Impact of Substitution Pattern and Chain Length on the Thermotropic Properties of Alkoxy-Substituted Triphenyl-Tristriazolotriazines †
Abstract
1. Introduction
2. Results
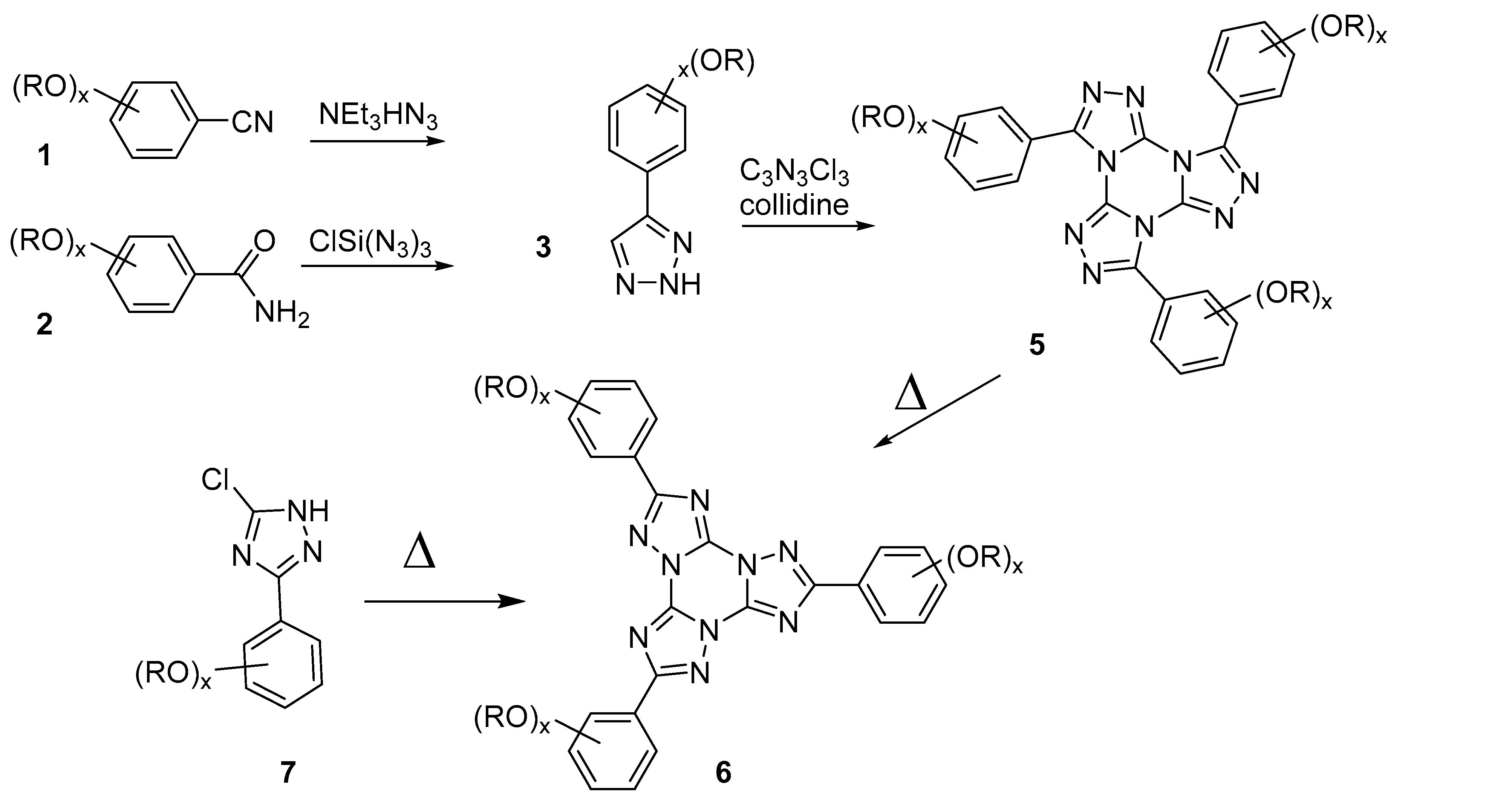
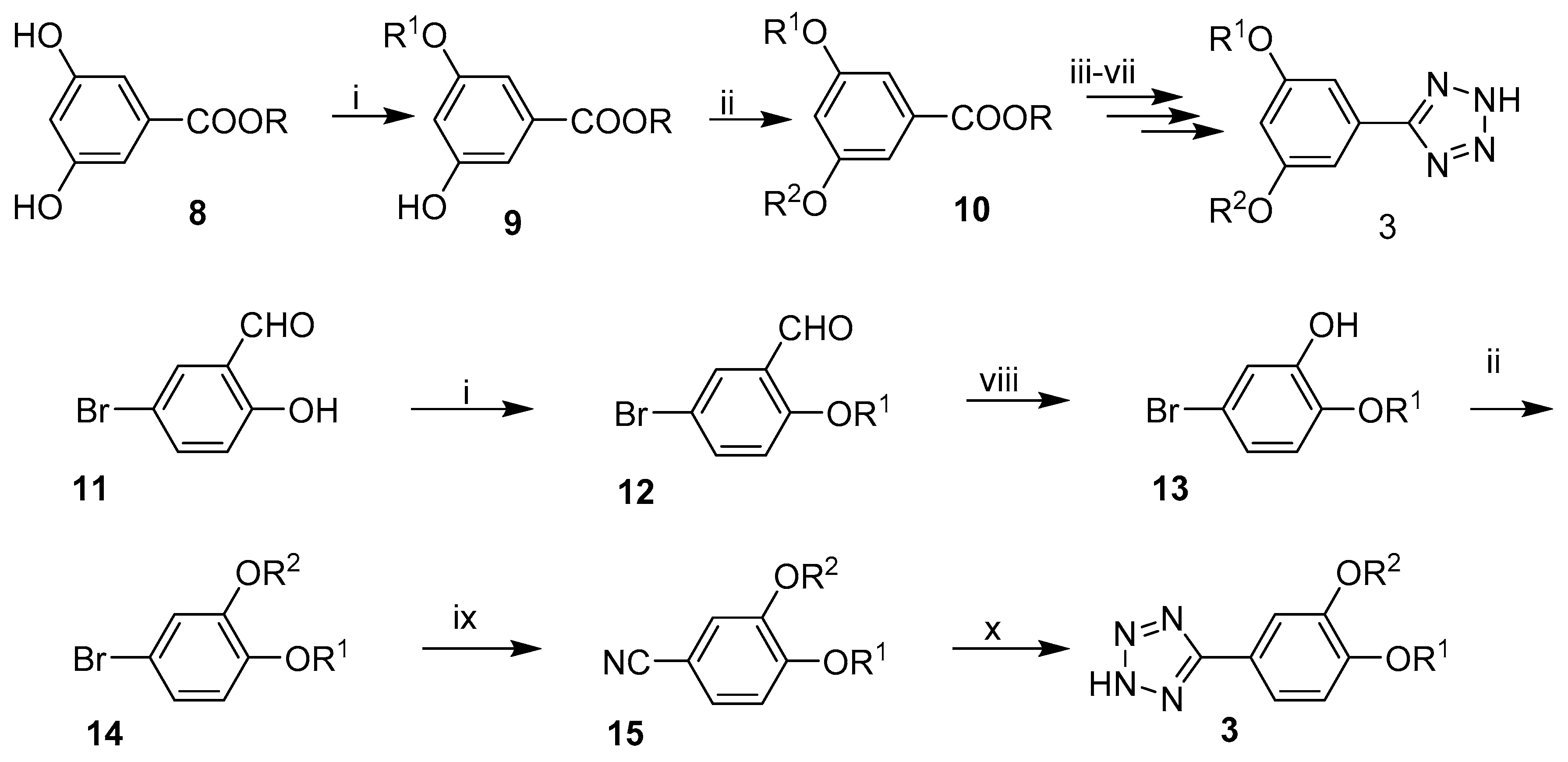
2.1. Synthesis
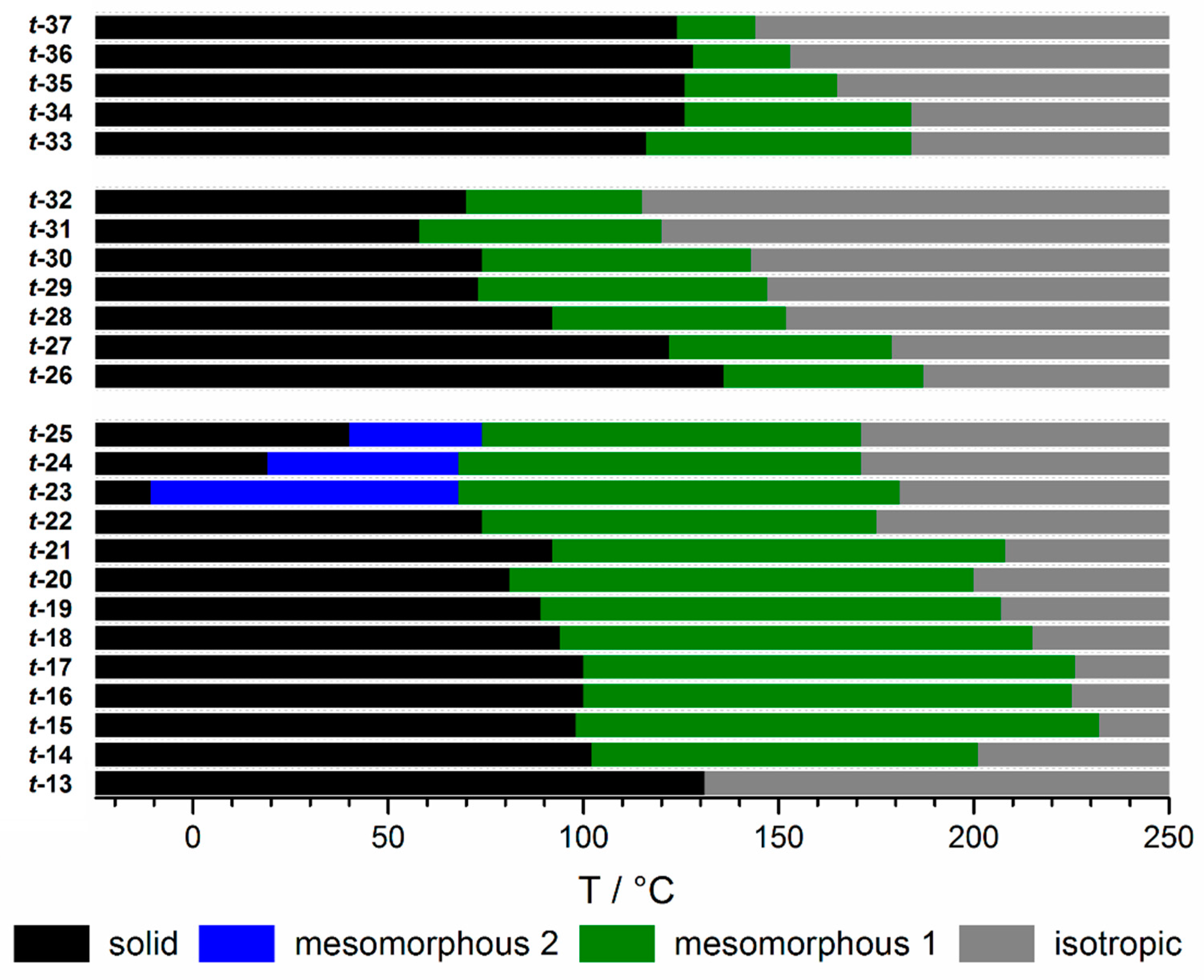
2.2. Thermal Properties of 1-n-alkyloxy-t-TTTs
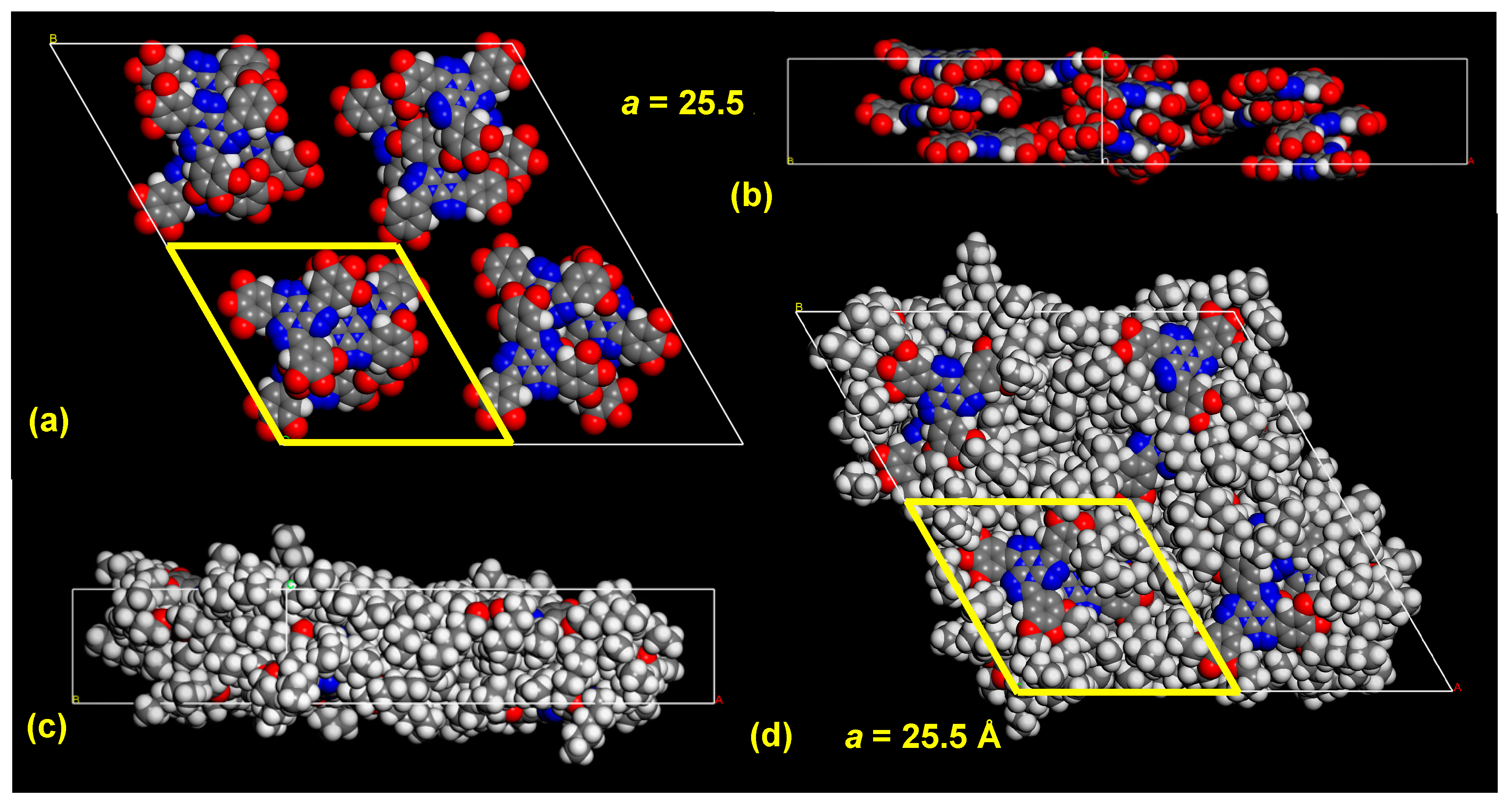
2.3. Thermal Properties of t-TTTs with Extended π-Systems and a 1-n-alkyloxy Periphery
2.4. Thermal Properties of t-TTTs with Branched Side Chains, Swallow-Tails or Chains of Different Lengths
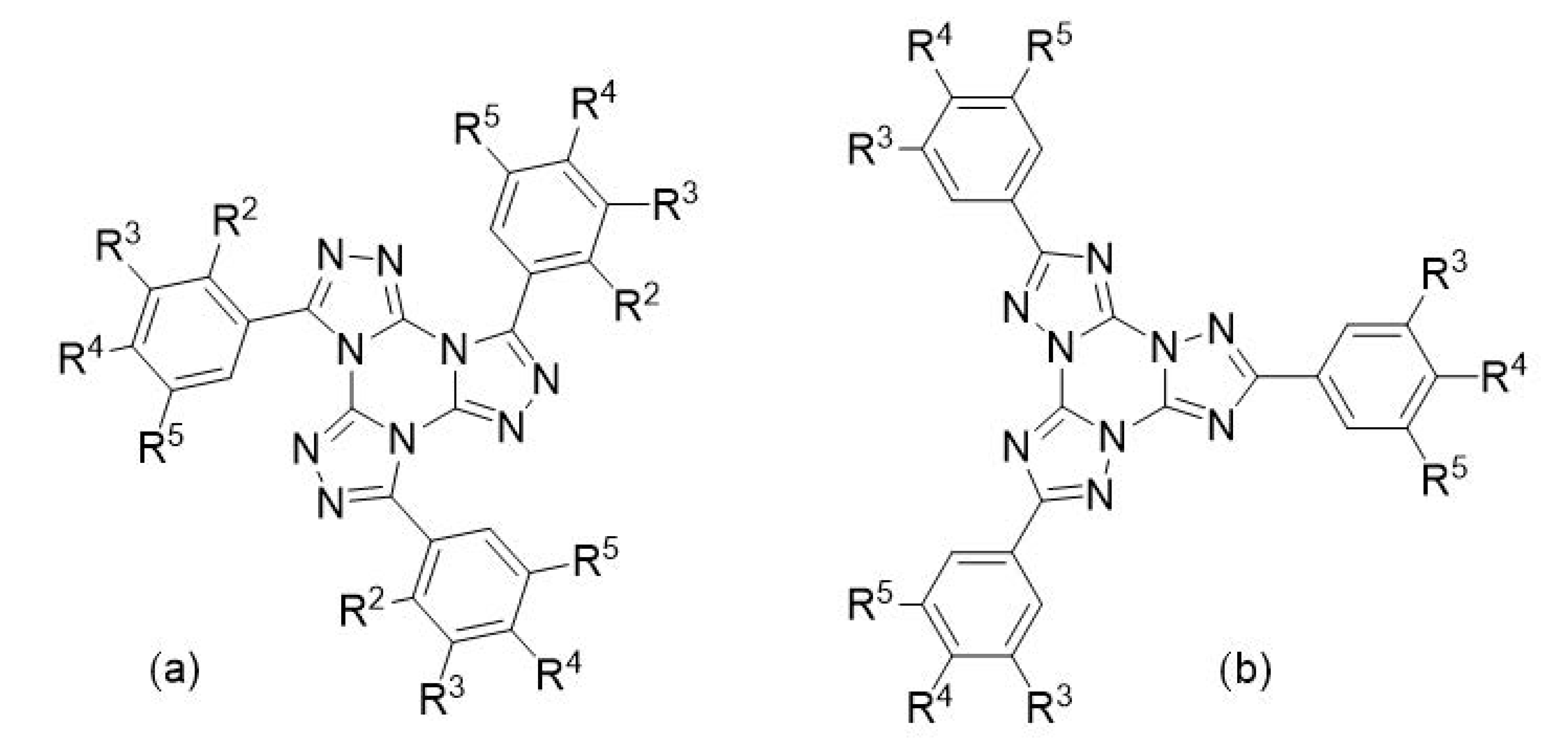
2.5. Thermal Properties of Alkoxyphenyl-Substituted TTTs with Radial Structure
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cristiano, R.; Gallardo, H.; Bertoluzzi, A.J.; Bechtold, I.H.; Campos, C.E.M.; Longo, R.L. Tristriazolotriazines: A core for luminescent discotic liquid crystals. Chem. Commun. 2008, 41, 5134–5136. [Google Scholar] [CrossRef]
- Cristiano, R.; Eccher, J.; Bechtold, I.H.; Tironi, C.N.; Vieira, A.A.; Molin, F.; Gallardo, H. Luminescent columnar liquid crystals based on tristriazolotriazine. Langmuir 2012, 28, 11590–11598. [Google Scholar] [CrossRef]
- Glang, S.; Schmitt, V.; Detert, H. Tristriazolotriazine—A novel heteroaromatic core for discotic liquid crystals. In Proceedings of the 36th German Topical Meeting on Liquid Crystals, Magdeburg, Germany, 12–14 March 2008; pp. 125–128. [Google Scholar]
- Glang, S.; Rieth, T.; Borchmann, D.; Fortunati, I.; Signorini, R.; Detert, H. Arylethynyl-Substituted Tristriazolotriazines: Synthesis, Optical Properties, and Thermotropic Behavior. Eur. J. Org. Chem. 2014, 2014, 3116–3126. [Google Scholar] [CrossRef]
- Bushby RJ Lozman, O.R. Discotic liquid crystals 25 years on. Curr. Opin. Colloid Interface Sci. 2002, 7, 343–354. [Google Scholar] [CrossRef]
- Laschat, S.; Baro, A.; Steinke, N.; Giesselmann, F.; Hägele, C.; Scalia, G.; Judele, R.; Kapatsina, E.; Sauer, S.; Schreivogel, A.; et al. Diskotische Flüssigkristalle: Von der maßgeschneiderten Synthese zur Kunststoffelektronik. Angew. Chem. 2007, 119, 4916–4973. [Google Scholar] [CrossRef]
- Kumar, S. Perspectives. In Chemistry of Discotic Liquid Crystals; CRC Press: Boca Raton, FL, USA, 2011; pp. 447–492. [Google Scholar]
- Sergeyev, S.; Pisula, W.; Geerts, Y.H. Discotic Liquid Crystals: A New Generation of Organic Semiconductors. Chem. Soc. Rev. 2008, 36, 1902–1929. [Google Scholar] [CrossRef] [PubMed]
- Wöhrle, T.; Wurzbach, I.; Kirres, J.; Kostidou, A.; Kapernaum, N.; Litterscheidt, J.; Haenle, J.C.; Staffeld, P.; Baro, A.; Giesselmann, F.; et al. Discotic Liquid Crystals. Chem. Rev. 2016, 116, 1139–1241. [Google Scholar] [CrossRef] [PubMed]
