Quantification of an Antibody-Conjugated Drug in Fat Plasma by an Affinity Capture LC-MS/MS Method for a Novel Prenyl Transferase-Mediated Site-Specific Antibody–Drug Conjugate
Abstract
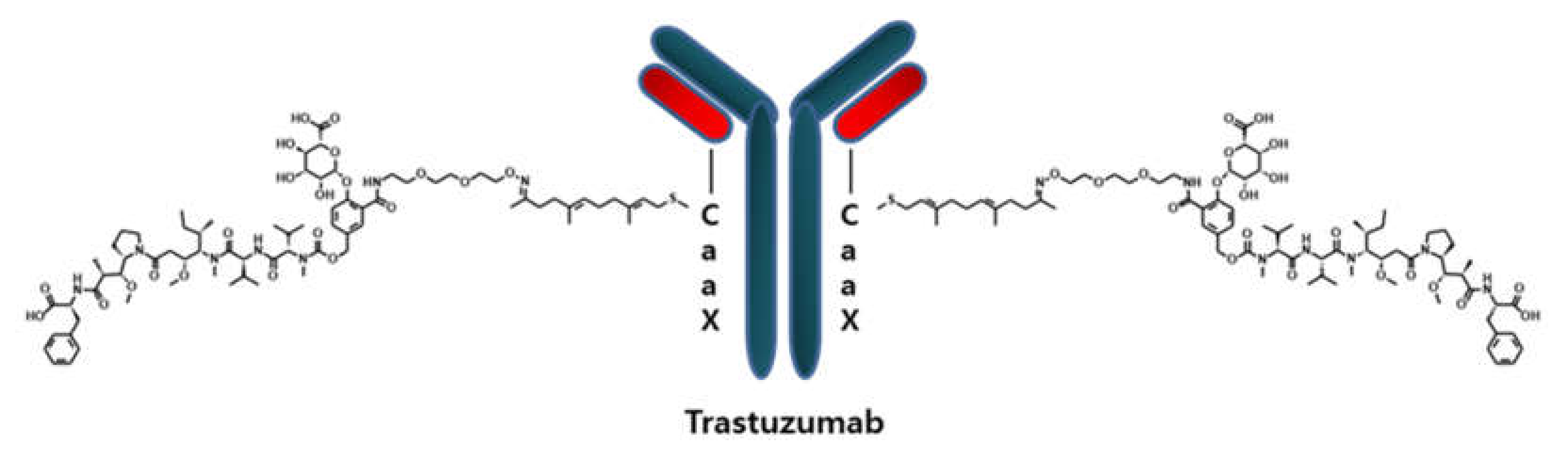
1. Introduction
2. Results and Discussions
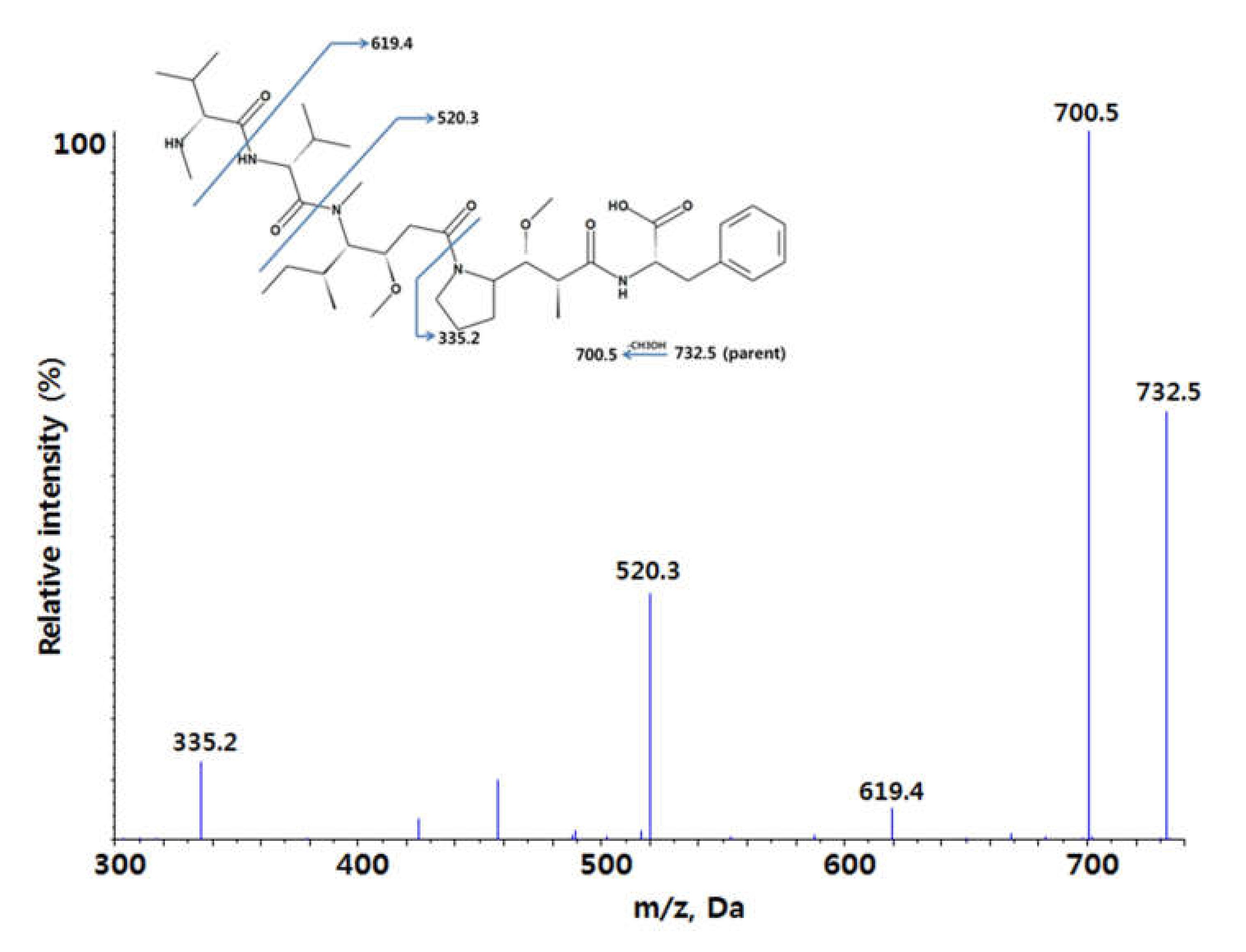
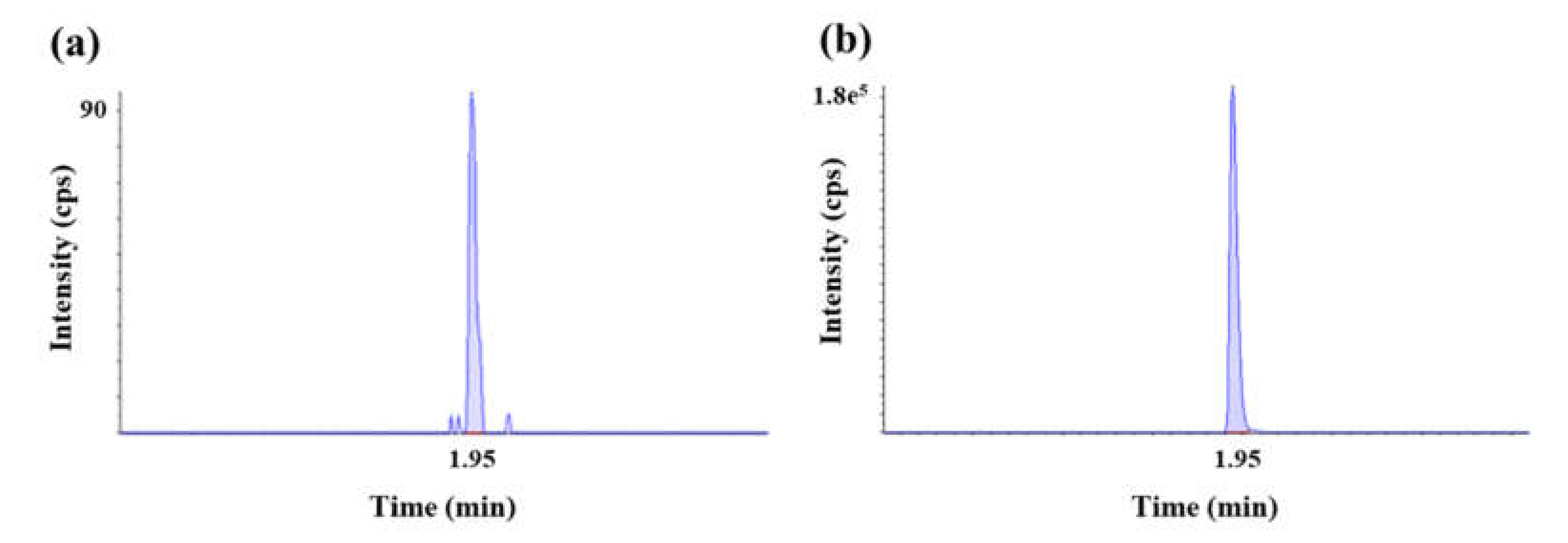
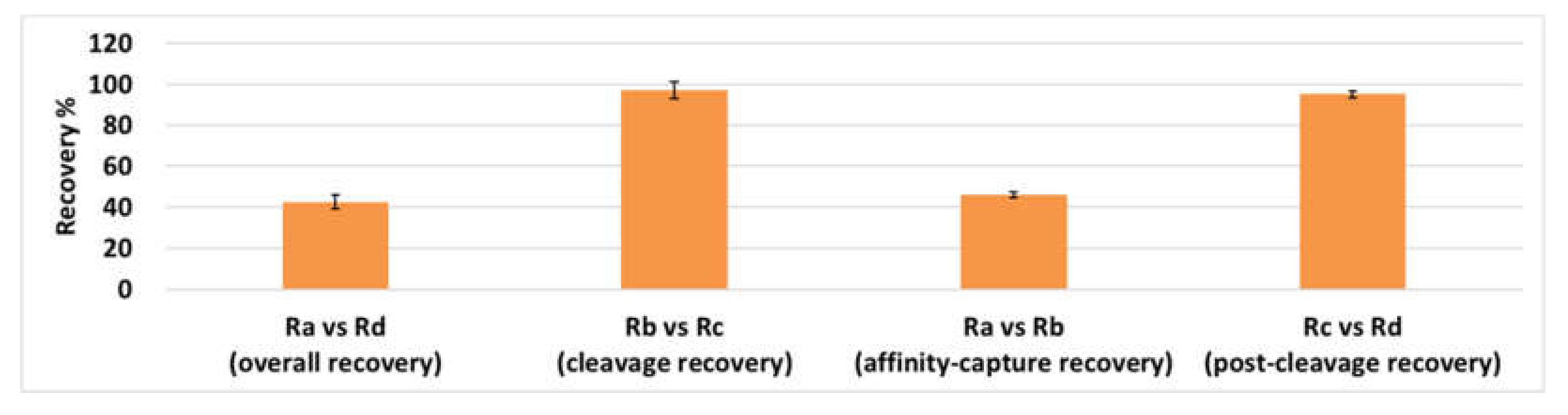
