Sambucus Nigra Extracts–Natural Antioxidants and Antimicrobial Compounds
Abstract
1. Introduction
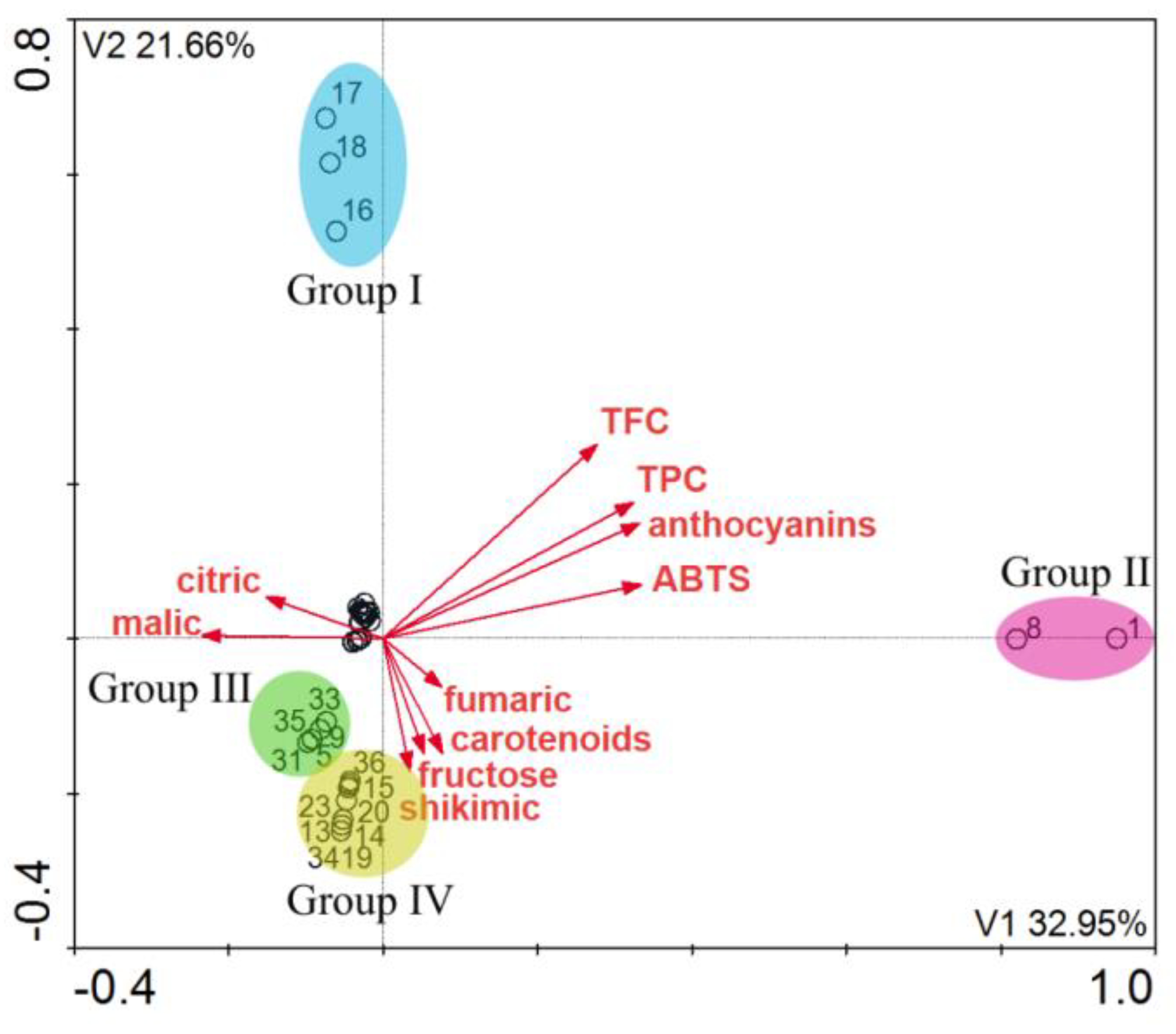
2. Results and Discussion
3. Materials and Methods
3.1. Research Material
3.2. Extraction Procedure
Chromatographic Analysis
3.3. Total Phenolic Content (TPC)
3.4. Total Chlorophyll Content
3.5. Total Anthocyanin Content (TAC)
3.6. Total Carotenoid Content
3.7. Sugar Content
3.8. MIC Measurement
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Charlebois, D.; Byers, P.L.; Finn, C.E.; Thomas, A.L. Elderberry: Botany, horticulture, potential. In Horticultural Reviews; Janick, J., Ed.; Wiley & Sons: Hoboken, NJ, USA, 2010; Volume 37, pp. 213–280. [Google Scholar]
- Fazio, A.; Plastina, P.; Meijerink, J.; Witkamp, R.F.; Gabriele, B. Comparative analyses of seeds of wild fruits of Rubus and Sambucus species from southern Italy: Fatty acid composition of the oil, total phenolic content, antioxidant and anti-inflammatory properties of the methanolic extracts. Food Chem. 2013, 140, 817–824. [Google Scholar] [CrossRef]
- Kaack, K.; Austed, T. Interaction of vitamin C and flavonoids in elderberry (Sambucus nigra L.) during juice processing. Plant Foods Hum. Nutr. 1998, 52, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.P.; Kaack, K.; Fretté, X.C. Selection of elderberry (Sambucus nigra L.) genotypes best suited for the preparation of elderflower extracts rich in flavonoids and phenolic acids. Eur. Food Res. Technol. 2008, 227, 293–305. [Google Scholar] [CrossRef]
- Salvador, Â.C.; Silvestre, A.J.; Rocha, S.M. Unveiling elderflowers (Sambucus nigra L.) volatile terpenic and norisoprenoids profile: Effects of different postharvest conditions. Food Chem. 2017, 229, 276–285. [Google Scholar] [CrossRef]
- Schmitzer, V.; Veberic, R.; Slatnar, A.; Stampar, F. Elderberry (Sambucus nigra L.) wine: A product rich in health promoting compounds. J. Agric. Food Chem. 2010, 58, 10143–10146. [Google Scholar] [CrossRef]
- Nurzyńska-Wierdak, R. Właściwości lecznicze i wykorzystanie w fizjoterapii niektórych gatunków roślin drzewiastych. Krzewy półkuli północnej. Ann. UMCS s EEE Hortic. 2016, 26, 27–46. (In Polish) [Google Scholar]
- Wierzbicki, A. Dziki bez czarny—Pozyskiwanie surowca i jego zastosowanie. Wiad. Zielar. 2002, 4, 8–10. (In Polish) [Google Scholar]
- Dyduch, J. Bez czarny—Charakterystyka biologiczna, wykorzystanie w ziołolecznictwie, kosmetyce i gospodarstwach domowych. Cz. I. Epistem. 2014, 25, 21–27. (In Polish) [Google Scholar]
- Ho, G.T.; Kase, E.T.; Wangensteen, H.; Barsett, H. Phenolic Elderberry Extracts, Anthocyanins, Procyanidins, and Metabolites Influence Glucose and Fatty Acid Uptake in Human Skeletal Muscle Cells. J. Agric. Food Chem. 2017, 65, 2677–2685. [Google Scholar] [CrossRef]
- Veberic, R.; Jakopic, J.; Stampar, F.; Schmitzer, V. European elderberry (Sambucus nigra L.) rich in sugars, organic acids, anthocyanins and selected polyphenols. Food Chem. 2009, 114, 511–515. [Google Scholar] [CrossRef]
