Enthalpic and Liquid-Phase Adsorption Study of Toluene–Cyclohexane and Toluene–Hexane Binary Systems on Modified Activated Carbons
Abstract
1. Introduction
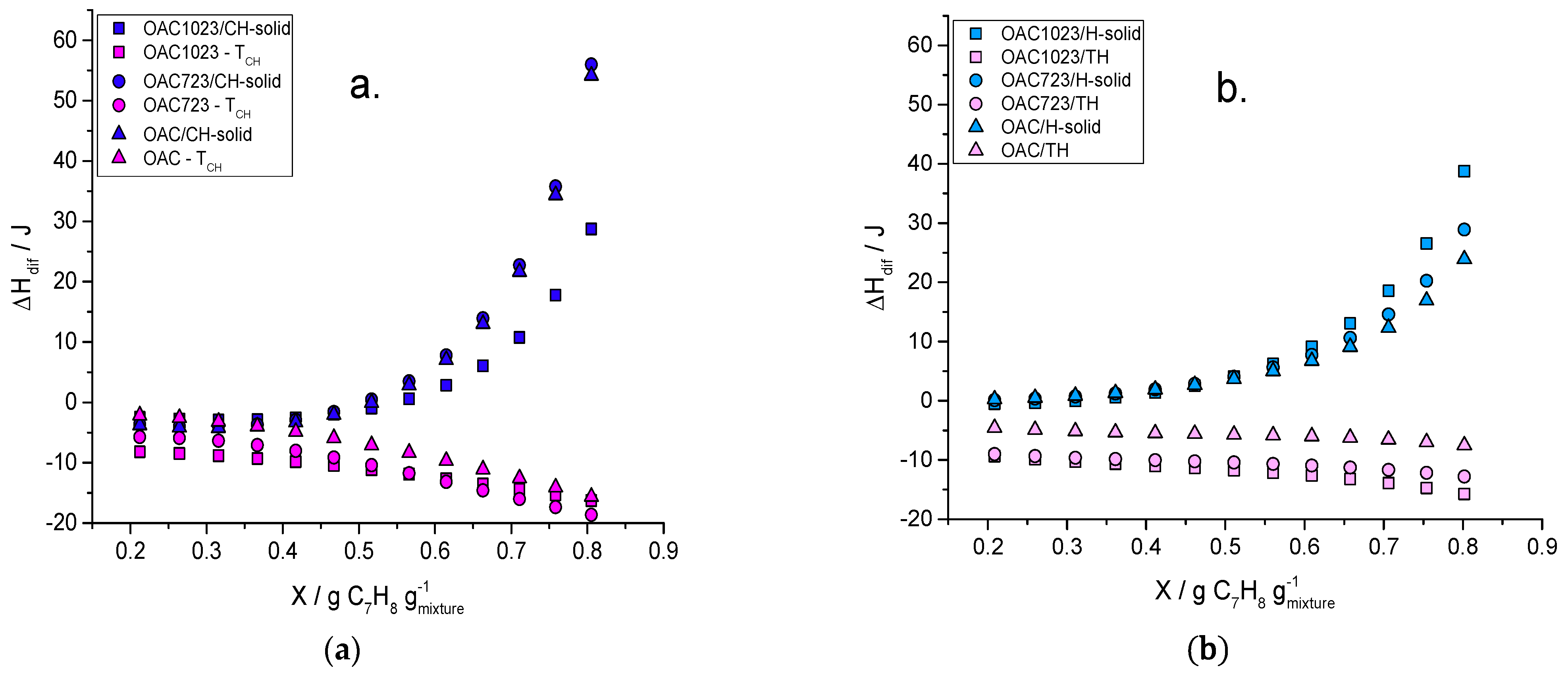
2. Results and Discussion
3. Materials and Methods
3.1. Samples
3.2. Physical Characterization of Samples
3.3. Chemical Characterization of Samples
3.4. Hydrocarbons Adsorption from Liquid Phase
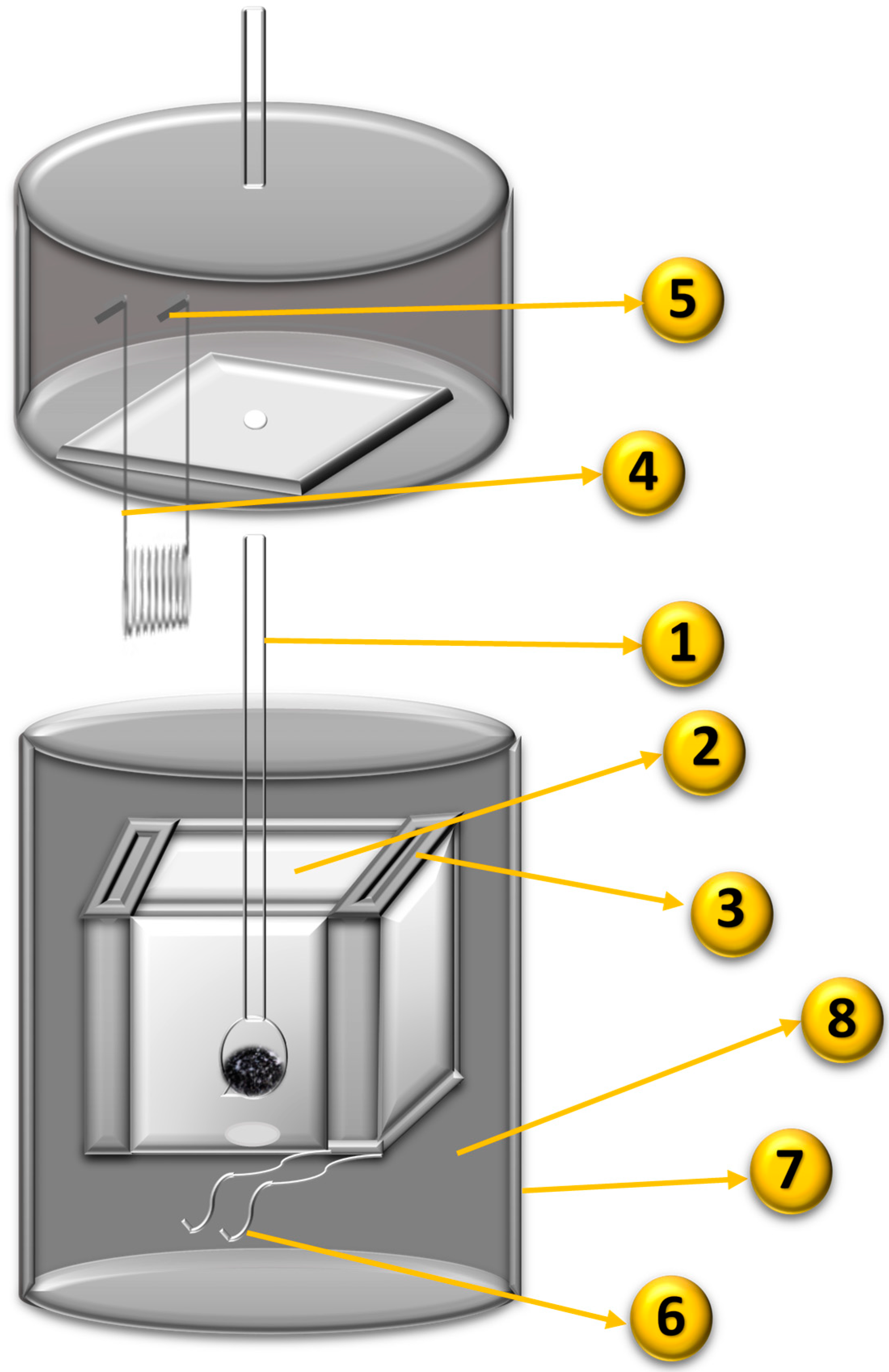
3.5. Calorimetric Evaluation of the Immersion Enthalpy for Hydrocarbons and Their Mixtures
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Piccin, J.S.; Cadaval, T.R.S.A.; De Pinto, L.A.A.; Dotto, G.L. Adsorption isotherms in liquid phase: Experimental, modeling, and interpretations. In Adsorption Processes for Water Treatment and Purification; Bonilla-Petriciolet, A., Mendoza-Castillo, D., Reynel-Ávila, H., Eds.; Springer: London, UK, 2017; pp. 19–51. ISBN 9783319581361. [Google Scholar]
- Rouquerol, J.; Rouquerol, F. Adsorption at the Liquid-Solid Interface: Thermodynamics and Methodology. In Adsorption by Powders and Porous Solids: Principles, Methodology and Applications, 2nd ed.; Rouquerol, J., Rouquerol, F., Sing, K., Llewellyn, P., Maurin, G., Eds.; Academic Press, Inc.: London, UK, 2014; pp. 105–158. ISBN 9780080970356. [Google Scholar]
- Giles, C.H.; MacEwan, T.H.; Nakhwa, S.N.; Smith, D. Studies in adsorption. Part XI. A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J. Chem. Soc. 1960, 3973–3993. [Google Scholar] [CrossRef]
- Giles, C.H.; Smith, D.; Huitson, A. A general treatment and classification of the solute adsorption isotherm. I. Theoretical. J. Colloid Interface Sci. 1974, 47, 755–765. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Sing, K.S.W.; Maurin, G.; Llewellyn, P. Introduction. In Adsorption by Powders and Porous Solids: Principles, Methodology and Applications; Rouquerol, F., Rouquerol, J., Sing, K., Llewellyn, P., Maurin, G., Eds.; Academic Press, Inc.: London, UK, 2014; pp. 1–24. ISBN 9780080970356. [Google Scholar]
- Limousin, G.; Gaudet, J.P.; Charlet, L.; Szenknect, S.; Barthès, V.; Krimissa, M. Sorption isotherms: A review on physical bases, modeling and measurement. Appl. Geochem. 2007, 22, 249–275. [Google Scholar] [CrossRef]
- Liu, L.; Luo, X.B.; Ding, L.; Luo, S.L. Application of Nanotechnology in the Removal of Heavy Metal From Water. In Nanomaterials for the Removal of Pollutants and Resource Reutilization; Luo, X.B., Deng, F., Eds.; Elsevier: London, UK, 2019; pp. 83–147. ISBN 9780128148389. [Google Scholar]
- Chen, X. Modeling of experimental adsorption isotherm data. Information 2015, 6, 14–22. [Google Scholar] [CrossRef]
