Electrocatalytic Assisted Performance Enhancement for the Na-S Battery in Nitrogen-Doped Carbon Nanospheres Loaded with Fe
Abstract
1. Introduction
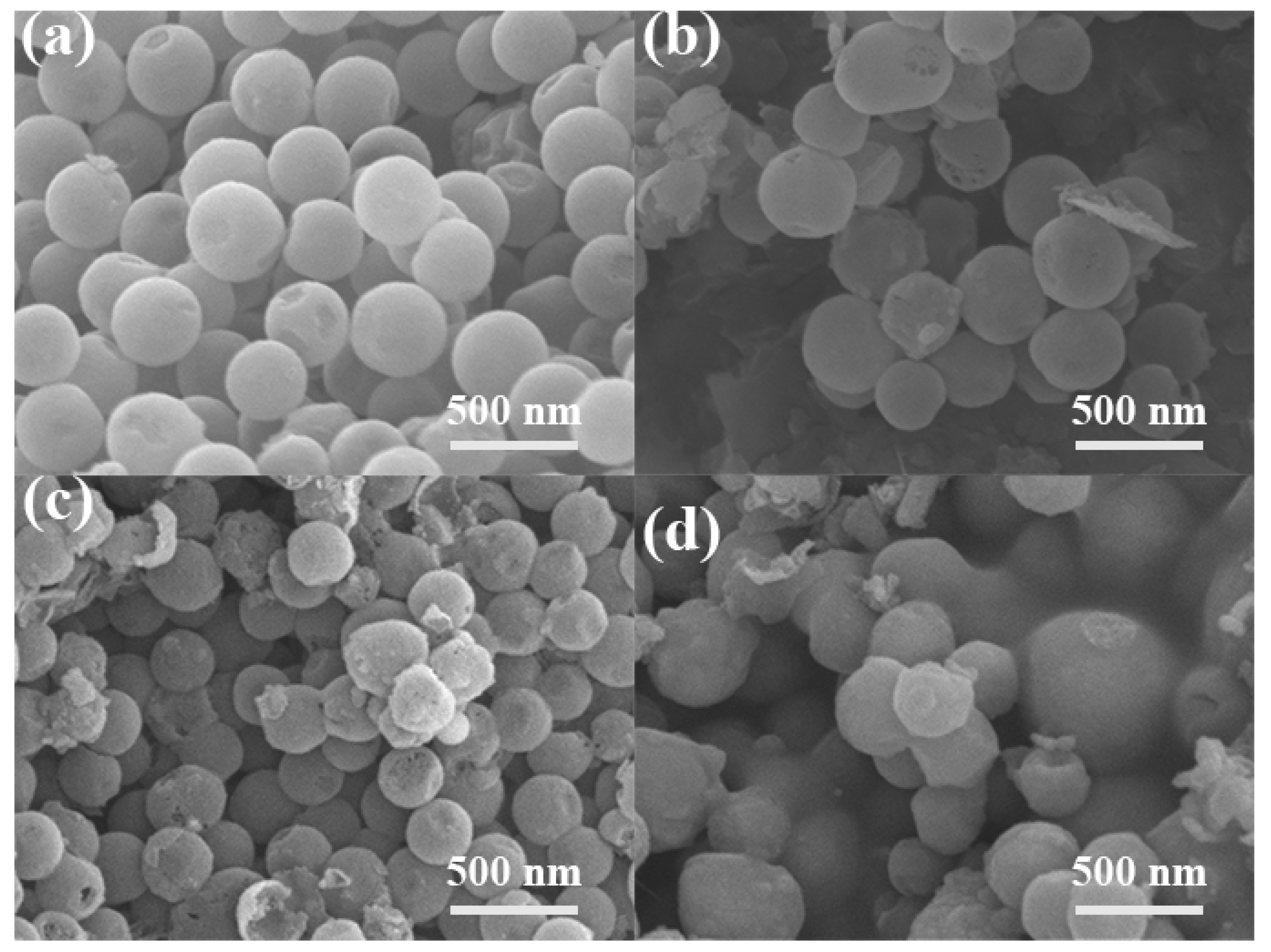
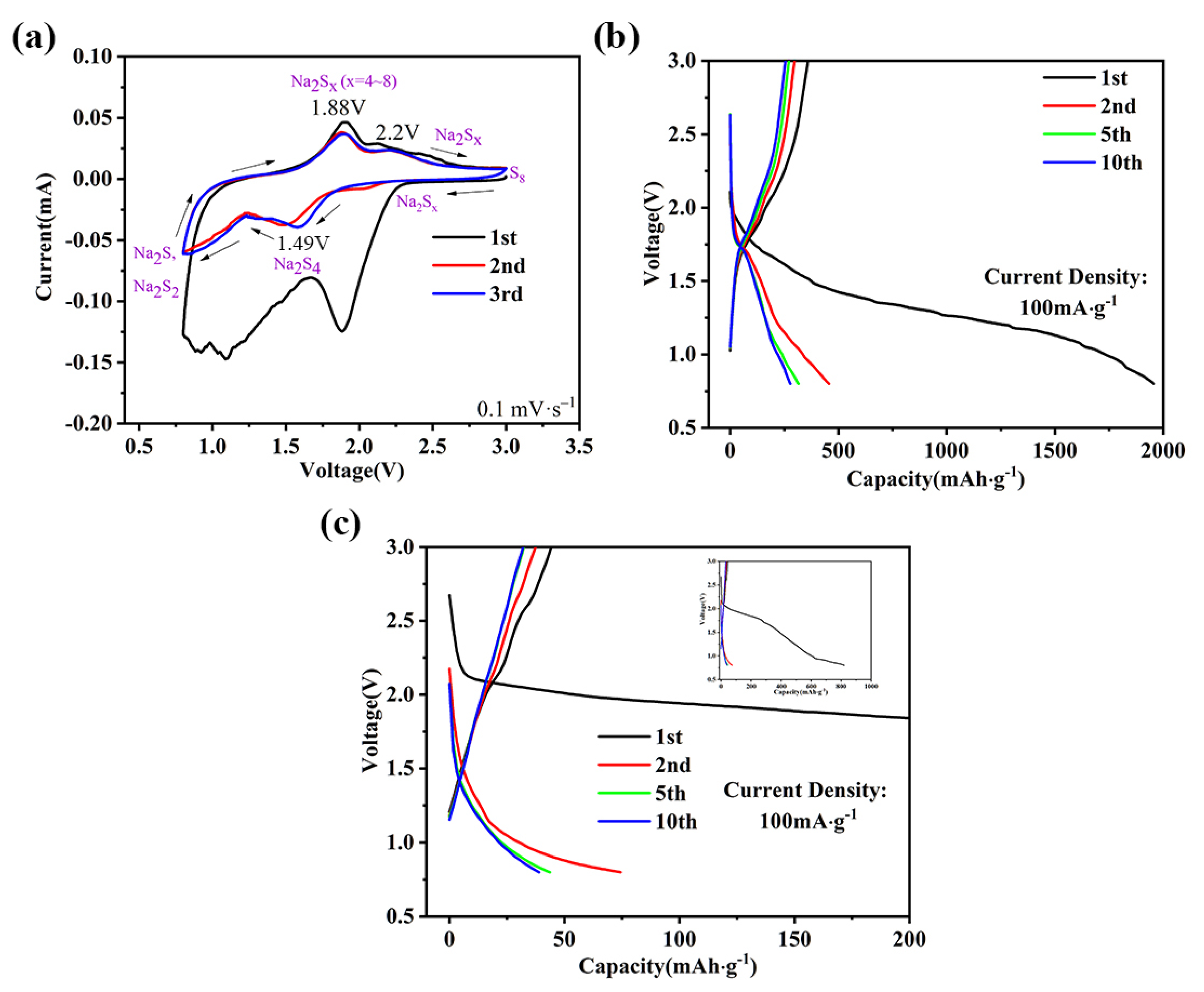
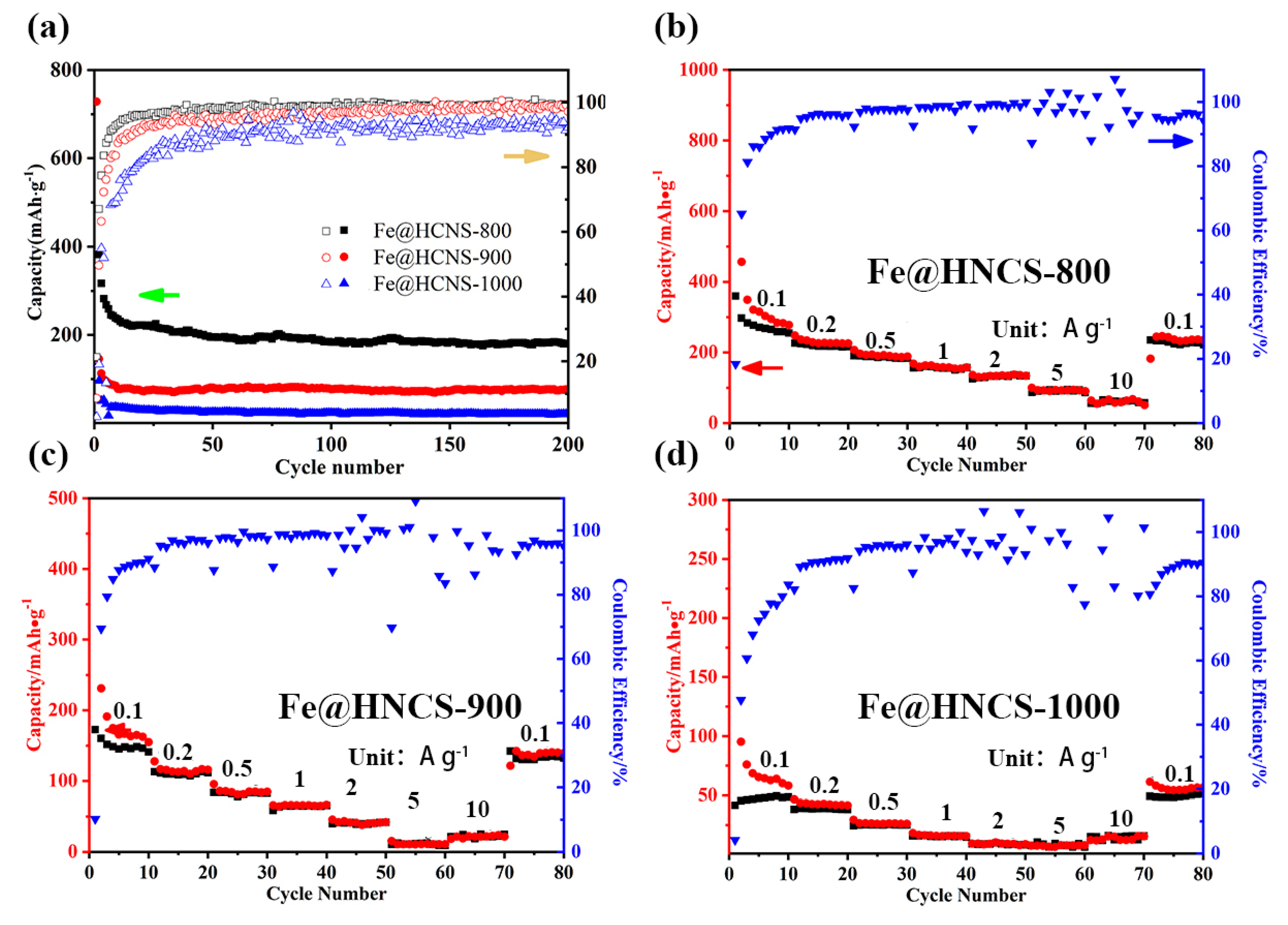
2. Results and Discussion
3. Experimental
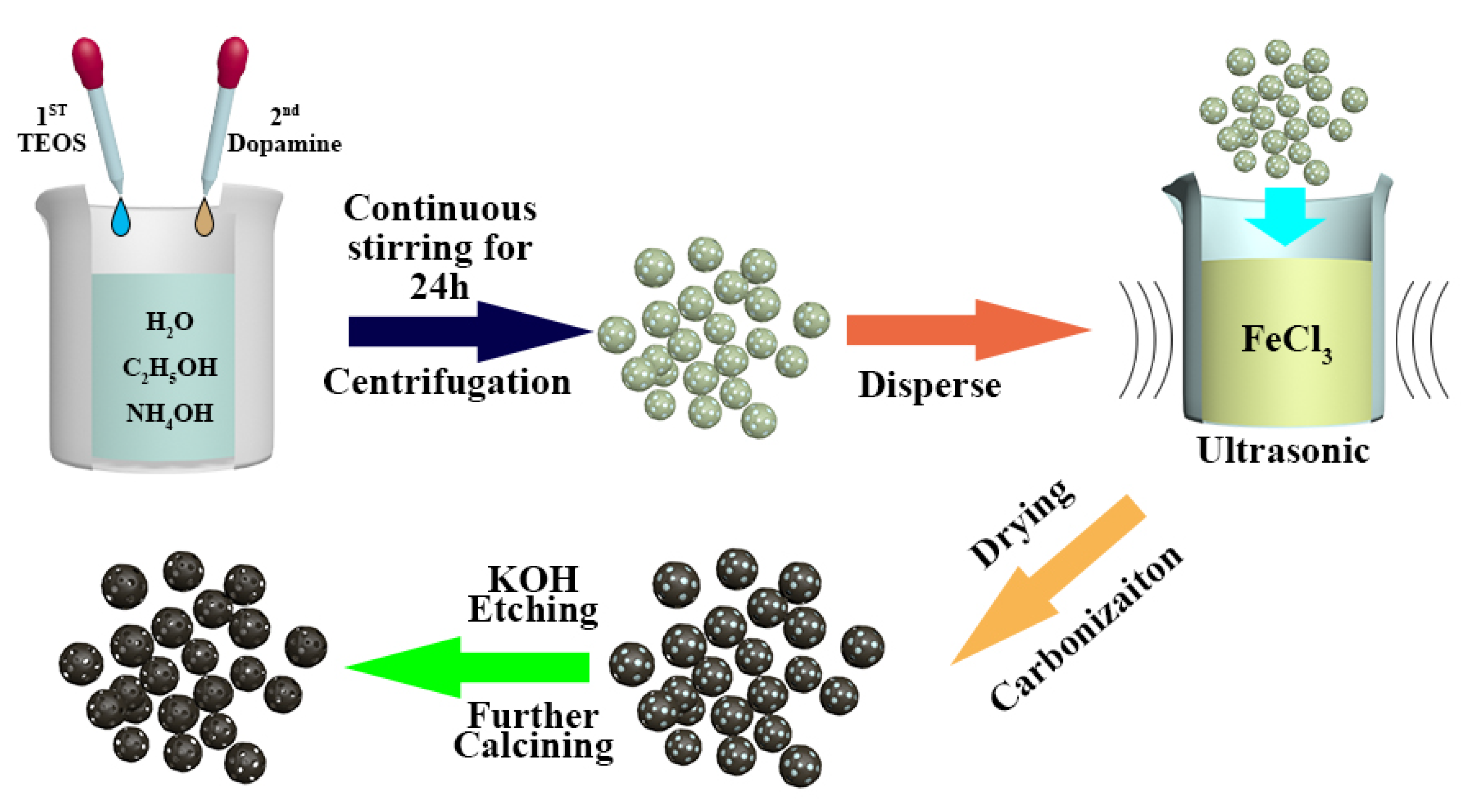
3.1. Preparation of HNCS and Fe@HNCS
3.2. Characterization
3.3. Electrochemical Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yao, L.; Lin, J.; Yang, H.; Wu, Q.; Wang, D.; Li, X.; Deng, L.; Zheng, Z. Two-dimensional hierarchically porous carbon nanosheets for flexible aqueous supercapacitors with high volumetric capacitance. Nanoscale 2019, 11, 11086. [Google Scholar] [CrossRef] [PubMed]
- Jayarama, S.; Jain, A.; Ulaganathan, M.; Edison, E.; Srinivasan, M.P.; Balasubramanian, R.; Aravindan, V.; Madhavi, S. Li-ion vs. Na-ion capacitors: A performance evaluation with coconut shell derived mesoporous carbon and natural plant based hard carbon. Chem. Eng. J. 2017, 316, 506–513. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Myung, S.-T.; Sun, Y.-K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Mei, J.; Wen, L.; Tong, Y.; Vasileff, A.; Wang, L.; Liang, J.; Sun, Z.; Dou, S. Nonlithium Metal-Sulfur Batteries: Steps Toward a leap. Adv. Mater. 2019, 31, 1802822. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Z.; Yao, L.; Chen, S.; Zhang, P.; Deng, L. Confined growth of NiCo2S4 nanosheets on carbon flakes derived from eggplant with enhanced performance for asymmetric supercapacitors. Chem. Eng. J. 2019, 366, 550–559. [Google Scholar] [CrossRef]
- Tang, W.; Wu, J.; Wang, X.; Xia, X.; Tu, J. Integrated carbon nanospheres arrays as anode materials for boosted sodium ion storage. Green Energy Environ. 2018, 3, 50–55. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical energy storage for the grid: a battery of choices. Science 2011, 334, 928. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Rogers, J.A.; Someya, T.; Huang, Y. Materials and mechanics for stretchable electronics. Science 2010, 327, 1603. [Google Scholar] [CrossRef]