- Akbulut, M.; Ercisli, S.; Tosun, M. Physico-chemical characteristics of some wild grown European elderberry (Sambucus nigra L.) genotypes. Pharmacogn. Mag. 2009, 5, 320–323. [Google Scholar]
- Kislichenko, V.S.; Vel’ma, V.V. Amino-acid composition of flowers, leaves and extract of Sambucus nigra flowers. Chem. Nat. Comp. 2006, 42, 125–126. [Google Scholar] [CrossRef]
- Gleńsk, M.; Gleńsk, M.; Gliński, J.A.; Włodarczyk, M. Determination of ursolic and oleanolic acid in Sambuci fructus. Chem. Biodivers. 2014, 11, 1939–1944. [Google Scholar] [CrossRef] [PubMed]
- Dulf, F.V.; Oroian, I.; Vodnar, D.C.; Socaciu, C.; Pintea, A. Lipid classes and fatty acid regiodistribution in triacylglycerols of seed oils of two Sambucus species (S. nigra L.; And, S. ebulus L.). Molecules 2013, 18, 11768–11782. [Google Scholar] [CrossRef] [PubMed]
- Viapiana, A.; Wesolowski, M. The Phenolic Contents and Antioxidant Activities of Infusions of Sambucus nigra L. Plant Foods Hum. Nutr. 2017, 72, 82–87. [Google Scholar] [CrossRef]
- Gleńsk, M.; Czapińska, E.; Woźniak, M.; Ceremuga, I.; Włodarczyk, M.; Terlecki, P.; Ziółkowski, P.; Seweryn, E. Triterpenoid acids as important antiproliferative constituents of European elderberry fruits. Nutr. Cancer 2017, 69, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Salvador ÂCRocha, S.M.; Silvestre, A.J.D. Lipophilic phytochemicals from elderberries (Sambucus nigra L.): Influence of ripening, cultivar and season. Ind. Crops Prod. 2015, 71, 15–23. [Google Scholar] [CrossRef]
- Kołodziej, B.; Maksymiec, N.; Drożdzal, K.; Antonkiewicz, J. Effect of traffic pollution on chemical composition of raw elderberry (Sambucus nigra L.). J. Elementol. 2012, 17, 67–78. [Google Scholar] [CrossRef]
- Nawirska-Olszańska, A. Roślina nie bez potencjału. Czarny bez—Chwast, a może jednak roślina lecznicza? Kierunek Spoż 2016, 2, 49–51. [Google Scholar]
- Liszka, K.D.; Najgebauer-Lejko, A.; Tabaszewska, M. Owoce czarnego bzu (Sambucus nigra L.)—Charakterystyka i możliwości wykorzystania w przemyśle spożywczym. [w:] Innowacyjne rozwiązania w technologii żywności i żywieniu człowieka. Tarko T, Drożdż I, Najgebauer-Lejko D, Duda Chodak A (red). PTTŻ Kraków 2016, 1, 102–109. (In Polish) [Google Scholar]
- Czech, A.; Rusinek, E.; Merska, M. Zawartość wybranych biopierwiastków w owocach i sokach owoców jagodowych. Probl. Hig. Epidemiol. 2011, 92, 836–839. (In Polish) [Google Scholar]
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Staniek, H.; Kidoń, M.; Łysiak, G.P. The content of selected minerals, bioactive compounds, and the antioxidant properties of the flowers and fruit of selected cultivars and wildly growing plants of Sambucus nigra L. Molecules 2020, 25, 876. [Google Scholar] [CrossRef] [PubMed]
- Assessment Report on Sambucus nigra L., Fructus; EMA/HMPC/44208/2012; EMA: London, UK, 2013.
