Cognitive Facilitation and Antioxidant Effects of an Essential Oil Mix on Scopolamine-Induced Amnesia in Rats: Molecular Modeling of In Vitro and In Vivo Approaches
Abstract
1. Introduction
2. Results and Discussion
2.1. The Chemical Composition of the Essential Oil Mix
2.2. Molecular Docking Simulations
2.3. Cholinesterase Inhibitory Effect of the Essential Oil Mix
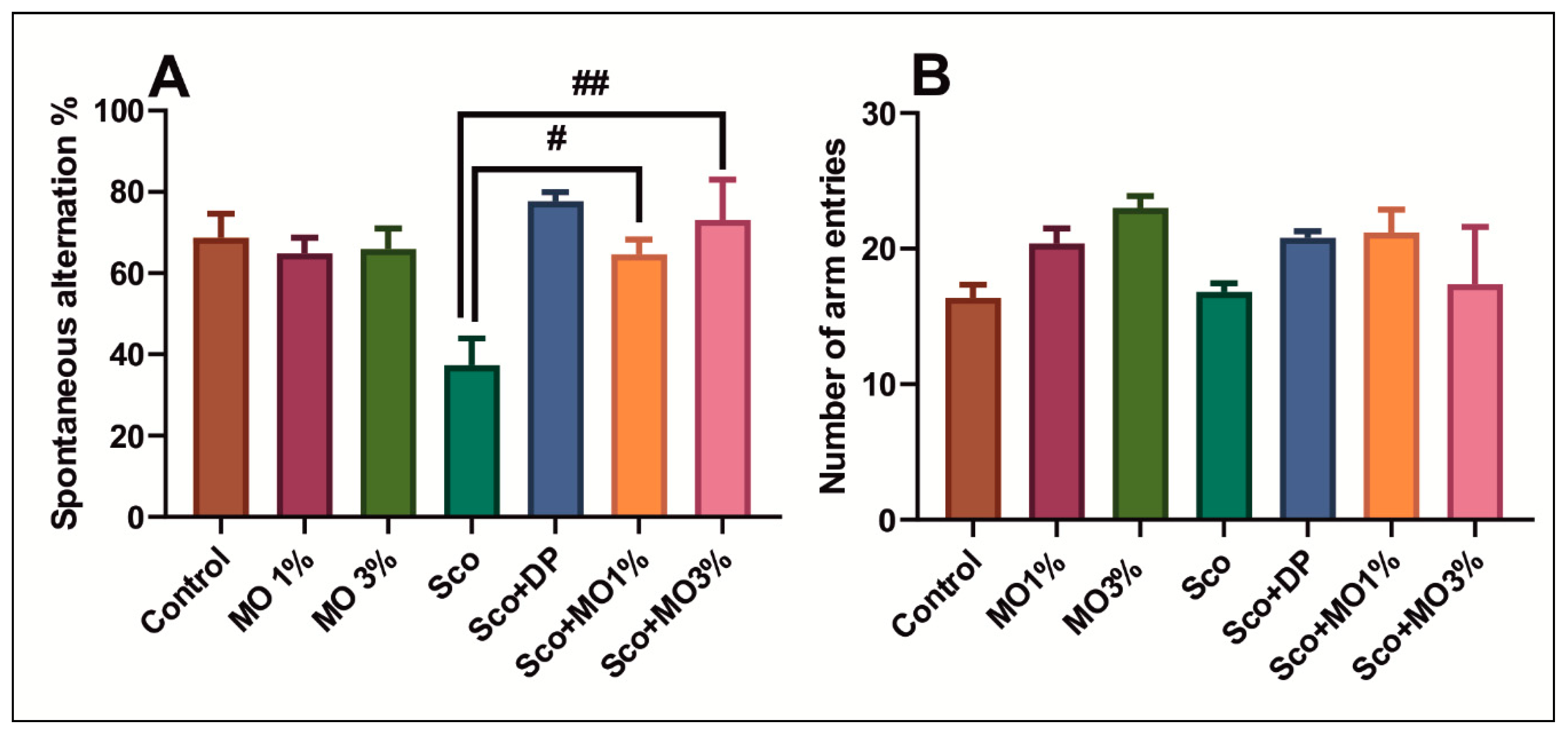
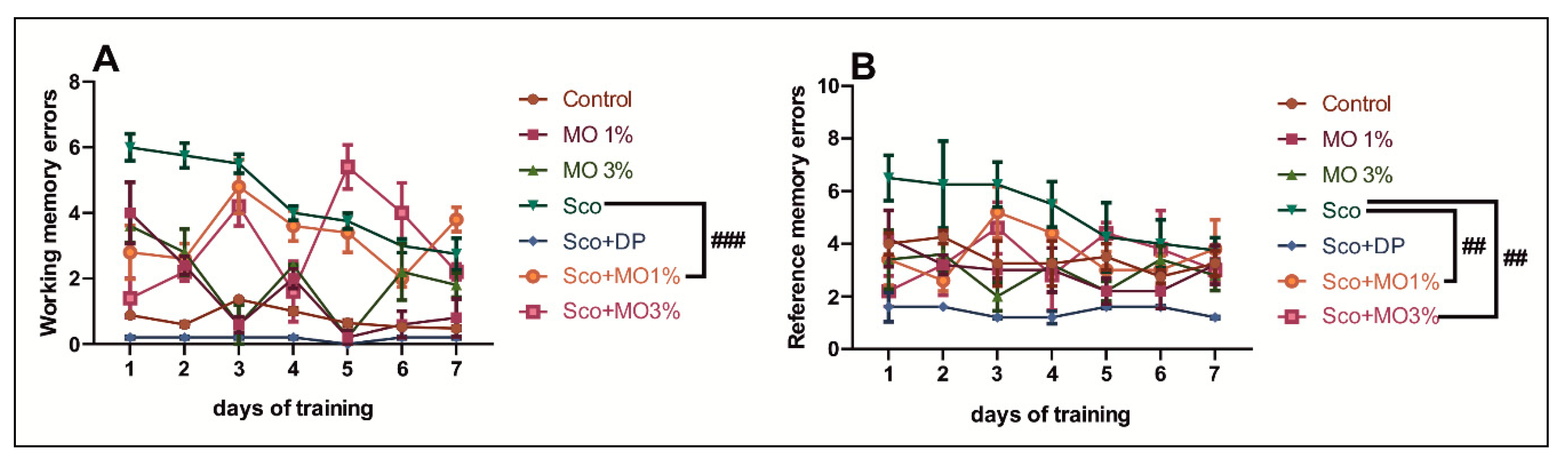
2.4. The Effects of the Essential Oil Mix on Cognitive Functions in Behavioral Tasks
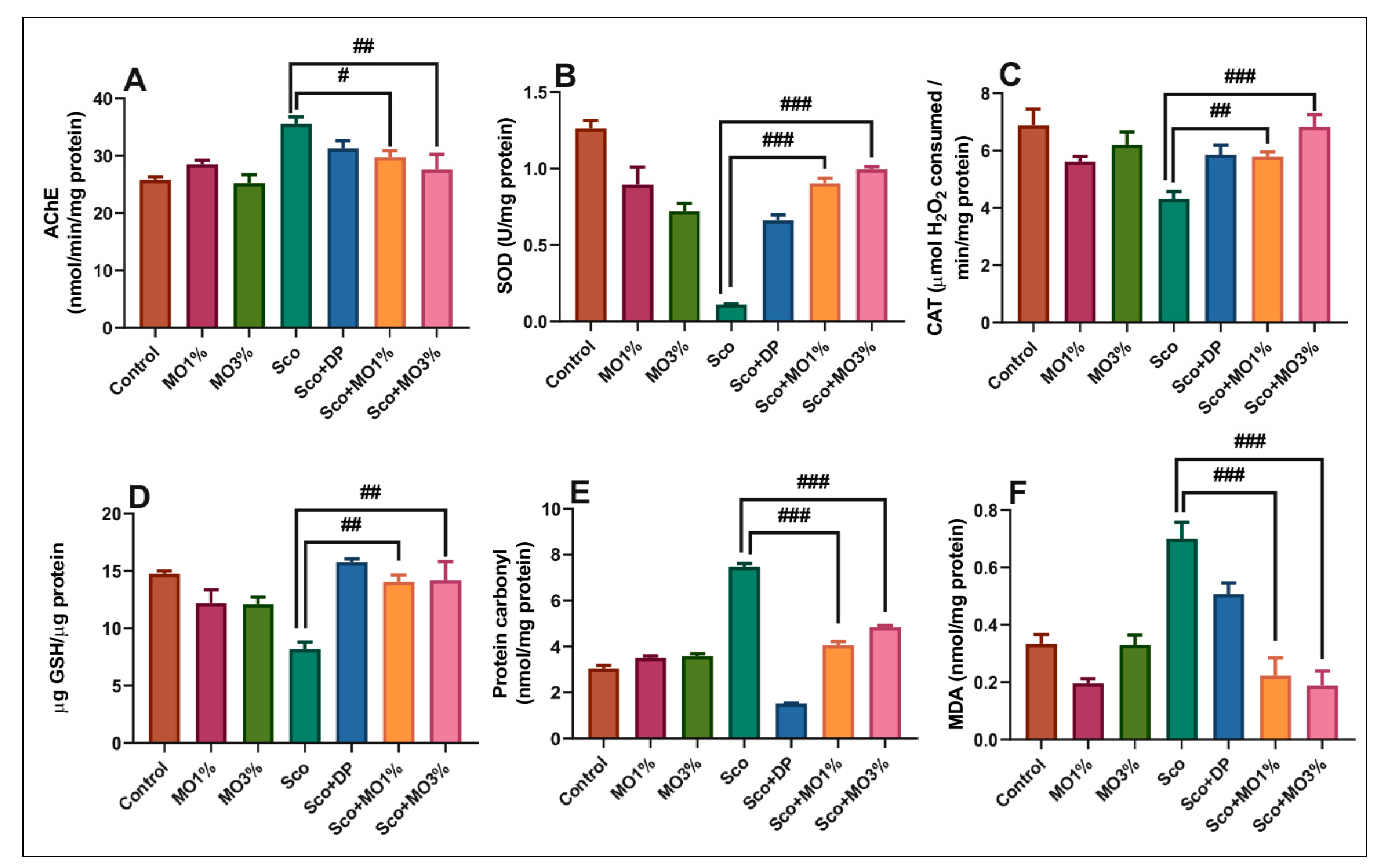
2.5. Effects of the Essential Oil Mix on Brain AChE in Sco-Treated Rats
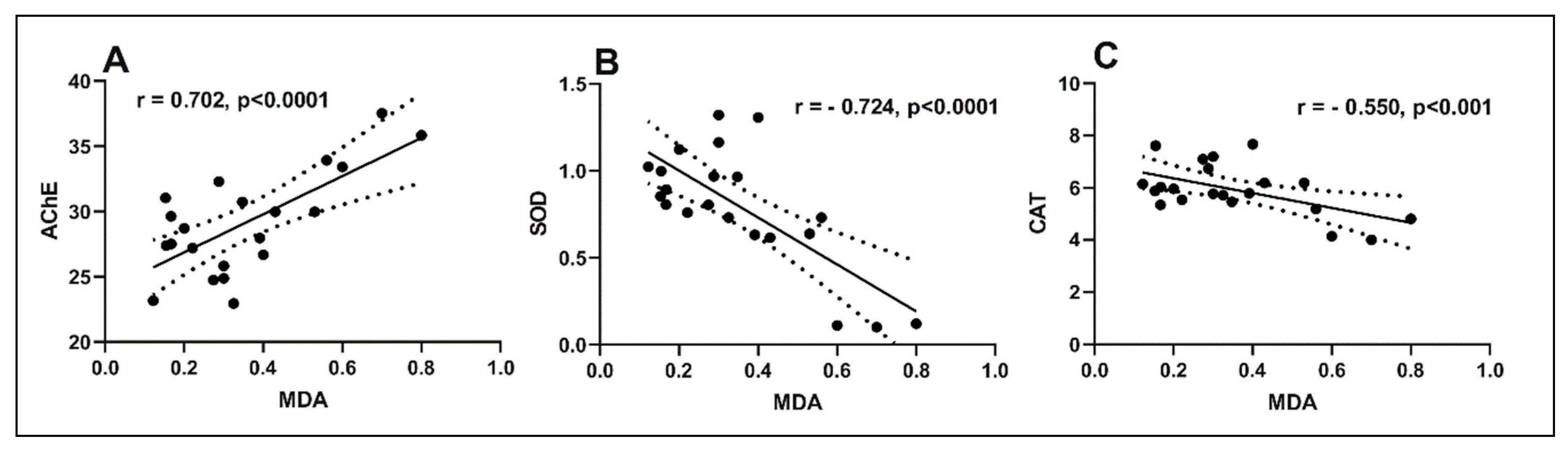
2.6. Effects of the Essential Oil Mix on Brain Oxidative Status in Sco-Treated Rats
3. Materials and Methods
3.1. Essential Oil and Plant Materials
3.2. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
3.3. Molecular Docking Experiments
3.4. Microtiter Enzyme Assays for AChE and BChE Inhibition
3.5. Animals
3.6. Drug Treatment and Group Division
3.7. Behavioral Analysis
3.7.1. Y-maze
3.7.2. Novel Object Recognition
3.7.3. Radial Arm Maze
3.8. Biochemical Parameters Assay
3.9. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tan, E.K.; Srivastava, A.K.; Arnold, W.D.; Singh, M.P.; Zhang, Y. Neurodegeneration: Etiologies and new therapies. Biomed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Esquerda-Canals, G.; Montoliu-Gaya, L.; Güell-Bosch, J.; Villegas, S. Mouse models of Alzheimer’s disease. J. Alzheimer’s Dis. 2017, 57, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- A Shah, A.; A Dar, T.; A Dar, P.; A Ganie, S.; A Kamal, M. A Current Perspective on the Inhibition of Cholinesterase by Natural and Synthetic Inhibitors. Curr. Drug Metab. 2017, 18, 96–111. [Google Scholar] [CrossRef] [PubMed]
- Haake, A.; Nguyen, K.; Friedman, L.; Chakkamparambil, B.; Grossberg, G.T. An update on the utility and safety of cholinesterase inhibitors for the treatment of Alzheimer’s disease. Expert Opin. Drug Saf. 2020, 19, 1–11. [Google Scholar] [CrossRef]