- Sathiya, M.; Rousse, G.; Ramesha, K.; Laisa, C.P.; Vezin, H.; Sougrati, M.T.; Doublet, M.-L.; Foix, D.; Gonbeau, D.; Walker, W.; et al. Reversible anionic redox chemistry in high-capacity layered-oxide electrodes. Nat. Mater. 2013, 12, 827–835. [Google Scholar] [CrossRef]
- Tan, G.; Wu, F.; Yuan, Y.; Chen, R.; Zhao, T.; Yao, Y.; Qian, J.; Liu, J.; Ye, Y.; Shahbazian-Yassar, R.; et al. Freestanding three-dimensional core-shell nanoarrays for lithium-ion battery anodes. Nat. Commun. 2016, 7, 11774. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Yao, L.; Li, Z.; Zhang, P.; Zhong, W.; Yuan, Q.; Deng, L. Hybrid hollow spheres of carbon @CoxNi1-xMoO4 as advanced electrodes for high-performance asymmetric supercapacitors. Nanoscale 2019, 11, 3281–3291. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Zhong, W.; Wang, J.; Zhang, P.; Fang, H.; Yao, L.; Liu, X.; Ren, X.; Li, Y. The enhancement of electrochemical capacitance of biomass-carbon by pyrolysis of extracted nanofibers. Electrochim. Acta 2017, 228, 398–406. [Google Scholar] [CrossRef]
- Väli, R.; Jänes, A.; Thomberg, T.; Lust, E. Synthesis and characterization of D-glucose derived nanospheric hard carbon negative electrodes for lithium- and sodium-ion batteries. Electrochim. Acta 2017, 253, 536–544. [Google Scholar] [CrossRef]
- Yang, C.; Yin, Y.; Guo, Y.; Wan, L. Electrochemical (de)lithiation of 1D sulfur chains in Li-S batteries: A model system study. J. Am. Chem. Soc. 2015, 137, 2215–2218. [Google Scholar] [CrossRef] [PubMed]
- Manthiram, A.; Fu, Y.; Chung, S.; Zu, C.; Su, Y. Rechargeable lithium-sulfur batteries. Chem. Rev. 2014, 114, 11751–11787. [Google Scholar] [CrossRef]
- Seh, Z.; Sun, Y.; Zhang, Q.; Cui, Y. Designing high-energy lithium-sulfur batteries. Chem. Soc. Rev. 2016, 45, 5605–5634. [Google Scholar] [CrossRef]
- Ji, X.; Lee, K.; Nazar, L. A highly ordered nanostructured carbonsulphur cathode for lithium-sulphur batteries. Nat. Mater. 2009, 8, 500–506. [Google Scholar] [CrossRef]
