Characterization of 18F-PM-PBB3 (18F-APN-1607) Uptake in the rTg4510 Mouse Model of Tauopathy
Abstract
1. Introduction
2. Results
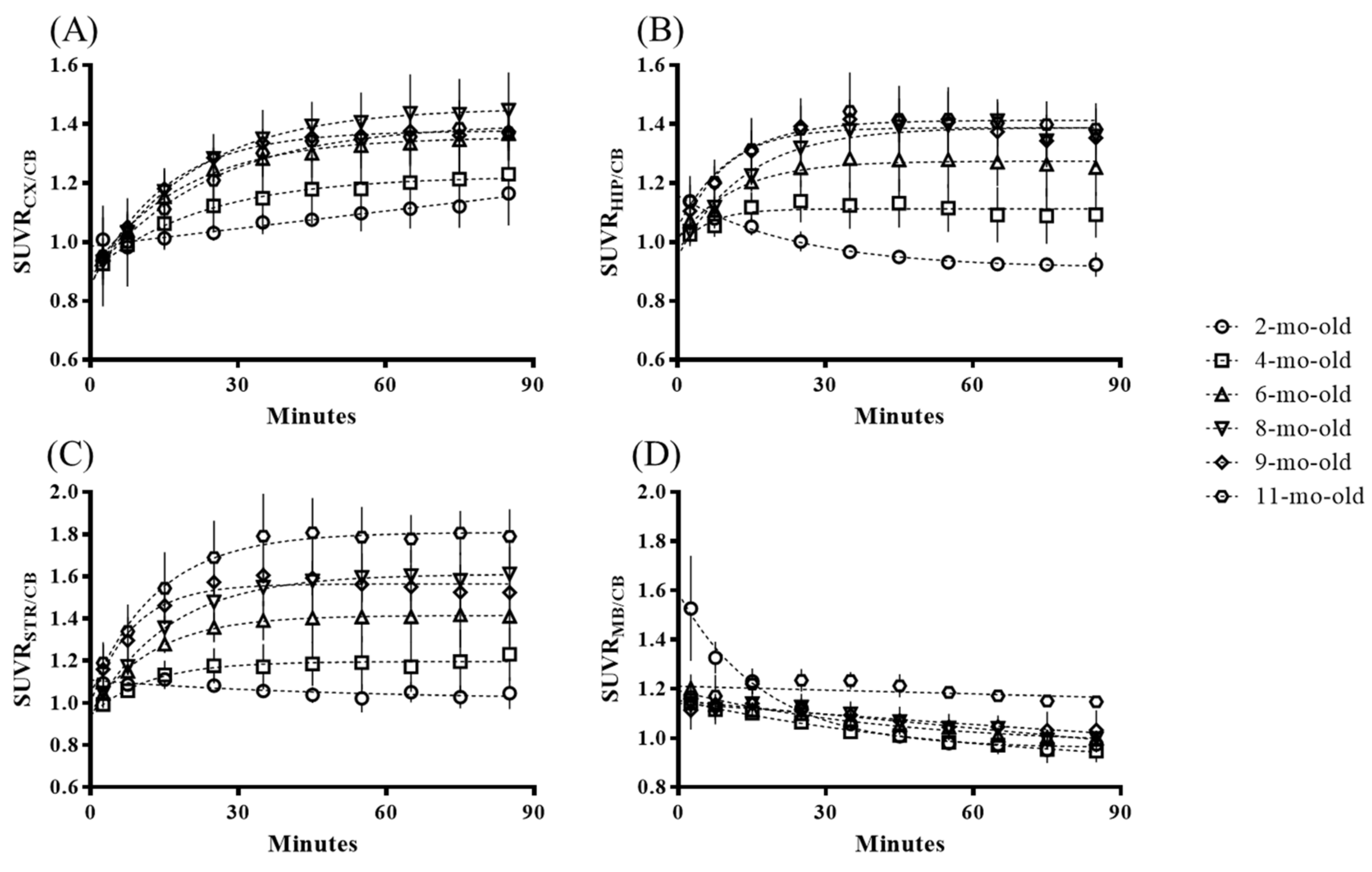
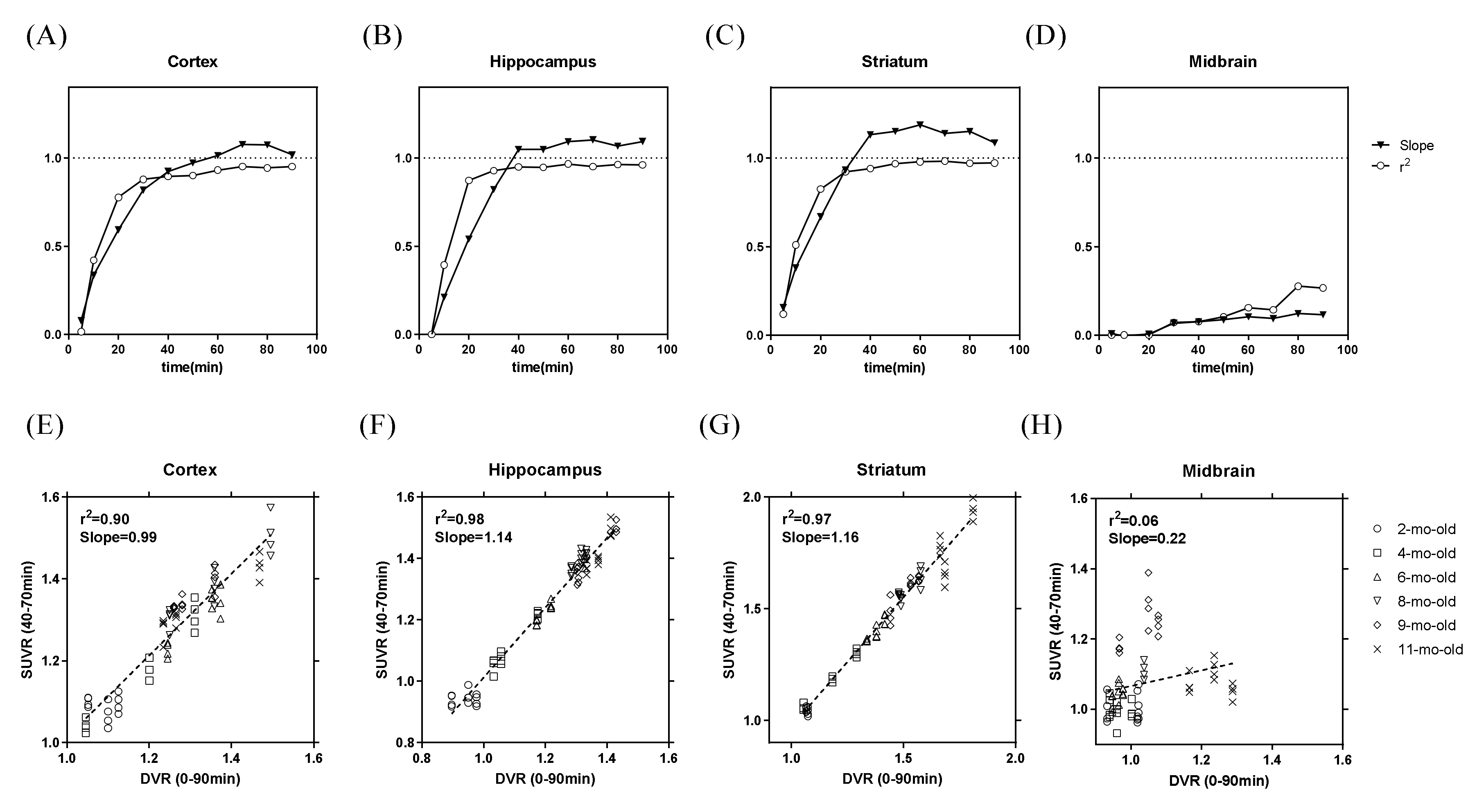
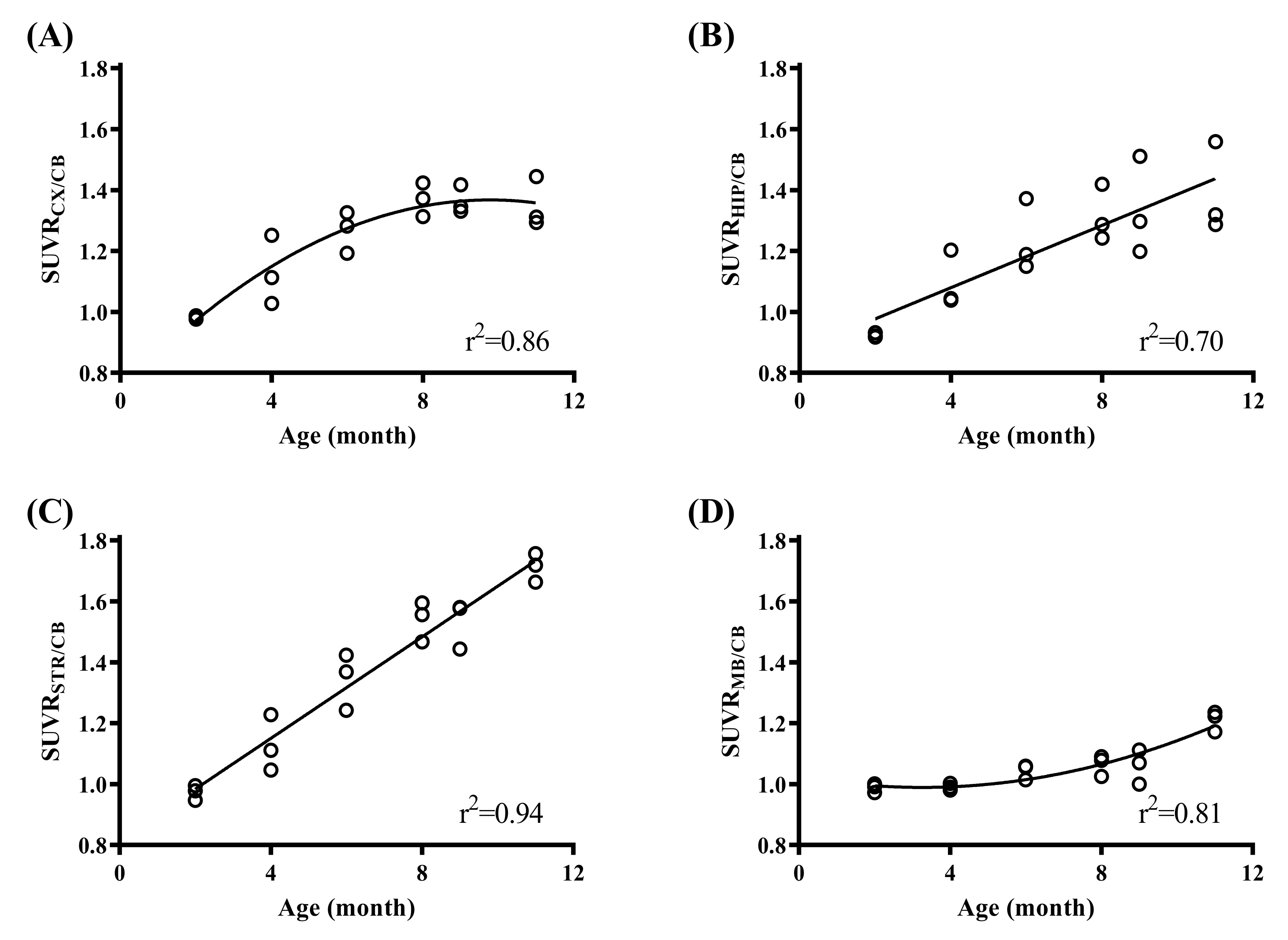
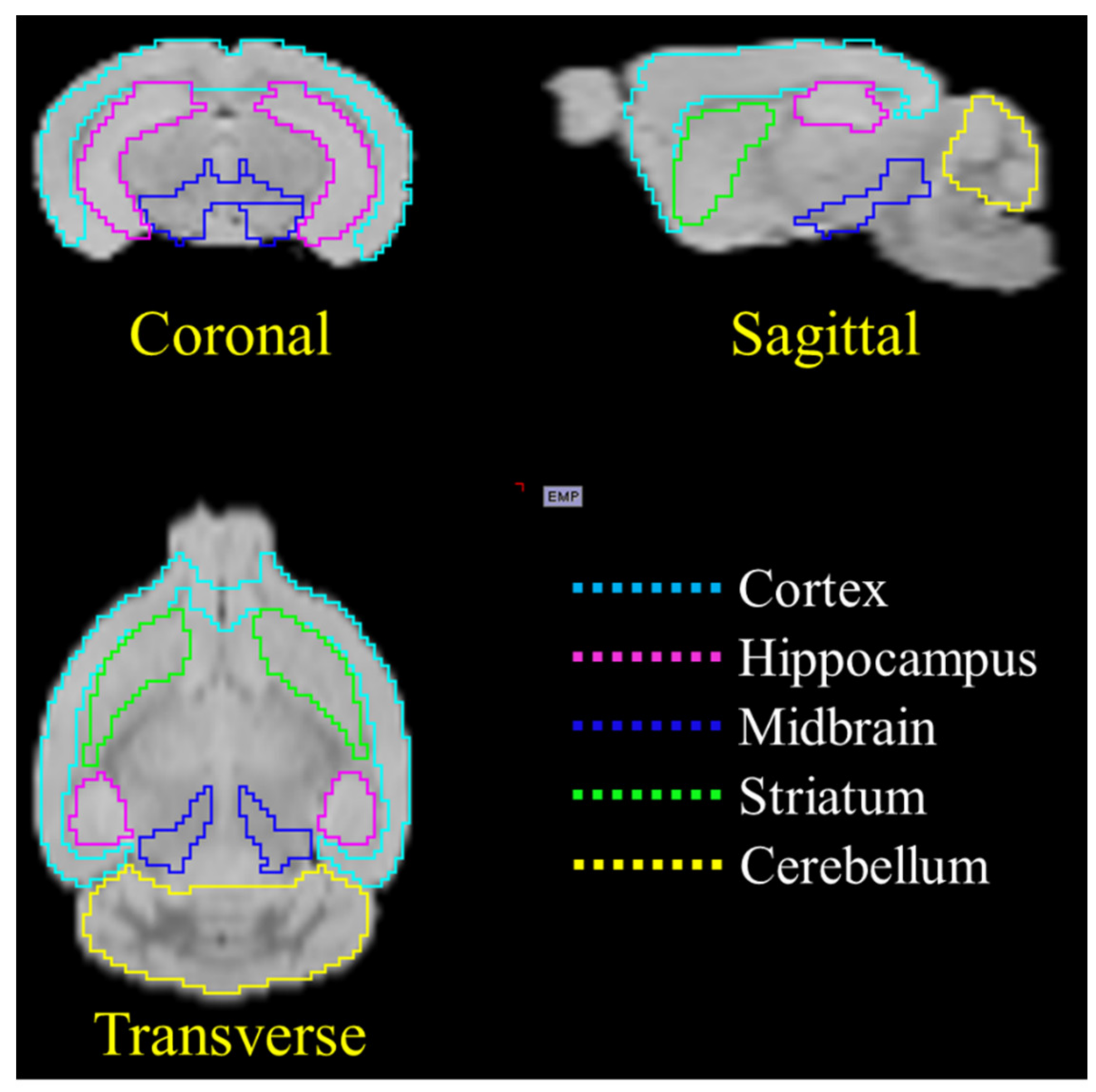
2.1. In Vivo Animal PET Studies
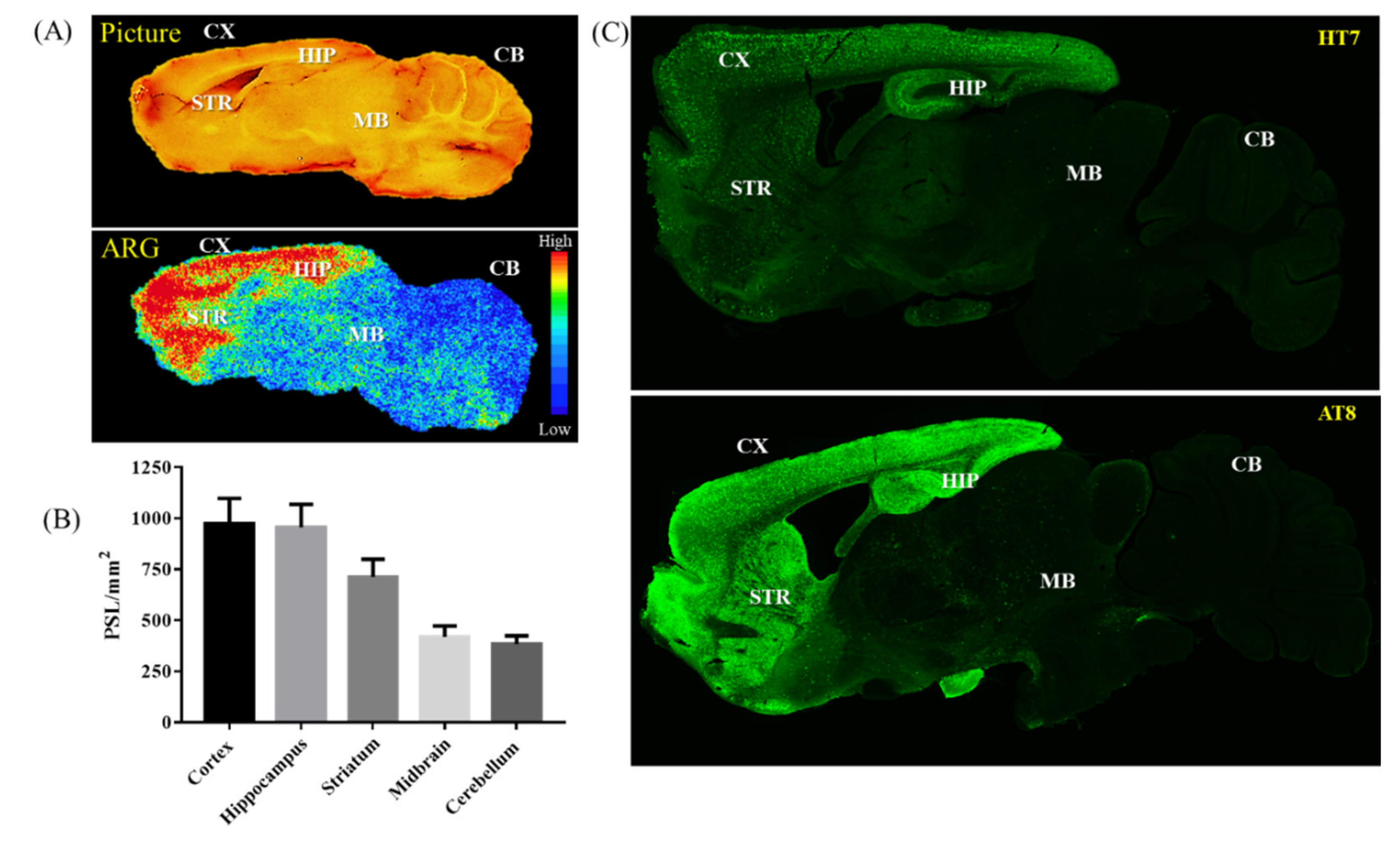
2.2. Ex Vivo Autoradiography
2.3. Immunofluorescence Staining
3. Discussion
4. Materials and Methods
4.1. Radiochemistry
4.2. Animals
4.3. In Vivo Animal PET Studies
4.4. Ex Vivo Autoradiography
4.5. Immunofluorescence Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ziegler-Graham, K.; Brookmeyer, R.; Johnson, E.; Arrighi, H.M. Worldwide variation in the doubling time of Alzheimer’s disease incidence rates. Alzheimer’s Dement. 2008, 4, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.C.; Storandt, M.; Miller, J.P.; McKeel, D.W.; Price, J.L.; Rubin, E.H.; Berg, L. Mild cognitive impairment represents early-stage Alzheimer disease. Arch. Neurol. 2001, 58, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Villemagne, V.L.; Fodero-Tavoletti, M.T.; Masters, C.L.; Rowe, C.C. Tau imaging: Early progress and future directions. Lancet Neurol. 2015, 14, 114–124. [Google Scholar] [CrossRef]