- Tonga, J.B.; Eilertsen, D.-E.; Solem, I.K.L.; Arnevik, E.A.; Korsnes, M.S.; Ulstein, I.D. Effect of self-efficacy on quality of life in people with Mild Cognitive Impairment and Mild Dementia: The mediating roles of depression and anxiety. Am. J. Alzheimer’s Dis. Other Dement. 2020, 35. [Google Scholar] [CrossRef]
- Cassidy, L.; Fernandez, F.; Johnson, J.B.; Naiker, M.; Owoola, A.G.; Broszczak, D.A. Oxidative stress in alzheimer’s disease: A review on emergent natural polyphenolic therapeutics. Complement. Ther. Med. 2020, 49, 102294. [Google Scholar] [CrossRef]
- Ayaz, M.; Sadiq, A.; Junaid, M.; Ullah, F.; Subhan, F.; Ahmed, J. Neuroprotective and Anti-Aging Potentials of Essential Oils from Aromatic and Medicinal Plants. Front. Aging Neurosci. 2017, 9. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S. Essential Oils: Extraction, Bioactivities, and Their Uses for Food Preservation. J. Food Sci. 2014, 79, R1231–R1249. [Google Scholar] [CrossRef]
- Benny, A.; Thomas, J. Essential Oils as Treatment Strategy for Alzheimer’s Disease: Current and Future Perspectives. Planta Med. 2019, 85, 239–248. [Google Scholar]
- De Araújo, J.S.F.; de Souza, E.L.; Oliveira, J.R.; Gomes, A.C.A.; Kotzebue, L.R.V.; da Silva Agostini, D.L.; de Oliveira, D.L.V.; Mazzetto, S.E.; da Silva, A.L.; Cavalcanti, M.T. Microencapsulation of sweet orange essential oil (Citrus aurantium var. dulcis) by liophylization using maltodextrin and maltodextrin/gelatin mixtures: Preparation, characterization, antimicrobial and antioxidant activities. Int. J. Biol. Macromol. 2020, 143, 991–999. [Google Scholar]
- Falls, N.; Singh, D.; Anwar, F.; Verma, A.; Kumar, V. Amelioration of neurodegeneration and cognitive impairment by lemon oil in experimental model of stressed mice. Biomed. Pharmacother. 2018, 106, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Oskouie, A.A.; Yekta, R.F.; Tavirani, M.R.; Kashani, M.S.; Goshadrou, F. Lavandula angustifolia effects on rat models of Alzheimer’s disease through the investigation of serum metabolic features using NMR metabolomics. Avicenna J. Med. Biotechnol. 2018, 10, 83–92. [Google Scholar]