- Kecili, R.; Hussain, C.M. Mechanism of adsorption on nanomaterials. In Nanomaterials in Chromatography: Current Trends in Chromatographic Research Technology and Techniques; Hussain, C.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 89–115. ISBN 9780128127926. [Google Scholar]
- Sing, K.S.W.; Rouquerol, F.; Rouquerol, J. Classical Interpretation of Physisorption Isotherms at the Gas-Solid Interface. In Adsorption by Powders and Porous Solids: Principles, Methodology and Applications; Rouquerol, F., Rouquerol, J., Sing, K., Llewellyn, P., Maurin, G., Eds.; Academic Press, Inc.: London, UK, 2014; pp. 159–189. ISBN 9780080970356. [Google Scholar]
- Laskar, I.I.; Hashisho, Z. Insights into modeling adsorption equilibria of single and multicomponent systems of organic and water vapors. Sep. Purif. Technol. 2020, 241, 116681–116703. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Haghseresht, F.; Lu, G.Q. Adsorption characteristics of phenolic compounds onto coal-reject-derived adsorbents. Energy Fuels 1998, 12, 1100–1107. [Google Scholar] [CrossRef]
- Mu, T.-H.; Sun, H.-N. Sweet Potato Leaf Polyphenols: Preparation, Individual Phenolic Compound Composition and Antioxidant Activity. In Polyphenols in Plants; Watson, R.R., Ed.; Academic Press, Inc.: London, UK, 2019; pp. 365–380. [Google Scholar]
- de Sá, A.; Abreu, A.S.; Moura, I.; Machado, A.V. Polymeric materials for metal sorption from hydric resources. In Water Purification; Grumezescu, A.M., Ed.; Academic Press, Inc.: London, UK, 2017; pp. 289–322. [Google Scholar]
- Wibowo, N.; Setyadhi, L.; Wibowo, D.; Setiawan, J.; Ismadji, S. Adsorption of benzene and toluene from aqueous solutions onto activated carbon and its acid and heat treated forms: Influence of surface chemistry on adsorption. J. Hazard. Mater. 2007, 146, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Amano, Y.; Machida, M.; Imazeki, F. Effect of Polarity of Activated Carbon Surface, Solvent and Adsorbate on Adsorption of Aromatic Compounds from Liquid Phase. Chem. Pharm. Bull. 2015, 63, 726–730. [Google Scholar] [CrossRef] [PubMed]
- Sheshdeh, R.K.; Abbasizadeh, S.; Nikou, M.R.K.; Badii, K.; Sharafi, M.S. Liquid Phase adsorption kinetics and equilibrium of toluene by novel modified-diatomite. J. Environ. Heal. Sci. Eng. 2014, 12, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Hindarso, H.; Ismadji, S.; Wicaksana, F.; Mudjijati; Indraswati, N. Adsorption of benzene and toluene from aqueous solution onto granular activated carbon. J. Chem. Eng. Data 2001, 46, 788–791. [Google Scholar] [CrossRef]
