3. Results and Discussion
To date, the nanoimprinted AAO template method transformed into one of the best advancements that have been demonstrated to significantly contribute to a decent fabrication of nanostructure arrays [
19,
20] and is also relatively low cost [
21] and simple to construct [
22].
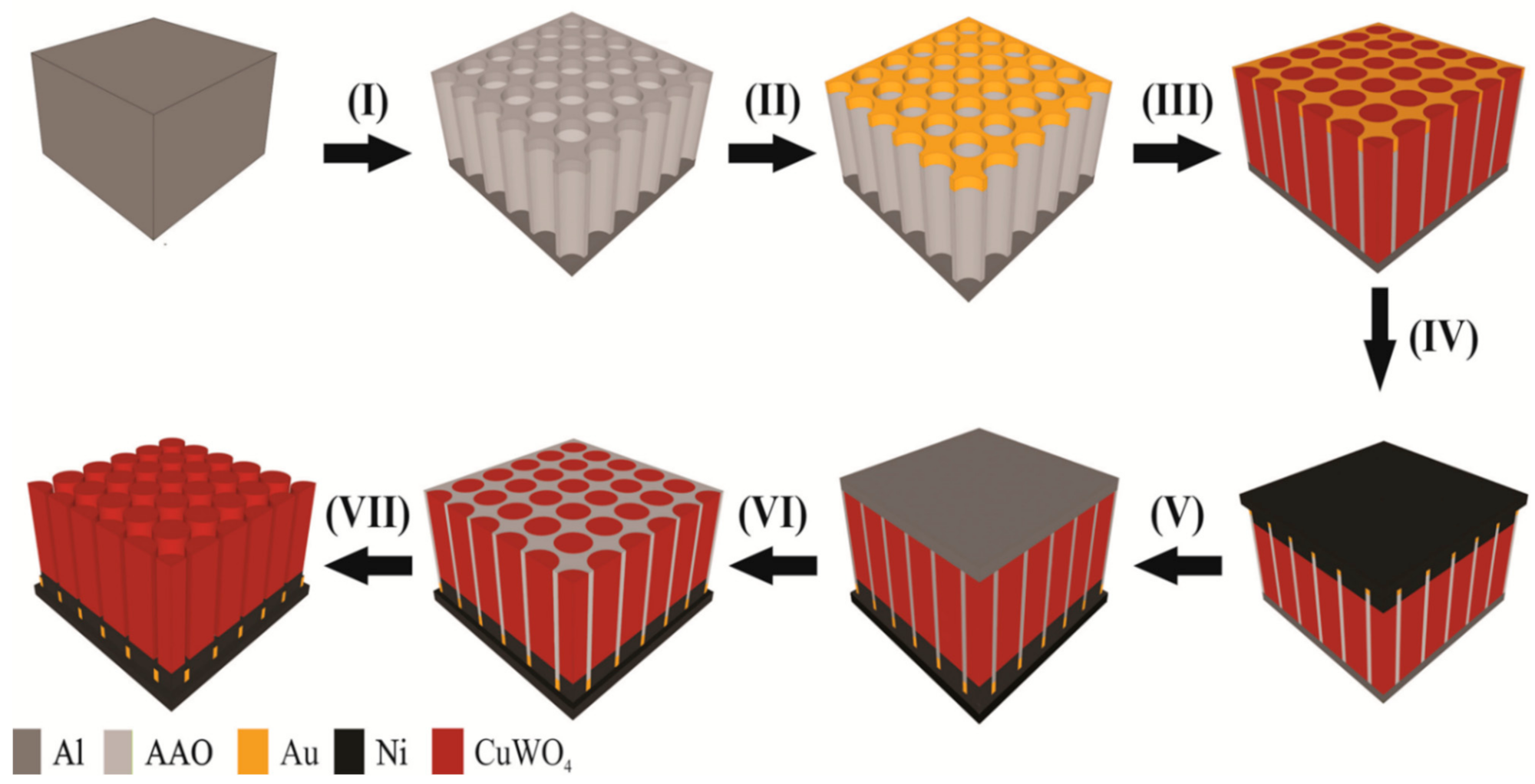
Figure 1 shows a detailed schematic step-by-step fabrication of the CuWO
4 nanorod arrays. The first step of this fabrication is using Ni sheet as the self-support on aluminum. The second step is anodization for as long as 30 min, submerged in an H
3PO
4 (5 wt%) solution with control pores between 100 nm and 200 nm.
