Characteristics of Dietary Fatty Acids Isolated from Historic Dental Calculus of the 17th- and 18th-Century Inhabitants of the Subcarpathian Region (Poland)
Abstract
1. Introduction
2. Materials and Methods
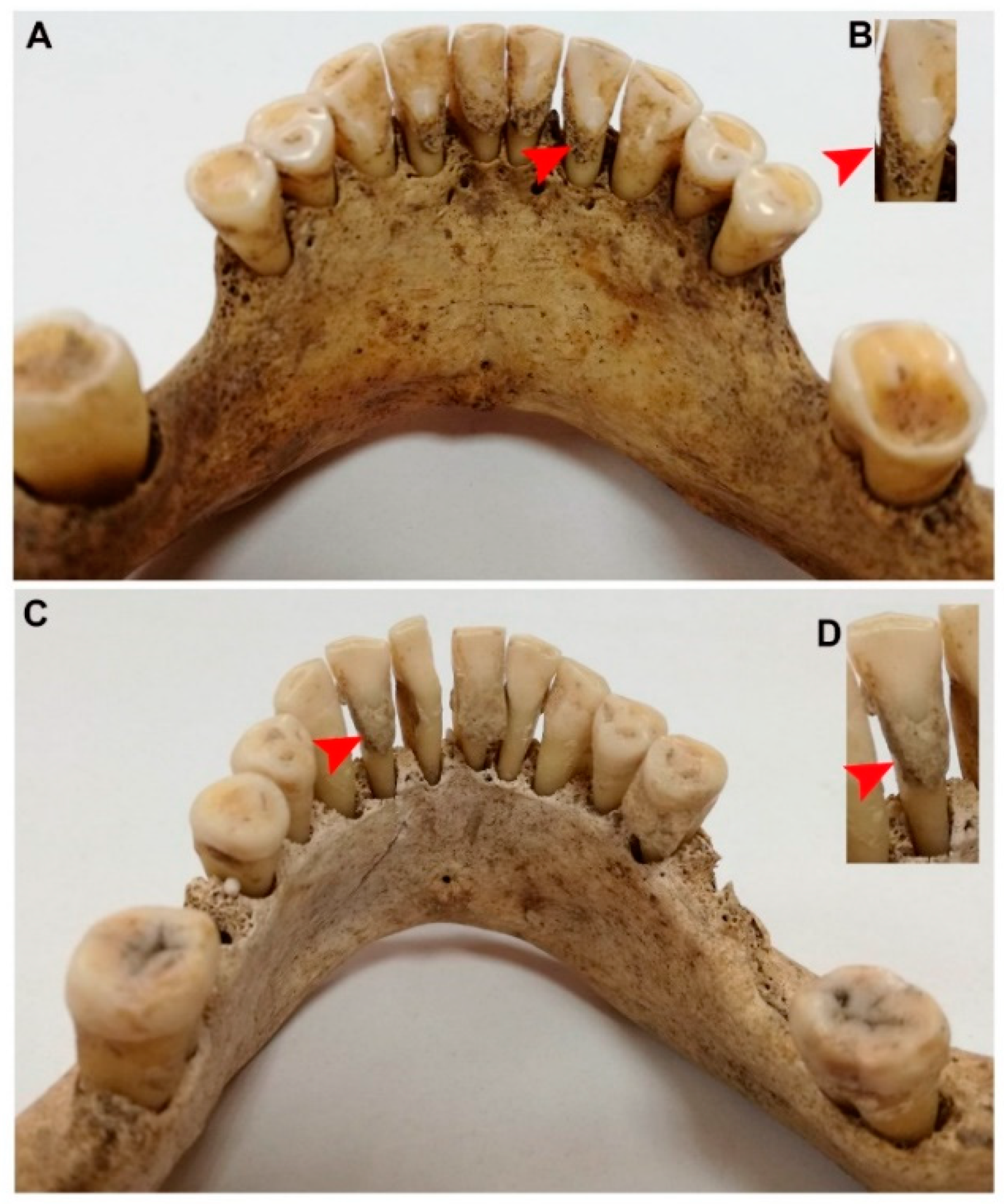
2.1. The Material and Its General Context
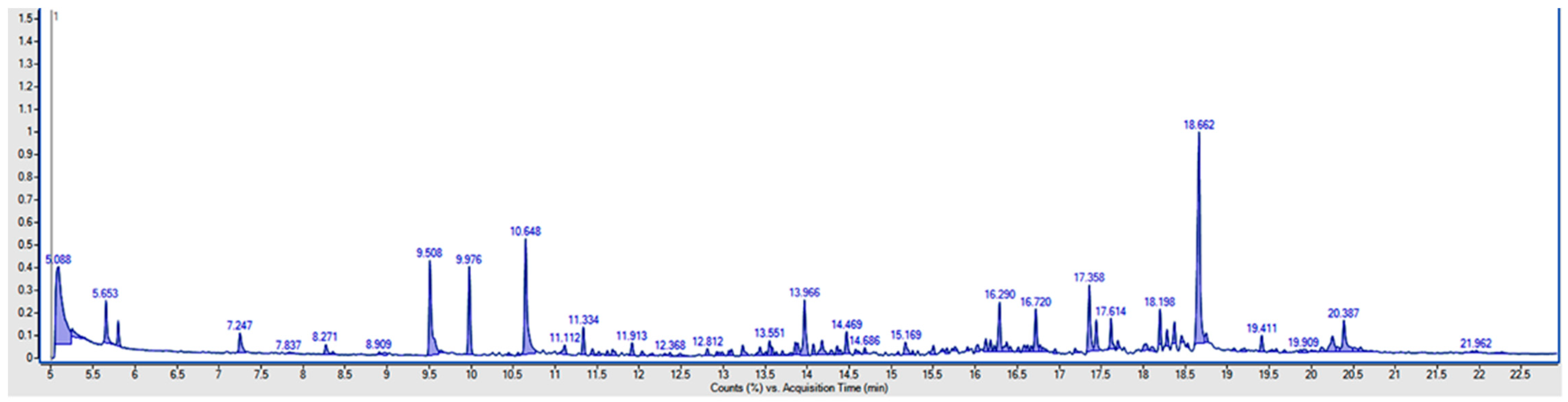
2.2. Analytical Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Velsko, I.M.; Yates, J.A.F.; Aron, F.; Hagan, R.W.; Frantz, L.A.F.; Loe, L.; Martinez, J.B.R.; Chaves, E.; Gosden, C.; Larson, G.; et al. Microbial differences between dental plaque and historic dental calculus are related to oral biofilm maturation stage. Microbiome 2019, 7, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Wade, W. Unculturable bacteria—the uncharacterized organisms that cause oral infections. J. R. Soc. Med. 2002, 95, 81–83. [Google Scholar] [CrossRef]
- Marsh, P.D. Microbiology of dental plaque biofilms and their role in oral health and caries. Dent. Clin. N. Am. 2010, 54, 441–454. [Google Scholar] [CrossRef]
- Marsh, P.D. Dental plaque as a biofilm and a microbial community—Implications for health and disease. BMC Oral Health 2006, 6, S14. [Google Scholar] [CrossRef]
- Adler, C.J.; Dobney, K.; Weyrich, L.S.; Kaidonis, J.; Walker, A.W.; Haak, W.; A Bradshaw, C.J.; Townsend, G.; Sołtysiak, A.; Alt, K.W.; et al. Sequencing ancient calcified dental plaque shows changes in oral microbiota with dietary shifts of the Neolithic and Industrial revolutions. Nat. Genet. 2013, 45, 450–455. [Google Scholar] [CrossRef]
- Buckley, S.; Usai, D.; Jakob, T.; Radini, A.; Hardy, K. Dental Calculus Reveals Unique Insights into Food Items, Cooking and Plant Processing in Prehistoric Central Sudan. PLoS ONE 2014, 9, e100808. [Google Scholar] [CrossRef] [PubMed]
- Warinner, C.; Hendy, J.; Speller, C.; Cappellini, E.; Fischer, R.; Trachsel, C.; Arneborg, J.; Lynnerup, N.; Craig, O.E.; Swallow, D.M.; et al. Direct evidence of milk consumption from ancient human dental calculus. Sci. Rep. 2015, 4, 7104. [Google Scholar] [CrossRef]
- Hendy, J.; Warinner, C.; Bouwman, A.; Collins, M.J.; Fiddyment, S.; Fischer, R.; Hagan, R.; Hofman, C.A.; Holst, M.; Chaves, E.; et al. Proteomic evidence of dietary sources in ancient dental calculus. Proc. R. Soc. B Boil. Sci. 2018, 285, 20180977. [Google Scholar] [CrossRef] [PubMed]
