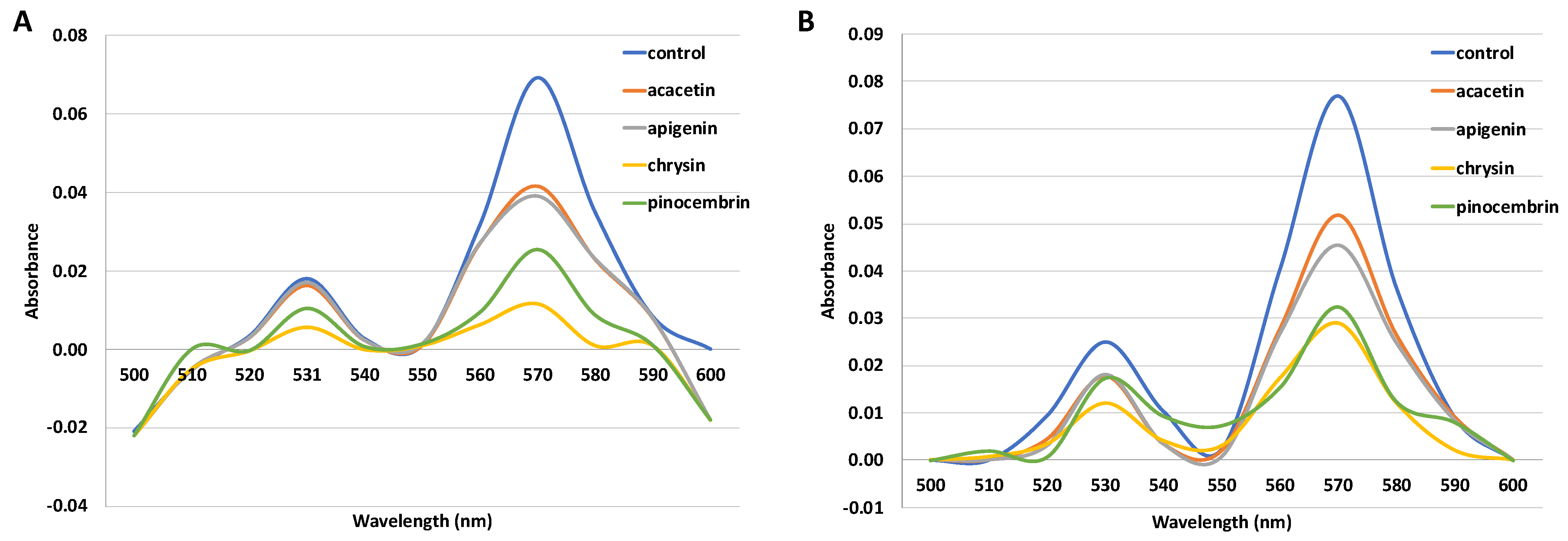
2.2. Hemochrome-Pyridine Assay
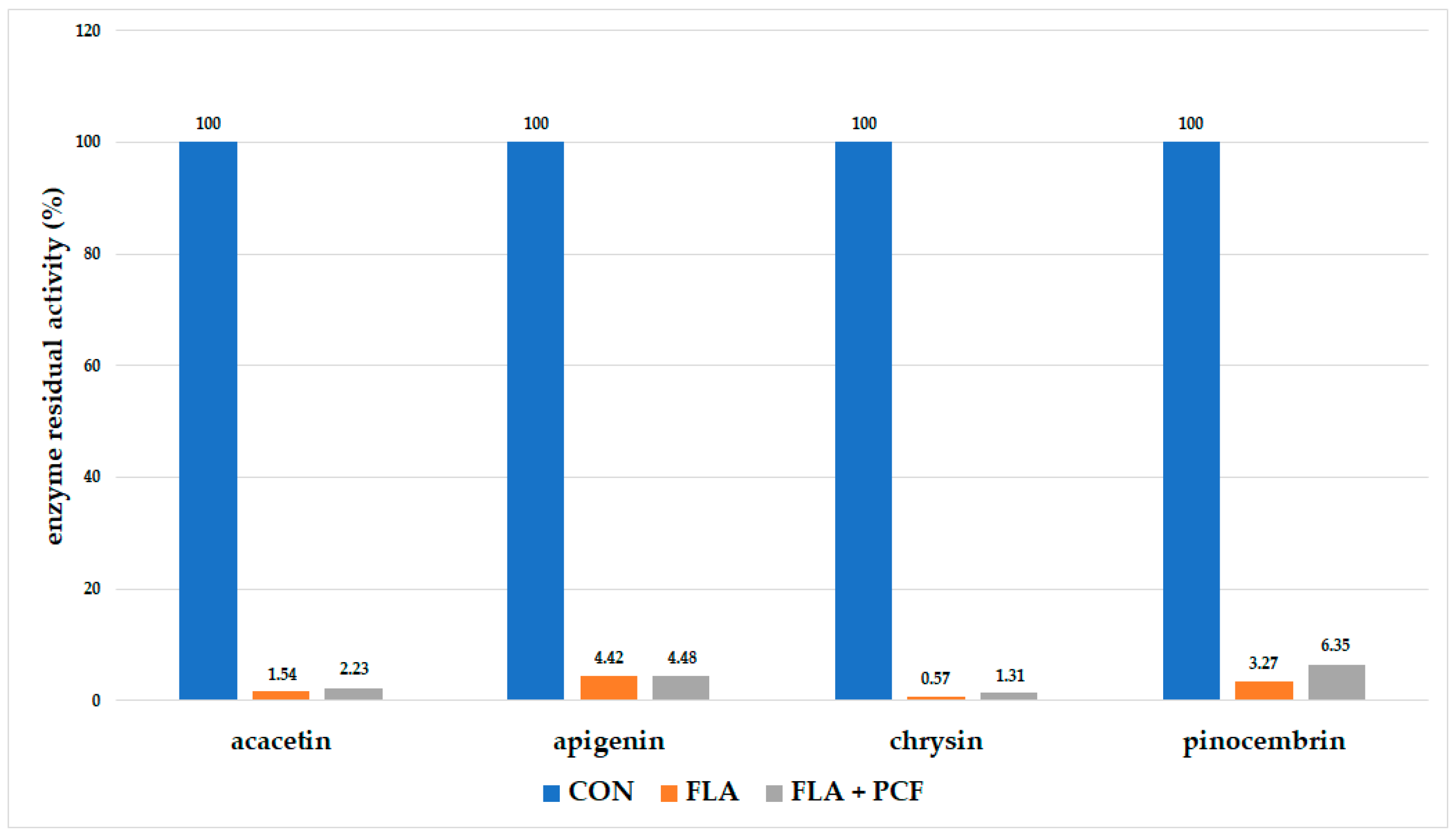
2.3. Pseudo-Irreversible Inhibition Assay
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Determination of Enzyme Kinetics and Residual Activity
4.2.2. Hemochromopyridine Assay
4.2.3. Pseudo-Irreversible Inhibition Assay
4.2.4. Results Processing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Papies, E.K.; Johannes, N.; Daneva, T.; Semyte, G.; Kauhanen, L.L. Using Consumption and Reward Simulations to Increase the Appeal of Plant-based Foods. Appetite 2020, 155, 104812. [Google Scholar] [CrossRef]
- Dwyer, J.T.; Peterson, J.J. Advances in Evaluation and Validity of the Dietary Intake of Specific Food Components Including Nutrients and Non-nutrients Measuring Flavonoid Intake: Need for Advanced Tools. Public Health Nutr. 2021, 5, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Jun, S.; Shin, S.; Joung, H. Estimation of Dietary Flavonoid Intake and Major Food Sources of Korean Adults. Br. J. Nutr. 2015, 115, 480–489. [Google Scholar] [CrossRef]
- Bhagwat, S.A.; Haytowitz, D.B.; Wasswa-Kintu, S.I.; Pehrsson, P.R. Process of Formulating USDA’s Expanded Flavonoid Database for the Assessment of Dietary Intakes: A New Tool for Epidemiological Research. Br. J. Nutr. 2015, 114, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Zujko, M.E.; Witkowska, A.M.; Waśkiewicz, A.; Mirończuk-Chodakowska, I. Dietary Antioxidant and Flavonoid Intakes Are Reduced in the Elderly. Oxid. Med. Cell. Longev. 2015, 2015, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Beking, K.; Vieira, A. An assessment of Dietary Flavonoid Intake in the UK and Ireland. Int. J. Food Sci. Nutr. 2011, 62, 17–19. [Google Scholar] [CrossRef]
- Khalil, M.I.; Sulaiman, S.A. The Potential Role of Honey and Its Polyphenols in Preventing Heart Diseases: A Review. Afr. J. Tradit. Complement. Altern. Med. 2010, 7, 315–321. [Google Scholar] [CrossRef]