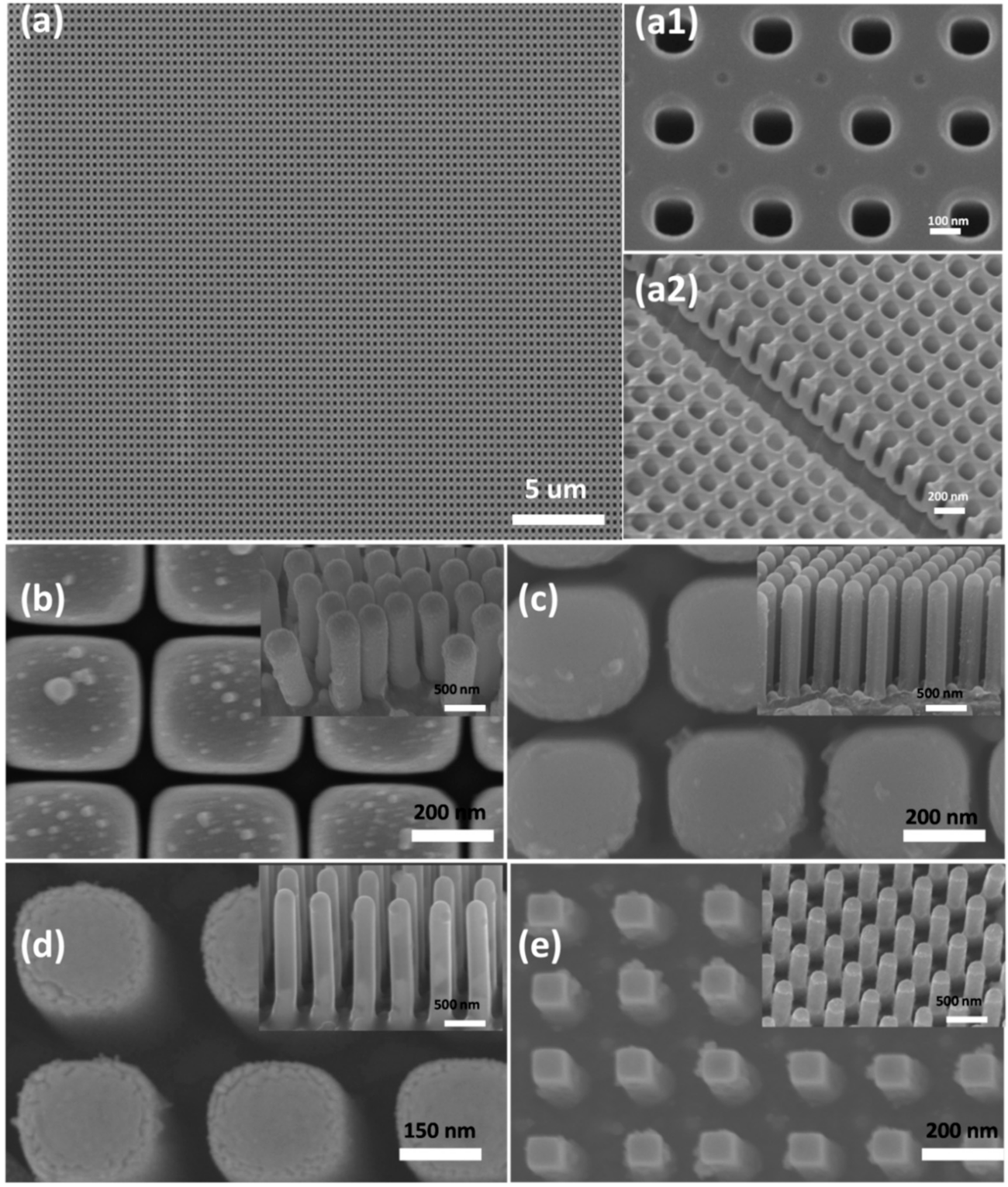
Figure 2a–c shows the SEM images of the AAO template and is an overview of the gap between two pores that is dependent on the length of time it was submerged in the H
3PO
4 (5 wt%) solution. The third step is the deposition of TiO
2 and Au. The fourth step is electrodeposition using three electrodes with a potential bias of −0.4 V versus Ag/AgCl and annealing at a temperature of 500 °C for 2 h. The fifth step is nickel plating and the sixth step is the removal of the back side of the AAO template. The last step is the removal of the template by submerging in an H
3PO
4 (5 wt%) solution for 3 h and at a temperature of 30 °C. A TiO
2 5 nm coating layer is added to all the samples using atomic layer deposition (ALD).
The CuWO
4 nanorod arrays obtained from AAO are shown in
Figure 2d,e. It can be seen that the morphology of the CuWO
4 nanorod arrays is well organized due to the high structure control of AAO. The length of the nanorod arrays that are relatively equivalent, in the range between 1 µm and 1.2 µm, and the relatively wide gap between two CuWO
4 nanorod arrays will significantly affect the performance of PEC. Electrolyte penetration can easily work on CuWO
4 nanorod arrays due to the absence of obstacles, resulting in a high hole and electron mobility. In addition, another advantage of AAO is the low contamination effect even when heated with CuWO
4 at temperatures exceeding 550 °C. SEM images shown in
Figure 2b,c clearly show no trace of AAO, which is proof that this way is very efficacious for the fabrication of this material and producing gaps between the CuWO
4 nanorod arrays of 50 nm, 100 nm, 150 nm, and 200 nm, respectively.
