Aptamer Laden Liquid Crystals Biosensing Platform for the Detection of HIV-1 Glycoprotein-120
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Cleaning and Chemical Modification of Glass Slides
2.3. Immobilization of B40t77 Aptamer on DMOAP-Coated Slides
2.4. Fabrication of B40t77 Aptamer-Based LC Biosensing Platform for gp-120 Detection
2.5. Selectivity of the Designed B40t77 Aptamer Based LCs Biosensor
2.6. Preparation of LC Optical Cell for Imaging Optical Images
2.7. Atomic Force Microscope (AFM) Characterization
3. Results and Discussion
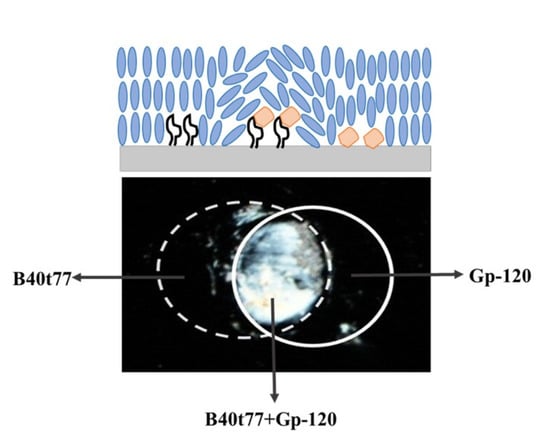
3.1. Schematic Illustrations for the Preparation and Sensing Strategy of B40t77 Aptamer-Based LC Biosensor
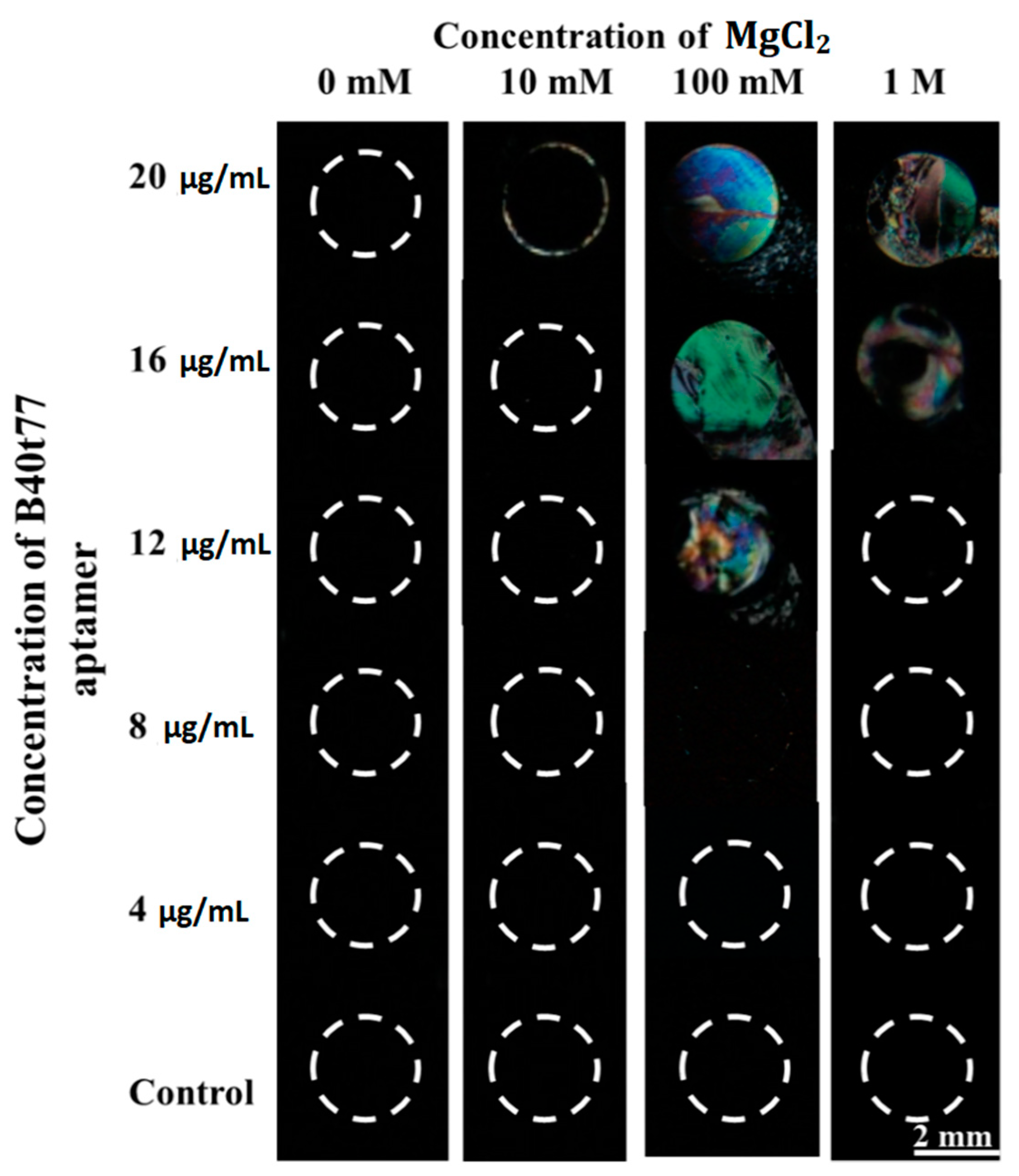
3.2. Optimization of B40t77 Aptamer Concentration
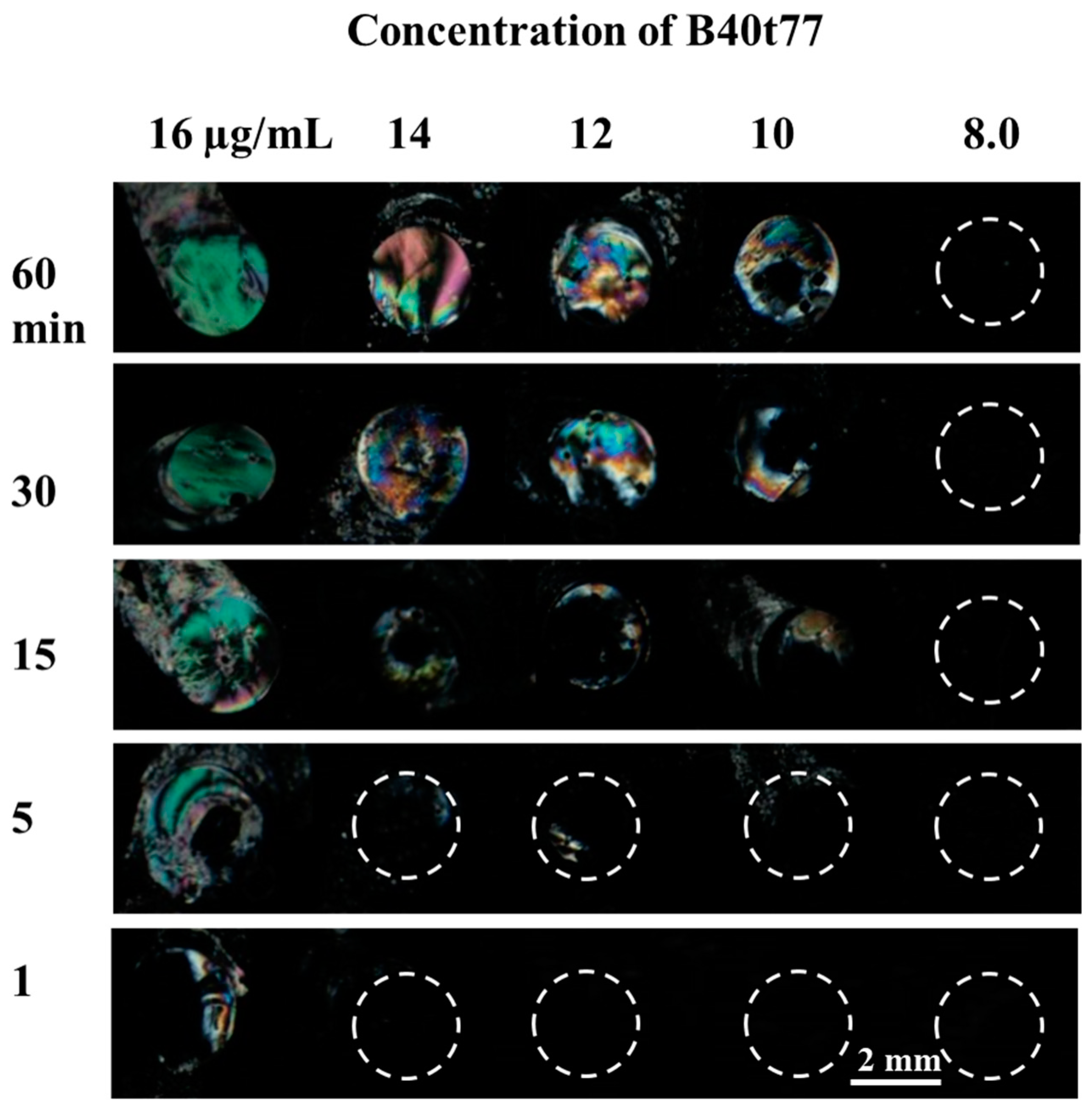
3.3. Effect of Time on Immobilization of Recognition Element on DMOAP-Coated Glass Slide Surface
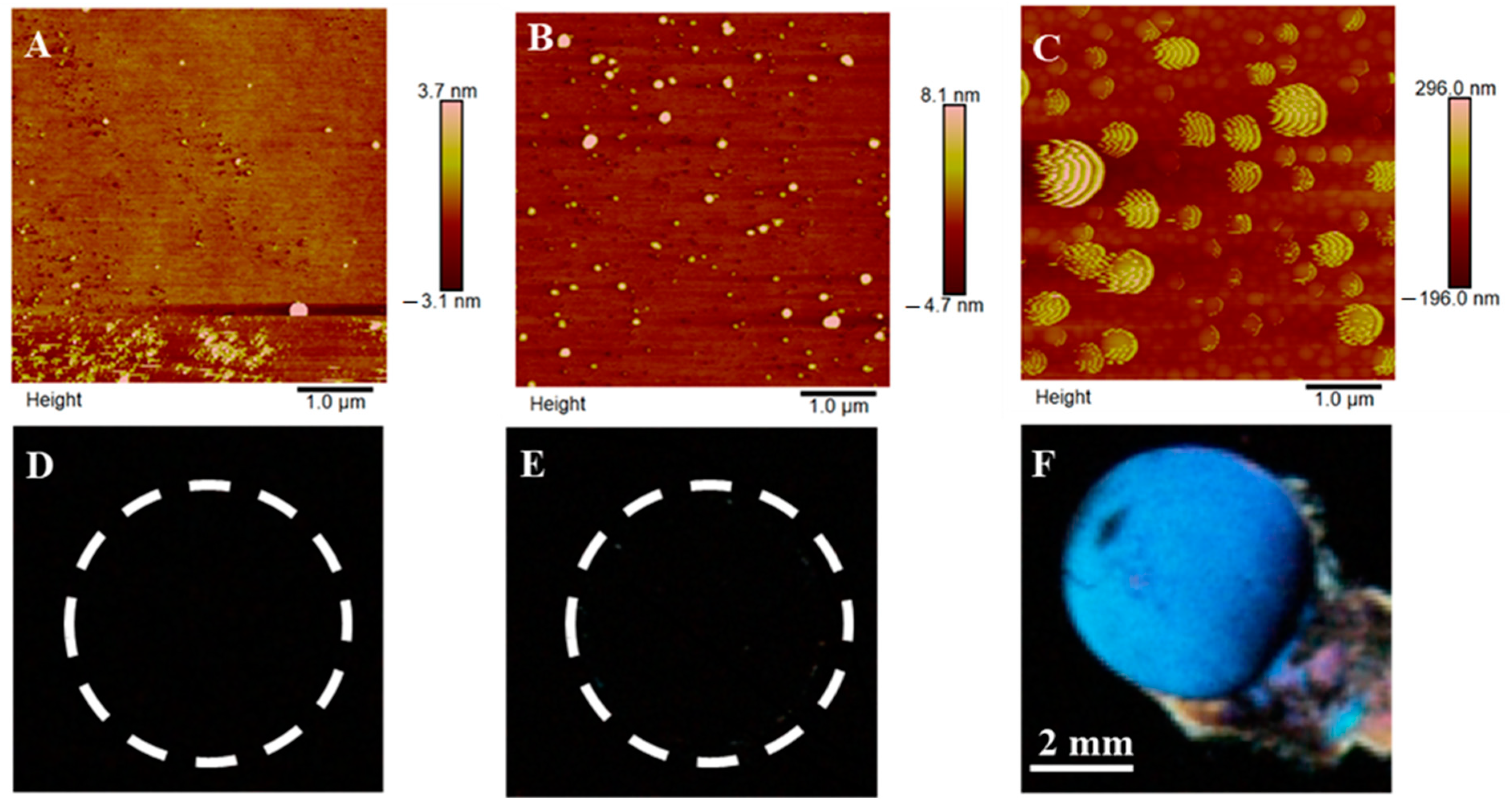
3.4. Characterization of the Designed Biosensing Platform
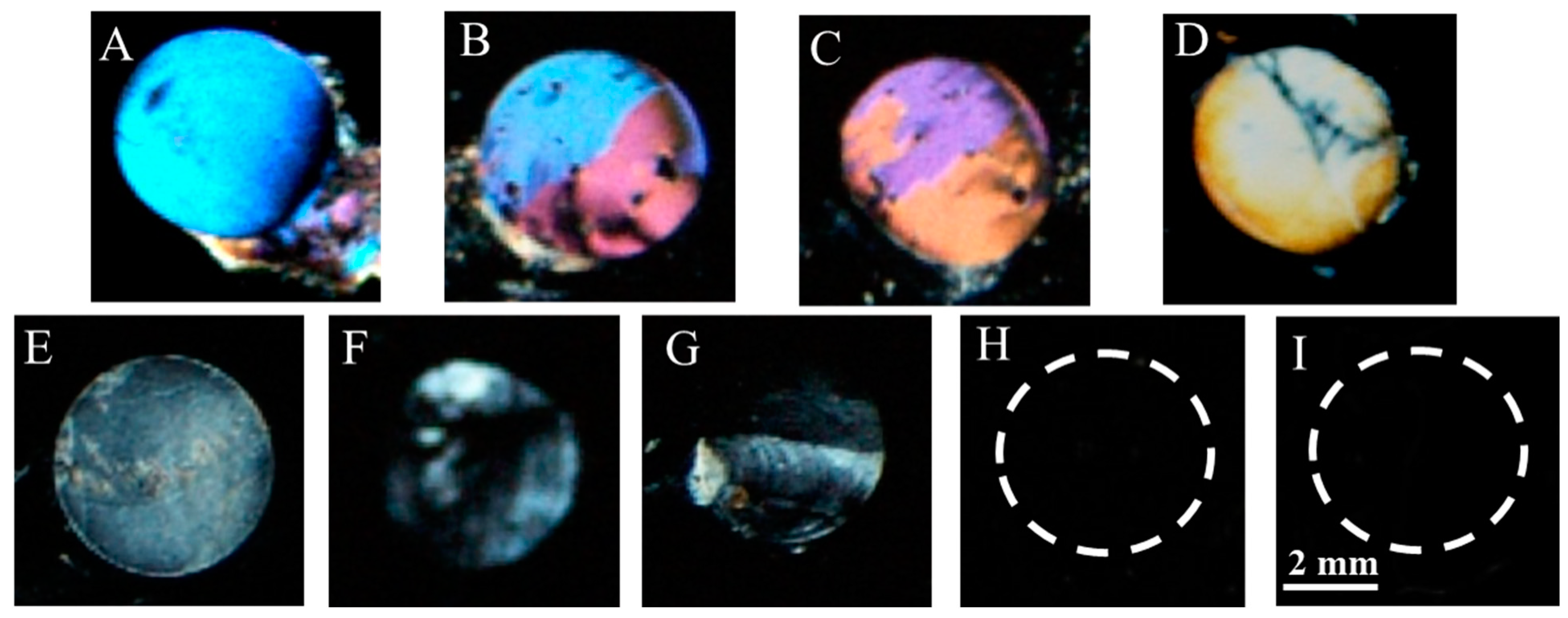
3.5. Detection of Target gp-120 by Surface-Immobilized Recognition Element B40t77Aptamer
3.6. Quantitative Analysis of Biosensor Performance
3.7. Selectivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kwong, P.D.; Wyatt, R.; Robinson, J.; Sweet, R.W.; Sodroski, J.; Hendrickson, W.A. Structure of an HIV gp 120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 1998, 393, 648–659. [Google Scholar] [CrossRef]