- Moro, A.M.; Brucker, N.; Charão, M.; Bulcão, R.; Freitas, F.; Baierle, M.; Nascimento, S.; Valentini, J.; Cassini, C.; Salvador, M.; et al. Evaluation of genotoxicity and oxidative damage in painters exposed to low levels of toluene. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2012, 746, 42–48. [Google Scholar] [CrossRef]
- Vulimiri, S.V.; Margaret Pratt, M.; Kulkarni, S.; Beedanagari, S.; Mahadevan, B. Reproductive and developmental toxicity of solvents and gases. In Reproductive and Developmental Toxicology; Gupta, R., Ed.; Academic Press, Inc.: Hopkinsville, KY, USA, 2017; pp. 379–396. ISBN 9780128042397. [Google Scholar]
- Clough, S.R. Toluene. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press, Inc.: Bethesda, MD, USA, 2014; pp. 595–598. ISBN 9780123864543. [Google Scholar]
- Gad, S.E. Cyclohexane. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press, Inc.: Bethesda, MD, USA, 2014; pp. 1106–1108. ISBN 9780123864543. [Google Scholar]
- Dada, E.A.; Achenie, L. Production of Cyclohexane from Hydrogenation of Benzene using Microreactor Technology. Comput. Aided Chem. Eng. 2012, 31, 240–244. [Google Scholar] [CrossRef]
- Clough, S.R. Hexane. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press, Inc.: Bethesda, MD, USA, 2014; pp. 900–904. ISBN 9780123864543. [Google Scholar]
- Farhad, K.; Brannagan, T.H. Neuropathy, Toxic. In Encyclopedia of the Neurological Sciences, 2nd ed.; Aminoff, M.J., Daroff, R.B., Eds.; Academic Press, Inc.: New York, NY, USA, 2014; pp. 511–515. ISBN 978-0-12-385158-1. [Google Scholar]
- Huang, H.; Xu, Y.; Feng, Q.; Leung, D.Y.C. Low temperature catalytic oxidation of volatile organic compounds: A review. Catal. Sci. Technol. 2015, 5, 2649–2669. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, B.; Creamer, A.E.; Cao, C.; Li, Y. Adsorption of VOCs onto engineered carbon materials: A review. J. Hazard. Mater. 2017, 338, 102–123. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Paek, D.; Kho, Y.L.; Choi, K.; Chae, H.J. Color vision impairments among shipyard workers exposed to mixed organic solvents, especially xylene. Neurotoxicol. Teratol. 2013, 37, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Betancur-Sánchez, A.M.; Vásquez-Trespalacios, E.M.; Sardi-Correa, C. Impaired colour vision in workers exposed to organic solvents: A systematic review. Arch. Soc. Española Oftalmol. 2017, 92, 12–18. [Google Scholar] [CrossRef]
- Park, H.; Park, H.D.; Jang, J.K. Exposure Characteristics of Construction Painters to Organic Solvents. Saf. Health Work 2016, 7, 63–71. [Google Scholar] [CrossRef]