- Pisula, W.; Muellen, K. Discotic liquid crystals as semi-conductors. In Handbook of Liquid Crystals, 2nd ed.; Goodby, J.W., Ed.; Wiley-VCH: Weinheim, Germany, 2014; Volume 8, pp. 627–673. [Google Scholar]
- Kaafarani, B.R. Discotic Liquid Crystals for Opto-Electronic Applications. Chem. Mater. 2011, 23, 378–396. [Google Scholar] [CrossRef]
- Detert, H.; Lehmann, M.; Meier, H. Star-shaped Conjugated Systems. Materials 2010, 3, 3218–3330. [Google Scholar] [CrossRef]
- Boden, N.; Bushby, R.J.; Clements, J.M.; Movaghar, B. Device applications of charge transport in discotic liquid crystals. J. Mater. Chem. 1999, 9, 2081–2086. [Google Scholar] [CrossRef]
- Volpi, R.; Camilo, A.C.S.; da Silva Filho, D.A.; Navarrete, J.T.L.; Gomez-Lor, B.; Delgado, M.C.R.; Linares, M. Modeling charge transport of discotic liquid crystalline triindoles: The role of peripheral substitution. Phys. Chem. Chem. Phys. 2017, 19, 24202–24208. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Wu, H.; Zhang, Z.; Zhang, A.; Yang, H.; Feng, Y.; Fang, Y.; Zhang, L.; Wang, Z.; Qu, W. Highly ordered columnar superlattice nanostructures with improved charge carrier mobility by thermotropic self-assembly of triphenylene-based discotics. J. Mater. Chem. C 2019, 7, 12463–12469. [Google Scholar] [CrossRef]
- Navarro, A.; Fernández-Liencres, M.P.; Garcia, G.; Granadino-Roldán, J.M.; Fernández-Gómez, M. A DFT approach to the charge transport related properties in columnar stacked π-conjugated N-heterocycle cores including electron donor and acceptor units. Phys. Chem. Chem. Phys. 2015, 17, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Brzeczek, A.; Ledwon, P.; Data, P.; Zassowski, P.; Golba, S.; Walczak, K.; Lapkowski, M. Synthesis and Properties of 1,3,5-Tricarbazolylbenzenes with Star-Shaped Architecture. Dyes Pigments 2015, 113, 640–648. [Google Scholar] [CrossRef]
- Bagnich, S.A.; Athanasopoulos, S.; Rudnick, A.; Schroegel, P.; Bauer, I.; Greenham, N.C.; Strohriegl, P.; Koehler, A. Excimer Formation by Steric Twisting in Carbazole and Triphenylamine-Based Host Materials. J. Phys. Chem. C 2015, 119, 2380–2387. [Google Scholar]
- Kozlov, O.V.; Luponosov, Y.N.; Ponomarenko, S.A.; Kausch-Busies, N.; Paraschuk, D.Y.; Olivier, Y.; Beljonne, D.; Cornil, J.; Pshenichnikov, M.S. Ultrafast Charge Generation Pathways in Photovoltaic Blends Based on Novel Star-Shaped Conjugated Molecules. Adv. Energy Mater. 2015, 5, 1401657. [Google Scholar] [CrossRef]
- Jiang, Y.; Yu, D.; Lu, L.; Zhan, C.; Wu, D.; You, W.; Wei, X.; Xie, Z.; Xiao, S. Tuning optical and electronic properties of star-shaped conjugated molecules with enlarged π-delocalization for organic solar cell application. J. Mater. Chem. A 2013, 1, 8270–8279. [Google Scholar] [CrossRef]