- Feng, Y.; Broder, C.C.; Kennedy, P.E.; Berger, E.A. HIV-1 entry cofactor: Functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science 1996, 272, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Hart, T.K.; Kirsh, R.; Ellens, H.; Sweet, R.W.; Lambert, D.M.; Petteway, S.R.; Leary, J.; Bugelski, P.J. Binding of soluble CD4 proteins to human immunodeficiency virus type 1 and infected cells induces release of envelope glycoprotein gp120. Proc. Natl. Acad. Sci. USA 1991, 88, 2189–2193. [Google Scholar] [CrossRef]
- Allan, J.S.; Coligan, J.E.; Barin, F.; Mclane, M.F.; Sodroski, J.G.; Rosen, C.A.; Haseltine, W.A.; Lee, T.H.; Essex, M. Major glycoprotein antigens that induce antibodies in AIDS patients are encoded by HTLV-III. Science 1985, 228, 1091–1094. [Google Scholar] [CrossRef]
- Rychert, J.; Strick, D.; Bazner, S.; Robinson, J.; Rosenberg, E. Detection of HIV gp120 in plasma during early HIV infection is associated with increased proinflammatory and immunoregulatory cytokines. AIDS Res. Hum. Retrovir. 2010, 26, 1139–1145. [Google Scholar] [CrossRef]
- Lalonde, J.M.; Do Kwon, Y.; Jones, D.M.; Sun, A.W.; Courter, J.R.; Soeta, T.; Kobayashi, T.; Princiotto, A.M.; Wu, X.; Schön, A.; et al. Structure-based design, synthesis, and characterization of dual hotspot small-molecule HIV-1 Entry Inhibitors. J. Med. Chem. 2012, 55, 4382–4396. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, B.; Shen, J.; Xiong, X.L.; Deng, S.X. Aptamer based bare eye detection of kanamycin by using a liquid crystal film on a glass support. Microchim. Acta 2017, 184, 3765–3771. [Google Scholar] [CrossRef]
- An, Z.; Jang, C.-H. Simple and Label-Free Liquid Crystal-based Optical Sensor for Highly Sensitive and Selective Endotoxin Detection by Aptamer Binding and Separation. ChemistrySelect 2019, 4, 1416–1422. [Google Scholar] [CrossRef]
- Popov, N.; Honaker, L.W.; Popova, M.; Usol’tseva, N.; Mann, E.K.; Jakli, A.; Popov, P. Thermotropic Liquid Crystal-Assisted Chemical and Biological Sensors. Materials 2017, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Zafiu, C.; Hussain, Z.; Küpcü, S.; Masutani, A.; Kilickiran, P.; Sinner, E.-K. Liquid crystals as optical amplifiers for bacterial detection. Biosens. Bioelectron. 2016, 80, 161–170. [Google Scholar] [CrossRef]
- Han, G.-R.; Song, Y.-J.; Jang, C.-H. Label-free detection of viruses on a polymeric surface using liquid crystals. Colloids Surf. B Biointerfaces 2014, 116, 147–152. [Google Scholar] [CrossRef]
- Kim, H.J.; Jang, C.-H. Liquid crystal-based aptasensor for the detection of interferon-γ and its application in the diagnosis of tuberculosis using human blood. Sens. Actuators B 2019, 282, 574–579. [Google Scholar] [CrossRef]