Figure 3a shows us the HRTEM images and SAED patterns of the CuWO
4 nanorod arrays with a nanorod array structure diameter of 200 nm, similar to that of previously reported CuWO
4 thin films. This is an important basis to propose a concept that irradiation can simply penetrate the surface and the gaps between the CuWO
4 nanorod arrays, thus causing a hydrogen evolution reaction in the counter electrode, and at the same time, an oxygen evolution reaction occurs in the CuWO
4 nanorod arrays. This is different from CuWO
4 thin films in which irradiation only occurs on the surface. To make the results of this research more credible, in
Figure 3b, a detailed electron diffraction tomography of the CuWO
4 nanorod arrays is shown to obtain a standard structure with a
cmcm space group and unit cell parameter of
a = 3.72 Å,
b = 5.51 Å, and
c = 3.98 Å. A high-quality single-crystal appears in this image, as was also visible in the structure of previous research. Furthermore, also shown in
Figure 3b, HRTEM using an aberration corrector on the projected crystal quality samples shows that the average gap that is formed between adjacent lattices is 0.22 nm, which in accordance with the area of the material (−110). This also gives information about the high crystallinity consistency, structure, and distribution of Cu and W within the CuWO
4 nanorod arrays. Subsequently, complimentary information about the elements from the fast Fourier transform (FFT) simulation is clearly shown in
Figure 3c, in which the atom O is randomly distributed in the structure that was formed. The XRD pattern of the electrode is shown in
Figure 3c, along with the Miller index of the single-crystal structure. For comparison, measurements were performed to both CuWO
4 thin films and CuWO
4 nanorod arrays where both samples were heated in air at a temperature of 550 °C for 2 h, and as a result of this fabrication method, XRD patterns are formed on the material [
11,
19], where the area of the formed CuWO
4 nanorod arrays material (001) is at a 24.21° angle and the area of the material (002) reflects an angle of 33.27°. To confirm the chemical composition of the CuWO
4 nanorod arrays further, an energy-dispersive X-ray (EDX) mapping is carried out on the constituent particles and is shown in
Figure 3e–g, based on the SEM image shown in
Figure 3d, and by giving the sequential signal information of Cu, W, and O. The increased crystal quality may be due to the thin coating layer of TiO
2 on the imprinted AAO templates that can supply an evenly distributed electric field and activation energy for electrochemical deposition to form the CuWO
4 nanorod arrays. Additionally, the thin coating layer of TiO
2 can be useful to reduce resistance. The composition from one of the CuWO
4 nanorod array samples was identified using X-ray photoelectron spectroscopy (XPS) likewise to that depicted in standard SI W
6+ [
10]. The Cu (2p) spectrum region is shown by a Cu (2p
3/2) peak and a Cu (2p
1/2) at 933.8 eV and 954.9 eV, respectively, which is also known as the Cu
2+ energy peak [
12]. Lastly, the O area (1s) that is shown with one peak (1s) is the oxide lattice domain and is generally used to depict the surface of hydroxide on the surface of metal oxide.
The distribution of the gaps between the CuWO
4 nanorod arrays for 50 nm, 100 nm, 150 nm, and 200 nm is, respectively, shown in
Figure 4a1–a4. It can be seen that for 50 nm the gaps between the CuWO
4 nanorod arrays are in the range of 48 nm up to 54 nm (
Figure 4a1), for 100 nm, the gaps are in the range of 94 nm up to 102 nm (
Figure 4a2), for 150 nm, the gaps are in the range of 148 nm up to 152 nm (
Figure 4a3), and for 200 nm, the gaps are in the range of 198 nm up to 202 nm (
Figure 4a4). This shows that the nanoimprinted AAO template is an effective technology in controlling CuWO
4 to become a nanostructure, which has never been performed previously. The result of the light absorber measurement using UV–Vis spectrophotometry on each distinct gap between the CuWO
4 nanorod arrays is shown in
Figure 4b. The results present an optimal condition that is obtained by each compartment, thus becoming the basis to claim an increased performance of the PEC water splitting procedure on these CuWO
4 nanorod arrays. The light absorber measurement carried out on the distinct gaps between the CuWO
4 nanorod arrays, in theory, provides a good chance to optimize the incoming light absorption ability of the photoanode. The absorption spectrum of the samples shown in
Figure 4b, shows an increase in incoming light absorption on wavelengths in the range of 300 nm up to 620 nm, which is related to the antireflection characteristic of the CuWO
4 nanorod array structure. Furthermore, the absorption spectrum of the CuWO
4 nanorod arrays shown in
Figure 4b presents absorption in the visible light range. Absorption tends to happen at around 500 nm for gaps between the CuWO
4 nanorod arrays of 50 nm and around 620 nm for gaps between the CuWO
4 nanorod arrays of 150 nm, which shows that the absorption originates from the CuWO
4 material.
To test the maximum carrier generation and the minimum carrier recombination of the CuWO
4 nanorod arrays on all the samples with distinct gaps between the CuWO
4 nanorod arrays, the PEC measurement was carried out on the photoanode of the CuWO
4 nanorod array and is shown in
Figure 4c. The working electrode measurement of the CuWO
4 nanorod arrays was carried out using three electrodes with an Ag/AgCl electrode reference and a Pt plate as the counter electrode. All the measurements were performed in 0.1 M Na
2SO
4 (pH 6.8). This procedure was conducted to minimalize the effects of outside bubbles that may occur during the measurement due to irradiation using a solar simulator.