- Metri, N.; Sallenave, X.; Beouch, L.; Plesse, C.; Goubard, F.; Chevrot, C. New star-shaped molecules derived from thieno[3, 2-b] thiophene unit and triphenylamine. Tetrahedron Lett. 2010, 51, 6673–6676. [Google Scholar] [CrossRef]
- Leriche, P.; Piron, F.; Ripaud, E.; Frere, P.; Allain, M.; Roncali, J. Star-shaped triazine–thiophene conjugated systems. Tetrahedron Lett. 2009, 50, 5673–5676. [Google Scholar] [CrossRef]
- Ponomarenko, S.A.; Tatarinova, E.A.; Muzafarov, A.M.; Kirchmeyer, S.; Brassat, L.; Mourran, A.; Moeller, M.; Setayesh, S.; De Leeuw, D. Star-shaped oligothiophenes for solution-processible organic electronics: Flexible aliphatic spacers approach. Chem. Mater. 2006, 18, 4101–4108. [Google Scholar] [CrossRef]
- Liu, C.-F.; Cheng, C.; Jiang, Y.; Lai, W.-Y.; Huang, W. Nitrogen-doped star-shaped polycyclic aromatic hydrocarbons based on fused triazatruxenes: Synthesis and optoelectronic properties. New J. Chem. 2017, 41, 13619. [Google Scholar] [CrossRef]
- El Sayed, M.T. Synthetic Routes to Electroactive Organic Discotic Aromatic Triazatruxenes. J. Heterocycl. Chem. 2018, 55, 21–43. [Google Scholar] [CrossRef]
- Lehmann, M.; Kestemont, G.; Aspe, R.G.; Buess-Herman, C.; Koch, M.H.J.; Debije, M.G.; Piris, J.; de Haas, M.P.; Warman, J.M.; Watson, M.D.; et al. High charge-carrier mobility in π-deficient discotic mesogens: Design and structure–property relationship. Chem. Eur. J. 2005, 11, 3349–3362. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Lee, W.-H.; Lee, R.-H. Solution processable star-shaped molecules with a triazine core and branching thienylenevinylenes for bulk heterojunction solar cells. RSC Adv. 2014, 4, 48150–48162. [Google Scholar] [CrossRef]
- Pradhan, B.; Pathak, S.K.; Gupta, R.K.; Gupta, M.; Pal, S.K.; Achalkumar, A.S. Star-shaped fluorescent liquid crystals derived from s-triazine and 1,3,4-oxadiazole moieties. J. Mater. Chem. C 2016, 4, 6117–6130. [Google Scholar] [CrossRef]
- Lukes, V.; Rapta, P.; Idzik, K.R.; Beckert, R.; Dunsch, L. Charged States of 1,3,5-Triazine Molecules as Models for Star-shaped Molecular Architecture: A DFT and spectroelectrochemcial Study. J. Phys. Chem. B 2011, 115, 3344–3353. [Google Scholar] [CrossRef]
- Ishi-i, T.; Moriyama, Y.; Kusakaki, Y. Turn-on-type emission enhancement and ratiometric emission color change based on the combination effect of aggregation and TICT found in the hexaazatriphenylene-triphenylamine dye in an aqueous environment. RSC Adv. 2016, 6, 86301. [Google Scholar] [CrossRef]
- Blas-Ferrando, V.M.; Ortiz, J.; Follana-Berna, J.; Fernandez-Lazaro, F.; Sastre-Santos, A.; Campos, A.; Mas-Torrent, M. Large-Size Star-Shaped Conjugated (Fused) Triphthalocyaninehexaazatriphenylene. Org. Lett. 2016, 18, 1466–1469. [Google Scholar] [CrossRef]