- Villemagne, V.L.; Doré, V.; Burnham, S.C.; Masters, C.L.; Rowe, C.C. Imaging tau and amyloid-β proteinopathies in Alzheimer disease and other conditions. Nat. Rev. Neurol. 2018, 14, 225–236. [Google Scholar] [CrossRef]
- Camus, V.; Payoux, P.; Barré, L.; Desgranges, B.; Voisin, T.; Tauber, C.; La Joie, R.; Tafani, M.; Hommet, C.; Chételat, G. Using PET with 18 F-AV-45 (florbetapir) to quantify brain amyloid load in a clinical environment. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 621–631. [Google Scholar] [CrossRef]
- Kung, H.F.; Choi, S.R.; Qu, W.; Zhang, W.; Skovronsky, D. 18F stilbenes and styrylpyridines for PET imaging of Aβ plaques in Alzheimer’s disease: A miniperspective. J. Med. Chem. 2009, 53, 933–941. [Google Scholar] [CrossRef]
- Morris, E.; Chalkidou, A.; Hammers, A.; Peacock, J.; Summers, J.; Keevil, S. Diagnostic accuracy of 18 F amyloid PET tracers for the diagnosis of Alzheimer’s disease: A systematic review and meta-analysis. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 374–385. [Google Scholar] [CrossRef]
- Thal, D.R.; Beach, T.G.; Zanette, M.; Heurling, K.; Chakrabarty, A.; Ismail, A.; Smith, A.P.; Buckley, C. [18F] flutemetamol amyloid positron emission tomography in preclinical and symptomatic Alzheimer’s disease: Specific detection of advanced phases of amyloid-β pathology. Alzheimer’s Dement. 2015, 11, 975–985. [Google Scholar] [CrossRef]