- Kennedy, D.; Scholey, A. The psychopharmacology of European herbs with cognition-enhancing properties. Curr. Pharm. Des. 2006, 12, 4613–4623. [Google Scholar] [CrossRef] [PubMed]
- Hasanein, P.; Felehgari, Z.; Emamjomeh, A. Preventive effects of Salvia officinalis L. against learning and memory deficit induced by diabetes in rats: Possible hypoglycaemic and antioxidant mechanisms. Neurosci. Lett. 2016, 622, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Fukumoto, S.; Yokogoshi, H. Components of lemon essential oil attenuate dementia induced by scopolamine. Nutr. Neurosci. 2009, 12, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Bu, Y.; Jeong, S.; Lim, J.; Kwon, Y.; Cha, D.S.; Kim, J.; Jeon, S.; Eun, J.; Jeon, H. Memory-enhancing effect of a supercritical carbon dioxide fluid extract of the needles of Abies koreana on scopolamine-induced amnesia in mice. Biosci. Biotechnol. Biochem. 2006, 70, 1821–1826. [Google Scholar] [CrossRef]
- Lee, G.-Y.; Lee, C.; Park, G.H.; Jang, J.-H. Amelioration of Scopolamine-Induced Learning and Memory Impairment by α-Pinene in C57BL/6 Mice. Evid. Based. Complement. Alternat. Med. 2017, 2017, 4926815. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Kumar, V.; Mal, M.; Houghton, P.J. In vitro acetylcholinesterase inhibitory activity of the essential oil from Acorus calamus and its main constituents. Planta Med. 2007, 73, 283–285. [Google Scholar] [CrossRef]
- De Souza, A.; Lopes, E.M.C.; da Silva, M.C.; Cordeiro, I.; Young, M.C.M.; Sobral, M.E.G.; Moreno, P.R.H. Chemical composition and acetylcholinesterase inhibitory activity of essential oils of Myrceugenia myrcioides(Cambess.) O. Berg and Eugenia riedelianaO. Berg, Myrtaceae. Rev. Bras. Farmacogn. 2010, 20, 175–179. [Google Scholar] [CrossRef]