- Segura, J.L.; Juarez, R.; Ramos, M.; Seoane, C. Hexaazatriphenylene (HAT) derivatives: From synthesis to molecular design, self-organization and device applications. Chem. Soc. Rev. 2015, 44, 6850–6885. [Google Scholar] [CrossRef]
- Gearba, R.I.; Lehmann, M.; Levin, J.; Ivanov, D.A.; Koch, M.H.J.; Barbera, J.; Debije, M.G.; Piris, J.; Geerts, Y.H. Tailoring Discotic Mesophases: Columnar Order Enforced with Hydrogen Bonds. Adv. Mater. 2003, 15, 1614–1618. [Google Scholar] [CrossRef]
- Cammidge, A.N.; Cook, M.J.; Harrison, K.J.; McKeown, N.B. Synthesis and characterisation of some 1,4,8,11,15,18,22,25-octa(alkoxymethyl)phthalocyanines; a new series of discotic liquid crystals. J. Chem. Soc. Perkin. 1991, 12, 3053–3058. [Google Scholar] [CrossRef]
- Tober, N.; Rieth, T.; Lehmann, M.; Detert, H. Synthesis, Thermal, and Optical Properties of Tris(5-aryl-1,3,4-oxadiazol-2-yl)-1,3,5-triazines, New Star-shaped Fluorescent Discotic Liquid Crystals. Chem. Eur. J. 2019, 25, 15295–15304. [Google Scholar] [CrossRef] [PubMed]
- Röder, N.; Marzalek, T.; Limbach, D.; Pisula, W.; Detert, H. Tetrakis(oxadiazolylphenyl)pyrazines: New St. Andrew’s Cross-Shaped Liquid Crystals. ChemPhysChem 2019, 20, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Glang, S.; Borchmann, D.; Rieth, T.; Detert, H. Tristriazolotriazines with π-Conjugated Segments: Star-shaped Fluorophors and Discotic Liquid Crystals. Adv. Sci. Technol. 2013, 77, 118–123. [Google Scholar] [CrossRef]
- Rieth, T.; Marszalek, T.; Pisula, W.; Detert, H. Thermotropic Properties and Molecular Packing of Discotic Tristriazolotriazines with Rigid Substituents. Chem. Eur. J. 2014, 20, 5000–5006. [Google Scholar] [CrossRef]
- Sperner, M.; Tober, N.; Detert, H. Tristriazolotriazines with Azobenzene Arms—Acidochromic Dyes and Discotic Liquid Crystals. Eur. J. Org. Chem. 2019, 4688–4693. [Google Scholar] [CrossRef]
- Detert, H. Tristriazolotriazines—Luminescent Discotic Liquid Crystals. Eur. J. Org. Chem. 2018, 2018, 4501–4507. [Google Scholar] [CrossRef]
- Herget, K.; Schollmeyer, D.; Detert, H. 3,7,11-Tris{4-[(1R,3S,4S)-neomenthyloxy]phenyl}tri[1,2,4]triazolo[4,3-a:4′,3′-c:4″,3″-e][1,3,5]triazine-chloroform-ethanol (1/1/1). Acta Cryst. E 2013, 69, 365. [Google Scholar] [CrossRef]
- Rieth, T.; Röder, N.; Lehmann, M.; Detert, H. Isomerisation of Liquid-Crystalline Tristriazolotriazines. Chem. Eur. J. 2018, 24, 93–96. [Google Scholar] [CrossRef]
- Dal-Bó, A.G.; López Cisneros, G.G.; Cercena, R.; Mendes, J.; Matos de Silveira, L.; Zapp, E.; Dominicano, K.G.; da Costa Duarte, R.; Rodembusch, F.S.; Allievi Frizon, T.E. Synthesis, electrochemical, thermal and photophysical characterization of photoactive discotic dyes based on the tris-[1,2,4]-triazolo-[1,3,5]-triazine core. Dyes Pigments 2016, 135, 49–56. [Google Scholar] [CrossRef]