- Rabinovici, G.; Jagust, W. Amyloid imaging in aging and dementia: Testing the amyloid hypothesis in vivo. Behav. Neurol. 2009, 21, 117–128. [Google Scholar] [CrossRef]
- Brendel, M.; Jaworska, A.; Probst, F.; Overhoff, F.; Korzhova, V.; Lindner, S.; Carlsen, J.; Bartenstein, P.; Harada, R.; Kudo, Y. Small-animal PET imaging of tau pathology with 18F-THK5117 in 2 transgenic mouse models. J. Nucl. Med. 2016, 57, 792–798. [Google Scholar] [CrossRef]
- Fodero-Tavoletti, M.T.; Okamura, N.; Furumoto, S.; Mulligan, R.S.; Connor, A.R.; McLean, C.A.; Cao, D.; Rigopoulos, A.; Cartwright, G.A.; O’Keefe, G. 18F-THK523: A novel in vivo tau imaging ligand for Alzheimer’s disease. Brain 2011, 134, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Chiotis, K.; Saint-Aubert, L.; Savitcheva, I.; Jelic, V.; Andersen, P.; Jonasson, M.; Eriksson, J.; Lubberink, M.; Almkvist, O.; Wall, A. Imaging in-vivo tau pathology in Alzheimer’s disease with THK5317 PET in a multimodal paradigm. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1686–1699. [Google Scholar] [CrossRef] [PubMed]
- Saint-Aubert, L.; Almkvist, O.; Chiotis, K.; Almeida, R.; Wall, A.; Nordberg, A. Regional tau deposition measured by [18 F] THK5317 positron emission tomography is associated to cognition via glucose metabolism in Alzheimer’s disease. Alzheimer’s Res. Ther. 