- Dohi, S.; Terasaki, M.; Makino, M. Acetylcholinesterase inhibitory activity and chemical composition of commercial essential oils. J. Agric. Food Chem. 2009, 57, 4313–4318. [Google Scholar] [CrossRef]
- Jewart, R.D.; Green, J.; Lu, C.; Cellar, J.; Tune, L.E. Cognitive, Behavioral, and Physiological Changes in Alzheimer Disease Patients as a Function of Incontinence Medications. Am. J. Geriatr. Psychiatry 2005, 13, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Potasiewicz, A.; Krawczyk, M.; Gzielo, K.; Popik, P.; Nikiforuk, A. Positive allosteric modulators of alpha 7 nicotinic acetylcholine receptors enhance procognitive effects of conventional anti-Alzheimer drugs in scopolamine-treated rats. Behav. Brain Res. 2020, 385, 112547. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, S.K.; Lee, D.R.; Choi, B.K.; Le, B.; Yang, S.H. Ameliorating effect of Citrus aurantium extracts and nobiletin on β-amyloid (1-42)-induced memory impairment in mice. Mol. Med. Rep. 2019, 20, 3448–3455. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wang, K.; Lu, C.; Dong, L.; Gao, L.; Yan, M.; Aibai, S.; Liu, X. Protective effect of lavender oil on scopolamine induced cognitive deficits in mice and H2O2 induced cytotoxicity in PC12 cells. J. Ethnopharmacol. 2016, 193, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Ozarowski, M.; Mikolajczak, P.L.; Bogacz, A.; Gryszczynska, A.; Kujawska, M.; Jodynis-Liebert, J.; Piasecka, A.; Napieczynska, H.; Szulc, M.; Kujawski, R.; et al. Rosmarinus officinalis L. leaf extract improves memory impairment and affects acetylcholinesterase and butyrylcholinesterase activities in rat brain. Fitoterapia 2013, 91, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L. Salvia (Sage): A Review of its Potential Cognitive-Enhancing and Protective Effects. Drugs R D 2017, 17, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Dodd, F.L.; Robertson, B.C.; Okello, E.J.; Reay, J.L.; Scholey, A.B.; Haskell, C.F. Monoterpenoid extract of sage (Salvia lavandulaefolia) with cholinesterase inhibiting properties improves cognitive performance and mood in healthy adults. J. Psychopharmacol. 2011, 25, 1088–1100. [Google Scholar] [CrossRef]