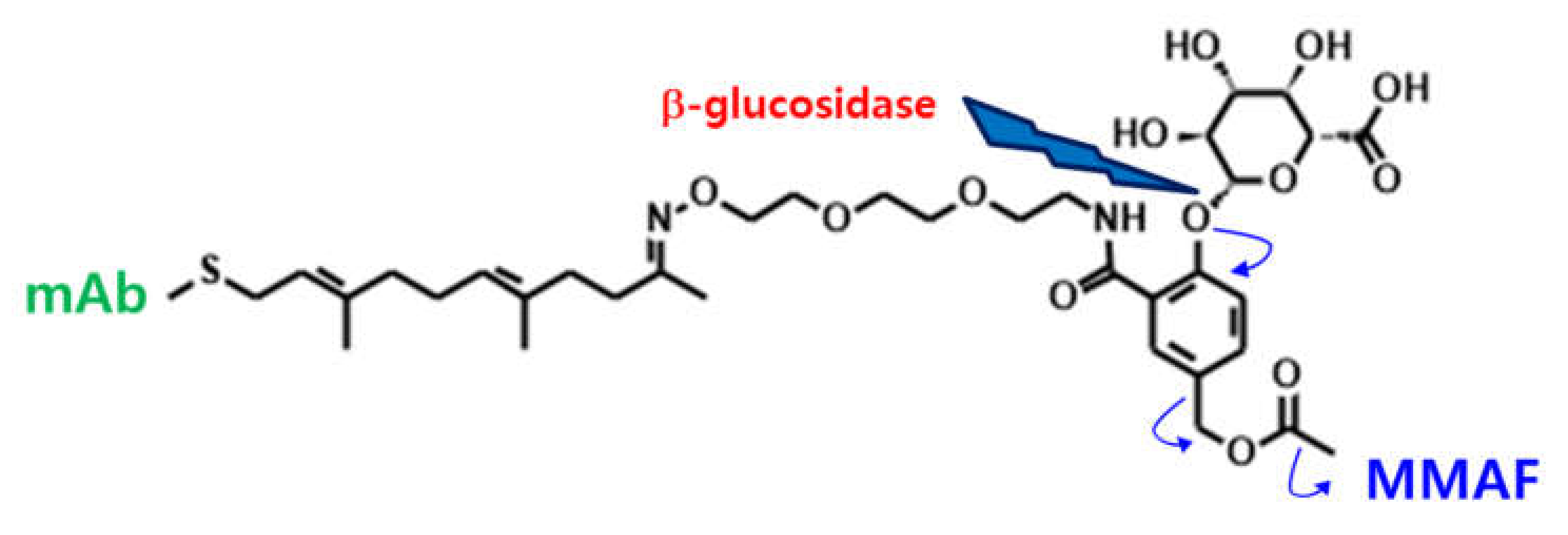
2.1. Method Development and Qualification
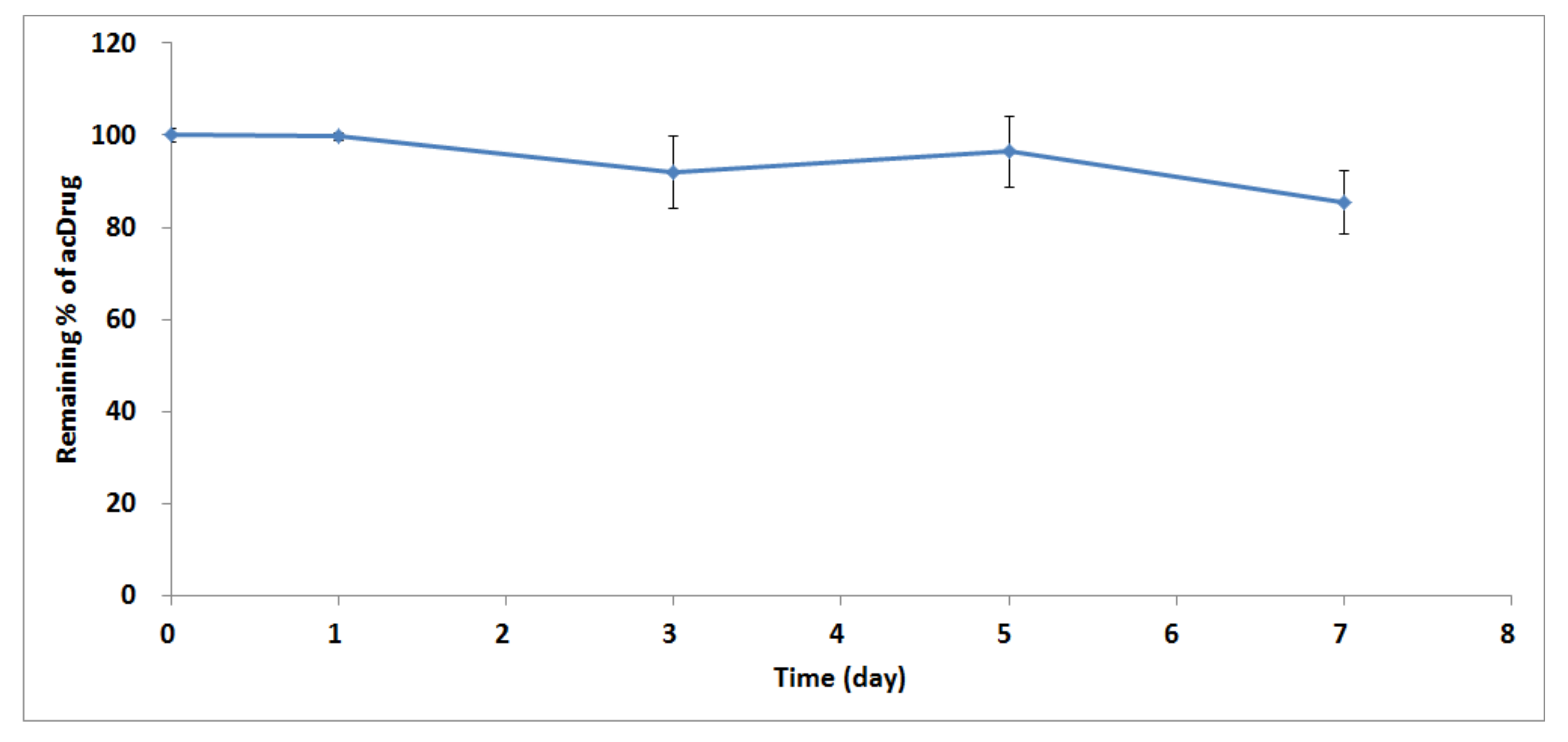
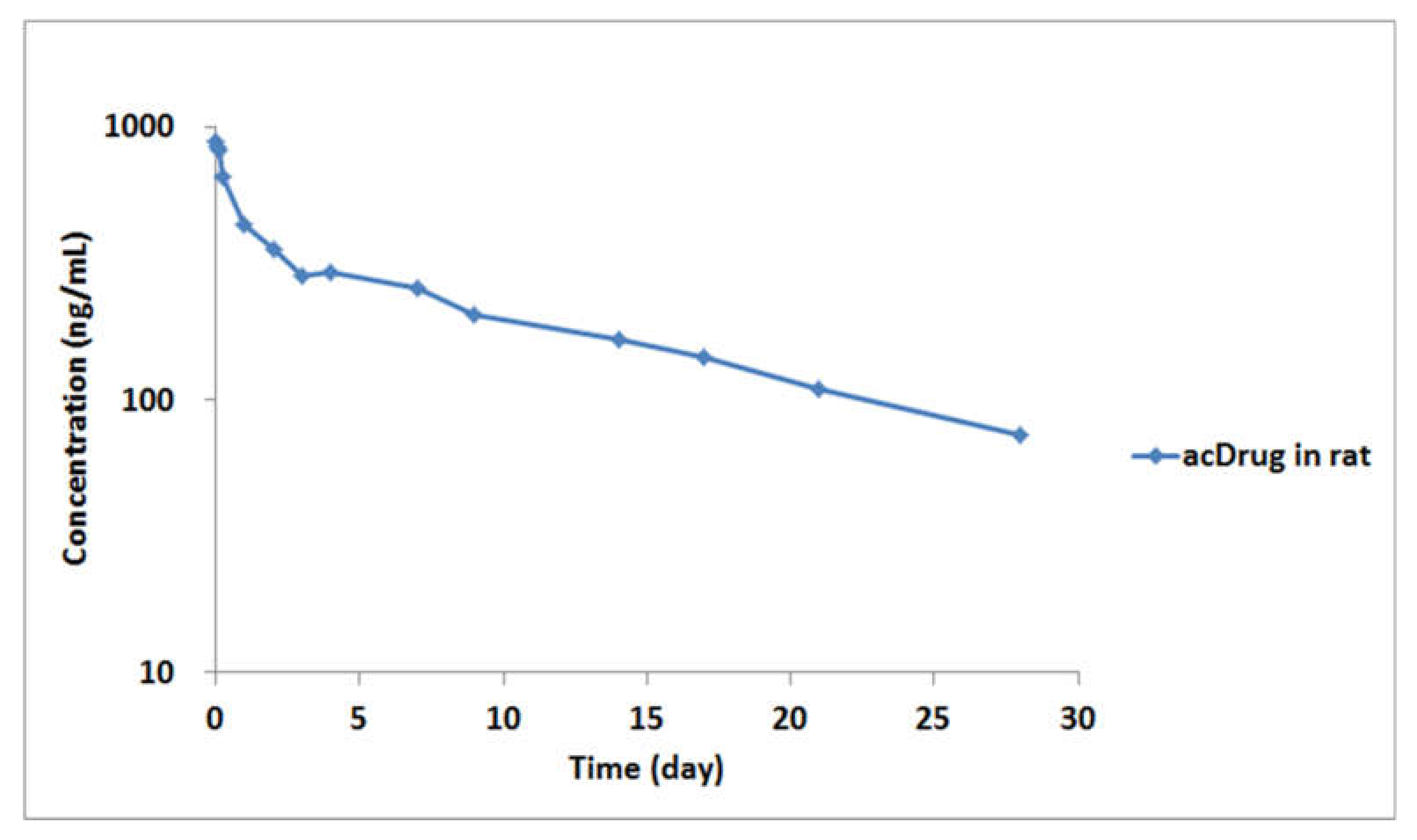
2.2. Application for In Vitro Linker Stability Study and Preclinical PK Study in Rats
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Preparation of Stocks, Standard (STD), and Quality Control (QC) Samples
3.3. Sample Preparation