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Harada, R.; Okamura, N.; Furumoto, S.; Furukawa, K.; Ishiki, A.; Tomita, N.; Tago, T.; Hiraoka, K.; Watanuki, S.; Shidahara, M. 18F-THK5351: A novel PET radiotracer for imaging neurofibrillary pathology in Alzheimer disease. J. Nucl. Med. 2016, 57, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.M.; Lee, S.-Y.; Seo, S.; Jeong, H.J.; Woo, S.-H.; Lee, H.; Lee, Y.-B.; Yeon, B.K.; Shin, D.H.; Park, K.H. Tau positron emission tomography using [18F] THK5351 and cerebral glucose hypometabolism in Alzheimer’s disease. Neurobiol. Aging 2017, 59, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Harada, R.; Ishiki, A.; Kai, H.; Sato, N.; Furukawa, K.; Furumoto, S.; Tago, T.; Tomita, N.; Watanuki, S.; Hiraoka, K. Correlations of 18F-THK5351 PET with postmortem burden of tau and astrogliosis in Alzheimer disease. J. Nucl. Med. 2018, 59, 671–674. [Google Scholar] [CrossRef]
- Ishikawa, A.; Tokunaga, M.; Maeda, J.; Minamihisamatsu, T.; Shimojo, M.; Takuwa, H.; Ono, M.; Ni, R.; Hirano, S.; Kuwabara, S. In vivo visualization of tau accumulation, microglial activation, and brain atrophy in a mouse model of tauopathy rTg4510. J. Alzheimer’s Dis. 2018, 61, 1037–1052. [Google Scholar] [CrossRef]