- Scholey, A.B.; Tildesley, N.T.J.; Ballard, C.G.; Wesnes, K.A.; Tasker, A.; Perry, E.K.; Kennedy, D.O. An extract of Salvia (sage) with anticholinesterase properties improves memory and attention in healthy older volunteers. Psychopharmacol. (Berl) 2008, 198, 127–139. [Google Scholar] [CrossRef]
- Bai, P.; Wang, K.; Zhang, P.; Shi, J.; Cheng, X.; Zhang, Q.; Zheng, C.; Cheng, Y.; Yang, J.; Lu, X.; et al. Development of chalcone-O-alkylamine derivatives as multifunctional agents against Alzheimer’s disease. Eur. J. Med. Chem. 2019, 183, 111737. [Google Scholar] [CrossRef]
- Kamat, P.K.; Kalani, A.; Rai, S.; Swarnkar, S.; Tota, S.; Nath, C.; Tyagi, N. Mechanism of Oxidative Stress and Synapse Dysfunction in the Pathogenesis of Alzheimer’s Disease: Understanding the Therapeutics Strategies. Mol. Neurobiol. 2016, 53, 648–661. [Google Scholar] [CrossRef]
- Swomley, A.M.; Butterfield, D.A. Oxidative stress in Alzheimer disease and mild cognitive impairment: Evidence from human data provided by redox proteomics. Arch. Toxicol. 2015, 89, 1669–1680. [Google Scholar] [CrossRef] [PubMed]
- Poprac, P.; Jomova, K.; Simunkova, M.; Kollar, V.; Rhodes, C.J.; Valko, M. Targeting Free Radicals in Oxidative Stress-Related Human Diseases. Trends Pharmacol. Sci. 2017, 38, 592–607. [Google Scholar] [CrossRef] [PubMed]
- Praticò, D. Oxidative stress hypothesis in Alzheimer’s disease: A reappraisal. Trends Pharmacol. Sci. 2008, 29, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.; Liaquat, L.; Ahmad, S.; Batool, Z.; Siddiqui, R.A.; Tabassum, S.; Shahzad, S.; Rafiq, S.; Naz, N. Naringenin protects AlCl3/D-galactose induced neurotoxicity in rat model of AD via attenuation of acetylcholinesterase levels and inhibition of oxidative stress. PLoS ONE 2020, 15, e0227631. [Google Scholar] [CrossRef]