- Brønnum–Hansen, K.; Hansen, S.H. High performance liquid chromatographic separation of anthocyanins of Sambucus nigra L. J. Chromatogr. 1983, 262, 385. [Google Scholar] [CrossRef]
- Wu, X.; Gu, L.; Prior, R.L.; McKay, S. Characterization of anthocyanins and proanthocyanidins in some cultivars of Ribes, Aronia, and Sambucus and their antioxidant capacity. J. Agric. Food Chem. 2014, 52, 7846. [Google Scholar] [CrossRef] [PubMed]
- Diviš, P.; Pořízka, J.; Vespalcová, M.; Matějíček, A.; Kaplan, J. Elemental composition of fruits from different Black elder (Sambucus nigra L.) culti-vars grown in the Czech Republic. J. Elem. 2015, 20, 549–557. [Google Scholar] [CrossRef]
- Turner, N.J.; Łuczaj, Ł.J.; Migliorini, P.; Pieroni, A.; Dreon, A.L.; Sacchetti, L.E. Edible and tended wild plants, traditional ecological knowledge and agroecology. Criti. Rev. Plant Sci. 2011, 30, 198–225. [Google Scholar] [CrossRef]
- Svanberg, I.; Łuczaj, Ł.; Pardo-de-Santayana, M.; Pieroni, A. History and current trends of ethnobiological research in Europe. In Ethnobiology; Anderson, E.N., Adams, K., Pearsall, D., Hunn, E., Turner, N., Eds.; Wiley-Blackwell: New York, NY, USA, 2011; pp. 189–212. [Google Scholar]
- Pawera, L.; Khomsan, A.; Zuhud, E.A.M.; Hunter, D.; Ickowitz, A.; Polesny, Z. Wild food plants and trends in their use: From knowledge and perceptions to drivers of change in West Sumatra, Indonesia. Foods 2011, 9, 1240. [Google Scholar] [CrossRef]
- Łuczaj, Ł.; Pieroni, A.; Tardío, J.; Pardo-De-Santayana, M.; Soukand, R.; Svanberg, I.; Kalle, R. Wild food plant use in 21st century Europe, the disapperance of old traditions and the search for new ciusines involving wild edibles. Acta Soc. Bot. Pol. 2012, 81, 359–370. [Google Scholar] [CrossRef]
- Hearst, C.; McCollum, G.; Nelson, D.; Ballard, L.M.; Millar, B.C.; Goldsmith, C.E.; Rooney, P.J.; Loughrey, A.; Moore, J.E.; Rao, J.R. Antibacterial activity of elder (Sambucus nigra L.) flower or berry against hospital pathogens. J. Med. Plant Res. 2010, 4, 1805–1809. [Google Scholar]
- Krawitz, C.; Mraheil, M.A.; Stein, M.; Imirzalioglu, C.; Domann, E.; Pleschka, S.; Hain, T. Inhibitory activity of a standardized elderberry liquid extract against clini-cally-relevant human respiratory bacterial pathogens and influenza A and B viruses. BMC Complement Altern. Med. 2011, 11, 16. [Google Scholar] [CrossRef]
- Galić, J.; Dragowić-Uzlec, V.; Levaj, B.; Bursać Kovecevic, V.; Plestić, S.; Arnautović, S. The Polyphenols Stability, Enzyme Activity and Physico-Chemical Parameters During Producing Wild Elderberry Concentrated Juice. Agri. Consp. Scient. 2019, 74, 181–186. [Google Scholar]
- Kozos, K.; Ochmian, I. Porównanie jakości kilku gatunków owoców o ciemnym zabarwieniu skórki uprawnych i pozyskanych ze stanowisk naturalnych. [w]: Badania i Rozwój Młodych Naukowców w Polsce. Nyćkowiak, J., Leśny J (red). Młodzi. Nauk. Poznań. 2015, 1, 75–81. (In Polish) [Google Scholar]
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Łysiak, G.P. Bioactive properties of Sambucus nigra L. as a functional ingredient for food and pharmaceutical industry. J. Funct. Foods 2018, 40, 377–390. [Google Scholar] [CrossRef]
- Ochmian, I.; Oszmianski, J.; Skupien, K. Chemical composition, phenolics, and firmness of small black fruits. Appl. Bot. Food Qual. 2009, 83, 64–69. [Google Scholar]
- Mudge, E.; Mudge, E.; Applequist, W.L.; Lister, P.F.; Townesmith, A.K.; Walker, K.M.; Brown, P.N. Variation of select flavonols and chlorogenic acid content of elderberry collected throughout the Eastern United States. J. Food Compost. Anal. 2016, 47, 52–59. [Google Scholar] [CrossRef]
- Leja, M.; Mareczek, A.; Nanaszko, B. Antyoksydacyjne właściwości owoców wybranych gatunków dziko rosnących drzew i krzewów. Rocz. AR. Pozn. 2007, 383, 327–331. (In Polish) [Google Scholar]
- Anton, A.M.; Pintea, A.M.; Rugină, D.O.; Sconţa, Z.M.; Hanganu, D.; Vlase, L.; Benedec, D. Preliminary studies on the chemical characterization an antioxidant capacity of polyphenols from Sambucus sp. Wstępne badania nad charakterystyką chemiczną i zdolnością antyoksydacyjną polifenoli firmy Sambucus sp. Dig. J. Nanometer Bios. 2013, 8, 973–980. [Google Scholar]
- Lee, J.; Finn, C.E. Anthocyanins and other polyphenolics in American elderberry (Sambucus canadensis) and European elderberry (S. nigra) cultivars. J. Sci. Food Agric. 2007, 87, 2665–2675. [Google Scholar] [CrossRef] [PubMed]
- Bronnum-Hansen, K.; Jacobsen, F.; Flink, J.M. Anthocyanin colourants from elderbeny (Sumbucus nigra L.). 1. Process considerations for production of liquid extract. J. Fd. Technol. 1985, 20, 703–711. [Google Scholar] [CrossRef]