- Mann, A.E.; Sabin, S.; Ziesemer, K.; Vågene, Å.J.; Schroeder, H.; Ozga, A.T.; Sankaranarayanan, K.; Hofman, C.A.; Yates, J.A.F.; Salazar-García, D.C.; et al. Differential preservation of endogenous human and microbial DNA in dental calculus and dentin. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Blatt, S.H.; Redmond, B.G.; Cassman, V.; Sciulli, P.W. Dirty teeth and ancient trade: Evidence of cotton fibres in human dental calculus from Late Woodland, Ohio. Int. J. Osteoarchaeol. 2010, 21, 669–678. [Google Scholar] [CrossRef]
- Hardy, K.; Buckley, S.; Collins, M.J.; Estalrrich, A.; Brothwell, D.; Copeland, L.; García-Tabernero, A.; García-Vargas, S.; De La Rasilla, M.; Lalueza-Fox, C.; et al. Neanderthal medics? Evidence for food, cooking, and medicinal plants entrapped in dental calculus. Naturwissenschaften 2012, 99, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Warinner, C.; Rodrigues, J.F.M.; Vyas, R.; Trachsel, C.; Shved, N.; Grossmann, J.; Radini, A.; Hancock, Y.; Tito, R.Y.; Fiddyment, S.; et al. Pathogens and host immunity in the ancient human oral cavity. Nat. Genet. 2014, 46, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Radini, A.; Buckley, S.; Rosas, A.; Estalrrich, A.; De La Rasilla, M.; Hardy, K. Neanderthals, trees and dental calculus: New evidence from El Sidrón. Antiquity 2016, 90, 290–301. [Google Scholar] [CrossRef]
- Velsko, I.M.; Overmyer, K.A.; Speller, C.; Klaus, L.; Collins, M.J.; Loe, L.; Frantz, L.A.F.; Sankaranarayanan, K.; Lewis, C.M.; Martinez, J.B.R.; et al. The dental calculus metabolome in modern and historic samples. Metabolomics 2017, 13, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Rogóż, J. Cmentarz-grób-szkielet. Badania antropologiczne szczątków kostnych dawnych mieszkańców Rzeszowa. In Ulica 3 Maja w Rzeszowie: Archeologia, Historia, Archeometria, Kafle Piecowe, Antropologia, Radiologia, Odontologia, Katalog; Rogóż, J., Mącik, H., Eds.; Wydawnictwo Uniwersytetu Rzeszowskiego; Fundacja Rzeszowskiego Ośrodka Archeologicznego, Oficyna Wydawnicza Zimowit: Rzeszów, Poland, 2019; pp. 165–206. ISBN 978-83-7996-686-8. [Google Scholar]
- Kocańda, P.; Ocadryga-Tokarczyk, E.; Tokarczyk, T.; Ur, R.I.A.; Archeologicznego, P.F.R.O. Wyniki badań archeologicznych prowadzonych w 2017 roku na ulicy 3 Maja w Rzeszowie, stanowisko 17. Mater. i Spraw. Rzesz. Ośrodka Archeol. 39, 149–162. [CrossRef]