- Chien, D.T.; Bahri, S.; Szardenings, A.K.; Walsh, J.C.; Mu, F.; Su, M.-Y.; Shankle, W.R.; Elizarov, A.; Kolb, H.C. Early clinical PET imaging results with the novel PHF-tau radioligand [F-18]-T807. J. Alzheimer’s Dis. 2013, 34, 457–468. [Google Scholar] [CrossRef]
- Chien, D.T.; Szardenings, A.K.; Bahri, S.; Walsh, J.C.; Mu, F.; Xia, C.; Shankle, W.R.; Lerner, A.J.; Su, M.-Y.; Elizarov, A. Early clinical PET imaging results with the novel PHF-tau radioligand [F18]-T808. J. Alzheimer’s Dis. 2014, 38, 171–184. [Google Scholar] [CrossRef]
- Xia, C.-F.; Arteaga, J.; Chen, G.; Gangadharmath, U.; Gomez, L.F.; Kasi, D.; Lam, C.; Liang, Q.; Liu, C.; Mocharla, V.P. [18F] T807, a novel tau positron emission tomography imaging agent for Alzheimer’s disease. Alzheimer’s Dement. 2013, 9, 666–676. [Google Scholar] [CrossRef]
- Hostetler, E.D.; Walji, A.M.; Zeng, Z.; Miller, P.; Bennacef, I.; Salinas, C.; Connolly, B.; Gantert, L.; Haley, H.; Holahan, M. Preclinical characterization of 18F-MK-6240, a promising PET tracer for in vivo quantification of human neurofibrillary tangles. J. Nucl. Med. 2016, 57, 1599–1606. [Google Scholar] [CrossRef]
- Vermeiren, C.; Motte, P.; Viot, D.; Mairet-Coello, G.; Courade, J.P.; Citron, M.; Mercier, J.; Hannestad, J.; Gillard, M. The tau positron-emission tomography tracer AV-1451 binds with similar affinities to tau fibrils and monoamine oxidases. Mov. Disord. 