- Vulić, J.J.; Vračar, L.O.; Šumić, Z.M. Chemical characteristics of cultivated elderberry fruit. Acta Period. Technol. 2008, 39, 85–90. [Google Scholar] [CrossRef]
- Młynarczyk, K.; Walkowiak-Tomczak, D. Wpływ zabiegów technologicznych i pochodzenia surowca na aktywność przeciwutleniającą i właściwości fizyczno-chemiczne soku z bzu czarnego. Nauka Przyr. Technol. 2017, 11, 385–395. (In Polish) [Google Scholar]
- Roschek, B.; Fink, R.C.; McMichael, M.D.; Li, D.; Alberte, R.S. Elderberry flavonoids bind to and prevent H1N1 infection in vitro. Phytochemistry 2009, 70, 1255–1261. [Google Scholar] [CrossRef]
- Piątkowska, E.; Witkowicz, R.; Pisulewska, E. Antocyjany—Charakterystyka, występowanie i oddziaływanie na organizm człowieka. Żywn. Nauka. Technol. Jakość. 2011, 4, 24–35. (In Polish) [Google Scholar]
- Bagchi, D.; Roy, S.; Patel, V. Safety and whole-body antioxidant potential of a novel anthocyanin-rich formulation of edible berries. Mol. Cell Biochem. 2006, 281, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Zielińska-Pisklak, M. Bez czarny (Sambucus nigra) domowy sposób nie tylko na grypę i przeziębienie. Lek. W. Polsce. 2013, 23, 48–54. (In Polish) [Google Scholar]
- Senica, M.; Stampar, F.; Mikulic-Petkovsek, M. Harmful (cyanogenic Glycoside) and Beneficial (phenolic) Compounds in Different Sambucus Species. J. Berry Res. 2019, 9, 395–409. [Google Scholar] [CrossRef]
- Appenteng, M.K.; Krueger, R.; Johnson, M.C.; Ingold, H.; Bell, R.; Thomas, A.L.; Greenlief, C.M. Cyanogenic glycoside analysis in american elderberry. Molecules 2021, 26, 1384. [Google Scholar] [CrossRef] [PubMed]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Ou, S.; Kwok, K. Ferulic acid: Pharmaceutical functions, preparation and applications in foods. J. Sci. Food Agric. 2004, 84, 1261–1269. [Google Scholar] [CrossRef]
- Papadopoulou, C.; Soulti, K.; Roussis, J.G. Potential Antimicrobial activity of red and white wine phenolic extracts against strains of Staphylococcus aureus, Escherichia coli and Candida albicans. Food Technol. Biotechnol. 2005, 43, 41–46. [Google Scholar]
- Przybylska-Balcerek, A.; Góral, T.; Kurasiak-Popowska, D.; Stuper-Szablewska, K. Phenolic acids in various genotypes of cereals grown in Poland in the years 2017–2018. Acad. J. Med. Plants 2019, 7, 092–099. [Google Scholar]
- Ozcan, T.A.; Akpinar-Bayizit, A.; Yilmaz-Ersan, L.; Delikanli, B. Phenolics in Human Health. Int. J. Chem. Eng. Appl. 2014, 5, 393–396. [Google Scholar] [CrossRef]
- Czechowska, S.K.; Markiewicz, R.; Borawska, M.H. Microbiological activity and cytotoxicity of selected phenolic acids in in vitro tests. Bromat. Chem. Toksykol. 1992, 3, 959–964. [Google Scholar]
- Nowak, H.; Kujawa, R.; Zadernowski, B.; Roczniak Kozłowska, H. Antioxidative and bactericidal properties of phenolic compounds in rapeseeds. Lipid/Fett. 1992, 94, 149–152. [Google Scholar] [CrossRef]
- Martins, N.; Barros, L.; Henriques, M.; Silva, S.; Ferreira, I.C.F.R. Activity of phenolic compounds from plant origin against Candida species. Ind. Crops Prod. 2015, 74, 648–670. [Google Scholar] [CrossRef]
- Haslam, E.; Lilley, T.H.; Warminski, E.; Liao, H.; Cai, Y.; Martin, R.; Goudling, P.N.; Luck, G. Polyphenol complexation. A study in molecular recognition. ACS Symp. Ser. 1992, 506, 8–50. [Google Scholar]
- Stachelska, M.A.; Jakubczyk, A.; Więtczak, B.; Tyl, S. Assessment of Yersinia Enterocolitica sensitivity to selected phenolic acids. Food Sci. Technol. Qual. 2012, 2, 88–98. [Google Scholar]
- Parus, A. Przeciwutleniające i farmakologiczne właściwości kwasów fenolowych. Post. Fitoter. 2013, 1, 48–53. [Google Scholar]
- Kędzia, B.; Hołderna-Kędzia, E. Toxicity and allergenic action of propolis. Adv. Phytother. 2001, 4, 282–291. [Google Scholar]
- Efenberger-Szmechtyk, M.; Nowak, A.; Czyżowska, A. Polifenole liści aroni jako naturalne związki przeciwdrobnoustrojowe. XII Konf. Nauk. cyklu Żywność XXI wieku Kraków wrzesień. 2016, 200, 22–23. (In Polish) [Google Scholar]
- Vieira, V.; Pires, T.C.S.P.; Calhelha, R.C.; Alves, M.J.; Ferreira, O.; Barros, L.; Ferreira, I.C.F.R. Phenolic profile, antioxidant and antibacterial properties of Juglans regia L. (walnut) leaves from the Northeast of Portugal. Ind. Crops.Prod. 2019, 134, 347–355. [Google Scholar] [CrossRef]
- Pernin, A.; Dubois-Brissonnet, F.; Roux, S.; Masson, M.; Bosc, V.; Noëlle Maillard, M. Phenolic compounds can delaythe oxidation of polyunsaturated fatty acids and the growth of Listeria monocytogenes: Structure-activityrelationships. J. Sci. Food Agric. 2018, 98, 5401–5408. [Google Scholar] [CrossRef] [PubMed]