- Zhou, G.; Paek, E.; Hwang, G.; Manthiram, A. Long-life Li/polysulphide batteries with high sulphur loading enabled by lightweight three-dimensional nitrogen/sulphur-codoped graphene sponge. Nat. Commun. 2015, 6, 7760. [Google Scholar] [CrossRef]
- Park, C.; Ahn, J.; Ryu, H.; Kim, K.; Ahn, H. Room-temperature solid-state sodium/sulfur battery. Electrochem. Solid State 2006, 9, A123–A125. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, B.; Lai, W.; Xu, Y.; Chou, S.; Liu, H.; Dou, S. Room-Temperature Sodium-Sulfur Batteries: A Comprehensive Review on Research Progress and Cell Chemistry. Adv. Energy Mater. 2017, 7, 1602829. [Google Scholar] [CrossRef]
- Lu, X.; Kirby, B.W.; Xu, W.; Li, G.; Kim, J.Y.; Lemmon, J.P.; Sprenkle, V.L.; Yang, Z. Advanced intermediate-temperature Na-S battery. Energy Environ. Sci. 2013, 6, 299–306. [Google Scholar] [CrossRef]
- Wen, Z.; Hu, Y.; Wu, X.; Han, J.; Gu, Z. Main Challenges for High Performance NAS Battery: Materials and Interfaces. Adv. Funct. Mater. 2013, 23, 1005. [Google Scholar] [CrossRef]
- Adelhelm, P.; Hartmann, P.; Bender, C.L.; Martin, B.; Christine, E.; Juergen, J. From lithium to sodium: Cell chemistry of room temperature sodium-air and sodium-sulfur batteries. Beilstein J. Nanotechnol. 2015, 6, 1016–1055. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Hu, X.; Tang, H.; Zhang, C.; Liu, S.; Yang, Y.-W.; Yang, Q.-H.; Luo, J. Processable and moldable sodium-metal anodes. Angew. Chem. Int. Ed. 2017, 56, 11921–11926. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, H.; Hu, X.; Matios, E.; Luo, J.; Zhang, Y.; Lu, X.; Li, W. Frogspawn-Coral-Like Hollow Sodium Sulfide Nanostructured Cathode for High-Rate Performance Sodium-Sulfur Batteries. Adv. Energy Mater. 2019, 9, 1803251. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.; Lai, W.; Chou, S.; Gu, Q.; Liu, H.; Zhao, D.; Dou, S. Achieving High-Performance Room-Temperature Sodium-Sulfur Batteries With S@Interconnected Mesoporous Carbon Hollow Nanospheres. J. Am. Chem. Soc. 2016, 138, 16576–16579. [Google Scholar] [CrossRef]