- Youn, K.; Lee, S.; Jun, M. Discovery of Nobiletin from Citrus Peel as a Potent Inhibitor of β-Amyloid Peptide Toxicity. Nutrients 2019, 11, 2648. [Google Scholar] [CrossRef]
- Mushtaq, A.; Anwar, R.; Ahmad, M. Lavandula stoechas (L) a very potent antioxidant attenuates dementia in scopolamine induced memory deficit mice. Front. Pharmacol. 2018, 9, 1375. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, Z.; Yue, Z.; Ding, L.; Zhou, Y.; Huang, Z.; Huang, H. Rosmarinic acid ameliorates H2O2-induced oxidative stress in L02 cells through MAPK and Nrf2 pathways. Rejuvenation Res. 2019, 22, 289–298. [Google Scholar] [CrossRef]
- Capatina, L.; Boiangiu, R.S.; Dumitru, G.; Napoli, E.M.; Ruberto, G.; Hritcu, L.; Todirascu-Ciornea, E. Rosmarinus officinalis Essential Oil Improves Scopolamine-Induced Neurobehavioral Changes via Restoration of Cholinergic Function and Brain Antioxidant Status in Zebrafish (Danio rerio). Antioxidants 2020, 9, 62. [Google Scholar] [CrossRef]
- Kolac, U.K.; Ustuner, M.C.; Tekin, N.; Ustuner, D.; Colak, E.; Entok, E. The anti-inflammatory and antioxidant effects of Salvia officinalis on lipopolysaccharide-induced inflammation in rats. J. Med. Food 2017, 20, 1193–1200. [Google Scholar] [CrossRef]
- Bahri, S.; Ben Ali, R.; Nahdi, A.; Mlika, M.; Abdennabi, R.; Jameleddine, S. Salvia officinalis attenuates bleomycin-induced oxidative stress and lung fibrosis in rats. Nutr. Cancer 2019, 1–11. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, J.; Cao, J.; Wang, D.; Liu, C.; Yang, R.; Li, X.; Sun, C. Antioxidant capacity, anticancer ability and flavonoids composition of 35 citrus (Citrus reticulata Blanco) varieties. Molecules 2017, 22, 1114. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wang, K.; Lu, C.; Dong, L.; Gao, L.; Yan, M.; Aibai, S.; Yang, Y.; Liu, X. The Protective Effect of Lavender Essential Oil and Its Main Component Linalool against the Cognitive Deficits Induced by D-Galactose and Aluminum Trichloride in Mice. Evid. -Based Complement. Altern. Med. 2017, 2017, 7426538. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. The therapeutic potential of rosemary (Rosmarinus officinalis) diterpenes for Alzheimer’s disease. Evidence-Based Complement. Altern. Med. 2016, 2016, 2680409. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef]
- Brus, B.; Košak, U.; Turk, S.; Pišlar, A.; Coquelle, N.; Kos, J.; Stojan, J.; Colletier, J.-P.; Gobec, S. Discovery, Biological Evaluation, and Crystal Structure of a Novel Nanomolar Selective Butyrylcholinesterase Inhibitor. J. Med. Chem. 2014, 57, 8167–8179. [Google Scholar] [CrossRef]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra Precision Glide: Docking and Scoring Incorporating a Model of Hydrophobic Enclosure for Protein−Ligand Complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef]
- Ellman, G.; Courtney, K.; Andres, V.J.; Feather-Stone, R. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharm. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Ionita, R.; Postu, P.A.; Mihasan, M.; Gorgan, D.L.; Hancianu, M.; Cioanca, O.; Hritcu, L. Ameliorative effects of Matricaria chamomilla L. hydroalcoholic extract on scopolamine-induced memory impairment in rats: A behavioral and molecular study. Phytomedicine 2018, 47, 113–120. [Google Scholar] [CrossRef]
- Jackson, L.L. VTE on an elevated T-maze. J. Comp. Psychol. 1943, 36, 99–107. [Google Scholar] [CrossRef]
- Postu, P.A.; Sadiki, F.Z.; El Idrissi, M.; Cioanca, O.; Trifan, A.; Hancianu, M.; Hritcu, L. Pinus halepensis essential oil attenuates the toxic Alzheimer’s amyloid beta (1-42)-induced memory impairment and oxidative stress in the rat hippocampus. Biomed. Pharmacother. 2019, 112, 108673. [Google Scholar] [CrossRef]