- Camargo, A.C.; Bismara Regitano-d’Arce, M.A.; Rasera, G.B.; Canniatti-Brazaca, S.G.; Prado-Silva, L.; Alvarenga, V.O.; Sant’Ana, A.S.; Shahidi, F. Phenolic acids and flavonoids of peanut by-products: Antioxidant capacity andantimicrobial effects. Food Chem. 2017, 237, 538–544. [Google Scholar] [CrossRef]
- Ali, R.M.; Houghton, P.J.; Raman, A.; Hoult, J.R.S. Antimicrobial and anti-inflammatory activities of extracts and constituents of Oroxylum indicum. Phytomedicine. 1998, 5, 375–381. [Google Scholar]
- Malterund, K.; Bremns, E.; Faegri, A.; Moe, T.; Sandanger, E. Flavonoids from the wood of Salix caprea as inhibitors of wood–destroying fungi. J. Nat. Prod. 1985, 48, 559–563. [Google Scholar] [CrossRef]
- Iwagawa, T.; Kawasaki, J.; Hase, T.; Sako, S.; Ōkubo, T.; Ishida, M.; Kim, M. An acetylated flavonol glycoside from Lasiobema japonica. Phytochemistry 1990, 29, 1013–1014. [Google Scholar] [CrossRef]
- Basile, A.; Giordano, S.; López-Sáez, J.A.; Cobianchi, R.C. Antibacterial activity of pure flavonoids isolated from mosses. Phytochemistry 1999, 52, 1479–1482. [Google Scholar] [CrossRef]
- Oksus, S.; Ayyildiz, H.; Johansson, C. 6-methoxylated and C-glycosyl flavonoids from Centaurea species. J. Nat. Prod. 1985, 47, 902–903. [Google Scholar] [CrossRef] [PubMed]
- Waage, S.K.; Hedin, P.A. Quercetin 3-O-galactosyl-(1Æ6)-glucoside, a compound from narrowleaf vetch with antibacterial activity. Phytochemistry 1985, 24, 243–245. [Google Scholar] [CrossRef]
- Liu, H.; Orjala, J.; Sticher, O.; Rali, T. Acylated flavonol glycosides from leaves of Stenochlaena palustris. J. Nat. Prod. 1999, 62, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Mitrokotsa, D.; Demetzos, C.; Harvala, C.; Mentis, A.; Perez, S.; Kokkinopoulo, D. Bioactive compounds from the buds of Plantanus orientalis and isolation of a new kaempferol glycoside. Planta. Med. 1993, 59, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hamburger, M.; Gueho, J.; Hostettmann, K. Antimicrobial flavonoids from Psiadia trinervia and their methylated and acetylated derivatives. Phytochemistry 1989, 28, 2323–2327. [Google Scholar] [CrossRef]
- Van Puyvelde, L.; De Kimpe, N.; Costa, J.; Munyjabo, V.; Nyirankuliza, S.; Hakizamungu, E.; Schamp, N. Isolation of flavonoids and chalcone from Helichrysum odoratissimum and synthesis of helichrysetin. J. Nat. Prod. 1989, 52, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P. Factors Influencing the Measurement of Bioavailability, Taking Calcium as a Model. J. Nutr. 2001, 131, 1376–1382. [Google Scholar] [CrossRef] [PubMed]
- Lafay, S.; Gil-Izquierdo, A. Bioavailability of phenolic acids. Phytochem. Rev. 2008, 7, 301–311. [Google Scholar] [CrossRef]
- Falcone Ferreyra, M.L.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Przybylska-Balcerek, A.; Frankowski, J.; Stuper-Szablewska, K. Bioactive compounds in sorghum. Eur. Food Res. Technol. 2019, 245, 1075–1080. [Google Scholar] [CrossRef]
- Stuper-Szablewska, K.; Kurasiak-Popowska, D.; Nawracała, J. Quantitative profile of phenolic acids and antioxidant activity of wheat grain exposed to stress. Eur. Food Res. Technol. 2019, 245, 1595–1603. [Google Scholar] [CrossRef]
- Johan, F.; Jafri, M.Z.; Lim, H.S.; Wan Maznah, W.O. Laboratory measurement: Chlorophyll-a concentration measurement with acetone method using spectrophotometer. In Proceedings of the 2014 IEEE International Conference on Industrial Engineering and Engineering Management, Selangor, Malaysia,, 9–12 December 2014; pp. 744–748. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by UV-visible spectroscopy. In Handbook of Food Analytical Chemistry—Pigment, Colorants, Flavors, Texture, and Bio-Active Food Components; Wrolstad, R.E., Ed.; John Wiley and Sons Inc.: New York, NY, USA, 2001; pp. 12–13. [Google Scholar]
- Gozdecka, G.; Kaniewska, J.; Domaradzki, M.; Jędryczka, K. Ocena zawartości wybranych składników bioaktywnych w przetworach z borówki czernicy, Żywność. Nauka Technol. Jakość 2015, 1, 170–180. [Google Scholar]
- Sztangret, J.; Korzeniowska, A.; Niemirowicz-Szczyt, K. Ocena plonowania oraz zawartość suchej masy i związków karotenoidowych w nowych mieszańcach dyni olbrzymiej (Cucurbita maxima Duch). Folia Hort. 2001, 13, 437–443. [Google Scholar]
- PN-EN 12136:2000, β-karotenu wg PN-90/A-75101/12; Fruit and Vegetable Juices-Determination of Total Carotenoid Content and Individual Carotenoid Fractions; Polish Committee for Standardization: Warsaw, Poland, 1 December 2013.