- Brake, J.M.; Daschner, M.K.; Luk, Y.Y.; Abbott, N.L. Biomolecular Interactions at Phospholipid-Decorated Surfaces of Liquid Crystals. Science 2003, 302, 2094–2097. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.N.; Orler, V.J.; Abbott, N.L. Ordering transitions triggered by specific binding of vesicles to protein-decorated interfaces of thermotropic liquid crystals. Langmuir 2012, 28, 6364–6376. [Google Scholar] [CrossRef]
- Hussain, Z.; Zafiu, C.; Küpcü, S.; Pivetta, L.; Hollfelder, N.; Masutani, A.; Kilickiran, P.; Sinner, E.K. Liquid crystal based sensors monitoring lipase activity: A new rapid and sensitive method for cytotoxicity assays. Biosens. Bioelectron. 2014, 56, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Rouhbakhsh, Z.; Verdian, A.; Rajabzadeh, G. Design of a liquid crystal-based aptasensing platform for ultrasensitive detection of tetracycline. Talanta 2020, 206, 120246. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Qazi, F.; Ahmed, M.I.; Usman, A.; Riaz, A.; Abbasi, A.D. Liquid crystals based sensing platform-technological aspects. Biosens. Bioelectron. 2016, 85, 110–127. [Google Scholar] [CrossRef]
- Du, J.Y.; Jiang, Q.F.; Lu, X.C.; Chen, L.C.; Zhang, Y.; Xiong, X.L. Detection of sulfadimethoxine using optical images of liquid crystals. Analyst 2019, 144, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Su, X.; Yang, D.; Luan, C. Label-free liquid crystal biosensor for cecropin B detection. Talanta 2018, 186, 60–64. [Google Scholar] [CrossRef]