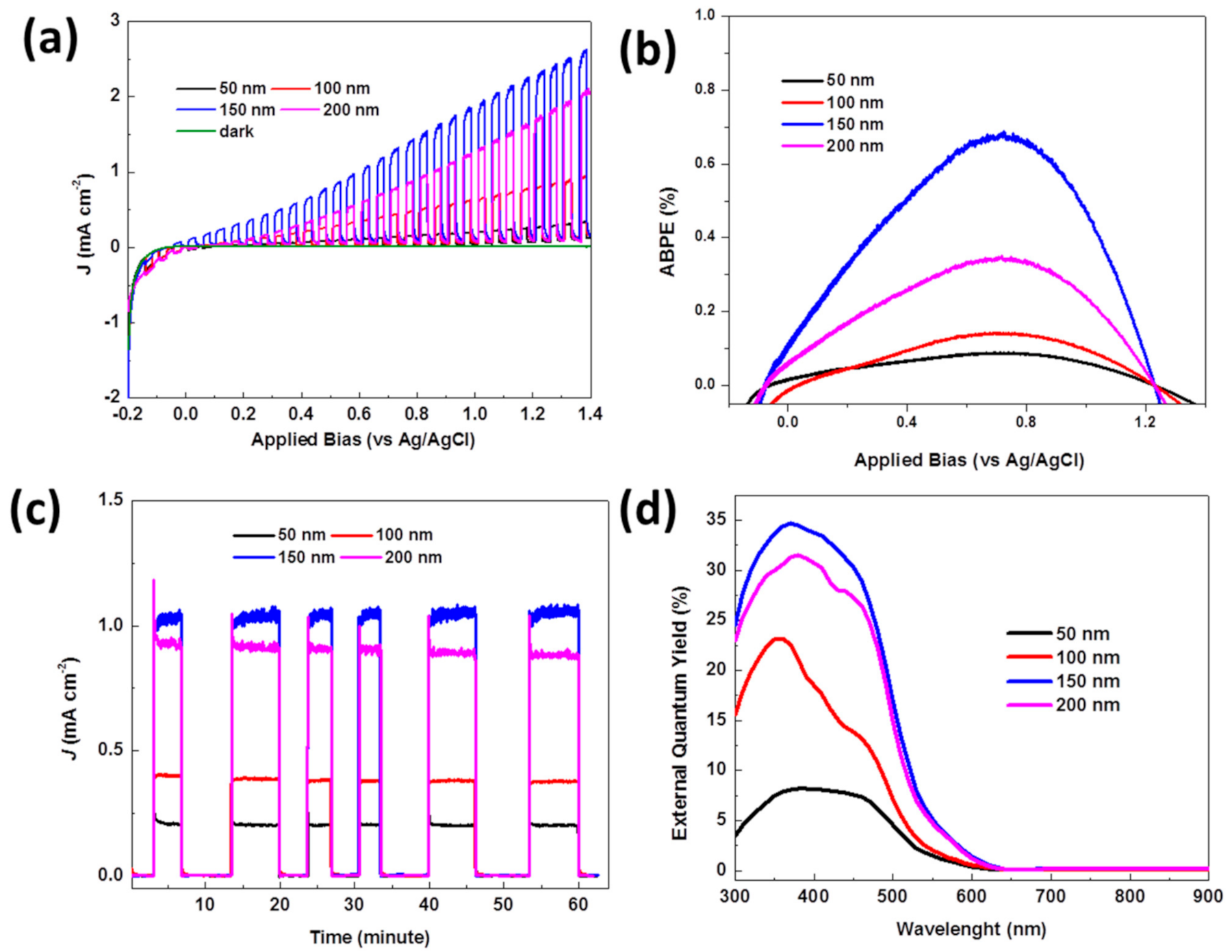
Figure 5a shows the characteristics of current density from irradiation on the photoanode of the CuWO
4 nanorod arrays versus the bias potential interval (
J-V) that is measured at a potential rate of 20 mV s
−1 and a potential range between −0.2 V to 1.4 V vs. Ag/AgCl in dark condition (shown by the green line) and an irradiation of AM 1.5 G, 100 mW cm
−2 on the photoanode of the CuWO
4 nanorod arrays. All the samples with distinct in-between gaps and diameters (shown in the SEM image in
Figure 2) were tested using the previously described measurement. The images clearly show an increase in irradiation current in areas with a positive bias voltage, which shows that CuWO
4 nanorod arrays are type-n semiconductors. For comparison, in the images given in
Figure S2 of the Supplementary Materials, all the measured samples of the CuWO
4 nanorod arrays show the same working area, which is in the positive voltage area. The fundamental difference that can be observed from this measurement is the magnitude of irradiation current density at intervals between 0 V and 0.4 V vs. Ag/AgCl. As described in previous research, there exists a possibility that a transient effect occurs between the standing current and membrane caused by an imperfect bonding between the particles of CuWO
4 that causes a decrease in the current density of the irradiation. Therefore, adding a very thin layer of TiO
2, approximately around ±5 nm (
Egap TiO
2 3.2 eV) using ALD on the surface of the CuWO
4 nanorod arrays, may increase the capacity of the light absorption spectrum of the CuWO
4 nanorod arrays. This is despite previous research findings, in which the photoanode of CuWO
4 obtained a maximum value of 3% to change the incoming light irradiation to hydrogen due to the large energy gap between the valence band and the conduction band. Nevertheless, the presence of the Au particles from the beginning of the fabrication process of the imprinted AAO template can also cause a surface plasmon resonance (SPR) effect. Hales et al. stated that the magnitude of the SPR effect depends on the size, morphology, and density of the Au particles [
23]. The Au particles that are scattered on the surface of the photoanode are also used as a conductor for the deposition of the CuWO
4 nanorod arrays in the early phase, as mentioned in the steps in
Figure 1. For comparison,
Figure S2 of the Supplementary Materials shows the photogenerated measurement on CuWO
4 thin films. CuWO
4 thin films were deposited on FTO glass with the same mechanism as the fabrication method of the CuWO
4 nanorod arrays. Likewise, the photogenerated measurement on CuWO
4 thin films used the same electrolyte and bias voltage limit range. It is clearly shown in
Figure S2 of the Supplementary Materials that the maximum irradiation current density of the applied bias potential thermodynamics is around 0.74 mA cm
−2, which is lower than the same measurement performed on the compartments of the CuWO
4 nanorod arrays. The logical reasoning behind this is that hole injection on CuWO
4 thin films to slow electrolytes and the weak current carrier enables the CuWO
4 thin films to have a lower performance toward its PEC water splitting measurement.
