Effect of Cornstalk Biochar Immobilized Bacteria on Ammonia Reduction in Laying Hen Manure Composting
Abstract
1. Introduction
2. Result
2.1. Effect of the Immobilized Mixed Bacteria of the Cornstalk Biochar Method on the NH3 Reduction of Composting
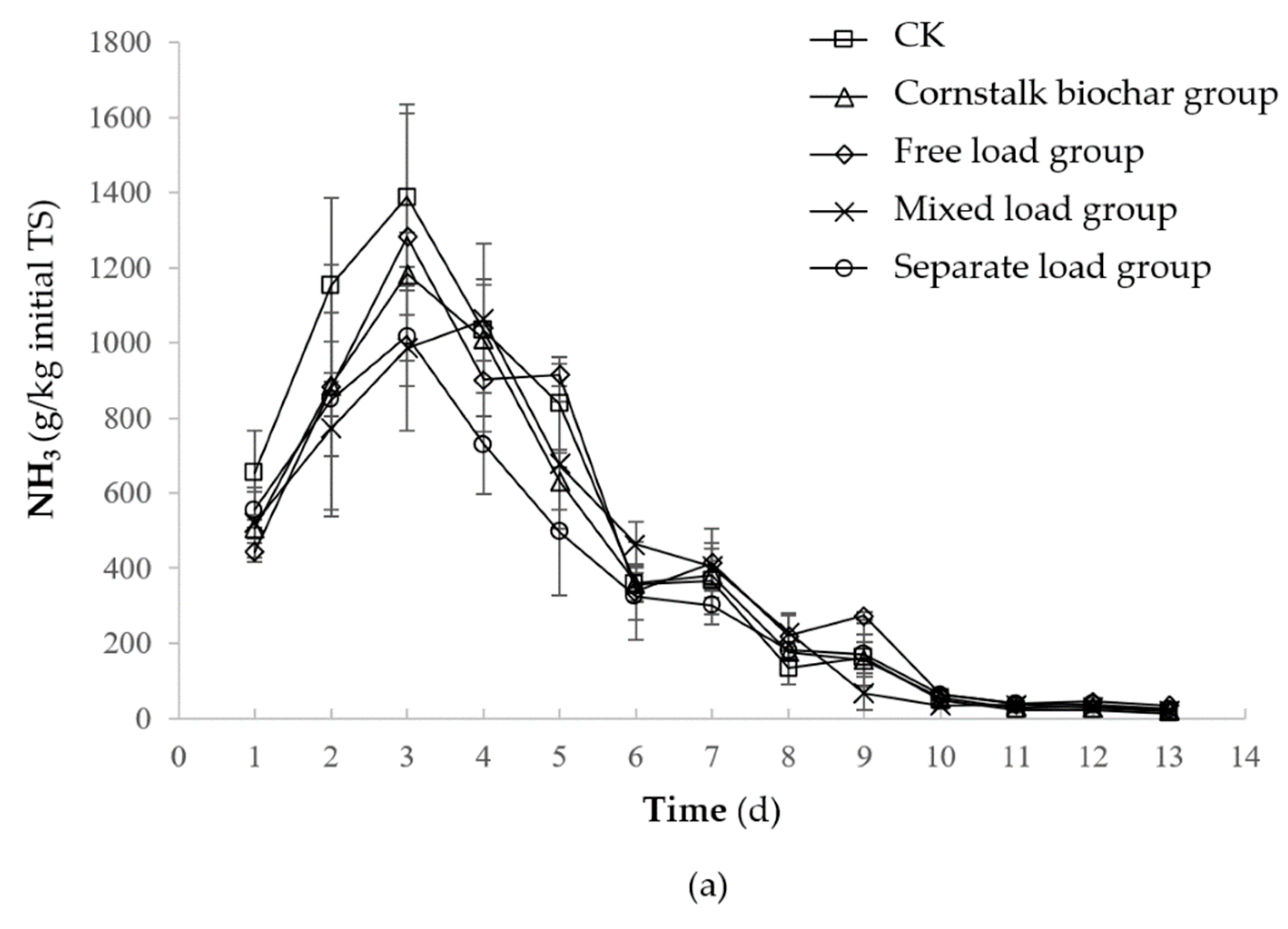
2.1.1. NH3 Emission
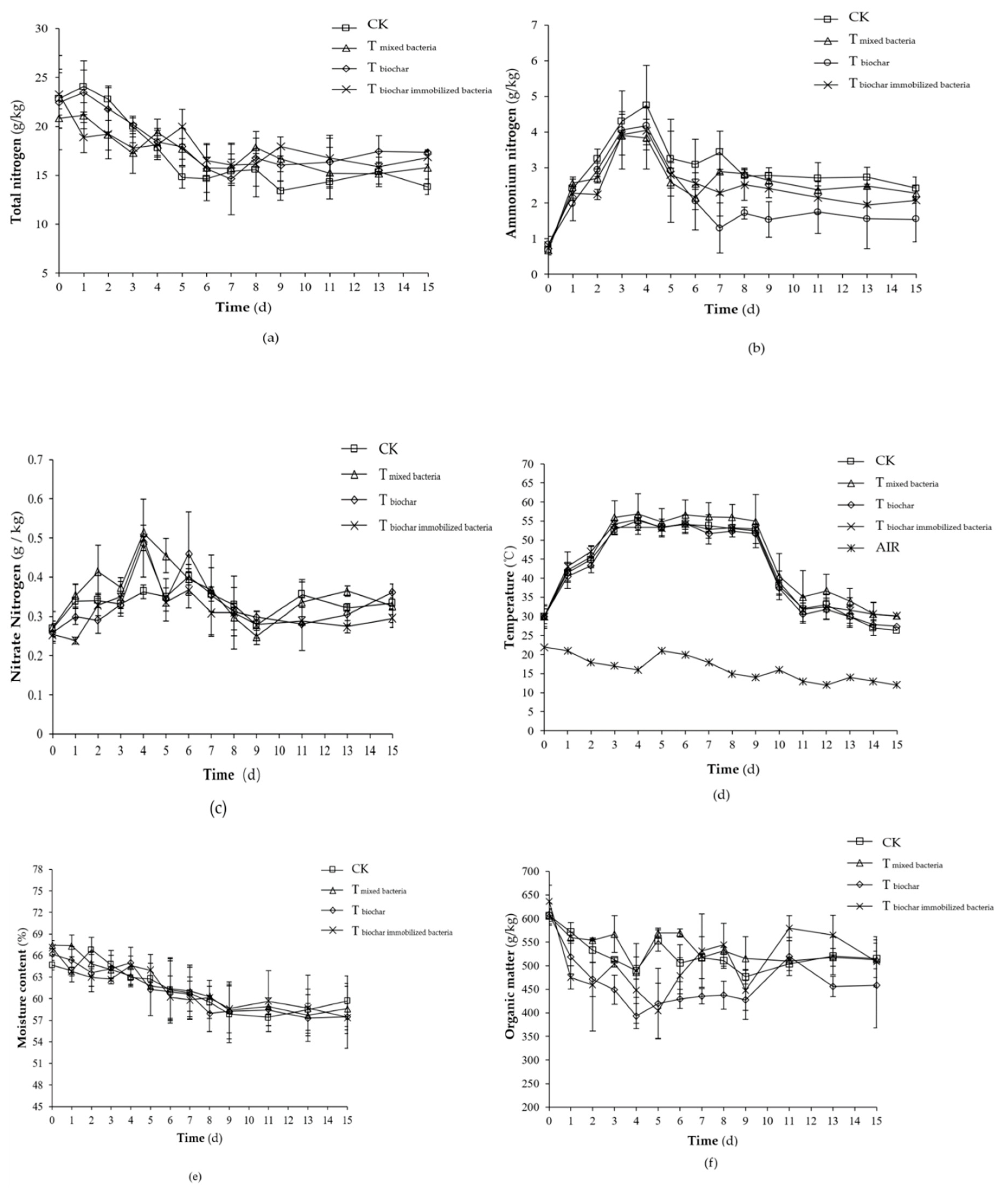
2.1.2. Changes of Physicochemical Factors
2.2. Simulated Composting Study on NH3 Emissions during Composting with Cornstalk Biochar Immobilized Mixed Bacteria
2.2.1. Changes of Physicochemical Factors
2.2.2. NH3 Emission
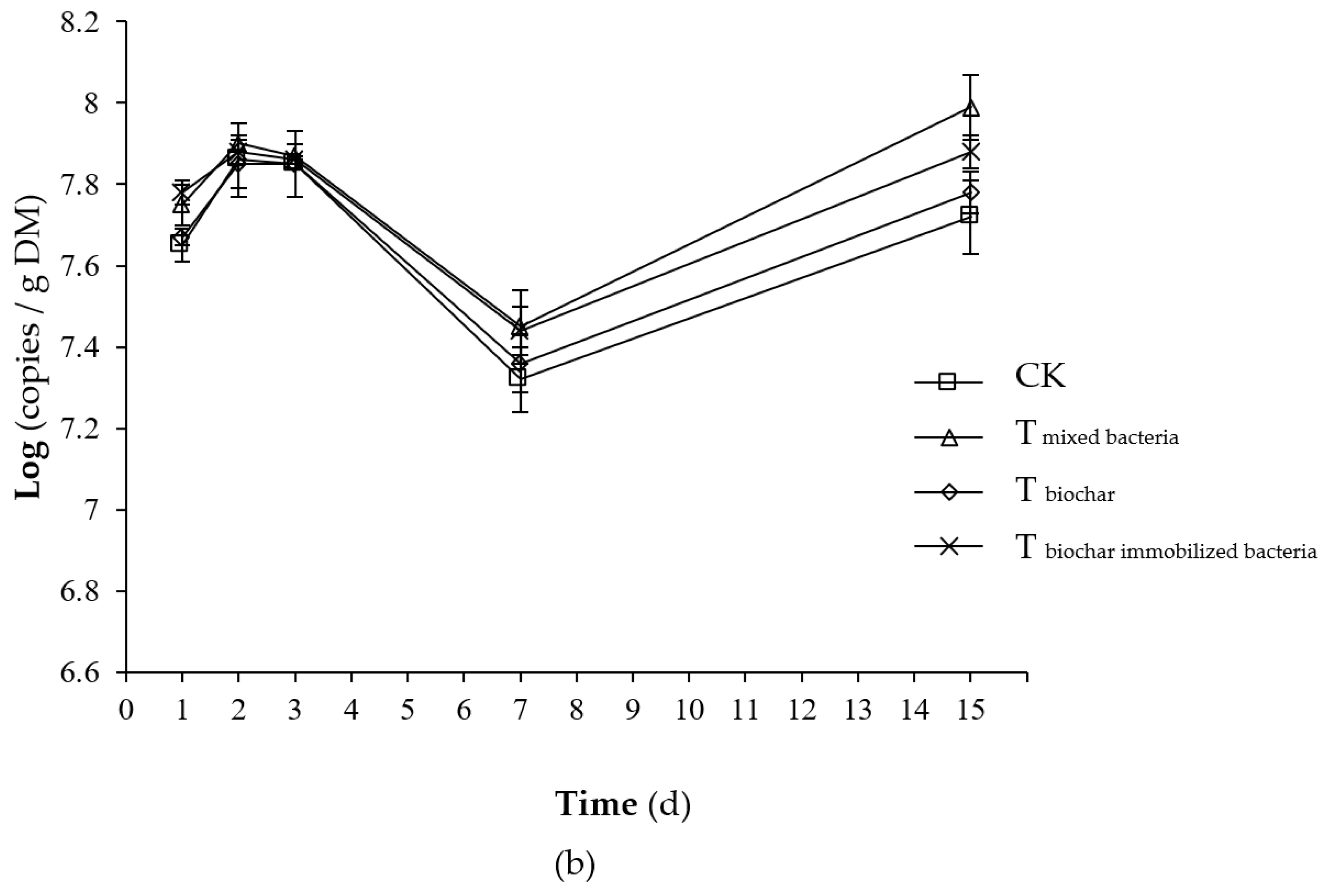
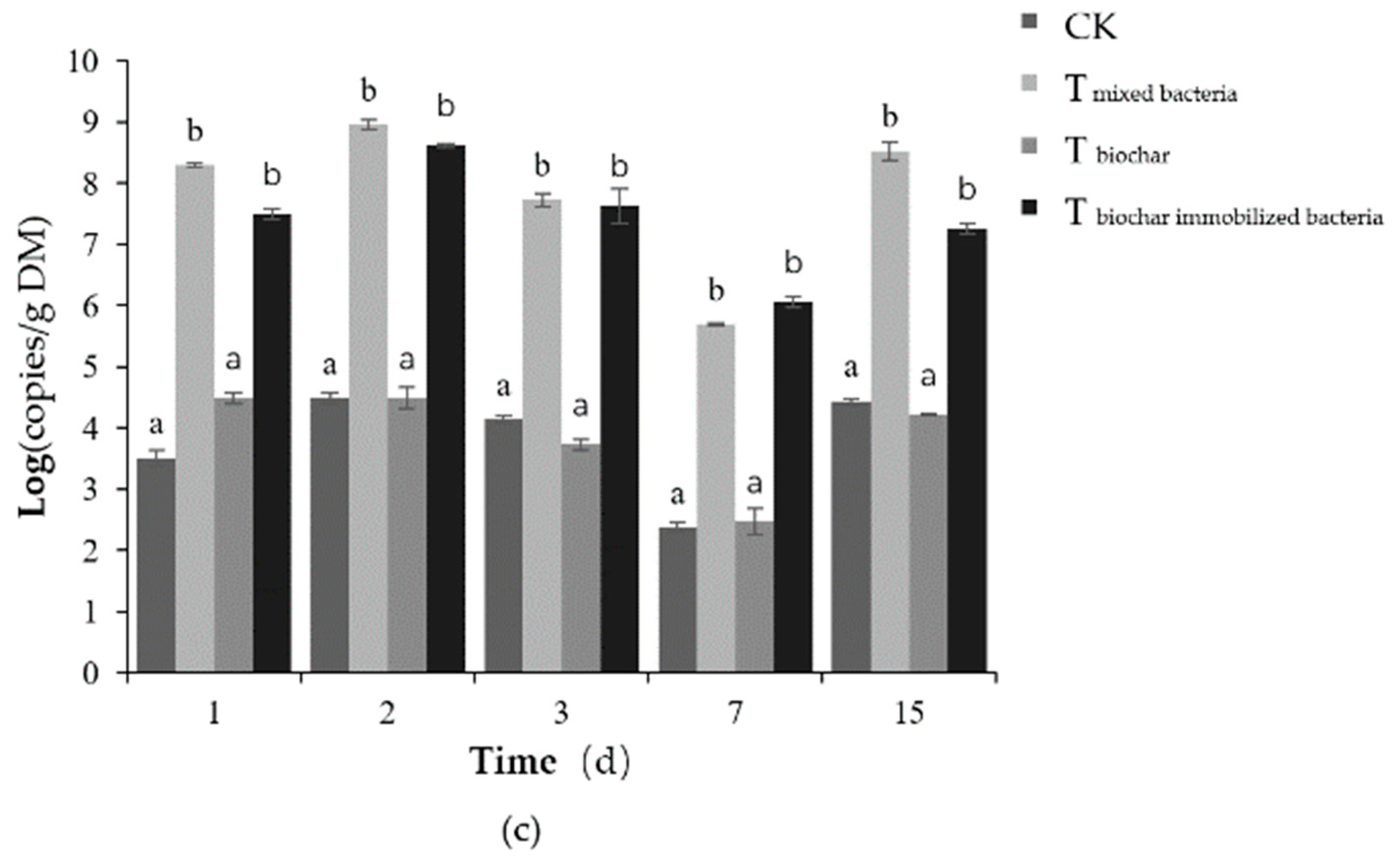
2.2.3. Total Number of Microorganisms
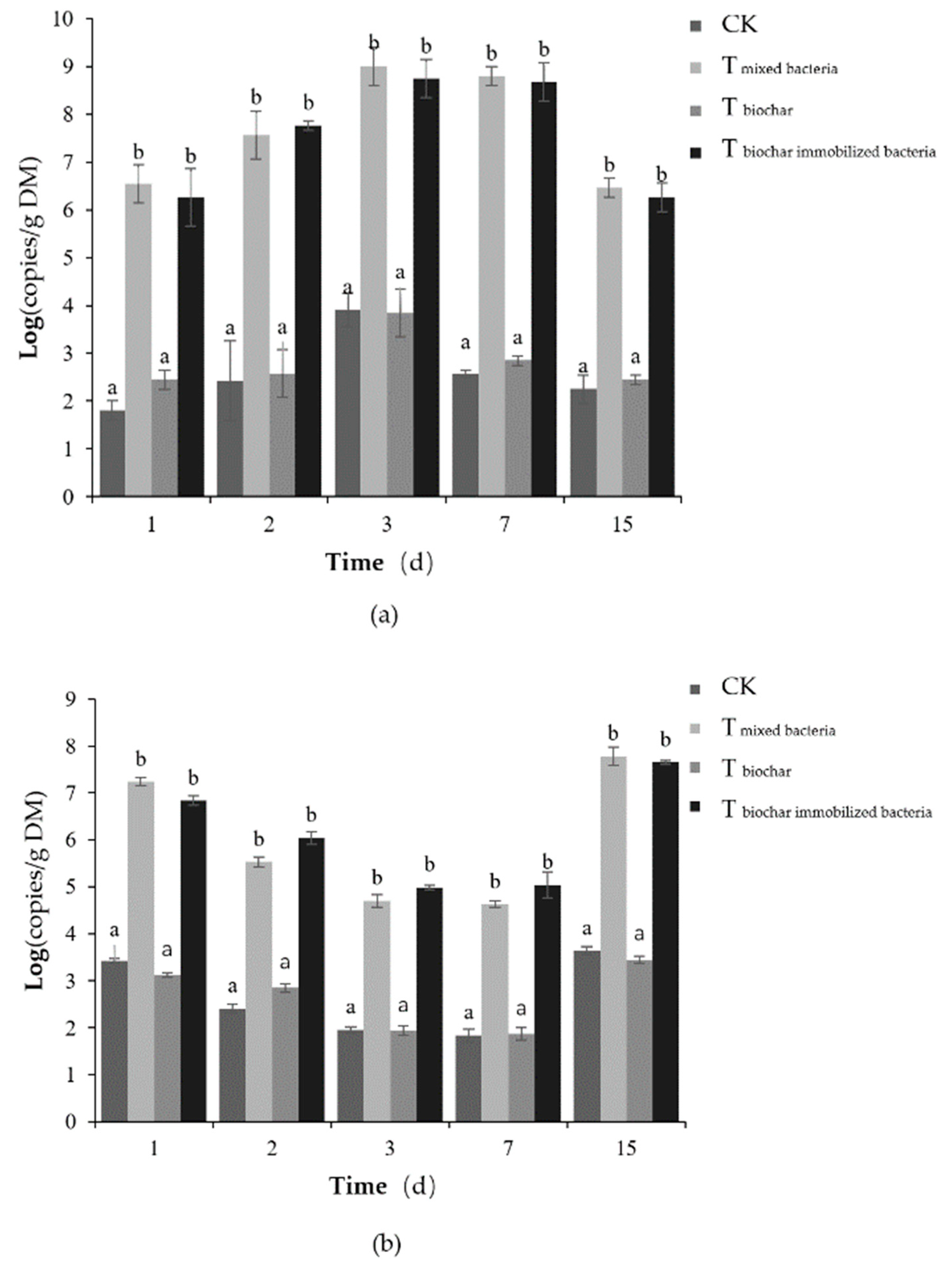
2.2.4. Number of Added Bacteria
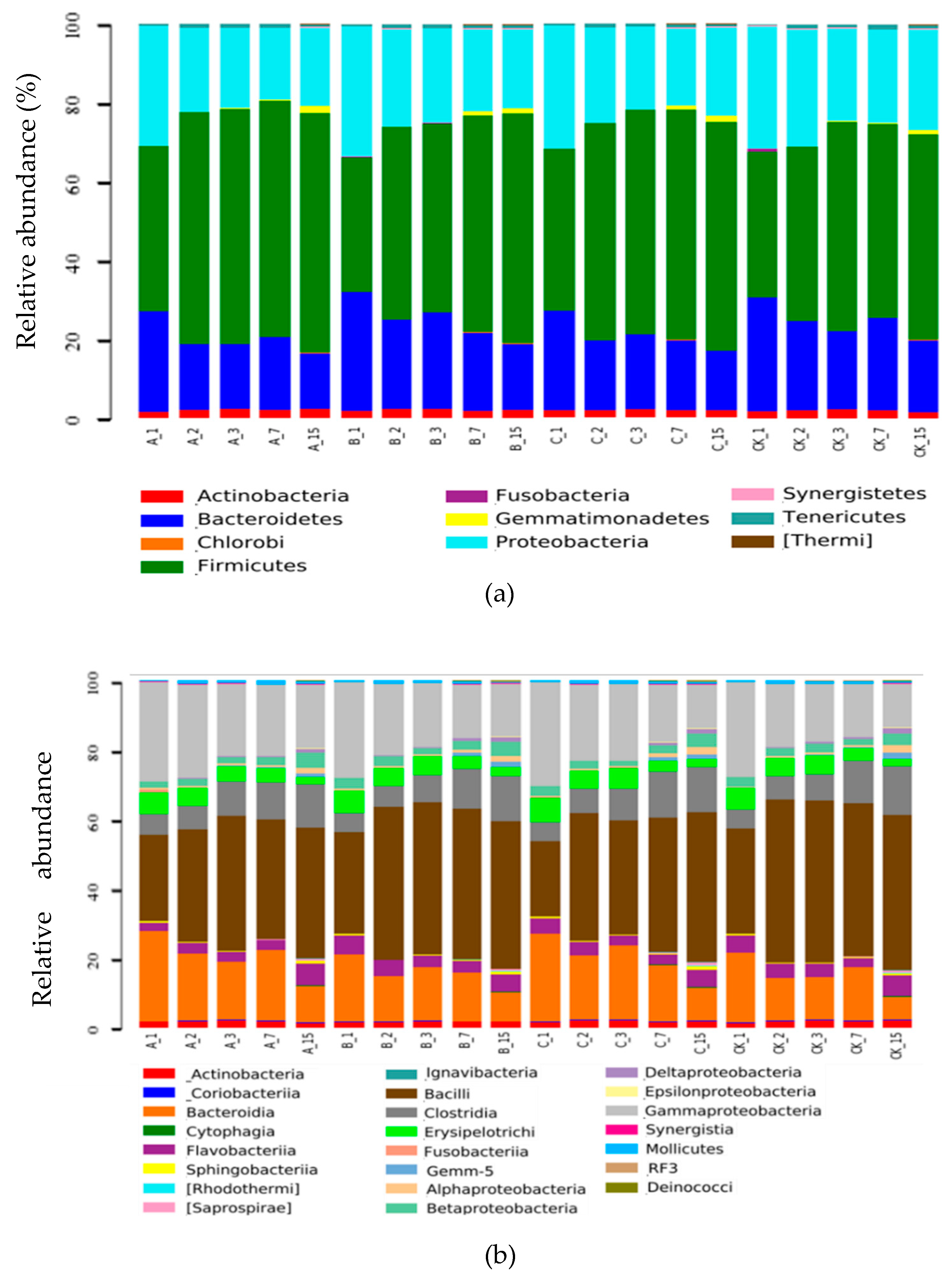
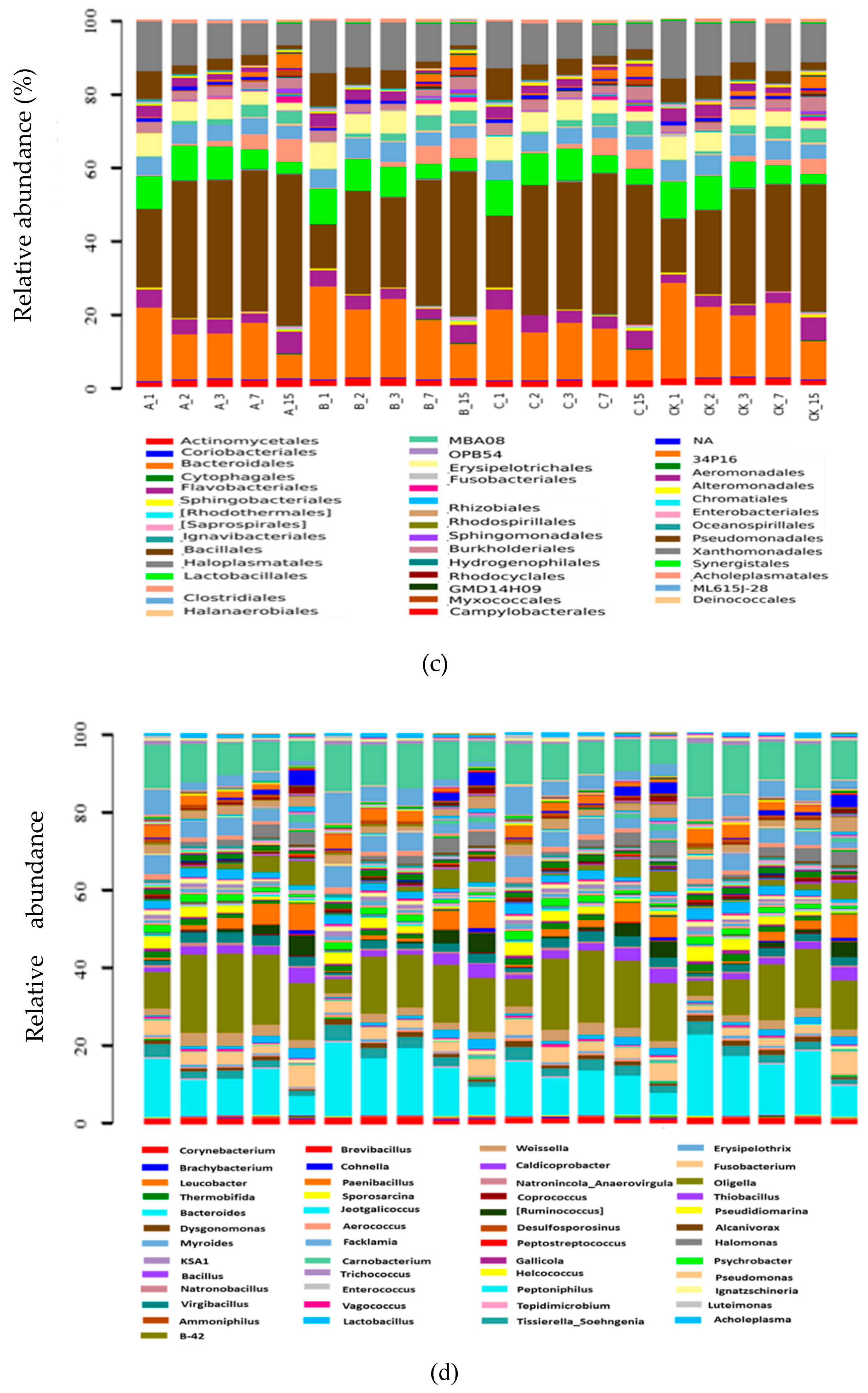
2.2.5. Composition of Bacterial Communities
2.2.6. Systematic Taxonomy Analysis
3. Discussion
4. Materials and Methods
4.1. Composting Materials
4.2. Experimental Design
4.2.1. Immobilized Mixed Bacteria of the Cornstalk Biochar Method
4.2.2. Simulated Composting Study on NH3 Emissions during Composting with Cornstalk Biochar Immobilized Mixed Bacteria
4.3. Sample Collection
4.3.1. Immobilized Mixed Bacteria of the Cornstalk Biochar Method
4.3.2. Simulated Composting Study on NH3 Emissions during Composting with Cornstalk Biochar Immobilized Mixed Bacteria
4.4. Bacteria Loading Rate Determination
4.5. Analytical Methods
4.5.1. Gaseous Measurements
4.5.2. Physicochemical Analyses
4.5.3. Total DNA Extraction and PCR
4.5.4. Detection of Bacterial and Fungal Diversity
Total DNA Extraction and PCR Amplification
Processing of Sequencing Data
4.6. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Windhorst, H.-W. A projection of the regional development of egg production until 2015. World’s Poult. Sci. J. 2008, 64, 356–376. [Google Scholar] [CrossRef]
- Magdelaine, P. Egg and egg product production and consumption in Europe and the rest of the world. In Improving the Safety and Quality of Eggs and Egg Products; Woodhead Publishing: Cambridge, UK, 2011; Volume 207, pp. 3–16. [Google Scholar]