3.4. LC-MS/MS Conditions
3.5. Method Qualification
3.6. Application for In Vitro Linker Stability Study and Preclinical PK Study in Rats
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Malik, P.; Phipps, C.; Edginton, A.; Blay, J. Pharmacokinetic considerations for antibody-drug conjugates against cancer. Pharm. Res. 2017, 34, 2579–2595. [Google Scholar] [CrossRef] [PubMed]
- Goldmacher, V.S.; Kovtun, Y.V. Antibody–drug conjugates: Using monoclonal antibodies for delivery of cytotoxic payloads to cancer cells. Ther. Deliv. 2011, 2, 397–416. [Google Scholar] [CrossRef] [PubMed]
- Perez, H.L.; Cardarelli, P.M.; Deshpande, S.; Gangwar, S.; Schroeder, G.M.; Vite, G.D.; Borzilleri, R.M. Antibody–drug conjugates: Current status and future directions. Drug Discov. Today 2014, 19, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.; Reichert, J.M. Antibody-drug conjugates: Present and future. MAbs 2014, 6, 15–17. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Kizlik-Masson, C.; Pèlegrin, A.; Watier, H.; Viaud-Massuard, M.-C.; Joubert, N. Antibody-drug conjugates: Design and development for therapy and imaging in and beyond cancer, LabEx MAbImprove industrial workshop, July 27–28, 2017, Tours, France. MAbs 2018, 10, 210–221. [Google Scholar] [CrossRef] [PubMed]
- Kaplon, H.; Muralidharan, M.; Schneider, Z.; Reichert, J.M. Antibodies to watch in 2020. MAbs 2019, 12, 219–238. [Google Scholar] [CrossRef] [PubMed]
- FINANCIAL TIMES. Available online: https://markets.ft.com/data/announce/detail?dockey=1323-14359225-2VKRVKGDQDIOR1DFULAJU4APGH (accessed on 23 December 2019).