- Hong-Li, W.; Sheng-Ao, J.; Sheng-Rong, L.; Qing-Yao, H.; Li, L.; Shi-Kang, T.; Cheng, H.; Li-Ping, Q.; Chang-Hong, C. Volatile organic compounds (VOCs) source profiles of on-road vehicle emissions in China. Sci. Total Environ. 2017, 607–608, 253–261. [Google Scholar] [CrossRef]
- Bates, M.N.; Reed, B.R.; Liu, S.; Eisen, E.A.; Hammond, S.K. Solvent exposure and cognitive function in automotive technicians. Neurotoxicology 2016, 57, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, L.; Moreno-Piraján, J.C. Instrumentación calorimétrica aplicada a la determinación de entalpias de inmersión de sólidos porosos. In Sólidos Porosos Preparación, Caracterización y Aplicaciones; Moreno-Piraján, J.C., Ed.; Ediciones Uniandes: Bogotá, Colombia, 2007; pp. 281–297. [Google Scholar]
- Moreno-Piraján, J.C.; Blanco, D.; Giraldo, L. Relation between the adsorbed quantity and the immersion enthalpy in catechol aqueous solutions on activated carbons. Int. J. Mol. Sci. 2012, 13, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Monje, D.; Giraldo, L.; Moreno-Piraján, J.C. Immersion enthalpy of benzene/cyclohexane and toluene/cyclohexane binary mixtures into modified activated carbons. J. Therm. Anal. Calorim. 2019, 138, 2565–2575. [Google Scholar] [CrossRef]
- Giraldo, L.; Moreno-Piraján, J.C. Enthalpic Contribution of Ni(II) in the Interaction between Carbonaceous Material and Aqueous Solution. J. Chem. 2017, 2017, 7308024. [Google Scholar] [CrossRef]
- Murillo, Y.S.; Giraldo, L.; Moreno-Piraján, J.C. Contribution enthalpic in the interaction of activated carbon with polar and apolar solvents. Arab. J. Chem. 2013, 6, 347–351. [Google Scholar] [CrossRef]
- Myers, A.L.; Monson, P.A. Physical adsorption of gases: The case for absolute adsorption as the basis for thermodynamic analysis. Adsorption 2014, 20, 591–622. [Google Scholar] [CrossRef]
- Garcia-Cuello, V.; Moreno-Piraján, J.C.; Giraldo-Gutiérrez, L.; Sapag, K.; Zgrablich, G. Determination of differential enthalpy and isotherm by adsorption calorimetry. Res. Lett. Phys. Chem. 2008, 2008, 127328. [Google Scholar] [CrossRef][Green Version]
- Llewellyn, P.L.; Maurin, G.; Poyet, T.; Dufau, N.; Denoyel, R.; Rouquerol, F. Do the differential enthalpies of adsorption vary between 77 K and 302 K? An experimental case study of argon and nitrogen on two faujasite type zeolites. Adsorption 2005, 11, 73–78. [Google Scholar] [CrossRef]
- Do, D.D.; Nicholson, D.; Fan, C. Development of equations for differential and integral enthalpy change of adsorption for simulation studies. Langmuir 2011, 27, 14290–14299. [Google Scholar] [CrossRef] [PubMed]