- Kocańda, P.; Ocadryga-Tokarczyk, E. Dzieje Ulicy 3 Maja w Rzeszowie w Świetle Badań Archeologicznych. In Ulica 3 Maja w Rzeszowie: Archeologia, Historia, Archeometria, Kafle Piecowe, Antropologia, Radiologia, Odontologia, Katalog; Rogóż, J., Mącik, H., Eds.; Wydawnictwo Uniwersytetu Rzeszowskiego; Fundacja Rzeszowskiego Ośrodka Archeologicznego, Oficyna Wydawnicza Zimowit: Rzeszów, Poland, 2019; pp. 11–66. ISBN 978-83-7996-686-8. [Google Scholar]
- Rogóż, J.; Ur, R.I.A. Nowożytne cmentarzysko z Placu Farnego oraz pochówki przy kościele Świętego Krzyża w Rzeszowie-wstępne informacje z badań antropologicznych. Mater. i Spraw. Rzesz. Ośrodka Archeol. 2018, 39, 163–183. [Google Scholar] [CrossRef]
- Noonan, M.J.; Tinnesand, H.V.; Buesching, C.D. Normalizing Gas-Chromatography-Mass Spectrometry Data: Method Choice can Alter Biological Inference. BioEssays 2018, 40, e1700210. [Google Scholar] [CrossRef]
- Salimon, J.; Omar, T.A.; Salih, N. Comparison of Two Derivatization Methods for the Analysis of Fatty Acids and Trans Fatty Acids in Bakery Products Using Gas Chromatography. Sci. World J. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Akoh, C.C.; Min, D.B. (Eds.) Food Lipids: Chemistry, Nutrition, and Biotechnology, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2008; ISBN 978-0-429-11032-0. [Google Scholar]
- Månsson, H.L. Fatty acids in bovine milk fat. Food Nutr. Res. 2008, 52, 1821. [Google Scholar] [CrossRef]
- Beare-Rogers, J.L.; Dieffenbacher, A.; Holm, J.V. Lexicon of lipid nutrition (IUPAC Technical Report). Pure Appl. Chem. 2001, 73, 685–744. [Google Scholar] [CrossRef]
- Smedman, A.E.; Gustafsson, I.-B.; Berglund, L.; Vessby, B.O. Pentadecanoic acid in serum as a marker for intake of milk fat: Relations between intake of milk fat and metabolic risk factors. Am. J. Clin. Nutr. 1999, 69, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Akoh, C.C. (Ed.) Food Lipids; CRC Press: Boca Raton, FL, USA, 2017; ISBN 978-1-4987-4487-4. [Google Scholar]
- Chow, C.K. (Ed.) Fatty Acids in Foods and Their Health Implications; CRC Press: Boca Raton, FL, USA, 2007; ISBN 978-0-429-12755-7. [Google Scholar]