Figure 4 nanorod arrays using the equation, which is
η =
J(1.23 −
Vapp)/
Plight, where
Vapp applies a bias potential vs. Ag/AgCl,
J is the external current density, and
Plight is the irradiation power. The results of the measurement are presented in
Figure 5b, showing a measurement of 0.06% (at 0.681 V vs. Ag/AgCl), 0.12% (at 0.683 V vs. Ag/AgCl), 0.72% (at 0.712 V vs. Ag/AgCl), and 0.38% (at 0.711 V vs. Ag/AgCl) for gaps between the CuWO
4 nanorod arrays of 50 nm, 100 nm, 150 nm, and 200 nm, respectively. From the measurements presented in
Figure 5b, it can be seen that the optimal electrode gap between the CuWO
4 nanorod arrays is 150 nm. As a result, the composition of the CuWO
4 nanorod arrays has a wider layer and is in direct contact with the electrolyte and, in turn, serves as an effective conductor of electrons from the interface of the working electrode to the counter electrode and vice versa, causing a quick charge transfer. Nevertheless, a gap too close or too far between two diamagnetic CuWO
4 nanorod arrays can significantly affect the created electric field [
24]. As a result, an optimal gap can have a significant effect on this diamagnetic material. Qualitatively, it can be interpreted that polarization of the induction current and the noncarrier of the magnetic field orbit can efficiently influence the irradiation conversion. As shown in
Figure 5c, the characteristics of AM 1.5G irradiation on the CuWO
4 nanorod arrays seem relatively stable within 60 min and in a dark–light condition. When the current resulted from irradiation reaches a value of 1.02 mA cm
−2 with 0.7 V vs. Ag/AgCl, this result is a significant improvement, compared to previous research. For comparison, we show the measurement of current irradiation of CuWO
4 thin films in
Figure S3 of the Supplementary Materials. This verifies our hypothesis that the photoanode of the CuWO
4 nanorod arrays significantly influences photogeneration, which is shown by CuWO
4 nanorod arrays exhibiting an absorption and photon collection through the bandgap transition between electrodes. The highest value comes from the electrode with an in-between gap of 150 nm, corresponding to the highest absorption curve shown in
Figure 4b.
As for the EQY (%) measurement on the photoanode of the CuWO
4 nanorod arrays for every sample, the measurement is carried out without the use of bias voltage, as shown in
Figure 5d. The EQY peak occurs at wavelengths around 410 nm. For additional information, this photon-to-current peak efficiency occurs for all the samples with distinct gaps between the CuWO
4 nanorod arrays. As stated before, the effect of the gap between the CuWO
4 nanorod arrays is very transparent. A close gap between the CuWO
4 nanorod arrays significantly influences a decrease in the efficiency value of the measurement. As a result, photon absorption of electrodes is effective within the relatively wide wavelength interval of visible light, which is in the range of 300 nm up to 620 nm. This is proof that nanostructures can significantly influence an increased response toward incoming light at wavelengths between 300 nm and 620 nm. The effect of the gap between the CuWO
4 nanorod arrays significantly impacts the performance of this photoelectrode.