- Foyet, H.S.; Asongalem, A.E.; Oben, E.K.; Cioanca, O.; Hancianu, M.; Hritcu, L. Effects of the Methanolic Extract of Vitellaria paradoxa Stem Bark Against Scopolamine-Induced Cognitive Dysfunction and Oxidative Stress in the Rat Hippocampus. Cell. Mol. Neurobiol. 2016, 36, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Olton, D.S.; Samuelson, R.J. Remembrance of places passed: Spatial memory in rats. J. Exp. Psychol. Anim. B 1976, 2, 97–116. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
Sample Availability: Not available. |



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boiangiu, R.S.; Brinza, I.; Hancianu, M.; Erdogan Orhan, I.; Eren, G.; Gündüz, E.; Ertas, H.; Hritcu, L.; Cioanca, O. Cognitive Facilitation and Antioxidant Effects of an Essential Oil Mix on Scopolamine-Induced Amnesia in Rats: Molecular Modeling of In Vitro and In Vivo Approaches. Molecules 2020, 25, 1519. https://doi.org/10.3390/molecules25071519
Boiangiu RS, Brinza I, Hancianu M, Erdogan Orhan I, Eren G, Gündüz E, Ertas H, Hritcu L, Cioanca O. Cognitive Facilitation and Antioxidant Effects of an Essential Oil Mix on Scopolamine-Induced Amnesia in Rats: Molecular Modeling of In Vitro and In Vivo Approaches. Molecules. 2020; 25(7):1519. https://doi.org/10.3390/molecules25071519
Chicago/Turabian StyleBoiangiu, Razvan Stefan, Ion Brinza, Monica Hancianu, Ilkay Erdogan Orhan, Gokcen Eren, Elife Gündüz, Halis Ertas, Lucian Hritcu, and Oana Cioanca. 2020. "Cognitive Facilitation and Antioxidant Effects of an Essential Oil Mix on Scopolamine-Induced Amnesia in Rats: Molecular Modeling of In Vitro and In Vivo Approaches" Molecules 25, no. 7: 1519. https://doi.org/10.3390/molecules25071519
APA StyleBoiangiu, R. S., Brinza, I., Hancianu, M., Erdogan Orhan, I., Eren, G., Gündüz, E., Ertas, H., Hritcu, L., & Cioanca, O. (2020). Cognitive Facilitation and Antioxidant Effects of an Essential Oil Mix on Scopolamine-Induced Amnesia in Rats: Molecular Modeling of In Vitro and In Vivo Approaches. Molecules, 25(7), 1519. https://doi.org/10.3390/molecules25071519