- Šmilauer, P.; Lepš, J. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University: Cambridge, UK, 2003. [Google Scholar]
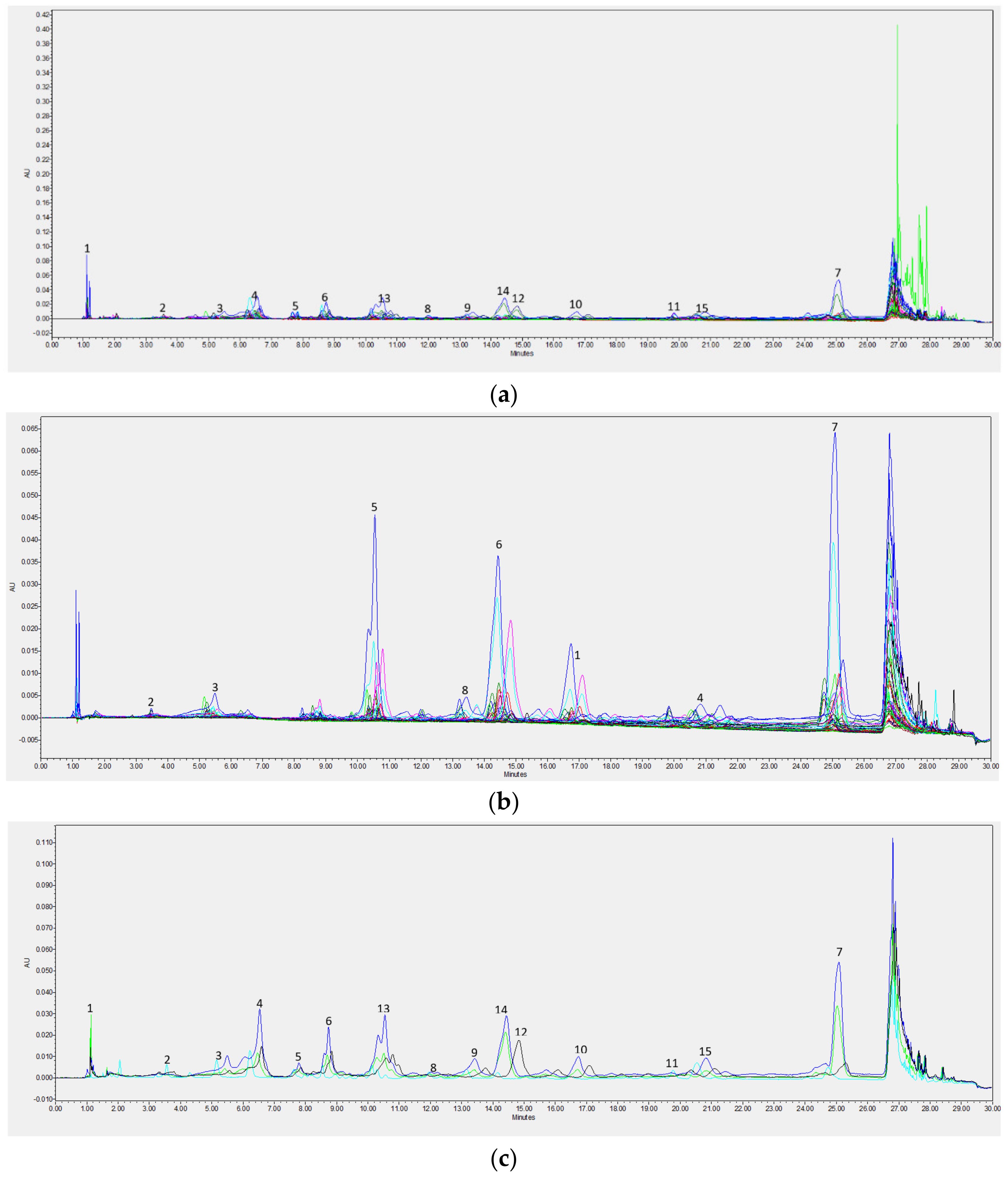
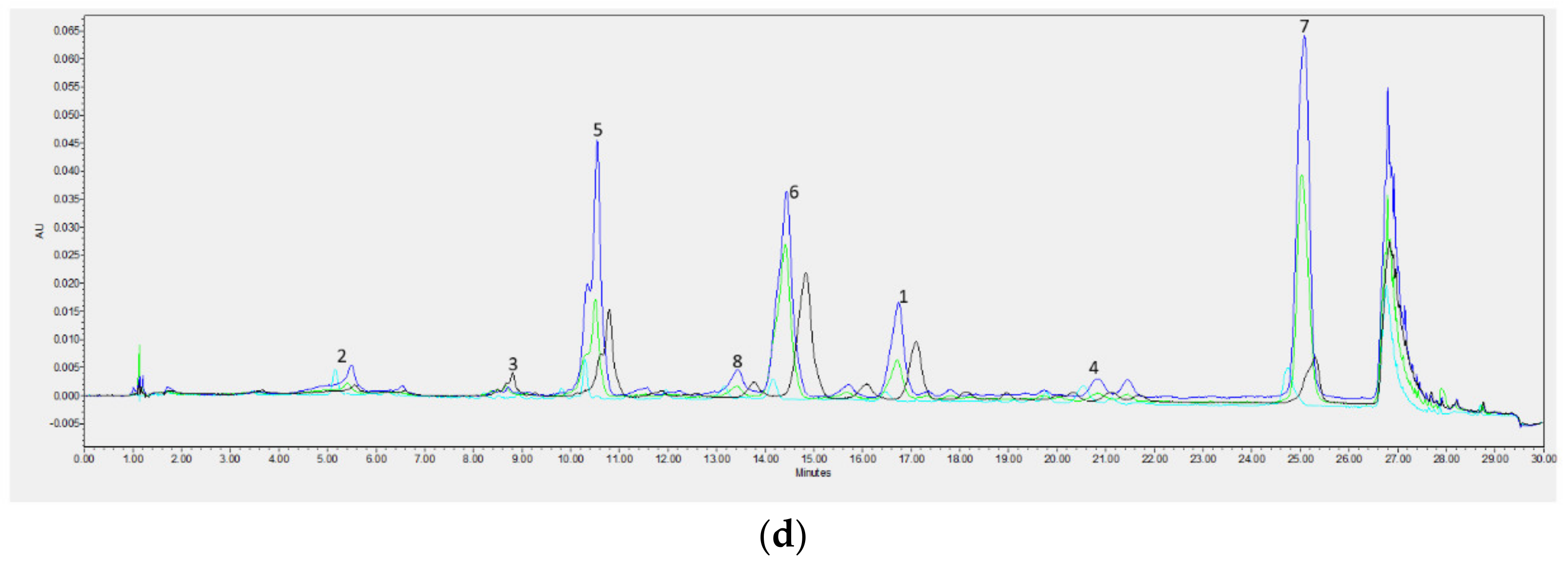


| Min | Max | Mean | SD | |
|---|---|---|---|---|
| ABTS+ (µmol Trolox equivalent/g extract) | 647.21 | 721.25 | 684.23 | 1.09.1975 |
| TPC (mg GAE/g extract) | 3.23 | 18.90 | 13.28 | 4.34 |
| TFC (mg RUTE/g extract) | 11.25 | 263.50 | 114.98 | 64.14 |
| [mg/g Extract] | Min | Max | Mean | SD |
|---|---|---|---|---|
| gallic | 0.34 | 8.32 | 3.34 | 1.82 |
| 4-hydroxybenzoic | 0.19 | 6.21 | 1.36 | 1.21 |
| vanillic | 0.02 | 0.25 | 0.13 | 0.06 |
| syringic | 0.42 | 3.09 | 1.61 | 0.75 |
| vanillin | 0.84 | 6.19 | 3.13 | 1.44 |
| benzoic | 2.09 | 30.96 | 12.36 | 5.98 |
| chlorogenic | 25.50 | 254.07 | 139.09 | 60.93 |
| protocatechuic | 0.24 | 1.61 | 0.89 | 0.38 |
| salicylic | 0.79 | 4.43 | 2.56 | 1.03 |
| caffeic | 0.26 | 5.53 | 2.03 | 1.40 |
| p-cumaric | 0.16 | 1.21 | 0.61 | 0.26 |
| ferulic | 11.20 | 67.09 | 34.63 | 14.84 |
| sinapic | 18.45 | 164.75 | 72.84 | 30.96 |
| t-cinnamic | 12.60 | 90.24 | 51.29 | 21.98 |
| rosmarinic | 0.16 | 2.24 | 0.79 | 0.41 |
| The content of flavonoids in the elderberry extract | ||||
| [mg/g extract] | Min | Max | Mean | SD |
| apigenin | 18.47 | 129.43 | 66.74 | 27.01 |
| catechin | 0.03 | 0.71 | 0.23 | 0.17 |
| kaempferol | 0.28 | 2.50 | 1.01 | 0.61 |
| luteolin | 2.76 | 49.13 | 15.85 | 10.24 |
| naringenin | 8.53 | 65.27 | 30.71 | 12.83 |
| quercetin | 97.38 | 504.88 | 306.60 | 125.02 |
| rutin | 124.98 | 2773.71 | 1105.39 | 604.12 |
| vitexin | 0.13 | 2.98 | 1.35 | 0.90 |
| [mg/g Extract] | Min | Max | Mean | SD |
|---|---|---|---|---|
| citric | 0.76 | 1.24 | 1.03 | 0.14 |
| malic | 0.23 | 0.37 | 0.29 | 0.05 |
| shikimic | 0.03 | 0.25 | 0.14 | 0.06 |
| fumaric | 0.03 | 0.12 | 0.07 | 0.03 |
| glucose | 3.08 | 7.40 | 4.89 | 1.44 |
| fructose | 3.88 | 9.38 | 5.91 | 1.77 |
| [mg/g Extract] | Min | Max | Mean | SD |
|---|---|---|---|---|
| Total carotenoids | 25.50 | 75.00 | 47.93 | 15.92 |
| Total chlorophyll | 0.08 | 0.54 | 0.29 | 0.14 |
| TAC | 75.18 | 149.78 | 109.81 | 22.62 |
| Tested Bacteria | Min | Max | Mean Value | |
|---|---|---|---|---|
| Pathogenic bacteria | E. coli (PCM 2793) | 0.05 | 0.5 | 0.275 |
| S. enteritidis (PCM 2548) | 0.1 | 0.5 | 0.3 | |
| L. inoccua (DSM 20649) | 0.1 | 0.5 | 0.3 | |