- Zhou, S.; Zhang, X.; Liao, X.-D.; Wu, Y.; Mi, J.-D.; Wang, Y. Effect of Different Proportions of Three Microbial Agents on Ammonia Mitigation during the Composting of Layer Manure. Molecules 2019, 24, 2513. [Google Scholar] [CrossRef]
- Chen, W.; Liao, X.; Wu, Y.; Liang, J.B.; Mi, J.; Huang, J.; Zhang, H.; Wu, Y.; Qiao, Z.; Li, X.; et al. Effects of different types of biochar on methane and ammonia mitigation during layer manure composting. Waste Manag. 2017, 61, 506–515. [Google Scholar] [CrossRef]
- Webster, A.B.; Thompson, S.A.; Hinkle, N.C.; Merka, W.C. In-House Composting of Layer Manure in a High-Rise, Tunnel-Ventilated Commercial Layer House During an Egg Production Cycle. J. Appl. Poult. Res. 2006, 15, 447–456. [Google Scholar] [CrossRef]
- Bernal, M.P.; Alburquerque, J.; Moral, R. Composting of animal manures and chemical criteria for compost maturity assessment. A review. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [CrossRef] [PubMed]
- Holm-Nielsen, J.B.; Al Seadi, T.; Oleskowicz-Popiel, P. The future of anaerobic digestion and biogas utilization. Bioresour. Technol. 2009, 100, 5478–5484. [Google Scholar] [CrossRef] [PubMed]
- Venglovsky, J.; Sasakova, N.; Placha, I. Pathogens and antibiotic residues in animal manures and hygienic and ecological risks related to subsequent land application. Bioresour. Technol. 2009, 100, 5386–5391. [Google Scholar] [CrossRef] [PubMed]
- Mitloehner, F.M.; Schenker, M.B. Environmental Exposure and Health Effects From Concentrated Animal Feeding Operations. Epidemiology 2007, 18, 309–311. [Google Scholar] [CrossRef]
- Kulcu, R.; Yaldiz, O. The composting of agricultural wastes and the new parameter for the assessment of the process. Ecol. Eng. 2014, 69, 220–225. [Google Scholar] [CrossRef]
- Huang, G.F.; Wu, Q.T.; Li, F.B.; Wong, J.W. Nitrogen transformations during pig manure composting. J. Environ. Sci. 2001, 13, 401. [Google Scholar]
- Zhang, Y.; Zhao, Y.; Chen, Y.; Lu, Q.; Li, M.; Wang, X.; Wei, Y.; Xie, X.; Wei, Z. A regulating method for reducing nitrogen loss based on enriched ammonia-oxidizing bacteria during composting. Bioresour. Technol. 2016, 221, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Le, P.D.; Aarnink, A.; Ogink, N.; Becker, P.M.; Verstegen, M.W.A. Odour from animal production facilities: its relationship to diet. Nutr. Res. Rev. 2005, 18, 3–30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, X.; Tian, Y.; Gong, X. Effects of brown sugar and calcium superphosphate on the secondary fermentation of green waste. Bioresour. Technol. 2013, 131, 68–75. [Google Scholar] [CrossRef]