- Li, Z.; Yuan, L.; Yi, Z.; Sun, Y.; Liu, Y.; Jiang, Y.; Shen, Y.; Xin, Y.; Zhang, Z.; Huang, Y. Insight into the Electrode Mechanism in Lithium-Sulfur Batteries with Ordered Microporous Carbon Confined Sulfur as the Cathode. Adv. Energy Mater. 2014, 4, 1301473. [Google Scholar] [CrossRef]
- Ghosh, A.; Kumar, A.; Roy, A.; Panda, M.R.; Kar, M.; MacFarlane, D.R.; Mitra, S. Three-Dimensionally Reinforced Freestanding Cathode for High-Energy Room-Temperature Sodium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2019, 11, 14101–14109. [Google Scholar] [CrossRef]
- Ma, D.; Li, Y.; Yang, J.; Mi, H.; Luo, S.; Deng, L.; Yan, C.; Rauf, M.; Zhang, P.; Sun, X.; et al. New Strategy for Polysulfide Protection Based on Atomic Layer Deposition of TiO2 onto Ferroelectric-Encapsulated Cathode: Toward Ultrastable FreeStanding Room Temperature Sodium-Sulfur Batteries. Adv. Funct. Mater. 2018, 28, 1705537. [Google Scholar] [CrossRef]
- Du, W.; Wu, Y.; Yang, T.; Guo, B.; Liu, D.; Bao, S.; Xu, M. Rational construction of rGO/VO2 nanoflowers as sulfur multifunctional hosts for room temperature Na-S batteries. Chem. Eng. J. 2020, 379, 122359. [Google Scholar] [CrossRef]
- Wang, N.; Wang, Y.; Bai, Z.; Fang, Z.; Zhang, X.; Xu, Z.; Ding, Y.; Xu, X.; Du, Y.; Dou, S.; et al. High-performance room-temperature sodium-sulfur battery enabled by electrocatalytic sodium polysulfides full conversion. Energy Environ. Sci. 2020. [Google Scholar] [CrossRef]
- Zhang, B.; Sheng, T.; Liu, Y.; Wang, Y.; Zhang, L.; Lai, W.; Wang, L.; Yang, J.; Gu, Q.; Chou, S.; et al. Atomic cobalt as an efficient electrocatalyst in sulfur cathodes for superior room-temperature sodium-sulfur batteries. Nat. Commun. 2018, 9, 4082. [Google Scholar] [CrossRef]
- Lim, W.; Mun, Y.; Cho, A.; Jo, C.; Lee, S.; Han, J.W.; Lee, J. Synergistic Effect of Molecular-Type Electrocatalysts with Ultrahigh Pore Volume Carbon Microspheres for Lithium-Sulfur Batteries. ACS Nano 2018, 12, 6013–6022. [Google Scholar] [CrossRef]