- Destrade, C.; Mondon, M.C.; Malthete, J. Hexasubstituted triphenylenes: A New Mesomorphic Order. J. Phys. Colloque 1979, 40, C3-17. [Google Scholar] [CrossRef][Green Version]
- Malthete, J.; Destrade, C.; Tinh, N.H.; Jacques, J.A. Pure Disc-Like Molecule with Cholesteric Properties. Mol. Cryst. Liq. Cryst 1981, 64, 233–238. [Google Scholar] [CrossRef]
- Ong, C.W.; Liao, S.C.; Chang, T.H.; Hsu, H.F. Rapid synthesis of new discotic liquid crystals based on diquinoxalino[2,3-a:2′,3′-c]phenazine containing hexakis(alkoxy) side arms. Tetrahedron Lett. 2003, 44, 1477–1480. [Google Scholar] [CrossRef]
- Bock, H.; Bebeau, A.; Seguy, I.; Jolinat, P.; Destruel, P. Crystal and Electronic Structure of a Fluorescent Columnar Liquid Crystalline Electron Transport Material. ChemPhysChem 2002, 3, 532–535. [Google Scholar] [CrossRef]
- Holst, H.C.; Pakula, T.; Meier, H. Liquid crystals in the series of 2,4,6-tristyryl-1,3,5-triazines. Tetrahedron 2004, 60, 6765–6775. [Google Scholar] [CrossRef]
- Foster, E.J.; Lavingueur, C.; Ke, Y.C.; Williams, V.E. Modular assembly of elliptical mesogens. Liq. Cryst. 2007, 34, 833–840. [Google Scholar]
- Paganuzzi, V.; Guatteri, P.; Riccardi, P.; Sacchelli, T.; Barbera, J.; Costa, M.; Dalcanale, E. Synthesis and Mesogenic Properties of Porphyrin Octaesters. Eur. J. Org. Chem. 1999, 1999, 1527–1539. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, W.Y.; Fan, Y.; Jian, W.P.; Liu, G.F. [5-(p-alkoxy)phenyl-10, 15, 20-tri-phenyl] porphyrin and their rare earth complex liquid crystalline. J. Phys. Org. Chem. 2007, 20, 229–235. [Google Scholar] [CrossRef]
- Bhyrappa, P.; Arunkumar, C.; Varghese, B.; Rao, D.S.S.; Prasad, S.K. Synthesis and mesogenic properties of β-tetrabrominated tetraalkyloxyporphyrins. Porphyr. Phthalocyanines 2008, 12, 54–64. [Google Scholar] [CrossRef]
- Lelj, F.; Morelli, G.; Ricciardi, G.; Roviello, A.; Sirigu, A. Discotic mesomorphism of 2,3,7,8,12,13,17,18-octakis (alkyl-thio) 5,10,15,20 tetraaza porphyrin and its complexes with some divalent transition metal ions. Synthesis and characterization. Liq. Cryst. 1992, 12, 941–960. [Google Scholar] [CrossRef]
- van der Pol, J.F.; Neeleman, E.; Zwikker, J.W.; Nolte, R.J.M.; Drenth, W.; Aerts, J.; Visser, R.; Picken, S. Liquid-crystalline phthalocyanines revisited. Liq. Cryst. 2006, 33, 1373–1387. [Google Scholar]
- Ban, K.; Nishizawa, K.; Ohta, K.; Shirai, H. Discotic liquid crystals of transition metal complexes 27: Supramolecular structure of liquid crystalline octakisalkylthiophthalocyanines and their copper complexes. J. Mater. Chem. 2000, 10, 1083–1090. [Google Scholar] [CrossRef]
- Huisgen, R.; Sturm, H.J.; Seidel, M. Ringöffnungen der Azole, V. Weitere Reaktionen der Tetrazole mit elektrophilen Agenzien. Chem. Ber. 1961, 94, 1555–1562. [Google Scholar] [CrossRef]