- Robertson, D.L.; Joyce, G.F. Selection in vitro of an RNA enzyme that specifically cleaves single-stranded DNA. Nature 1990, 344, 467–468. [Google Scholar] [CrossRef]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Khoshbin, Z.; Verdian, A.; Housaindokht, M.R.; Izadyar, M.; Rouhbakhsh, Z. Aptasensors as the future of antibiotics test kits-a case study of the aptamer application in the chloramphenicol detection. Biosens. Bioelectron. 2018, 122, 263–283. [Google Scholar] [CrossRef]
- Toh, S.Y.; Citartan, M.; Gopinath, S.C.B.; Tang, T.H. Aptamers as a replacement for antibodies in enzyme-linked immunosorbent assay. Biosens. Bioelectron. 2015, 64, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Wandtke, T.; Wozniak, J.; Kopinski, P. Aptamers in diagnostics and treatment of viral infections. Viruses 2015, 7, 751–780. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Hu, R.; Bao, T.; Zhang, X.; Wang, S. An insertion approach electrochemical aptasensor for mucin 1 detection based on exonuclease-assisted target recycling. Biosens. Bioelectron. 2015, 71, 13–17. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Zhang, Q.; Xiong, X.; Deng, S. Detection of pulmonary surfactant protein A by using an aptamer-based liquid crystal biosensor. Anal. Methods 2018, 10, 2895–2900. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, Q.; Wu, W. Graphene-Based Steganographic Aptasensor for Information Computing and Monitoring Toxins of Biofilm in Food. Front. Microbiol. 