- Coats, S.; Williams, M.; Kebble, B.; Dixit, R.; Tseng, L.; Yao, N.-S.; Tice, D.A.; Soria, J.-C. Antibody Drug Conjugates: Future Directions in Clinical and Translational Strategies to Improve the Therapeutic Index. Clin. Cancer Res. 2019, 25, 5441–5448. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.j.; Choi, H.J.; Yun, M.; Kang, Y.; Jung, J.E.; Ryu, Y.; Kim, T.Y.; Cha, Y.j.; Cho, H.S.; Min, J.J. Enzymatic prenylation and oxime ligation for the synthesis of stable and homogeneous protein–drug conjugates for targeted therapy. Angew. Chem. Int. Ed. 2015, 54, 12020–12024. [Google Scholar] [CrossRef] [PubMed]
- Myler, H.; Rangan, V.S.; Wang, J.; Kozhich, A.; Cummings, J.A.; Neely, R.; Dail, D.; Liu, A.; Wang, B.; Vezina, H.E. An integrated multiplatform bioanalytical strategy for antibody–drug conjugates: A novel case study. Bioanalysis 2015, 7, 1569–1582. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Xu, K.; Saad, O.M.; Dere, R.C.; Carrasco-Triguero, M. Bioanalytical assay strategies for the development of antibody–drug conjugate biotherapeutics. Bioanalysis 2013, 5, 201–226. [Google Scholar] [CrossRef] [PubMed]
- Gorovits, B.; Alley, S.C.; Bilic, S.; Booth, B.; Kaur, S.; Oldfield, P.; Purushothama, S.; Rao, C.; Shord, S.; Siguenza, P. Bioanalysis of antibody–drug conjugates: American association of pharmaceutical scientists antibody–drug conjugate working group position paper. Bioanalysis 2013, 5, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Rago, B.; Tumey, L.N.; Wei, C.; Barletta, F.; Clark, T.; Hansel, S.; Han, X. Quantitative conjugated payload measurement using enzymatic release of antibody–drug conjugate with cleavable linker. Bioconjug. Chem. 2017, 28, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Grafmuller, L.; Wei, C.; Ramanathan, R.; Barletta, F.; Steenwyk, R.; Tweed, J. Unconjugated payload quantification and DAR characterization of antibody–drug conjugates using high-resolution MS. Bioanalysis 2016, 8, 1663–1678. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gu, H.; Liu, A.; Kozhich, A.; Rangan, V.; Myler, H.; Luo, L.; Wong, R.; Sun, H.; Wang, B. Antibody–drug conjugate bioanalysis using LB-LC–MS/MS hybrid assays: Strategies, methodology and correlation to ligand-binding assays. Bioanalysis 2016, 8, 1383–1401. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Lee, M.W.; Shin, Y.G. Qualification and application of a liquid chromatography–quadrupole time-of-flight mass spectrometric method for the determination of trastuzumab in rat plasma. Biomed. Chromatogr. 2016, 30, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Stephan, J.P.; Kozak, K.R.; Wong, W.L.T. Challenges in developing bioanalytical assays for characterization of antibody–drug conjugates. Bioanalysis 2011, 3, 677–700. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.-W.; Yu, A.-M. Conference report: New analytical technologies for biological discovery. Bioanalysis 2010, 2, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Ramagiri, S.; Garofolo, F. Large molecule bioanalysis using Q-TOF without predigestion and its data processing challenges. Bioanalysis 2012, 4, 529–540. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| QC | Theoretical Concentration (ng/mL) | Mean Concentration (ng/mL) | Accuracy (%) | Precision (% CV) | n |