- Pinzon, L.C.; Uy, M.M.; Hong Sze, K.; Wang, M.; Keung Chu, I. Isolation and Characterization of Antimicrobial, Anti-inflammatory and Chemopreventive Flavones from Premna odorata Blanco. J. Med. Plants Res. 2011, 5, 2729–2735. [Google Scholar]
- Kraft, C.; Jenett-Siems, K.; Siems, K.; Jakupović, J.; Mavi, S.; Bienzle, U.; Eich, E. In Vitro Antiplasmodial Evaluation of Medicinal Plants from Zimbabwe. Phyther. Res. 2003, 17, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Agrawal, P.; Yim, D.; Agarwal, C.; Agarwal, R. Acacetin Inhibits Cell Growth and Cell Cycle Progression, and Induces Apoptosis in Human Prostate Cancer Cells: Structure-activity Relationship with Linarin and Linarin Acetate. Carcinogenesis 2005, 26, 845–854. [Google Scholar] [CrossRef]
- Hsu, Y.L.; Kuo, P.L.; Liu, C.F.; Lin, C.C. Acacetin-induced Cell Cycle Arrest and Apoptosis in Human Non-small Cell Lung Cancer A549 Cells. Cancer Lett. 2004, 212, 53–60. [Google Scholar] [CrossRef]
- Sung, B.; Chung, H.Y.; Kim, N.D. Role of Apigenin in Cancer Prevention via the Induction of Apoptosis and Autophagy. J. Cancer Prev. 2016, 21, 216–222. [Google Scholar] [CrossRef]
- McKay, D.L.; Blumberg, J.A. A Review of the Bioactivity and Potential Health Benefits of Chamomile Tea (Matricaria Recutita L.). Phytother. Res. 2006, 20, 519–530. [Google Scholar] [CrossRef]
- Villa-Rodriguez, J.A.; Kerimi, A.; Abranko, L.; Tumova, S.; Ford, L.; Blackburn, R.S.; Rayner, C.; Williamson, G. Acute Metabolic Actions of the Major Polyphenols in Chamomile: An In Vitro Mechanistic Study on Their Potential to Attenuate Postprandial Hyperglycaemia. Sci. Rep. 2018, 8, 5471. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.; Barker, G.; Wall, C.A.; Lappas, M. Dietary Phytophenols Curcumin, Naringenin and Apigenin Reduce Infection-induced Inflammatory and Contractile Pathways in Human Placenta, Foetal Membranes and Myometrium. Mol. Hum. Reprod. 2013, 19, 451–462. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, Y.; Lin, L.; Zhou, J. Apigenin Suppresses the Apoptosis of H9C2 Rat Cardiomyocytes Subjected to Myocardial Ischemia-reperfusion Injury via Upregulation of the PI3K/Akt Pathway. Mol. Med. Rep. 2018, 18, 1560–1570. [Google Scholar] [CrossRef]