2020, 10, 3139. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Tang, L.; Zeng, G.; Zhou, Y.; Zhang, Y.; Long, B.; Fang, S.; Chen, S.; Yu, J. Current Progress in Aptasensors for Heavy Metal Ions Based on Photoelectrochemical Method: A Review. Curr. Anal. Chem. 2018, 14, 4–12. [Google Scholar] [CrossRef]
- Labib, M.; Berezovski, M.V. Electrochemical aptasensors for microbial and viral pathogens. Adv. Biochem. Eng. Biotechnol. 2014, 140, 155–181. [Google Scholar] [CrossRef]
- Dey, A.K.; Griffiths, C.; Lea, S.M.; James, W. Structural characterization of an anti-gp120 RNA aptamer that neutralizes R5 strains of HIV-1. RNA 2005, 11, 873–884. [Google Scholar] [CrossRef]
- Dey, A.K.; Khati, M.; Tang, M.; Wyatt, R.; Lea, S.M.; James, W. An aptamer that neutralizes R5 strains of human immunodeficiency virus type 1 blocks gp120-CCR5 interaction Title. J. Virol. 2005, 79, 13806–13810. [Google Scholar] [CrossRef]
- John, S.V.; Khati, M.; Mamba, B.B.; Arotiba, O.; Rotherham, L.S. Towards HIV Detection: Novel Poly (propylene) Dendrimer-Streptavidin Platform for Electrochemical DNA and gp-120 aptamer biosensors. Int. J. Electrochem. Sci. 2014, 9, 5425–5437. [Google Scholar]
- Xue, C.Y.; Kun-Lin, Y. Dark-to-bright optical responses of liquid crystals supported on solid surfaces decorated with proteins. Langmuir 2008, 24, 563–567. [Google Scholar] [CrossRef]