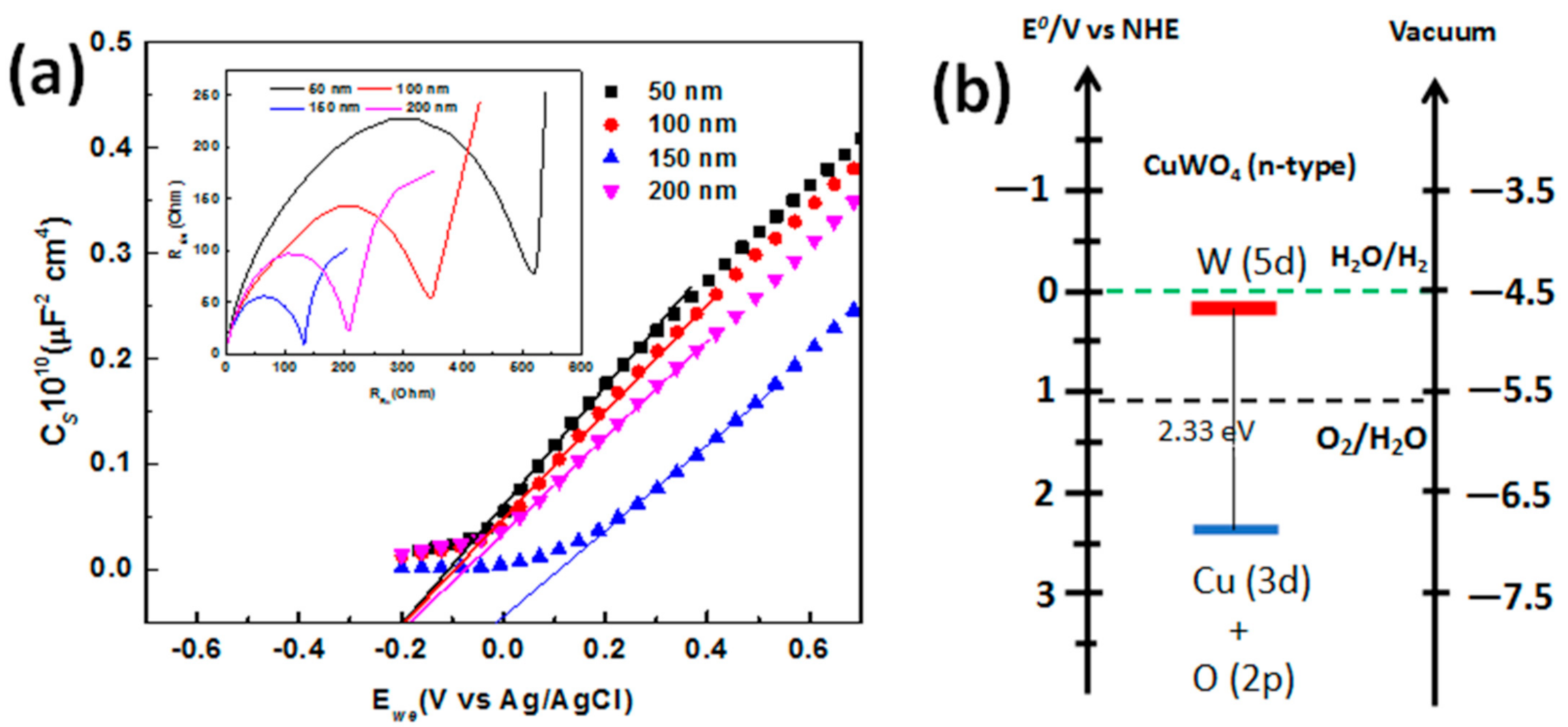
Next, a measurement to describe the electronic structure of the CuWO
4 nanorod arrays composite is needed. For this, a relevant Mott–Schottky (M–S) plot is used and also by observing the electrochemical impedance, which, in this research, starts from a frequency of 1 Hz up to 1 MHz with a bias potential interval of −0.4 V up to +0.7 V vs. Ag/AgCl. The capacitance value of the measurement is presented in
Figure 6a at a frequency of 1 kHz. From this, it is shown that the M–S plot from each electrode with a distinct gap between the CuWO
4 nanorod arrays in the PEC water splitting procedure has a linear profile and a positive slope that demonstrates features of a type-n semiconductor. These data are then used for the M–S relation, in which the typical potential of the flat band V
FB is in the range of 0.2 V vs. Ag/AgCl up to 0.19 V vs. Ag/AgCl, which is in reference to previous research [
24,
25]. From the results, we obtained a plot and is linearly projected on each distinct flat bond potential of −0.16 V (vs. Ag/AgCl), −0.14 V (vs. Ag/AgCl), −0.104 V (vs. Ag/AgCl), and 0.46 V (vs. Ag/AgCl) in the open-circuit voltage under 100 mW cm
−2 irradiation for each electrode with distinct gaps between the CuWO
4 nanorod arrays of 50 nm, 100 nm, 150, and 200 nm, respectively. Then, the density of the majority charge carrier is calculated from the gradient of the M–S plot by using the equation d(
Csc−2)/dE = 2/
eεoεND, where the dielectric constant is 83 for the CuWO
4 nanorod arrays and the effective electron mass is 70 [
24,
26]. From the calculation, the density of the majority charge carrier of 0.84 × 10
20 cm
−3, 0.97 × 10
20 cm
−3, 2.86 × 10
20 cm
−3, and 1.35 × 10
20 cm
−3 are obtained for distinct gaps between the CuWO
4 nanorod arrays of 50 nm, 100 nm, 150, and 200 nm, respectively. The results show a higher majority charge carrier than that reported in previous research [
24,
26].