- El-Ahl, A.; Elmorsy, S.; Elbeheery, A. A novel approach for the synthesis of 5-substituted tetrazole derivatives from primary amides in mild one-step method. Tetrahedron Lett. 1997, 38, 1257–1260. [Google Scholar] [CrossRef]
- Tartakovsky, V.A.; Frumkin, A.E.; Churakov, A.M.; Strelenko, Y.A. New approaches to synthesis of tris[1,2,4]triazolo[1,3,5]triazines. Russ. Chem. Bull. Int. Ed. 2005, 54, 719–726. [Google Scholar] [CrossRef]
- Limbach, D.; Detert, H.; Schollmeyer, D. 2,6,10-Trichloro-tris([1,2,4]triazolo)[1,5-a:1′,5′-c:1″,5″-e]-1,3,5-triazine. IUCR Data 2018, 3, x180212. [Google Scholar] [CrossRef]
- Rieth, T.; Glang, S.; Borchmann, D.; Detert, H. 3,5-Dialkoxy substituted triphenyl-tristriazolotriazines: Fluorescent discotic liquid crystals. Mol. Cryst. Liq. Cryst. 2015, 610, 89–99. [Google Scholar] [CrossRef]
- Simulations were performed using Accelrys Materials Studio 2017 R2 using the CompassII force field. Module: Forcite; Force Field: COMPASSII, Algorithm: Smart; Quality: Medium (Energy 0,001 kcal/mol; Force 0.5 kcal/mol/A); Summation method: Ewald
- Cammidge, A.N. The effect of size and shape variation in discotic liquid crystals based on triphenylene cores. Philos. Trans. R. Soc. Math. Phys. Eng. Sci. 2006, 364, 2697–2708. [Google Scholar] [CrossRef]
- Kumar, S.; Varschney, S.K. A Room-Temperature Discotic Nematic Liquid Crystal. Angew. Chem. Int Ed. 2000, 39, 3140–3142. [Google Scholar] [CrossRef]
- Bisoy, H.K.; Kumar, S. Room-temperature electron-deficient discotic liquid crystals: Facile synthesis and mesophase characterization. New J. Chem. 2008, 32, 1974–1980. [Google Scholar] [CrossRef]
- Sergeyev, S.; Pouzet, E.; Debever, O.; Levin, J.; Gierschner, J.; Cornil, J.; Aspe, R.G.; Geerts, Y.H. Liquid crystalline octaalkoxycarbonyl phthalocyanines: Design, synthesis, electronic structure, self-aggregation and mesomorphism. J. Mater. Chem. 2007, 17, 1774–1784. [Google Scholar] [CrossRef]
- Liu, C.Y.; Fechtenkötter, A.; Watson, M.D.; Müllen, K.; Bard, A.J. Room Temperature Discotic Liquid Crystalline Thin Films of Hexa-peri-hexabenzocoronene: Synthesis and Optoelectronic Properties. Chem. Mater. 2003, 15, 124–130. [Google Scholar] [CrossRef]
- Pisula, W.; Kastler, M.; Wasserfallen, D.; Mondeshki, M.; Piris, J.; Schnell, I.; Müllen, K. Relation between Supramolecular Order and Charge Carrier Mobility of Branched Alkyl Hexa-peri-hexabenzocoronenes. Chem. Mater. 2006, 18, 3634–3640. [Google Scholar] [CrossRef]
- Seo, S.H.; Jones, T.V.; Seyler, H.; Peters, J.O.; Kim, T.H.; Chang, J.Y.; Tew, G.N. Liquid Crystalline Order from ortho-Phenylene Ethynylene Macrocycles. J. Am. Chem. Soc. 2006, 128, 9264–9265. [Google Scholar] [CrossRef] [PubMed]
- van de Craats, A.M.; Bunk, O.; Nielsen, M.M.; Watson, M.; Müllen, K.; Chanzy, H.D.; Sirringhaus, H.; Friend, R.H. Meso-Epitaxial Solution-Growth of Self-Organizing Discotic Liquid-Crystalline Semiconductors. Adv. Mat. 2003, 15, 495–499. [Google Scholar] [CrossRef]