2018, 33, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, M.; Shimada, H.; Suhara, T.; Shinotoh, H.; Ji, B.; Maeda, J.; Zhang, M.-R.; Trojanowski, J.Q.; Lee, V.M.-Y.; Ono, M. Imaging of tau pathology in a tauopathy mouse model and in Alzheimer patients compared to normal controls. Neuron 2013, 79, 1094–1108. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Sahara, N.; Kumata, K.; Ji, B.; Ni, R.; Koga, S.; Dickson, D.W.; Trojanowski, J.Q.; Lee, V.M.; Yoshida, M. Distinct binding of PET ligands PBB3 and AV-1451 to tau fibril strains in neurodegenerative tauopathies. Brain 2017, 140, 764–780. [Google Scholar] [CrossRef] [PubMed]
- Shimada, H.; Kitamura, S.; Shinotoh, H.; Endo, H.; Niwa, F.; Hirano, S.; Kimura, Y.; Zhang, M.-R.; Kuwabara, S.; Suhara, T. Association between Aβ and tau accumulations and their influence on clinical features in aging and Alzheimer’s disease spectrum brains: A [11C] PBB3-PET study. Alzheimer’s Dement. 2017, 6, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Kitamura, S.; Shimada, H.; Sahara, N.; Takuwa, H.; Yasymasa, Y.; Trojanowski, J.; Virginia, L.; Tetsuya, S.; Zhang, M.-R.; et al. Development of novel tau PET tracers, [18F]AMPBB3 and [18F]PM-PBB3. Presented at 11th Human Amyloid Imaging, Miami, FL, USA, 15–17 January 2017; p. 34. Available online: https://hai.worldeventsforum.com/past_editions/ (accessed on 31 March 2020).