- Morand, P.; Peres, G.; Robin, P.; Yulipriyanto, H.; Baron, S. Gaseous emissions from composting bark/manure mixtures. Compost Sci Util 2005, 13, 14–26. [Google Scholar] [CrossRef]
- Wang, A.; Wang, Y.; Liao, X.-D.; Wu, Y.; Liang, J.B.; Laudadio, V.; Tufarelli, V. Sodium butyrate mitigates in vitro ammonia generation in cecal content of laying hens. Environ. Sci. Pollut. Res. 2016, 23, 16272–16279. [Google Scholar] [CrossRef]
- Jiang, T.; Schuchardt, F.; Li, G.; Guo, R.; Zhao, Y. Effect of C/N ratio, aeration rate and moisture content on ammonia and greenhouse gas emission during the composting. J. Environ. Sci. 2011, 23, 1754–1760. [Google Scholar] [CrossRef]
- Suzelle, B.; Denis, C.; Maher, T.; William, K. Effect of carbon source on compost nitrogen and carbon losses. Bioresource Technol. 2002, 83, 189–194. [Google Scholar]
- Veeken, A.; De Wilde, V.; Hamelers, B. Passively Aerated Composting of Straw-Rich Pig Manure: Effect of Compost Bed Porosity. Compos. Sci. Util. 2002, 10, 114–128. [Google Scholar] [CrossRef]
- Mahimairaja, S.; Bolan, N.; Hedley, M.; MacGregor, A. Losses and transformation of nitrogen during composting of poultry manure with different amendments: An incubation experiment. Bioresour. Technol. 1994, 47, 265–273. [Google Scholar] [CrossRef]
- Yan, Z.; Liu, X.; Yuan, Y.; Liao, Y.; Li, X. Deodorization study of the swine manure with two yeast strains. Biotechnol. Bioprocess Eng. 2013, 18, 135–143. [Google Scholar] [CrossRef]
- Gutarowska, B.; Matusiak, K.; Borowski, S.; Rajkowska, A.; Brycki, B. Removal of odorous compounds from poultry manure by microorganisms on perlite–bentonite carrier. J. Environ. Manage. 2014, 141, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bi, L.; Liao, Y.; Lu, D.; Zhang, H.; Liao, X.-D.; Liang, J.B.; Wu, Y. Influence and characteristics of Bacillus stearothermophilus in ammonia reduction during layer manure composting. Ecotoxicol. Environ. Saf. 2019, 180, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Philippe, F.; Cabaraux, J.-F.; Nicks, B. Ammonia emissions from pig houses: Influencing factors and mitigation techniques. Agric. Ecosyst. Environ. 2011, 141, 245–260. [Google Scholar] [CrossRef]