- Budig, H.; Lunkwitz, R.; Paschke, R.; Tschierske, C.; Nuetz, U.; Diele, S.; Pelzl, G. The influence of heteroatoms and branchings on the liquid-crystalline properties of cyclotriveratrylene derivatives. J. Mater. Chem. 1996, 6, 1283–1289. [Google Scholar] [CrossRef]
- Lee, M.; Kim, J.-W.; Peleshanko, S.; Larson, K.; Yoo, Y.-S.; Vaknin, D.; Markutsya, S.; Tsukruk, V.V. Amphiphilic hairy disks with branched hydrophilic tails and a hexa-peri-hexabenzocoronene core. J. Am. Chem. Soc. 2002, 124, 9121–9128. [Google Scholar] [CrossRef]
- De, J.; Gupta, S.P.; Bala, I.; Kumar, S.; Pal, S.K. Phase Behavior of a New Class of Anthraquinone-Based Discotic Liquid Crystals. Langmuir 2017, 33, 13849–13860. [Google Scholar] [CrossRef]
- Pathak, S.K.; Nath, S.; De, J.; Pal, S.K.; Achalkumar, A.S. Contrasting effects of heterocycle substitution and branched tails in the arms of star-shaped molecules. New J. Chem. 2017, 41, 4680–4688. [Google Scholar] [CrossRef]
- Feng, C.; Ding, Y.-H.; Han, X.-D.; Yu, W.-H.; Xiang, S.-K.; Wang, B.-Q.; Hu, P.; Li, L.-C.; Chen, X.-Z.; Zhao, K.-Q. Triphenylene 2, 3-dicarboxylic imides as luminescent liquid crystals: Mesomorphism, optical and electronic properties. Dyes Pigments 2017, 139, 87–96. [Google Scholar] [CrossRef]
- Laschat, S.; Baro, A.; Woehrle, T.; Kirres, J. Playing with nanosegregation in discotic crown ethers: From molecular design to OFETs, nanofibers and luminescent materials. Liq. Cryst. Today 2016, 25, 48–60. [Google Scholar] [CrossRef]
- Kirres, J.; Knecht, F.; Seubert, P.; Baro, A.; Laschat, S. δ-Methyl Branching in the Side Chain Makes the Difference: Access to Room-Temperature Discotics. ChemPhysChem 2016, 17, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Varshney, S.K.; Prasad, V.; Takezoe, H. Spontaneous Achiral Symmetry Breaking in Liquid Crystalline Phases. Liq. Cryst. 2011, 38, 53–60. [Google Scholar] [CrossRef]
- Mahoney, S.J.; Ahmida, M.M.; Kayal, H.; Fox, N.; Shimizu, Y.; Eichhorn, S.H. Synthesis, mesomorphism and electronic properties of nonaflate and cyano-substituted pentyloxy and 3-methylbutyloxy triphenylenes. J. Mater. Chem. 2009, 19, 9221–9232. [Google Scholar] [CrossRef]
- Stackhouse, P.J.; Hird, M. Influence of branched chains on the mesomorphic properties of symmetrical and unsymmetrical triphenylene discotic liquid crystals. Liq. Cryst. 2008, 35, 597–607. [Google Scholar] [CrossRef]
- Weissflog, W.; Wiegeleben, A.; Diele, S.; Demus, D. Liquid crystalline swallow-tailed compounds I. Cryst. Res. Technol. 1984, 19, 583–591. [Google Scholar] [CrossRef]
- Weissflog, W.; Letko, I.; Pelzl, G.; Diele, S. Liq. Cryst. 1995, 18, 867–870. [CrossRef]