- Huang, C.-C.; Hsiao, I.-T.; Lin, K.-J.; Lian, C.-F.; Hsu, J.-L.; Huang, K.-L. Optimal scanning time for 18F-APN-1607 (18F-PMPBB3) Tau PET Imaging. Presented at 13th Human Amyloid Imaging, Miami, FL, USA, 16–18 January 2019; p. 143. Available online: https://hai.worldeventsforum.com/past_editions/ (accessed on 31 March 2020).
- Santacruz, K.; Lewis, J.; Spires, T.; Paulson, J.; Kotilinek, L.; Ingelsson, M.; Guimaraes, A.; DeTure, M.; Ramsden, M.; McGowan, E. Tau suppression in a neurodegenerative mouse model improves memory function. Science 2005, 309, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, M.; Kotilinek, L.; Forster, C.; Paulson, J.; McGowan, E.; SantaCruz, K.; Guimaraes, A.; Yue, M.; Lewis, J.; Carlson, G.; et al. Age-dependent neurofibrillary tangle formation, neuron loss, and memory impairment in a mouse model of human tauopathy (P301L). J. Neurosci. 2005, 25, 10637–10647. [Google Scholar] [CrossRef]
- Braak, H.; Alafuzoff, I.; Arzberger, T.; Kretzschmar, H.; Del Tredici, K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006, 112, 389–404. [Google Scholar] [CrossRef]
- Ni, R.; Ji, B.; Ono, M.; Sahara, N.; Zhang, M.-R.; Aoki, I.; Nordberg, A.; Suhara, T.; Higuchi, M. Comparative in vitro and in vivo quantifications of pathologic tau deposits and their association with neurodegeneration in tauopathy mouse models. J. Nucl. Med. 2018, 59, 960–966. [Google Scholar] [CrossRef]
- Villemagne, V.L.; Furumoto, S.; Fodero-Tavoletti, M.T.; Mulligan, R.S.; Hodges, J.; Harada, R.; Yates, P.; Piguet, O.; Pejoska, S.; Doré, V. In vivo evaluation of a novel tau imaging tracer for Alzheimer’s disease. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 816–826. [Google Scholar] [CrossRef]
- Logan, J.; Fowler, J.S.; Volkow, N.D.; Wang, G.J.; Ding, Y.S.; Alexoff, D.L. Distribution volume ratios without blood sampling from graphical analysis of PET data. J. Cereb. Blood Flow Metab. 1996, 16, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Wu, F.; Zhang, Y.; Gong, C.-X.; Iqbal, K.; Liu, F. Expression of Tau Pathology-Related Proteins in Different Brain Regions: A Molecular Basis of Tau Pathogenesis. Front. Aging Neurosci. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, S.N.; Baker, S.L.; Okamura, N.; Furukawa, K.; Ishiki, A.; Furumoto, S.; Tashiro, M.; Yanai, K.; Arai, H.; Kudo, Y. Dynamic PET measures of tau accumulation in cognitively normal older adults and Alzheimer’s disease patients measured using [18F] THK-5351. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, B.J.; Klunk, W.E.; Mathis, C.A.; Hoge, J.A.; Ziolko, S.K.; Lu, X.; Meltzer, C.C.; Schimmel, K.; Tsopelas, N.D.; DeKosky, S.T. Simplified quantification of Pittsburgh Compound B amyloid imaging PET studies: A comparative analysis. J. Nucl. Med. 2005, 46, 1959–1972. [Google Scholar] [PubMed]
- Sahara, N.; Maeda, J.; Ishikawa, A.; Tokunaga, M.; Suhara, T.; Higuchi, M. Microglial activation during pathogenesis of tauopathy in rTg4510 mice: Implications for the early diagnosis of tauopathy. J. Alzheimer’s Dis. 2018, 64, S353–S359. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Braunstein, K.; Zhang, J.; Lau, A.; Sibener, L.; Deeble, C.; Wong, P. The neuritic plaque facilitates pathological conversion of tau in an Alzheimer’s disease mouse model. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef]