| Food-spoilage bacteria | P. fluorescens (PCM 2123) | 0.1 | 0.5 | 0.3 |
| P. fragii (PCM 1856) | 0.05 | 0.5 | 0.275 | |
| P. mirabilis (PCM 1361) | 0.05 | 0.5 | 0.275 | |
| Control bacteria | M. luteus (PCM 525) | 0.05 | 0.5 | 0.275 |
| Axes | 1 | 2 | 3 | 4 | Total Eigenvalues |
|---|---|---|---|---|---|
| Eigenvalue | 0.3279 | 0.2567 | 0.0821 | 0.0458 | 2.115 |
| Length of gradient | 2.896 | 2.156 | 1.391 | 1.097 | |
| Polyphenols and flavonoids– samples correlations | 0.919 | 0.907 | 0.544 | 0.655 | |
| Polyphenols and flavonoids– samples relation | 35.7 | 37.1 | 0.0 | 0.0 |
| Axes | 1 | 2 | 3 | 4 | Total Eigenvalues |
|---|---|---|---|---|---|
| Eigenvalue | 0.3721 | 0.1697 | 0.6512 | 0.0329 | 2.059 |
| Length of gradient | 3.088 | 1.873 | 1.255 | 1.066 | |
| Polyphenols and flavonoids–correlations in samples | 0.951 | 0.742 | 0.421 | 0.235 | |
| Polyphenols and flavonoids–correlations in samples | 41.2 | 25.8 | 0.0 | 0.0 |
| No. | Location | Voivodeship | Mass of Extract [g] |
|---|---|---|---|
| 1 | 51°24′99′′N 21°58′16′′E | Lublin Voivodeship | 0.25 |
| 2 | 52°01′93′′N 17°78′44′′E | Greater Poland Voivodeship | 0.19 |
| 3 | 52°65′67′′N 16°95′29′′E | Greater Poland Voivodeship | 0.65 |
| 4 | 53°31′56′′N 20°67′35′′E | Greater Poland Voivodeship | 0.39 |
| 5 | 52°99′64′′N 18°70′72′′E | Kuyavian-Pomeranian Voivodeship | 0.20 |
| 6 | 49°39′96′′N 22°44′98′′E | Podkarpackie Voivodeship | 0.79 |
| 7 | 49°27′54′′N 19°86′88′′E | Lesser Poland Voivodeship | 0.48 |
| 8 | 51°25′05′′N 22°57′01′′E | Lublin Voivodeship | 0.10 |
| 9 | 51°62′26′′N 17°94′28′′E | Greater Poland Voivodeship | 0.69 |
| 10 | 50°29′68′′N 16°65′20′′E | Lower Silesian Voivodeship | 0.44 |
| 11 | 53°92′82′′N 14°44′89′′E | West Pomeranian Voivodeship | 0.80 |
| 12 | 53°91′31′′N 14°52′00′′E | West Pomeranian Voivodeship | 1.05 |
| 13 | 53°47′30′′N 17°89′64′′E | Kuyavian-Pomeranian Voivodeship | 1.83 |
| 14 | 53°48′46′′N 18°07′17′′E | Kuyavian-Pomeranian Voivodeship | 0.74 |
| 15 | 53°77′66′′N 20°47′65′′E | Warmian-Masurian Voivodeship | 0.61 |
| 16 | 53°39′84′′N 20°94′62′′E | Warmian-Masurian Voivodeship | 0.16 |
| 17 | 53°58′34′′N 20°28′16′′E | Warmian-Masurian Voivodeship | 1.04 |
| 18 | 54°21′38′′N 21°74′16′′E | Warmian-Masurian Voivodeship | 0.28 |
| 19 | 53°81′29′′N 20°35′80′′E | Warmian-Masurian Voivodeship | 0.40 |
| 20 | 53°59′70′′N 19°85′43′′E | Warmian-Masurian Voivodeship | 1.08 |
| 21 | 54°47′25′′N 16°63′07′′E | West Pomeranian Voivodeship | 0.12 |