- Cunningham, C.; Ivshina, I.; Lozinsky, V.; Kuyukina, M.; Philp, J. Bioremediation of diesel-contaminated soil by microorganisms immobilised in polyvinyl alcohol. Int. Biodeterior. Biodegradation 2004, 54, 167–174. [Google Scholar] [CrossRef]
- Seruga, P.; Krzywonos, M.; Pyżanowska, J.; Urbanowska, A.; Pawlak-Kruczek, H.; Niedzwiecki, L. Removal of Ammonia from the Municipal Waste Treatment Effluents using Natural Minerals. Molecules 2019, 24, 3633. [Google Scholar] [CrossRef]
- Scholz, M.; Martin, R. Ecological equilibrium on biological activated carbon. Water Res. 1997, 31, 2959–2968. [Google Scholar] [CrossRef]
- Li, Y.; Yi, L.; Ma, P.; Zhou, L. Industrial Wastewater Treatment by the Combination of Chemical Precipitation and Immobilized Microorganism Technologies. Environ. Eng. Sci. 2007, 24, 736–744. [Google Scholar] [CrossRef]
- Hockaday, W.; Grannas, A.M.; Kim, S.; Hatcher, P.G. The transformation and mobility of charcoal in a fire-impacted watershed. Geochim. et Cosmochim. Acta. 2007, 71, 3432–3445. [Google Scholar] [CrossRef]
- Wu, Y.-M.; Yang, J.; Fan, X.-L.; Fu, S.; Sun, M.-T.; Guo, R.-B. Elimination of methane in exhaust gas from biogas upgrading process by immobilized methane-oxidizing bacteria. Bioresour. Technol. 2017, 231, 124–128. [Google Scholar] [CrossRef]
- Talha, A.; Goswami, M.; Giri, B.S.; Sharma, A.; Rai, B.N.; Singh, R.S. Bioremediation of Congo red dye in immobilized batch and continuous packed bed bioreactor by Brevibacillus parabrevis using coconut shell bio-char. Bioresour. Technol. 2018, 252, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Yuan, M.; Qian, L. Enhanced bioremediation of PAH-contaminated soil by immobilized bacteria with plant residue and biochar as carriers. J. Soils Sediments 2012, 12, 1350–1359. [Google Scholar] [CrossRef]
- Michitsch, R.; Gordon, R.; Jamieson, R.; Stratton, G. Bacterial Pathogen Occurrence and Persistence in Livestock Mortality Biopiles. Resources 2017, 6, 49. [Google Scholar] [CrossRef]