- Naemura, S. Polar and nonpolar contributions to liquid-crystal orientations on substrates. J. Appl. Phys. 1980, 51, 6149–6159. [Google Scholar] [CrossRef]
- Chen, C.H.; Yang, K.L. Detection and quantification of DNA adsorbed on solid surfaces by using liquid crystals. Langmuir 2010, 26, 1427–1430. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Yang, K.L. Liquid crystal-based immunoassays for detecting hepatitis B antibody. Anal. Biochem. 2012, 421, 321–323. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbasi, A.D.; Hussain, Z.; Yang, K.-L. Aptamer Laden Liquid Crystals Biosensing Platform for the Detection of HIV-1 Glycoprotein-120. Molecules 2021, 26, 2893. https://doi.org/10.3390/molecules26102893
Abbasi AD, Hussain Z, Yang K-L. Aptamer Laden Liquid Crystals Biosensing Platform for the Detection of HIV-1 Glycoprotein-120. Molecules. 2021; 26(10):2893. https://doi.org/10.3390/molecules26102893
Chicago/Turabian StyleAbbasi, Amna Didar, Zakir Hussain, and Kun-Lin Yang. 2021. "Aptamer Laden Liquid Crystals Biosensing Platform for the Detection of HIV-1 Glycoprotein-120" Molecules 26, no. 10: 2893. https://doi.org/10.3390/molecules26102893
APA StyleAbbasi, A. D., Hussain, Z., & Yang, K.-L. (2021). Aptamer Laden Liquid Crystals Biosensing Platform for the Detection of HIV-1 Glycoprotein-120. Molecules, 26(10), 2893. https://doi.org/10.3390/molecules26102893