- Majid, T.; Ali, Y.O.; Venkitaramani, D.V.; Jang, M.-K.; Lu, H.-C.; Pautler, R.G. In vivo axonal transport deficits in a mouse model of fronto-temporal dementia. NeuroImage: Clin. 2014, 4, 711–717. [Google Scholar] [CrossRef]
- Tzourio-Mazoyer, N.; Landeau, B.; Papathanassiou, D.; Crivello, F.; Etard, O.; Delcroix, N.; Mazoyer, B.; Joliot, M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002, 15, 273–289. [Google Scholar] [CrossRef]
- Weng, C.-C.; Chen, Z.-A.; Chao, K.-T.; Ee, T.-W.; Lin, K.-J.; Chan, M.-H.; Hsiao, T.; Yen, T.-C.; Kung, M.-P.; Hsu, C.-H. Quantitative analysis of the therapeutic effect of magnolol on MPTP-induced mouse model of Parkinson’s disease using in vivo 18F-9-fluoropropyl-(+)-dihydrotetrabenazine PET imaging. PLoS ONE 2017, 12. [Google Scholar] [CrossRef]
- Lammertsma, A.A.; Hume, S.P. Simplified reference tissue model for PET receptor studies. Neuroimage 1996, 4, 153–158. [Google Scholar] [CrossRef]
- Wu, Y.; Carson, R.E. Noise reduction in the simplified reference tissue model for neuroreceptor functional imaging. J. Cereb. Blood Flow Metab. 2002, 22, 1440–1452. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the precursor of 18F-PM-PBB3 (18F-APN-1607) and its standards are available from the authors. |


| 2-mo-old | 4-mo-old | 6-mo-old | 8-mo-old | 9-mo-old | 11-mo-old | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CX | 0.98 | ± | 0.01 | 1.13 | ± | 0.11 | 1.27 | ± | 0.07* | 1.37 | ± | 0.06** | 1.36 | ± | 0.05** | 1.35 | ± | 0.08* |
| HIP | 0.92 | ± | 0.01 | 1.10 | ± | 0.09 | 1.24 | ± | 0.12* | 1.32 | ± | 0.09* | 1.34 | ± | 0.16* | 1.39 | ± | 0.15* |
| STR | 0.97 | ± | 0.02 | 1.13 | ± | 0.09 | 1.34 | ± | 0.09* | 1.54 | ± | 0.07** | 1.53 | ± | 0.08** | 1.71 | ± | 0.05*** |
| MB | 0.99 | ± | 0.02 | 0.99 | ± | 0.01 | 1.04 | ± | 0.03 | 1.06 | ± | 0.03 | 1.06 | ± | 0.06 | 1.21 | ± | 0.03** |
| * p < 0.05; ** p < 0.01; *** p < 0.001 | ||||||||||||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weng, C.-C.; Hsiao, I.-T.; Yang, Q.-F.; Yao, C.-H.; Tai, C.-Y.; Wu, M.-F.; Yen, T.-C.; Jang, M.-K.; Lin, K.-J. Characterization of 18F-PM-PBB3 (18F-APN-1607) Uptake in the rTg4510 Mouse Model of Tauopathy. Molecules 2020, 25, 1750. https://doi.org/10.3390/molecules25071750
Weng C-C, Hsiao I-T, Yang Q-F, Yao C-H, Tai C-Y, Wu M-F, Yen T-C, Jang M-K, Lin K-J. Characterization of 18F-PM-PBB3 (18F-APN-1607) Uptake in the rTg4510 Mouse Model of Tauopathy. Molecules. 2020; 25(7):1750. https://doi.org/10.3390/molecules25071750
Chicago/Turabian StyleWeng, Chi-Chang, Ing-Tsung Hsiao, Qing-Fang Yang, Cheng-Hsiang Yao, Chin-Yin Tai, Meng-Fang Wu, Tzu-Chen Yen, Ming-Kuei Jang, and Kun-Ju Lin. 2020. "Characterization of 18F-PM-PBB3 (18F-APN-1607) Uptake in the rTg4510 Mouse Model of Tauopathy" Molecules 25, no. 7: 1750. https://doi.org/10.3390/molecules25071750
APA StyleWeng, C.-C., Hsiao, I.-T., Yang, Q.-F., Yao, C.-H., Tai, C.-Y., Wu, M.-F., Yen, T.-C., Jang, M.-K., & Lin, K.-J. (2020). Characterization of 18F-PM-PBB3 (18F-APN-1607) Uptake in the rTg4510 Mouse Model of Tauopathy. Molecules, 25(7), 1750. https://doi.org/10.3390/molecules25071750