- Guo, R.; Li, G.; Jiang, T.; Schuchardt, F.; Chen, T.; Zhao, Y.; Shen, Y. Effect of aeration rate, C/N ratio and moisture content on the stability and maturity of compost. Bioresour. Technol. 2012, 112, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.; Hernandez, T.; Costa, F. Study on water extract of sewage sludge composts. Soil Sci. Plant Nutr. 1991, 37, 399–408. [Google Scholar] [CrossRef]
- Shehata, E.; Liu, Y.; Feng, Y.; Cheng, D.; Li, Z. Changes in Arsenic and Copper Bioavailability and Oxytetracycline Degradation during the Composting Process. Molecules 2019, 24, 4240. [Google Scholar] [CrossRef] [PubMed]
- Botero, L.M.; Brown, K.B.; Brumefield, S.; Burr, M.; Castenholz, R.W.; Young, M.; McDermott, T.R. Thermobaculum terrenum gen. nov., sp. nov.: a non-phototrophic gram-positive thermophile representing an environmental clone group related to the Chloroflexi (green non-sulfur bacteria) and Thermomicrobia. Arch. Microbiol. 2004, 181, 269–277. [Google Scholar] [CrossRef]
- Asano, R.; Otawa, K.; Ozutsumi, Y.; Yamamoto, N.; Abdel-Mohsein, H.S.; Nakai, Y. Development and analysis of microbial characteristics of an acidulocomposting system for the treatment of garbage and cattle manure. J. Biosci. Bioeng. 2010, 110, 419–425. [Google Scholar] [CrossRef]
- Lim, L.Y.; Bong, C.P.C.; Chua, L.S.; Lee, C.T. Physicochemical profile of microbial-assisted composting on empty fruit bunches of oil palm trees. Environ. Sci. Pollut. Res. 2015, 22, 19814–19822. [Google Scholar] [CrossRef]
- Guizelini, B.P.; Vandenberghe, L.P.S.; Sella, S.R.B.R.; Soccol, C.R. Study of the influence of sporulation conditions on heat resistance of Geobacillus stearothermophilus used in the development of biological indicators for steam sterilization. Arch. Microbiol. 2012, 194, 991–999. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.C.; Zhang, H.; Shi, H.; Hu, T.; Ngo, H.H. Characteristics of nitrogen transformation and microbial community in an aerobic composting reactor under two typical temperatures. Bioresour. Technol. 2013, 137, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Jia, X.; Xu, P.; Huang, X.; Gu, W.; Zhang, F.; Yang, S.; Tang, S. Improved composting of poultry feces via supplementation with ammonia oxidizing archaea. Bioresour. Technol. 2012, 120, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Martins, O.; Dewes, T. Loss of nitrogenous compounds during composting of animal wastes. Bioresour. Technol. 1992, 42, 103–111. [Google Scholar] [CrossRef]