- Khaoula, H.; Imen, B.; Foued, M.; Jemni, A. Study of the adsorption interaction potential in a microporous solid. Int. J. Adv. Res. 2016, 4, 60–69. [Google Scholar] [CrossRef]
- Ternero-Hidalgo, J.J.; Rosas, J.M.; Palomo, J.; Valero-Romero, M.J.; Rodríguez-Mirasol, J.; Cordero, T. Functionalization of activated carbons by HNO3 treatment: Influence of phosphorus surface groups. Carbon 2016, 101, 409–419. [Google Scholar] [CrossRef]
- Vinke, P.; van der Eijk, M.; Verbree, M.; Voskamp, A.F.; van Bekkum, H. Modification of the surfaces of a gas activated carbon and a chemically activated carbon with nitric acid, hypochlorite, and ammonia. Carbon 1994, 32, 675–686. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R.; Freitas, M.M.A.; Órfão, J.J.M. Modification of the surface chemistry of activated carbons. Carbon 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Hernández-Monje, D.; Giraldo, L.; Moreno-Piraján, J.C. Interaction between Hydrocarbons C6 and Modified Activated Carbons: Correlation between Adsorption Isotherms and Immersion Enthalpies. ACS Omega 2019, 4, 19595–19604. [Google Scholar] [CrossRef] [PubMed]
- Montes-Morán, M.A.; Suárez, D.; Angel Menéndez, J.; Fuente, E. The Basicity of Carbons. In Novel Carbon Adsorbents; Tascón, J.M.D., Ed.; Elsevier: Oxford, UK, 2012; pp. 173–203. ISBN 9780080977447. [Google Scholar]
- Barkauskas, J.; Dervinyte, M. An investigation of the functional groups on the surface of activated carbons. J. Serb. Chem. Soc. 2004, 69, 363–375. [Google Scholar] [CrossRef]
- Bandosz, T.J.; Jagiello, J.; Contescu, C.; Schwarz, J.A. Characterization of the surfaces of activated carbons in terms of their acidity constant distributions. Carbon 1993, 31, 1193–1202. [Google Scholar] [CrossRef]
- Nielsen, L.; Biggs, M.J.; Skinner, W.; Bandosz, T.J. The effects of activated carbon surface features on the reactive adsorption of carbamazepine and sulfamethoxazole. Carbon 2014, 80, 419–432. [Google Scholar] [CrossRef]
- Fuente, E.; Menéndez, J.A.; Suárez, D.; Montes-Morán, M.A. Basic surface oxides on carbon materials: A global view. Langmuir 2003, 19, 3505–3511. [Google Scholar] [CrossRef]
- Webster, C.E.; Drago, R.S.; Zerner, M.C.; Gaines, V. Molecular Dimensions for Adsorptives. J. Am. Chem. Soc. 1998, 120, 5509–5516. [Google Scholar] [CrossRef]
- Shi, Q.; Yang, X.; Wu, L.; Liu, H.; Zhang, J.; Zhang, F.; Long, C. Binary adsorption equilibrium and breakthrough of toluene and cyclohexane on macroporous and hypercrosslinked polymeric resins. Microporous Mesoporous Mater. 2018, 271, 73–82. [Google Scholar] [CrossRef]