- Mani, R.; Natesan, V. Chrysin: Sources, Beneficial Pharmacological Activities, and Molecular Mechanism of Action. Phytochemistry 2018, 145, 187–196. [Google Scholar] [CrossRef]
- Sanderson, J.T.; Hordijk, J.; Denison, M.S.; Springsteel, M.F.; Nantz, M.H.; van den Berg, M. Induction and Inhibition of Aromatase (CYP19) Activity by Natural and Synthetic Flavonoid Compounds in H295R Human Adrenocortical Carcinoma Cells. Toxicol. Sci. 2004, 82, 70–79. [Google Scholar] [CrossRef]
- Cho, H.; Cheol-Won, Y.; Woo-Kyu, P.; Jae-Yang, K.; Kyoung, S.K.; Park, Y.; Lee, S.; Kim, B.K. Modulation of the Activity of Pro-inflammatory Enzymes, COX-2 and iNOS, by Chrysin Derivatives. Pharmacol. Res. 2004, 49, 37–43. [Google Scholar] [CrossRef]
- Woodman, O.L.; Chan, E.C.H. Vascular and Antioxidant Actions of Flavonols and Flavones. Clin. Exp. Pharmacol. Physiol. 2004, 31, 786–790. [Google Scholar] [CrossRef]
- Ham, D.; Jun, S.; Kang, M.; Paik, H.Y.; Joung, H.; Shin, S. Consumption of Korean Foods with High Flavonoid Contents Reduces the Likelihood of Having Elevated C-Reactive Protein Levels: Data from the 2015–2017 Korea National Health and Nutrition Examination Survey. Nutrients 2019, 11, 2370. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, P.; Qu, X.A.; Sanseau, P.; Yang, L. Systematic Prediction of Drug Combinations based on Clinical Side-effects. Sci. Rep. 2014, 4, 7160. [Google Scholar] [CrossRef] [PubMed]
- Strandell, J.; Bate, A.; Lindquist, M.; Edwards, I.R. Drug-drug Interactions-A Preventable Patient Safety Issue? Br. J. Clin. Pharmacol. 2008, 65, 144–146. [Google Scholar] [CrossRef]
- Becker, M.L.; Kallewaard, M.; Caspers, P.W.J.; Visser, L.E.; Leufkens, H.G.M.; Stricker, B.H.C. Hospitalisations and Emergency Department Visits due to Drug-drug Interactions: A Literature Review. Pharmacopedemiol. Drug Saf. 2007, 16, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Rendić, S.; Guengerich, F.P. Survey of Human Oxidoreductases and Cytochrome P450 Enzymes Involved in the Metabolism of Xenobiotic and Natural Chemicals. Chem. Res. Toxicol. 2015, 28, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Bojić, M. Preclinical Cytochrome P450 Inhibition and Interaction Studies of New Drug Candidates. Farm. Glas. 2015, 71, 229–242. [Google Scholar]