- Sánchez-Monedero, M.; Roig, A.; Paredes, C.; Bernal, M.P. Nitrogen transformation during organic waste composting by the Rutgers system and its effects on pH, EC and maturity of the composting mixtures. Bioresour. Technol. 2001, 78, 301–308. [Google Scholar] [CrossRef]
- Zeng, G.; Zhang, J.; Chen, Y.; Yu, Z.; Yu, M.; Li, H.; Liu, Z.-F.; Chen, M.; Lu, L.; Hu, C. Relative contributions of archaea and bacteria to microbial ammonia oxidation differ under different conditions during agricultural waste composting. Bioresour. Technol. 2011, 102, 9026–9032. [Google Scholar] [CrossRef]
- Kuroda, K.; Hanajima, D.; Fukumoto, Y.; Suzuki, K.; Kawamoto, S.; Shima, J.; Haga, K. Isolation of thermophilic ammonium-tolerant bacterium and its application to reduce ammonia emission during composting of animal wastes. Biosci. Biotechnol. Biochem. 2004, 68, 286–292. [Google Scholar] [CrossRef]
- Kowalchuk, G.A.; Naoumenko, Z.S.; Derikx, P.J.; Felske, A.; Stephen, J.R.; Arkhipchenko, I.A. Molecular analysis of ammonia-oxidizing bacteria of the beta subdivision of the class Proteobacteria in compost and composted materials. Appl. Environ. Microbiol. 1999, 65, 396. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Z.-Y.; Jiang, Y.-F.; Qian, H.-F.; Zhang, W.; Chen, J. Assessment of the Bacterial Community for Denitrifying Removal of Nitric Oxide in a Rotating Drum Biofilter by Denaturing Gradient Gel Electrophoresis. Environ. Eng. Sci. 2009, 26, 1189–1196. [Google Scholar] [CrossRef]
- Yamada, T.; Araki, S.; Ikeda-Ohtsubo, W.; Okamura, K.; Hiraishi, A.; Ueda, H.; Ueda, Y.; Miyauchi, K.; Endo, G. Community structure and population dynamics of ammonia oxidizers in composting processes of ammonia-rich livestock waste. Syst. Appl. Microbiol. 2013, 36, 359–367. [Google Scholar] [CrossRef]
- Chandna, P.; Nain, L.; Singh, S.; Kuhad, R.C. Assessment of bacterial diversity during composting of agricultural byproducts. BMC Microbiol. 2013, 13, 99. [Google Scholar] [CrossRef]