- Pei, J.; Zhang, J.S. Determination of adsorption isotherm and diffusion coefficient of toluene on activated carbon at low concentrations. Build. Environ. 2012, 48, 66–76. [Google Scholar] [CrossRef]
- Pei, J.; Zhang, J. Modeling of sorbent-based gas filters: Development, verification and experimental validation. Build. Simul. 2010, 3, 75–86. [Google Scholar] [CrossRef]
- Seo, J.; Kato, S.; Ataka, Y.; Chino, S. Performance test for evaluating the reduction of VOCs in rooms and evaluating the lifetime of sorptive building materials. Build. Environ. 2009, 44, 207–215. [Google Scholar] [CrossRef]
- Iovino, P.; Canzano, S.; Capasso, S.; Di Natale, M.; Erto, A.; Lama, A.; Musmarra, D. Single and competitive adsorption of toluene and naphthalene onto activated carbon. Chem. Eng. Trans. 2013, 32, 67–72. [Google Scholar] [CrossRef]
- Zhou, K.; Ma, W.; Zeng, Z.; Ma, X.; Xu, X.; Guo, Y.; Li, H.; Li, L. Experimental and DFT study on the adsorption of VOCs on activated carbon/metal oxides composites. Chem. Eng. J. 2019, 372, 1122–1133. [Google Scholar] [CrossRef]
- Thongsai, N.; Jaiyong, P.; Kladsomboon, S.; In, I.; Paoprasert, P. Utilization of carbon dots from jackfruit for real-time sensing of acetone vapor and understanding the electronic and interfacial interactions using density functional theory. Appl. Surf. Sci. 2019, 487, 1233–1244. [Google Scholar] [CrossRef]
- Shimoyama, T.; Tashima, K.; Ruike, M. Grand canonical Monte Carlo simulation study of cyclohexane, oxane, 1,4-dioxane, and 1,3,5-trioxane confined in carbon slit pore. Colloids Surf. A Physicochem. Eng. Asp. 2017, 533, 255–266. [Google Scholar] [CrossRef]
- García-Rivero, M.; Saucedo-Castañeda, G.; Gutiérrez-Rojas, M. Organic solvents improve hydrocarbon desorption and biodegradation in highly contaminated weathered soils. J. Environ. Eng. Sci. 2007, 6, 389–395. [Google Scholar] [CrossRef]
- Anuradha, S.; Raj, K.J.A.; Elangovan, T.; Viswanathan, B. Adsorption of VOC on steam activated carbon derived from coconut shell charcoal. Indian J. Chem. Technol. 2014, 21, 345–349. [Google Scholar]
- Bedolla, P.O.; Feldbauer, G.; Wolloch, M.; Eder, S.J.; Dörr, N.; Mohn, P.; Redinger, J.; Vernes, A. Effects of van der Waals interactions in the adsorption of isooctane and ethanol on Fe(100) surfaces. J. Phys. Chem. C 2014, 118, 17608–17615. [Google Scholar] [CrossRef]
- Erto, A.; Chianese, S.; Lancia, A.; Musmarra, D. On the mechanism of benzene and toluene adsorption in single-compound and binary systems: Energetic interactions and competitive effects. Desalin. Water Treat. 2017, 86, 259–265. [Google Scholar] [CrossRef]