- Gismondi, A.; Baldoni, M.; Gnes, M.; Scorrano, G.; D’Agostino, A.; Di Marco, G.; Calabria, G.; Petrucci, M.; Müldner, G.; Von Tersch, M.; et al. A multidisciplinary approach for investigating dietary and medicinal habits of the Medieval population of Santa Severa (7th–15th centuries, Rome, Italy). PLoS ONE 2020, 15, e0227433. [Google Scholar] [CrossRef] [PubMed]
- Mennel, S. All Manners of Food; Basil Blackwell, Inc.: New York, NY, USA, 1985. [Google Scholar]
- Tannahill, R. Food in History; Crown Publishers, Inc.: New York, NY, USA, 1989. [Google Scholar]
| Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 | Sample 6 | Sample 7 | |
|---|---|---|---|---|---|---|---|
| Sex | F | F | F | M | F | M | M |
| Age | maturus | maturus–senilis | adultus | maturus | adultus | adultus | maturus |
| Grave no. | 2 ind. 2 | 3 | 12 | 13 | 23 | 35 ind. 1 | 1, HC |
| Fatty Acid % | |||||||
| Tetradecanoic acid | 19.7 | 13.0 | − | − | 6.6 | 10.3 | − |
| Pentadecanoic acid | 24.2 | 13.0 | 23.5 | 11.1 | 12.3 | 31.1 | 11.6 |
| Hexadecanoic acid | 48.2 | 56.6 | 53.0 | 61.1 | 65.9 | 37.9 | 58.1 |
| Octadecanoic acid | 7.9 | 17.4 | 23.5 | 27.8 | 15.2 | 20.7 | 30.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogóż, J.; Podbielska, M.; Szpyrka, E.; Wnuk, M. Characteristics of Dietary Fatty Acids Isolated from Historic Dental Calculus of the 17th- and 18th-Century Inhabitants of the Subcarpathian Region (Poland). Molecules 2021, 26, 2951. https://doi.org/10.3390/molecules26102951
Rogóż J, Podbielska M, Szpyrka E, Wnuk M. Characteristics of Dietary Fatty Acids Isolated from Historic Dental Calculus of the 17th- and 18th-Century Inhabitants of the Subcarpathian Region (Poland). Molecules. 2021; 26(10):2951. https://doi.org/10.3390/molecules26102951
Chicago/Turabian StyleRogóż, Joanna, Magdalena Podbielska, Ewa Szpyrka, and Maciej Wnuk. 2021. "Characteristics of Dietary Fatty Acids Isolated from Historic Dental Calculus of the 17th- and 18th-Century Inhabitants of the Subcarpathian Region (Poland)" Molecules 26, no. 10: 2951. https://doi.org/10.3390/molecules26102951
APA StyleRogóż, J., Podbielska, M., Szpyrka, E., & Wnuk, M. (2021). Characteristics of Dietary Fatty Acids Isolated from Historic Dental Calculus of the 17th- and 18th-Century Inhabitants of the Subcarpathian Region (Poland). Molecules, 26(10), 2951. https://doi.org/10.3390/molecules26102951