- Smits, N.A.C.; Hefting, M.; Agterveld, M.K.-V.; Laanbroek, H.; Paalman, A.; Bobbink, R. Nitrification along a grassland gradient: Inhibition found in matgrass swards. Soil Boil. Biochem. 2010, 42, 635–641. [Google Scholar] [CrossRef]
- Zhang, K.; Gu, J.; Wang, X.; Yin, Y.; Zhang, X.; Zhang, R.; Tuo, X.; Zhang, L. Variations in the denitrifying microbial community and functional genes during mesophilic and thermophilic anaerobic digestion of cattle manure. Sci. Total. Environ. 2018, 634, 501–508. [Google Scholar] [CrossRef]
- Sims, A.P.; Folkes, B.F. A kinetic study of the assimilation of [15 N]-ammonia and the synthesis of amino acids in an exponentially growing culture of Candida utilis. In Proceedings of the Royal Society of London. Series B. Biological Sciences; The Royal Society: London, UK; Volume 159, pp. 479–502.
- Ferguson, A.R.; Sims, A.P. The Regulation of Glutamine Metabolism in Candida utilis: the Role of Glutamine in the Control of Glutamine Synthetase. J. Gen. Microbiol. 1974, 80, 159–171. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liang, G.; Liao, X.; Du, G.; Jian, C. A new strategy to enhance glutathione production by multiple HO-induced oxidative stresses in Candida utilis. Bioresource Technol. 2009, 100, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.; De Neergaard, A.; Jensen, L.S. Potential of aeration flow rate and bio-char addition to reduce greenhouse gas and ammonia emissions during manure composting. Chemosphere 2014, 97, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Hua, L.; Wu, W.; Liu, Y.; McBride, M.B.; Chen, Y. Reduction of nitrogen loss and Cu and Zn mobility during sludge composting with bamboo charcoal amendment. Environ. Sci. Pollut. Res. 2008, 16, 1–9. [Google Scholar] [CrossRef]
- Chen, Y.-X.; Huang, X.-D.; Han, Z.; Huang, X.; Hu, B.; Shi, D.; Wu, W. Effects of bamboo charcoal and bamboo vinegar on nitrogen conservation and heavy metals immobility during pig manure composting. Chemosphere 2010, 78, 1177–1181. [Google Scholar] [CrossRef]
- Lehmann, J. A handful of carbon. Nature 2007, 447, 143–144. [Google Scholar] [CrossRef]
- Saito, Y.; Mori, M.; Shida, S.; Arima, T. Formaldehyde adsorption and desorption properties of wood-based charcoal. J WOOD SCI 2000, 46, 596–601. [Google Scholar]