- Selmi, T.; Sanchez-Sanchez, A.; Gadonneix, P.; Jagiello, J.; Seffen, M.; Sammouda, H.; Celzard, A.; Fierro, V. Tetracycline removal with activated carbons produced by hydrothermal carbonisation of Agave americana fibres and mimosa tannin. Ind. Crops Prod. 2018, 115, 146–157. [Google Scholar] [CrossRef]
- Mbarki, F.; Selmi, T.; Kesraoui, A.; Seffen, M.; Gadonneix, P.; Celzard, A.; Fierro, V. Hydrothermal pre-treatment, an efficient tool to improve activated carbon performances. Ind. Crops Prod. 2019, 140, 111717–111727. [Google Scholar] [CrossRef]
- Salame, I.I.; Bandosz, T.J. Surface chemistry of activated carbons: Combining the results of temperature-programmed desorption, Boehm, and potentiometric titrations. J. Colloid Interface Sci. 2001, 240, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Estupiñán, P.; Legnoverde, M.S.; Simonetti, S.; Díaz Compañy, A.; Juan, A.; Giraldo, L.; Moreno-Piraján, J.C.; Basaldella, E.I. Influence of functionalization, surface area and charge distribution of SBA15-based adsorbents on CO (II) and NI (II) removal from aqueous solutions. J. Environ. Chem. Eng. 2020, 8, 103671–103700. [Google Scholar] [CrossRef]
- Jagiełło, J. Stable Numerical Solution of the Adsorption Integral Equation Using Splines. Langmuir 1994, 10, 2778–2785. [Google Scholar] [CrossRef]
- Contescu, A.; Contescu, C.; Putyera, K.; Schwarz, J.A. Surface acidity of carbons characterized by their continuous pK distribution and Boehm titration. Carbon 1997, 35, 83–94. [Google Scholar] [CrossRef]
- Moreno-Piraján, J.C.; Giraldo, L.; García-Cuello, V.; Vargas Delgadillo, D.P.; Rodríguez-Estupiñán, P.; Murillo-Acevedo, Y.; Cantillo, M. Thermodynamic of the Interactions Between Gas-Solid and Solid-Liquid on Carbonaceous Materials. In Thermodynamics—Interaction Studies—Solids, Liquids and Gases; Moreno-Piraján, J.C., Ed.; InTech Open: London, UK, 2011; pp. 164–195. [Google Scholar]

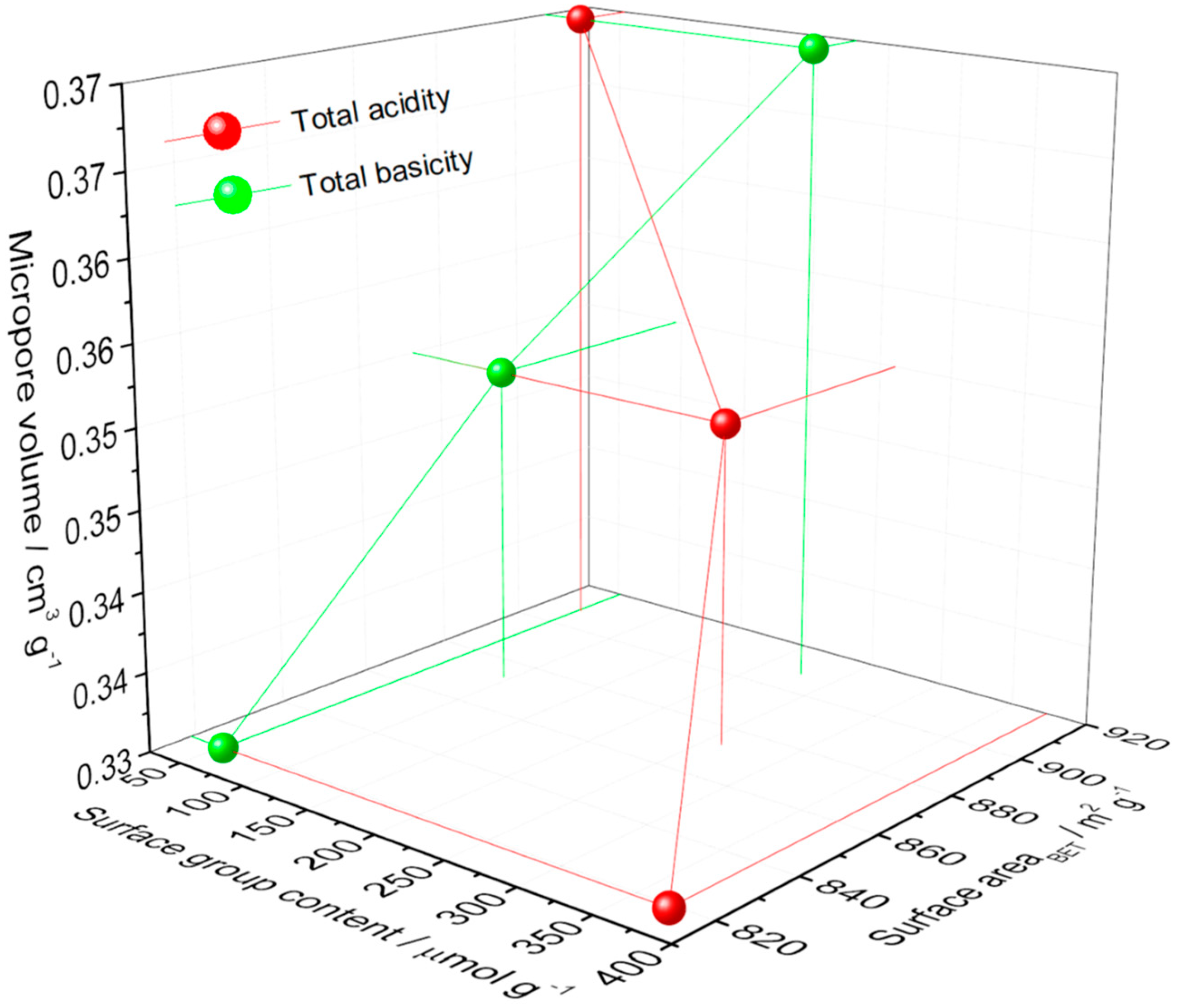

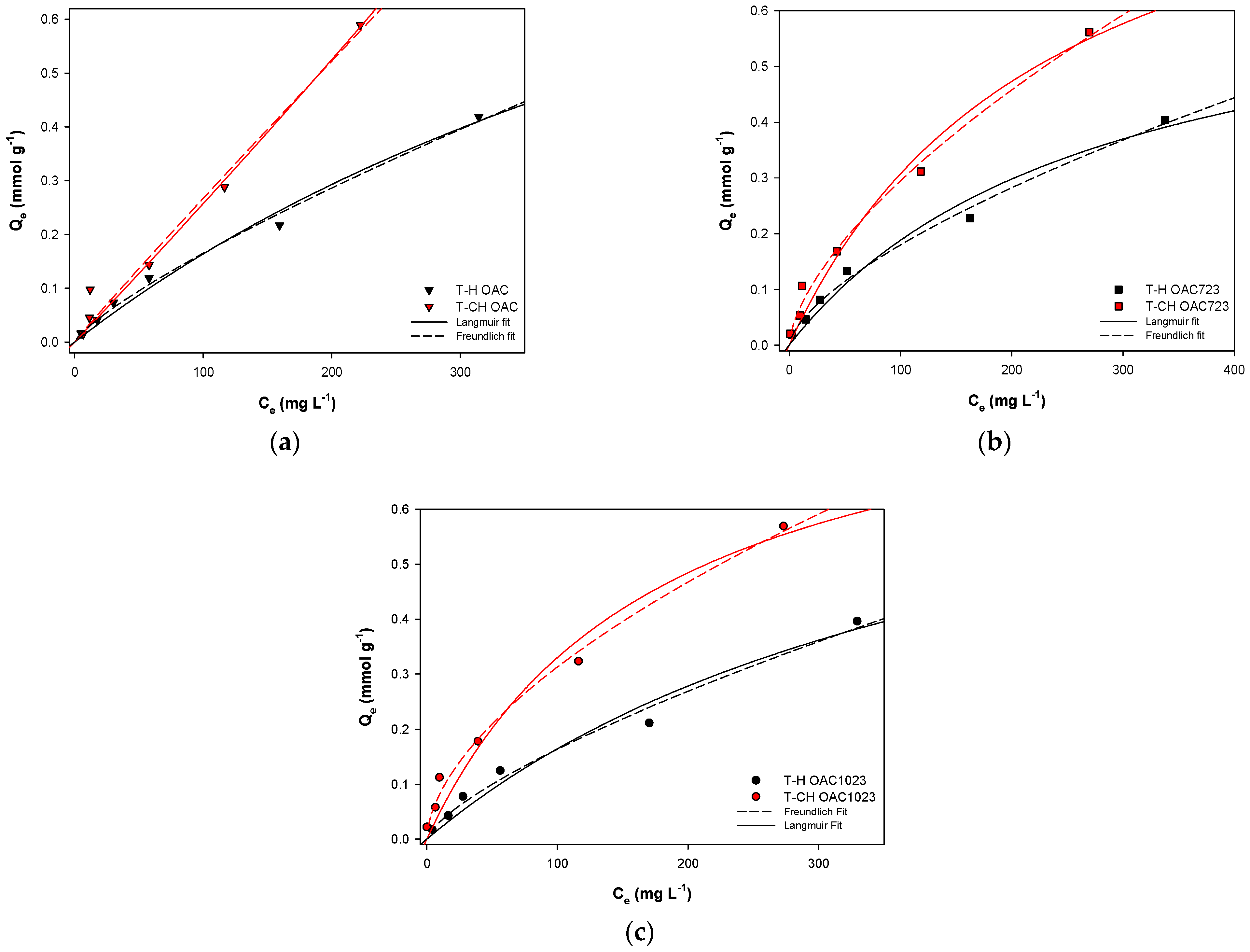
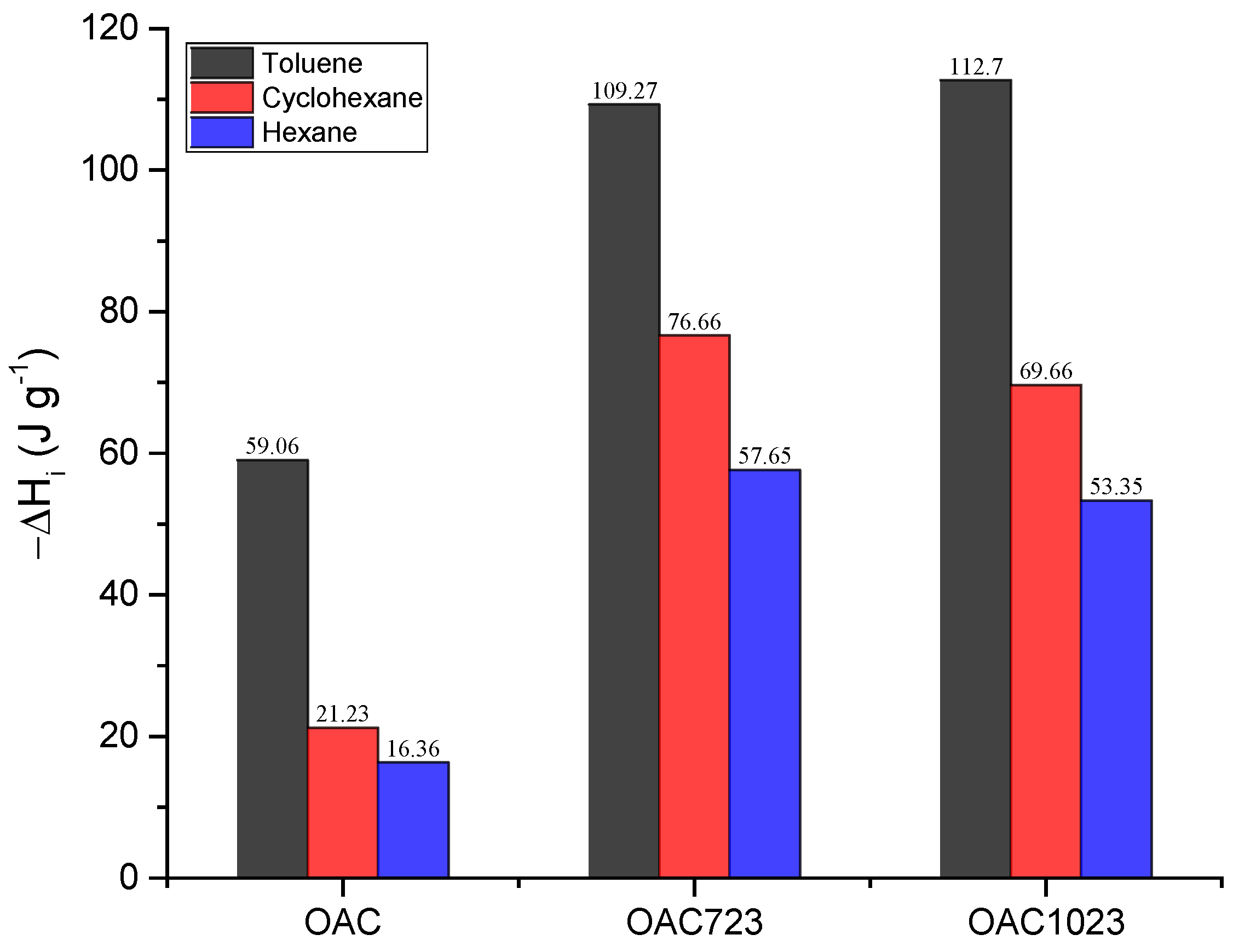
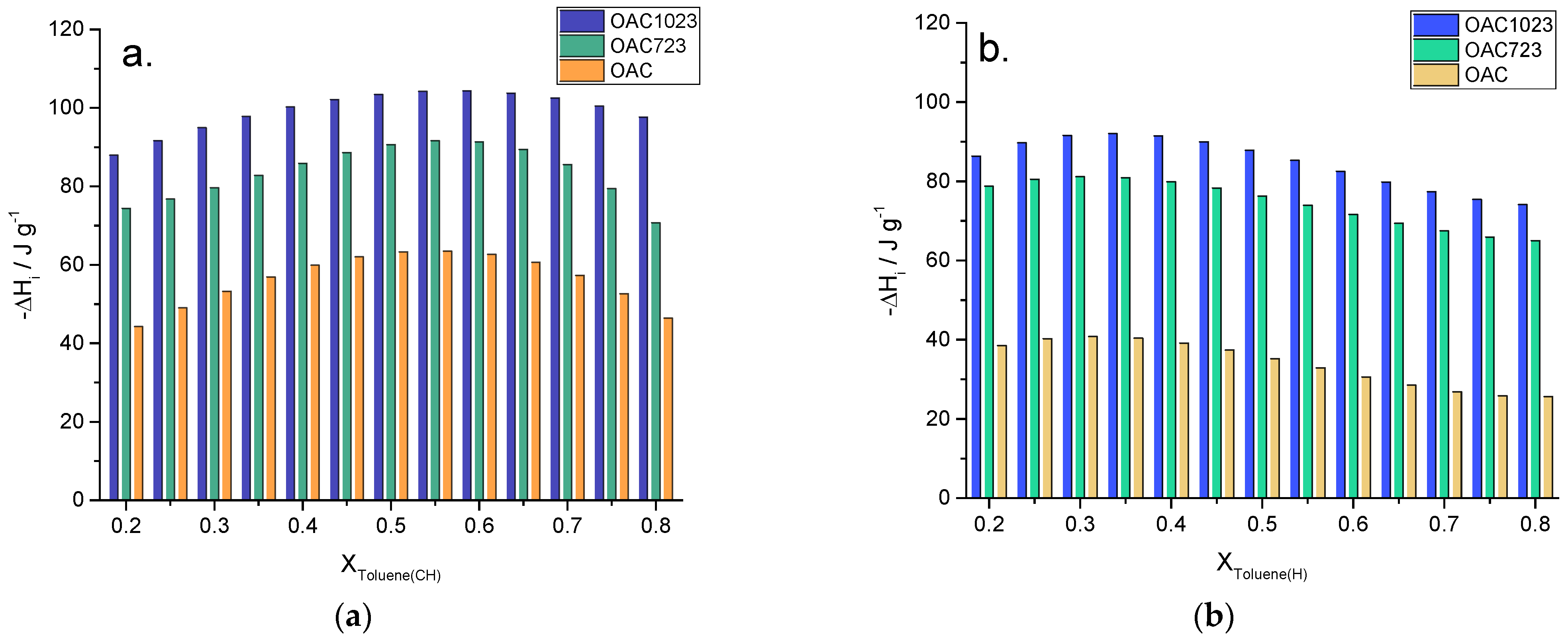

| Model | Langmuir [8,9,10,11,12] | Freundlich [8,11,12,13,14,15,16] |
|---|---|---|
| Statements | The surface has finite active centers with the equal probability to adsorb a single molecule. The energy of adsorption in monolayer is the highest. More pressure is needed for formation of successive layers. | Empirical model for reversible and non-ideal adsorption. Different energies of adsorption for the active sites. Heterogeneous surface where multilayer can be formed. |