Figure 6a also clearly demonstrates that the CuWO
4 nanorod arrays have the behavior and conductivity of a type-n semiconductor, and the highest value of the majority charge carrier belongs to the CuWO
4 nanorod array with an in-between gap of 150 nm. As shown in the inset of
Figure 6a, the Nyquist plot is measured in the frequency range of 10
6 Hz up to 1 MHz with a bias of 0 V vs. Ag/AgCl for two sets of electrodes in PEC. In addition, this is to show that a Nyquist plot resulting in a half circle at the highest frequency is the fundamental characteristic of the process of charge transfer throughout the process of measurement. Furthermore, the diameter of the half circle formed by the Nyquist plot is relatively equal to the charge transfer resistance [
27]. The overall electrochemical measurements show that the modification of the CuWO
4 nanorod arrays has a positive impact on the electronic nature of the CuWO
4, which is indicated by the increase in doping density and area of band banding on the interface of the photoanode and electrolyte. Additionally, an observation on charge transfer resistance can be made on the overall electrochemical measurements, that is, the smaller the resistance, the more useful it is to promote charge separation in water molecules and charge carrier transfer as a result of irradiation. This provides a higher efficiency signal for utilizing charge generated from photogeneration of the CuWO
4 nanorod arrays, compared to that of CuWO
4 thin films.
On the other hand, determining the proper bandgap of the CuWO
4 nanorod arrays based on bandgap values obtained using the Tauc plot method is shown in
Figure 6b. It is known that a water splitting reaction must be at the right range of standard reduction and oxidation potential because CuWO
4 nanorod arrays have valence bands and conduction bands within that range, thus making CuWO
4 nanorod arrays a very good material for the PEC water splitting procedure. To summarize, the small distance between the Fermi level and the valence band indicates that CuWO
4 nanorod arrays are the desired type-n semiconductor. The estimated bandgap has a slightly lower value than that of previous research that is closely related to material defect [
13], in which the energy was obtained in vacuum conditions, and surface defect was disregarded through an ion milling procedure. This procedure causes a significant difference in bandgap that is measured with the electrochemical technique, compared to the procedure that we carried out.
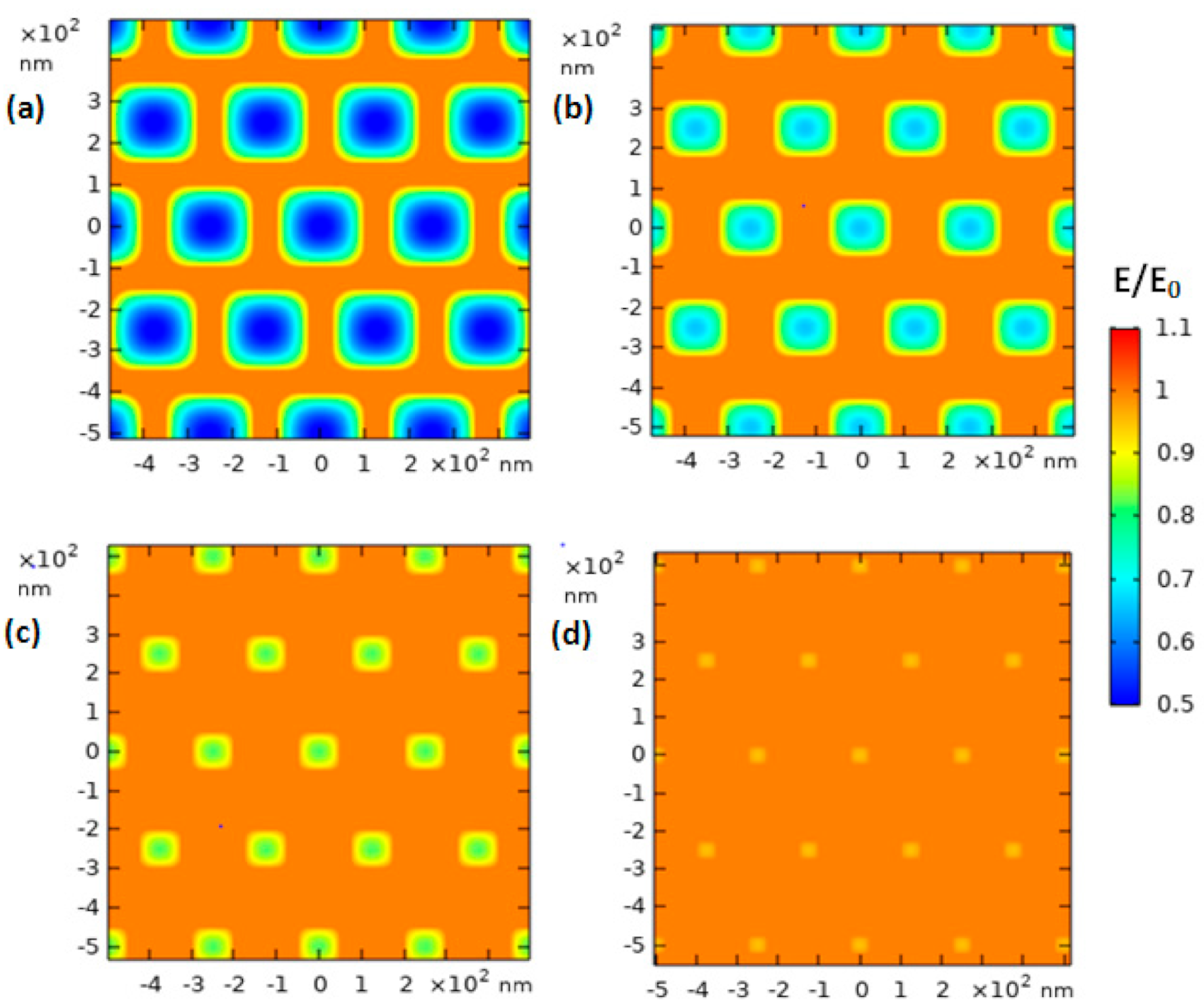
We carried out a simulation of the selected material to further understand the interaction between the photon and the CuWO
4 nanorod arrays using the FDTD method and is shown in
Figure 7 for each distinct gap between the CuWO
4 nanorod arrays. From the outcome of this simulation, information on the electric field distribution of photon irradiation with a specific wavelength of 410 nm on areas of the CuWO
4 nanorod arrays is obtained and captured from the top layer. The distinct gaps between the CuWO
4 nanorod arrays observed ranged from small to large gaps. The density of electric field coupling on all types of material can produce a strong energy contour, thus having a big influence on the efficiency signal of spectroscopy. As a result, the distribution of the CuWO