- Henderson, L.; Yue, Q.Y.; Bergquist, C.; Gerden, B.; Arlett, P. St John’s wort (Hypericum perforatum): Drug Interactions and Clinical Outcomes. Br. J. Clin. Pharmacol. 2002, 54, 349–356. [Google Scholar] [CrossRef]
- Šarić Mustapić, D.; Debeljak, Ž.; Maleš, Ž.; Bojić, M. The Inhibitory Effect of Flavonoid Aglycones on the Metabolic Activity of CYP3A4 Enzyme. Molecules 2018, 23, 2553. [Google Scholar] [CrossRef]
- Kondža, M.; Rimac, H.; Maleš, Ž.; Turčić, P.; Ćavar, I.; Bojić, M. Inhibitory Effect of Acacetin, Apigenin, Chrysin and Pinocembrin on Human Cytochrome P450 3A4. Croat. Chem. Acta 2020, 93, 33–39. [Google Scholar] [CrossRef]
- Lozić, M.; Rimac, H.; Bojić, M. Citokrom P450 i Metabolizam lijekova–značenje i novosti. Farm. Glas. 2016, 72, 747–760. [Google Scholar]
- Galetin, A.; Clarke, S.E.; Houston, J.B. Multisite Kinetic Analysis of Interactions between Prototypical CYP3A4 Subgroup Substrates: Midazolam, Testosterone, and Nifedipine. Drug Metab. Dispos. 2003, 31, 1108–1116. [Google Scholar] [CrossRef]
- Wang, X.; Morris, M.E. Effects of the Flavonoid Chrysin on Nitrofurantoin Pharmacokinetics in Rats: Potential Involvement of ABCG2. Drug Metab. Dispos. 2007, 35, 268–274. [Google Scholar] [CrossRef]
- Mück, W.; Ochmann, K.; Rohde, G.; Unger, S.; Kuhlmann, J. Influence of Erythromycin Pre- and Co-treatment on Single-dose Pharmacokinetics of the HMG-CoA Reductase Inhibitor Cerivastatin. Eur. J. Clin. Pharmacol. 1998, 53, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Wrighton, S.A.; Ring, B.J. Inhibition of Human CYP3A Catalyzed 1′-Hydroxy Midazolam Formation by Ketoconazole, Nifedipine, Erythromycin, Cimetidine, and Nizatidine. Pharm. Res. 1994, 11, 921–924. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Braidy, N.; Habtemariam, S.; Orhan, I.E.; Daglia, M.; Manay, A.; Gortzi, O.; Nabavi, S.M. Neuroprotective Effects of Chrysin: From Chemistry to Medicine. Neurochem. Int. 2015, 90, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Dasmahapatra, A.K.; Khan, S.I.; Khan, I.A. Anti-aromatase Activity of the Constituents from Damiana (Turnera diffusa). J. Ethnopharmacol. 2008, 120, 387–393. [Google Scholar] [CrossRef]
- Hostetler, G.A.; Ralston, R.A.; Schwartz, S.J. Flavones: Food Sources, Bioavailability, Metabolism, and Bioactivity. Adv. Nutr. 2017, 8, 423–435. [Google Scholar] [CrossRef]
- Xu, Y.; Xin, Y.; Lu, C.; Fu, J.; Luo, L.; Yin, Z. Synergistic Effects of Apigenin and Paclitaxel on Apoptosis of Cancer Cells. PLoS ONE 2011, 6, e29169. [Google Scholar] [CrossRef]
- Shankar, E.; Goel, A.; Gupta, K.; Gupta, S. Plant Flavone Apigenin: An Emerging Anticancer Agent. Curr. Pharmacol. Rep. 2017, 3, 423–446. [Google Scholar] [CrossRef] [PubMed]
- Foti, E.S.; Rock, D.A.; Pearson, J.T.; Wahlstrom, J.L.; Wienkers, L.C. Mechanism-Based Inactivation of Cytochrome P450 3A4 by Mibefradil through Heme Destruction. Drug Metab. Dispos. 2011, 39, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P.; Martin, M.V.; Sohl, C.D.; Cheng, Q. Measurement of Cytochrome P450 and NADPH–cytochrome P450 Reductase. Nat. Protoc. 2009, 4, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Sohl, C.D.; Cheng, Q.; Guengerich, F.P. Chromatographic Assays of Drug Oxidation by Human Cytochrome P450 3A4. Nat. Protoc. 2009, 4, 1252–1257. [Google Scholar] [CrossRef] [PubMed]
- Flink, E.B.; Watson, C.J. A Method for the Quantitative Determination of Hemoglonin and Related Heme Pigments in Feces, Urine, and Blood Plasa. J. Biol. Chem. 1942, 146, 171–178. [Google Scholar] [CrossRef]
- Paul, K.G.; Theorell, H.; Åkeson, Å.; Virtanen, A.I.; Sörensen, N.A. The Molar Light Absorption of Pyridine Ferroprotoporphrin (Pyridine Haemochromogen). Acta Chem. Scand. 1953, 7, 1284–1287. [Google Scholar] [CrossRef]