|---|---|---|---|---|---|
| Low QC | 239.67 | 260.61 | 108.74 | 7.09 | 10 |
| High QC | 479.33 | 543.29 | 113.34 | 3.18 | 10 |
| Statistics | Dilution QC (4793.35 ng/mL) |
|---|---|
| Mean concentration (ng/mL) | 5558.85 |
| Accuracy (%) | 115.97 |
| Precision (% CV) | 4.77 |
| n | 4 |
| Assessments | Theoretical Concentration (ng/mL) | Calculated Concentration (ng/mL) | Accuracy (%) | Precision (%CV) | n |
|---|---|---|---|---|---|
| Short-term (room temperature, 1 day) | 239.67 | 254.72 | 106.28 | 3.21 | 3 |
| 479.33 | 541.53 | 107.34 | 6.12 | 3 | |
| Long-term (−80 ℃, 10 months) | 239.67 | 239.43 | 99.90 | 3.26 | 3 |
| 479.33 | 541.71 | 113.01 | 4.99 | 3 | |
| Freeze-thaw (−80 ℃, 3 cycles) | 239.67 | 250.69 | 104.6 | 3.58 | 3 |
| 479.33 | 537.66 | 112.17 | 7.78 | 3 |
| Administration | AUC (ng/day/mL) | CL (mL×day/kg) | Alpha HL (day) | Beta HL (day) | Cmax (ng/mL) | V1 (mL/kg) | Vss (mL/kg) |
|---|---|---|---|---|---|---|---|
| Intravenous (IV) | 6679.43 | 4.31 | 0.37 | 12.13 | 890.42 | 32.35 | 72.35 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, B.i.; Park, M.-H.; Byeon, J.-J.; Shin, S.-H.; Choi, J.; Park, Y.; Park, Y.-H.; Chae, J.; Shin, Y.G. Quantification of an Antibody-Conjugated Drug in Fat Plasma by an Affinity Capture LC-MS/MS Method for a Novel Prenyl Transferase-Mediated Site-Specific Antibody–Drug Conjugate. Molecules 2020, 25, 1515. https://doi.org/10.3390/molecules25071515
Lee Bi, Park M-H, Byeon J-J, Shin S-H, Choi J, Park Y, Park Y-H, Chae J, Shin YG. Quantification of an Antibody-Conjugated Drug in Fat Plasma by an Affinity Capture LC-MS/MS Method for a Novel Prenyl Transferase-Mediated Site-Specific Antibody–Drug Conjugate. Molecules. 2020; 25(7):1515. https://doi.org/10.3390/molecules25071515
Chicago/Turabian StyleLee, Byeong ill, Min-Ho Park, Jin-Ju Byeon, Seok-Ho Shin, Jangmi Choi, Yuri Park, Yun-Hee Park, Jeiwook Chae, and Young G. Shin. 2020. "Quantification of an Antibody-Conjugated Drug in Fat Plasma by an Affinity Capture LC-MS/MS Method for a Novel Prenyl Transferase-Mediated Site-Specific Antibody–Drug Conjugate" Molecules 25, no. 7: 1515. https://doi.org/10.3390/molecules25071515
APA StyleLee, B. i., Park, M.-H., Byeon, J.-J., Shin, S.-H., Choi, J., Park, Y., Park, Y.-H., Chae, J., & Shin, Y. G. (2020). Quantification of an Antibody-Conjugated Drug in Fat Plasma by an Affinity Capture LC-MS/MS Method for a Novel Prenyl Transferase-Mediated Site-Specific Antibody–Drug Conjugate. Molecules, 25(7), 1515. https://doi.org/10.3390/molecules25071515