- Zhang, B.; Sheng, T.; Wang, Y.; Chou, S.; Davey, K.; Dou, S.; Qiao, S. Long-Life Room-Temperature Sodium-Sulfur Batteries by Virtue of Transition-Metal-Nanocluster-Sulfur Interactions. Angew. Chem. Int. Ed. 2019, 58, 1484–1488. [Google Scholar] [CrossRef]
- Yang, Y.; Zhong, Y.; Shi, Q.; Wang, Z.; Sun, K.; Wang, H. Electrocatalysis in Lithium Sulfur Batteries under Lean Electrolyte Conditions. Angew. Chem. Int. Ed. 2018, 57, 15549–15552. [Google Scholar] [CrossRef]
- Liu, Y.; Han, M.; Xiong, Q.; Zhang, S.; Zhao, C.; Gong, W.; Wang, G.; Zhang, H.; Zhao, H. Dramatically Enhanced Ambient Ammonia Electrosynthesis Performance by In-Operando Created Li-S Interactions on MoS2 Electrocatalyst. Adv. Energy Mater. 2019, 9, 1803935. [Google Scholar] [CrossRef]
- Du, L.; Wu, Q.; Yang, L.; Wang, X.; Che, R.; Lyu, Z.; Chen, W.; Wang, X.; Hua, Z. Efficient synergism of electrocatalysis and physical confinement leading to durable high-power lithium-sulfur batteries. Nano Energy 2019, 57, 34–40. [Google Scholar] [CrossRef]
- Xiao, Z.; Yang, Z.; Wang, L.; Nie, H.; Zhong, M.; Lai, Q.; Xu, X.; Zhang, L.; Huang, S.A. Lightweight TiO2/Graphene Interlayer, Applied as a Highly Effective Polysulfide Absorbent for Fast, Long-Life Lithium-Sulfur Batteries. Adv. Mater. 2015, 27, 2891–2898. [Google Scholar] [CrossRef]
- Lei, T.; Chen, W.; Huang, J.; Yan, C.; Sun, H.; Wang, C.; Zhang, W.; Li, Y.; Xiong, J. Multi-Functional Layered WS2 Nanosheets for Enhancing the Performance of Lithium-Sulfur Batteries. Adv. Energy Mater. 2017, 7, 1601843–1601850. [Google Scholar] [CrossRef]
- Song, Y.; Cai, W.; Kong, L.; Cai, J.; Zhang, Q.; Sun, J. Rationalising Electrocatalysis of Li-S Chemistry by Mediator Design: Progress and Prospects. Adv. Energy. Mater. 2019, 1901075. [Google Scholar] [CrossRef]