| 22 | 51°30′05′′N 16°83′01′′E | Lower Silesian Voivodeship | 0.37 |
| 23 | 54°16′88′′N 17°49′22′′E | Pomeranian Voivodeship | 0.50 |
| 24 | 53°26′97′′N 16°46′70′′E | West Pomeranian Voivodeship | 0.16 |
| 25 | 52°39′91′′N 16°71′89′′E | Greater Poland Voivodeship | 1.38 |
| 26 | 52°97′29′′N 16°54′44′′E | Greater Poland Voivodeship | 0.18 |
| 27 | 53°27′61′′N 15°46′33′′E | West Pomeranian Voivodeship | 0.71 |
| 28 | 52°77′12′′N 16°87′97′′E | Greater Poland Voivodeship | 0.94 |
| 29 | 52°80′71′′N 17°19′73′′E | Greater Poland Voivodeship | 0.11 |
| 30 | 51°76′81′′N 15°87′48′′E | Lubusz Voivodeship | 1.05 |
| 31 | 52°10′76′′N 19°94′47′′E | Łódź Voivodeship | 0.56 |
| 32 | 53°24′68′′N 17°01′70′′E | Greater Poland Voivodeship | 0.22 |
| 33 | 52°04′58′′N 18°36′86′′E | Greater Poland Voivodeship | 0.57 |
| 34 | 52°53′95′′N 16°26′42′′E | Greater Poland Voivodeship | 0.16 |
| 35 | 51°76′87′′N 19°45′69′′E | Łódź Voivodeship | 0.52 |
| 36 | 52°47′75′′N 16°87′72′′E | Greater Poland Voivodeship | 2.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przybylska-Balcerek, A.; Szablewski, T.; Szwajkowska-Michałek, L.; Świerk, D.; Cegielska-Radziejewska, R.; Krejpcio, Z.; Suchowilska, E.; Tomczyk, Ł.; Stuper-Szablewska, K. Sambucus Nigra Extracts–Natural Antioxidants and Antimicrobial Compounds. Molecules 2021, 26, 2910. https://doi.org/10.3390/molecules26102910
Przybylska-Balcerek A, Szablewski T, Szwajkowska-Michałek L, Świerk D, Cegielska-Radziejewska R, Krejpcio Z, Suchowilska E, Tomczyk Ł, Stuper-Szablewska K. Sambucus Nigra Extracts–Natural Antioxidants and Antimicrobial Compounds. Molecules. 2021; 26(10):2910. https://doi.org/10.3390/molecules26102910
Chicago/Turabian StylePrzybylska-Balcerek, Anna, Tomasz Szablewski, Lidia Szwajkowska-Michałek, Dariusz Świerk, Renata Cegielska-Radziejewska, Zbigniew Krejpcio, Elżbieta Suchowilska, Łukasz Tomczyk, and Kinga Stuper-Szablewska. 2021. "Sambucus Nigra Extracts–Natural Antioxidants and Antimicrobial Compounds" Molecules 26, no. 10: 2910. https://doi.org/10.3390/molecules26102910
APA StylePrzybylska-Balcerek, A., Szablewski, T., Szwajkowska-Michałek, L., Świerk, D., Cegielska-Radziejewska, R., Krejpcio, Z., Suchowilska, E., Tomczyk, Ł., & Stuper-Szablewska, K. (2021). Sambucus Nigra Extracts–Natural Antioxidants and Antimicrobial Compounds. Molecules, 26(10), 2910. https://doi.org/10.3390/molecules26102910