4 nanorod arrays produced by the scattering of the nanorod arrays is in line with nano metal particle arrays. Jain et al. [
28] explained that bipolar coupling caused by the in-between gaps will result in a decay of the plasmon coupling, and this will become irrelevant on metal, material form, and the dielectric constant of all types of material. Moreover, the increase of intensity in the near field indicates a faster decay as the gap of the CuWO
4 nanorod arrays becomes bigger. In other words, if the gaps between the CuWO
4 nanorod arrays are made to be bigger, then the electric field coupling becomes weaker. This weakening of the coupling may be one of the reasons a bandgap shift occurs in the CuWO
4 nanorod arrays. However, the bandgap shift that occurs in the CuWO
4 nanorod arrays can be controlled by setting the gaps between the CuWO
4 nanorod arrays of the samples to 150 nm and 200 nm based on the conventional quantum confinement effect. In other words, the results give information that a bandgap shift can be achieved by controlling the diameter and the in-between gaps on the conventional quantum confinement effect even though the obtained parameters are relatively bigger than that of the Bohr radius. Due to the reasons above, along with the relative energy density of the CuWO
4 nanorod arrays, which is not significantly different, the quantum effect shows that a cumulative change in thickness of the CuWO
4 nanorod arrays is not accounted for. However, the bandgap shift is specifically tied to the interaction between the CuWO
4 nanorod arrays after irradiation and the uniformity of the structure of the material. The stability of the bandgap is in relevance to the power of the near-field light, where this is used to increase the advantages of the optical properties of the CuWO
4 nanorod arrays to be used as material for the application of photoelectric.
The FDTD simulation on the CuWO
4 nanorod arrays gives information on the interaction connection between the photon and the geometric parameter of the CuWO
4 nanorod arrays that possess distinct gaps between the arrays, thus resulting in optimal electric field intensity. The simulation that was taken from irradiation on the top layer of the CuWO
4 nanorod arrays shows the abundant distribution of counter anode along the layer that differentiates CuWO
4 nanorod arrays with the medium. It can be seen in
Figure 7 (also see
Figure S3 of the Supplementary Materials) that as the gaps between the CuWO
4 nanorod arrays become bigger, the electric field becomes weaker; both of them were carried out at close to 700 nm. Likewise, as the gaps between the CuWO
4 nanorod arrays become smaller, the electric field becomes stronger. Nevertheless, the electric field that occurs on the CuWO
4 nanorod arrays also affects the magnetic field; thus, it has relevance toward the penetration of the electron charge of electrolytes on the CuWO
4 nanorod arrays. As a result, a suitable gap between the CuWO
4 nanorod arrays is needed to obtain a magnetic field and an electric field that significantly contributes toward the performance of the PEC water splitting procedure. The results of this experiment and simulation clearly show that CuWO
4 nanorod arrays greatly contribute toward the efficiency of CuWO
4 thin films.