- Yan, Z.; Xiao, J.; Lai, W.; Wang, L.; Gebert, F.; Wang, Y.; Gu, Q.; Liu, H.; Chou, S.; Liu, H.; et al. Nickel sulfide nanocrystals on nitrogen-doped porous carbon nanotubes with high-efficiency electrocatalysis for room-temperature sodium-sulfur batteries. Nat. Commun. 2019, 10, 4793. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Guo, C.; Liu, X.; Liu, J.; Vasileff, A.; Jiao, Y.; Zheng, Y.; Qiao, S. Emerging Two-Dimensional Nanomaterials for Electrocatalysis. Chem. Rev. 2018, 118, 6337–6408. [Google Scholar] [CrossRef]
- Wu, G.; More, K.L.; Johnston, C.M.; Zelenay, P. High-Performance Electrocatalysts for Oxygen Reduction Derived from Polyaniline, Iron, and Cobalt. Science 2011, 332, 443–447. [Google Scholar] [CrossRef]
- Proietti, E.; Jaouen, F.; Lefèvre, M.; Larouche, N.; Tian, J.; Herranz, J.; Dodelet, J.-P. Iron-Based Cathode Catalyst with Enhanced Power Density in Polymer Electrolyte Membrane Fuel Cells. Nat. Commun. 2011, 2, 416. [Google Scholar] [CrossRef]
- Jiang, W.-J.; Gu, L.; Li, L.; Zhang, Y.; Zhang, X.; Zhang, L.-J.; Wang, J.-Q.; Hu, J.-S.; Wei, Z.; Wan, L.-J. Understanding the High Activity of Fe-N-C Electrocatalysts in Oxygen Reduction: Fe/Fe3C Nanoparticles Boost the Activity of Fe-Nx. J. Am. Chem. Soc. 2016, 138, 3570–3578. [Google Scholar] [CrossRef]
- Zhu, X.; Qian, F.; Liu, Y.; Matera, D.; Wu, G.; Zhang, S.; Chen, J. Controllable synthesis of magnetic carbon composites with high porosity and strong acid resistance from hydrochar for efficient removal of organic pollutants: An overlooked influence. Carbon 2016, 99, 338–347. [Google Scholar] [CrossRef]
- Yao, L.; Yang, J.; Zhang, P.; Deng, L. In situ surface decoration of Fe3C/Fe3O4/C nanosheets: Towards bi-functional activated carbons with supercapacitance and efficient dye adsorption. Bioresour. Technol. 2018, 256, 208–215. [Google Scholar] [CrossRef]
- Zhong, W.; Chen, J.; Zhang, P.; Deng, L.; Yao, L.; Ren, X.; Li, Y.; Mi, H.; Sun, L. Air plasma etching towards rich active sites in Fe/N-porous carbon for oxygen reduction reaction with superior catalytic performance. J. Mater. Chem. A 2017, 5, 16605–16610. [Google Scholar] [CrossRef]