| Equation | nm (mmol g−1): maximum adsorption capacity b (L mmol−1): Langmuir constant associated with adsorption energy Ce (mmol L−1): Concentration at equilibrium | Kf (mmol g−1 * mmol L−1): Freundlich constant related to adsorption capacity Ce (mmol L−1): Concentration at equilibrium 1/n: related to surface heterogeneity |
| Samples | OAC | OAC723 | OAC1023 | ||
|---|---|---|---|---|---|
| pKa < 7 | pKa 2–4 | Range | 2.7–3.5 | 3.2–4.0 | 3.6–4.8 |
| Peak | 3.15 | 3.58 | 4.16 | ||
| Content (mmol g−1) | 0.08 | 0.02 | 0.07 | ||
| pKa 4–7 | Range | 4.7–5.6 | 4.8–5.8 | 5.4–6.4 | |
| Peak | 5.14 | 5.29 | 5.95 | ||
| Content (mmol g−1) | 0.03 | 0.04 | 0.04 | ||
| pKa > 7 | pKa 7–8 | Range | 7.0–8.0 | ||
| Peak | 7.43 | ||||
| Content (mmol g−1) | 0.05 | ||||
| pKa 10–12 | Range | 10.2–11.2 | 10.6–11.6 | 10.8–11.8 | |
| Peak | 10.7 | 11.09 | 11.27 | ||
| Content (mmol g−1) | 0.59 | 1.02 | 1.01 | ||
| Model↓ | System → | Toluene–Hexane | Toluene–Cyclohexane | ||||
|---|---|---|---|---|---|---|---|
| OAC | OAC723 | OAC1023 | OAC | OAC723 | OAC1023 | ||
| Langmuir | nm (mmol g−1) | 0.416 | 0.714 | 0.903 | 0.724 | 1.026 | 0.910 |
| b (L mmol−1) | 0.007 | 0.004 | 0.002 | 0.005 | 0.004 | 0.006 | |
| R2 | 0.986 | 0.977 | 0.970 | 0.915 | 0.974 | 0.967 | |
| Freundlich | Kf (mmol g−1) (mmol L−1) | 0.004 | 0.009 | 0.006 | 0.003 | 0.016 | 0.022 |
| 1/n | 0.795 | 0.652 | 0.715 | 0.968 | 0.636 | 0.580 | |
| R2 | 0.993 | 0.993 | 0.986 | 0.979 | 0.992 | 0.992 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Monje, D.; Giraldo, L.; Moreno-Piraján, J.C. Enthalpic and Liquid-Phase Adsorption Study of Toluene–Cyclohexane and Toluene–Hexane Binary Systems on Modified Activated Carbons. Molecules 2021, 26, 2839. https://doi.org/10.3390/molecules26102839
Hernández-Monje D, Giraldo L, Moreno-Piraján JC. Enthalpic and Liquid-Phase Adsorption Study of Toluene–Cyclohexane and Toluene–Hexane Binary Systems on Modified Activated Carbons. Molecules. 2021; 26(10):2839. https://doi.org/10.3390/molecules26102839
Chicago/Turabian StyleHernández-Monje, Diana, Liliana Giraldo, and Juan Carlos Moreno-Piraján. 2021. "Enthalpic and Liquid-Phase Adsorption Study of Toluene–Cyclohexane and Toluene–Hexane Binary Systems on Modified Activated Carbons" Molecules 26, no. 10: 2839. https://doi.org/10.3390/molecules26102839
APA StyleHernández-Monje, D., Giraldo, L., & Moreno-Piraján, J. C. (2021). Enthalpic and Liquid-Phase Adsorption Study of Toluene–Cyclohexane and Toluene–Hexane Binary Systems on Modified Activated Carbons. Molecules, 26(10), 2839. https://doi.org/10.3390/molecules26102839