- Bojić, M.; Barbero, L.; Dolgos, H.; Freisleben, A.; Gallemann, D.; Riva, S.; Guengerich, F.P. Time- and NADPH-dependent Inhibition of Cytochrome P450 3A4 by the Cyclopentapeptide Cilengitide: Significance of the Guanidine Group and Accompanying Spectral Changes. Drug Metab. Dispos. 2014, 42, 1438–1446. [Google Scholar] [CrossRef]
| Acacetin | Apigenin | Chrysin | Pinocembrin | |||||
|---|---|---|---|---|---|---|---|---|
| Parameter | NIF | TSN | NIF | TSN | NIF | TSN | NIF | TSN |
| IC50 (µM) | 7.5 ± 2.7 | 10.9 ± 0.3 | 8.4 ± 1.1 | 11.4 ± 0.4 | 2.5 ± 0.6 | 0.6 ± 0.5 | 4.3 ± 1.1 | 5.0 ± 0.6 |
| Ki (µM) | 12.1 ± 5.6 | 6 ± 3 | 20.2 ± 12.7 | 1.5 ± 0.8 | 2.4 ± 1.0 | 0.6 ± 0.3 | 5.1 ± 1.6 | 1.2 ± 0.3 |
| kinact (min−1) | 0.10 ± 0.02 | 0.036 ± 0.006 | 0.11 ± 0.04 | 0.11 ± 0.01 | 0.07 ± 0.01 | 0.065 ± 0.005 | 0.04 ± 0.01 | 0.018 ± 0.001 |
| kinact/Ki (min−1 µM−1) | 0.008 | 0.006 | 0.005 | 0.073 | 0.029 | 0.108 | 0.008 | 0.015 |
| Flavonoid | Heme Concentration (%) | Heme Concentration with the Addition of SOD and CAT |
|---|---|---|
| acacetin | 48.8 ± 0.4 | 63.3 ± 0.5 |
| apigenin | 45.1 ± 1.7 | 55.1 ± 2.9 |
| chrysin | 2.9 ± 0.1 | 5.5 ± 0.5 |
| pinocembrin | 25.3 ± 0.4 | 35.3 ± 1.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondža, M.; Bojić, M.; Tomić, I.; Maleš, Ž.; Rezić, V.; Ćavar, I. Characterization of the CYP3A4 Enzyme Inhibition Potential of Selected Flavonoids. Molecules 2021, 26, 3018. https://doi.org/10.3390/molecules26103018
Kondža M, Bojić M, Tomić I, Maleš Ž, Rezić V, Ćavar I. Characterization of the CYP3A4 Enzyme Inhibition Potential of Selected Flavonoids. Molecules. 2021; 26(10):3018. https://doi.org/10.3390/molecules26103018
Chicago/Turabian StyleKondža, Martin, Mirza Bojić, Ivona Tomić, Željan Maleš, Valentina Rezić, and Ivan Ćavar. 2021. "Characterization of the CYP3A4 Enzyme Inhibition Potential of Selected Flavonoids" Molecules 26, no. 10: 3018. https://doi.org/10.3390/molecules26103018
APA StyleKondža, M., Bojić, M., Tomić, I., Maleš, Ž., Rezić, V., & Ćavar, I. (2021). Characterization of the CYP3A4 Enzyme Inhibition Potential of Selected Flavonoids. Molecules, 26(10), 3018. https://doi.org/10.3390/molecules26103018