- Shi, J.; Zhou, X.; Xu, P.; Qiao, J.; Chen, Z.; Liu, Y. Nitrogen and Sulfur Co-doped Mesoporous Carbon Materials as Highly Efficient Electrocatalysts for Oxygen Reduction Reaction. Electrochim. Acta 2014, 145, 259–269. [Google Scholar] [CrossRef]
- Yao, L.; Wu, Q.; Zhang, P.; Zhang, J.; Wang, D.; Li, Y.; Ren, X.; Mi, H.; Deng, L.; Zheng, Z. Scalable 2D Hierarchical Porous Carbon Nanosheets for Flexible Supercapacitors with Ultrahigh Energy Density. Adv. Mater. 2018, 30, 1706054. [Google Scholar] [CrossRef]
- Deng, L.; Young, R.J.; Kinloch, I.A.; Zhu, Y.; Eichhorn, S.J. Carbon nanofibers produced from electrospun cellulose nanofibers. Carbon 2013, 58, 66–75. [Google Scholar] [CrossRef]
- Yang, Y.; Qiu, M.; Liu, L.; Su, D.; Pi, Y.; Yan, G. Nitrogen-Doped Hollow Carbon Nanospheres Derived from Dopamine as High-Performance Anode Materials for Sodium-Ion Batteries. Nano 2016, 11, 1650124. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Resonant Raman spectroscopy of disordered, amorphous, and diamond like carbon. Phys. Rev. B 2001, 64, 075414 1–075414 13. [Google Scholar] [CrossRef]
- Väli, R.; Jänes, A.; Thomberg, T.; Lust, E. D-Glucose Derived Nanospheric Hard Carbon Electrodes for Room-Temperature Sodium-Ion Batteries. J. Electrochem. Soc. 2016, 163, A1619–A1626. [Google Scholar] [CrossRef]
- Zoromba, M.S.; Abdel-Aziz, M.H.; Bassyouni, M.; Gutub, S.; Demko, D.; Abdelkader, A. Electrochemical activation of graphene at low temperature: The synthesis of three-dimensional nanoarchitectures for high-performance supercapacitors and capacitive deionisation. ACS Sustain. Chem. Eng. 2017, 5, 4573–4581. [Google Scholar] [CrossRef]
- Abdelkader, A.M.; Fray, D.J. Controlled electrochemical doping of graphene-based 3D nanoarchitecture electrodes for supercapacitors and capacitive deionisation. Nanoscale 2017, 9, 14548–14557. [Google Scholar] [CrossRef]
- Qiao, Z.; Zhang, H.; Karakalos, S.; Hwang, S.; Xue, J.; Chen, M.; Su, D.; Wu, G. 3D Polymer Hydrogel for High-Performance Atomic Iron-Rich Catalysts for Oxygen Reduction in Acidic Media. Appl. Catal. B 2017, 219, 629–639. [Google Scholar] [CrossRef]