- Zhang, L.; Sun, X. Changes in physical, chemical, and microbiological properties during the two-stage co-composting of green waste with spent mushroom compost and biochar. Bioresour. Technol. 2014, 171, 274–284. [Google Scholar] [CrossRef]
- Wang, J.; Du, X.; Zhang, Y.; Li, T.; Liao, X. Effect of Substrate on Identification of Microbial Communities in Poultry Carcass Composting and Microorganisms Associated with Poultry Carcass Decomposition. J. Agric. Food Chem. 2016, 64, 6838–6847. [Google Scholar] [CrossRef] [PubMed]
- Belyaeva, O.; Haynes, R.J. Chemical, microbial and physical properties of manufactured soils produced by co-composting municipal green waste with coal fly ash. Bioresour. Technol. 2009, 100, 5203–5209. [Google Scholar] [CrossRef]
- Rynk, R. Monitoring moisture in composting systems. Biocycle 2000, 41, 53. [Google Scholar]
- Bremner, J.M. Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 1960, 55, 11. [Google Scholar] [CrossRef]
- Miao, L.; Wang, S.; Li, B.; Cao, T.; Zhang, F.; Wang, Z.; Peng, Y. Effect of carbon source type on intracellular stored polymers during endogenous denitritation (ED) treating landfill leachate. Water Res. 2016, 100, 405–412. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Marchant-Forde, J.N.; Zhang, X.; Wang, Y. Effect of Cornstalk Biochar Immobilized Bacteria on Ammonia Reduction in Laying Hen Manure Composting. Molecules 2020, 25, 1560. https://doi.org/10.3390/molecules25071560
Zhang H, Marchant-Forde JN, Zhang X, Wang Y. Effect of Cornstalk Biochar Immobilized Bacteria on Ammonia Reduction in Laying Hen Manure Composting. Molecules. 2020; 25(7):1560. https://doi.org/10.3390/molecules25071560
Chicago/Turabian StyleZhang, Huaidan, Jeremy N. Marchant-Forde, Xinyi Zhang, and Yan Wang. 2020. "Effect of Cornstalk Biochar Immobilized Bacteria on Ammonia Reduction in Laying Hen Manure Composting" Molecules 25, no. 7: 1560. https://doi.org/10.3390/molecules25071560
APA StyleZhang, H., Marchant-Forde, J. N., Zhang, X., & Wang, Y. (2020). Effect of Cornstalk Biochar Immobilized Bacteria on Ammonia Reduction in Laying Hen Manure Composting. Molecules, 25(7), 1560. https://doi.org/10.3390/molecules25071560