- Deng, D.; Chen, X.; Yu, L.; Wu, X.; Liu, Q.; Liu, Y.; Yang, H.; Tian, H.; Hu, Y.; Du, P.; et al. A single iron site confined in a graphene matrix for the catalytic oxidation of benzene at room temperature. Sci. Adv. 2015, 1, e1500462. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, D.; Qin, X.; Lin, K.; Kang, F.; Li, B.; Shanmukaraj, D.; Rojo, T.; Armand, M.; Wang, G. A room-temperature sodium–sulfur battery with high capacity and stable cycling performance. Nat. Commun. 2018, 9, 3870. [Google Scholar] [CrossRef]
- Yu, X.; Manthiram, A. Capacity enhancement and discharge mechanisms of room-temperature sodium-sulfur batteries. ChemElectronChem 2014, 1, 1275–1280. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Abdelkader, A.; Demko, D.; Deng, L.; Zhang, P.; He, T.; Wang, Y.; Huang, L. Electrocatalytic Assisted Performance Enhancement for the Na-S Battery in Nitrogen-Doped Carbon Nanospheres Loaded with Fe. Molecules 2020, 25, 1585. https://doi.org/10.3390/molecules25071585
Zhu J, Abdelkader A, Demko D, Deng L, Zhang P, He T, Wang Y, Huang L. Electrocatalytic Assisted Performance Enhancement for the Na-S Battery in Nitrogen-Doped Carbon Nanospheres Loaded with Fe. Molecules. 2020; 25(7):1585. https://doi.org/10.3390/molecules25071585
Chicago/Turabian StyleZhu, Jianhui, Amr Abdelkader, Denisa Demko, Libo Deng, Peixin Zhang, Tingshu He, Yanyi Wang, and Licong Huang. 2020. "Electrocatalytic Assisted Performance Enhancement for the Na-S Battery in Nitrogen-Doped Carbon Nanospheres Loaded with Fe" Molecules 25, no. 7: 1585. https://doi.org/10.3390/molecules25071585
APA StyleZhu, J., Abdelkader, A., Demko, D., Deng, L., Zhang, P., He, T., Wang, Y., & Huang, L. (2020). Electrocatalytic Assisted Performance Enhancement for the Na-S Battery in Nitrogen-Doped Carbon Nanospheres Loaded with Fe. Molecules, 25(7), 1585. https://doi.org/10.3390/molecules25071585