- Diele, S.; Oelsner, S.; Kuschel, F.; Hisgen, B.; Ringsdorf, H.; Zentel, R. X-ray investigations of liquid crystalline homo- and copolysiloxanes with paired mesogens. Makromol. Chem. 1987, 188, 1993–2000. [Google Scholar] [CrossRef]
- Pisula, W.; Tomovic, Z.; Kolb, U.; Muellen, K. Melt Processing of Hexa-peri-hexabenzocoronene on the Water Surface. Langmuir 2011, 27, 1524–1529. [Google Scholar] [CrossRef]
- Wicklein, A.; Lang, A.; Muth, M.; Thelakkat, M. Swallow-tail substituted liquid crystalline perylene bisimides: Ssynsthesis and thermotropic properties. J. Am. Chem. Soc. 2009, 131, 14442–14453. [Google Scholar] [CrossRef]
- Liu, F.; Prehm, M.; Zeng, X.; Ungar, G.; Tschierske, C. Two-and three-dimensional liquid-crystal phases from axial bundles of rodlike polyphiles: Segmented cylinders, crossed columns, and ribbons between sheets. Angew. Chem. Int. Ed. 2011, 50, 10599–10602. [Google Scholar] [CrossRef] [PubMed]
- Weck, M.; Dunn, A.R.; Matsumoto, K.; Coates, G.W.; Lobkovsky, E.B.; Grubbs, R.H. Influence of perfluoroarene–arene interactions on the phase behavior of liquid crystalline and polymeric materials. Angew. Chem. Int. Ed. 1999, 38, 2741–2745. [Google Scholar] [CrossRef]
- Henderson, P.; Kumar, S.; Rego, J.A.; Ringsdorf, H.; Schumacher, P. The synthesis of alkoxybromotriphenylenes: New discotic liquid crystals and valuable precursors to ‘mixed tail’ discotics. Chem. Commun. 1995, 10, 1059–1060. [Google Scholar] [CrossRef]
- Kumar, S.; Manickam, M. Novel unsymmetrical triphenylene discotic liquid crystals: First synthesis of 1, 2, 3, 6, 7, 10, 11-heptaalkoxytriphenylenes. Chem. Commun. 1998, 14, 1427–1428. [Google Scholar] [CrossRef]
- Kumar, S.; Naido, J.J.; Shankar Rao, D.S. Novel dibenzo[fg,op]naphthacene discotic liquid crystals: A versatile rational synthesis. J. Mater. Chem. 2002, 12, 1335–1341. [Google Scholar] [CrossRef]
- Adib, Z.A.; Clarkson, G.J.; McKeown, N.B.; Treacher, K.E.; Gleeson, H.F.; Stennet, A.S. Molecular assemblies of novel amphiphilic phthalocyanines: An investigation into the self-ordering properties of complex functional materials. J. Mater. Chem. 1998, 8, 2371–2378. [Google Scholar] [CrossRef]
- Yuksel, F.; Attila, D.; Ahsen, V. Synthesis and characterization of liquid crystalline unsymmetrically substituted phthalocyanines. Polyhedron 2007, 26, 4551–4556. [Google Scholar] [CrossRef]
- Kumar, S.; Naidu, J.J. Novel hexasubstituted triphenylene discotic liquid crystals having three different types of peripheral substituent. Liq. Cryst. 2002, 29, 899–906. [Google Scholar] [CrossRef]
- Cammidge, A.N.; Gopee, H. Antiaromatic twinned triphenylene discotics showing nematic phases and 2-dimensional π-overlap in the solid state. J. Mater. Chem. 2001, 11, 2773–2783. [Google Scholar] [CrossRef]

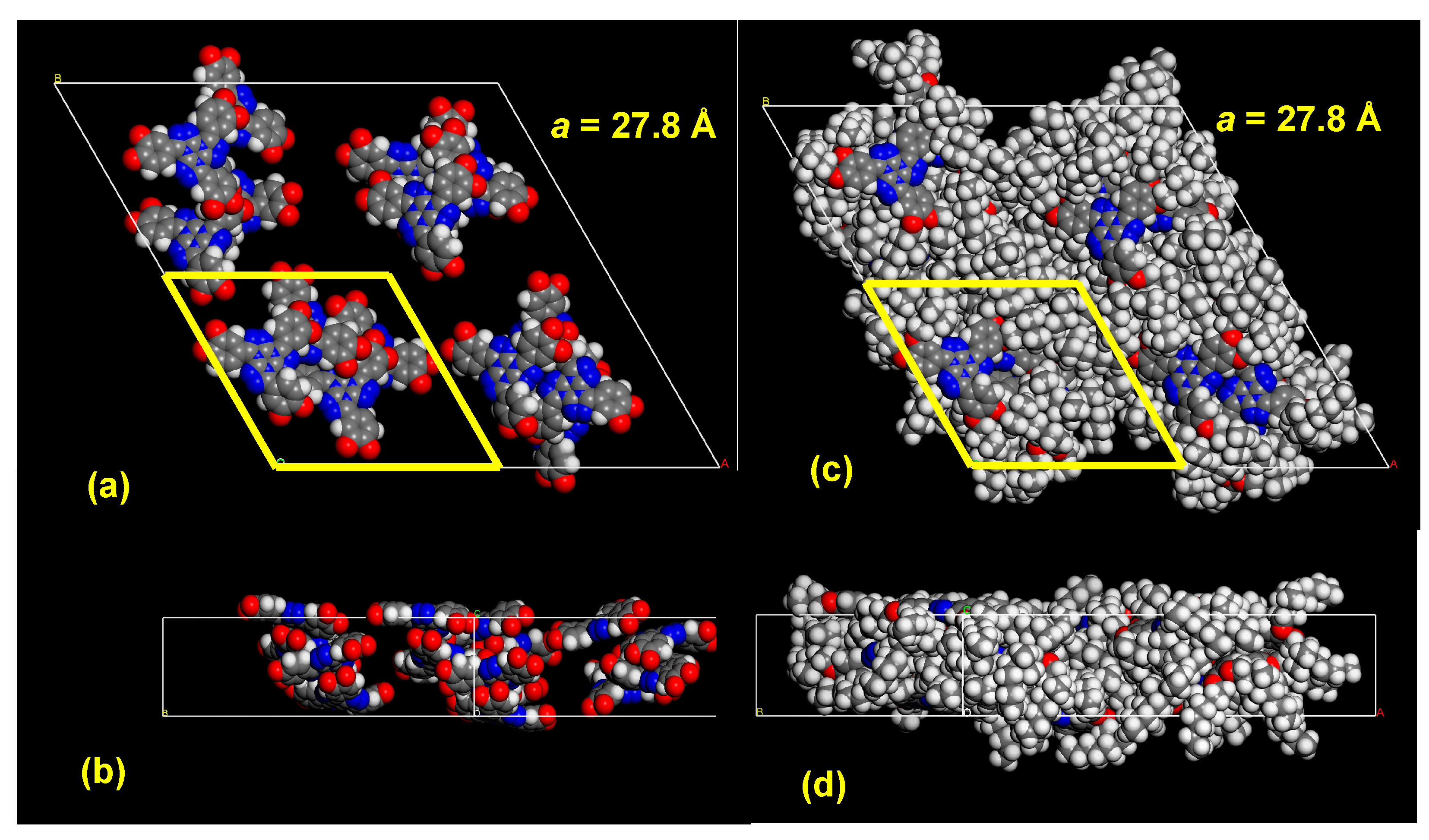
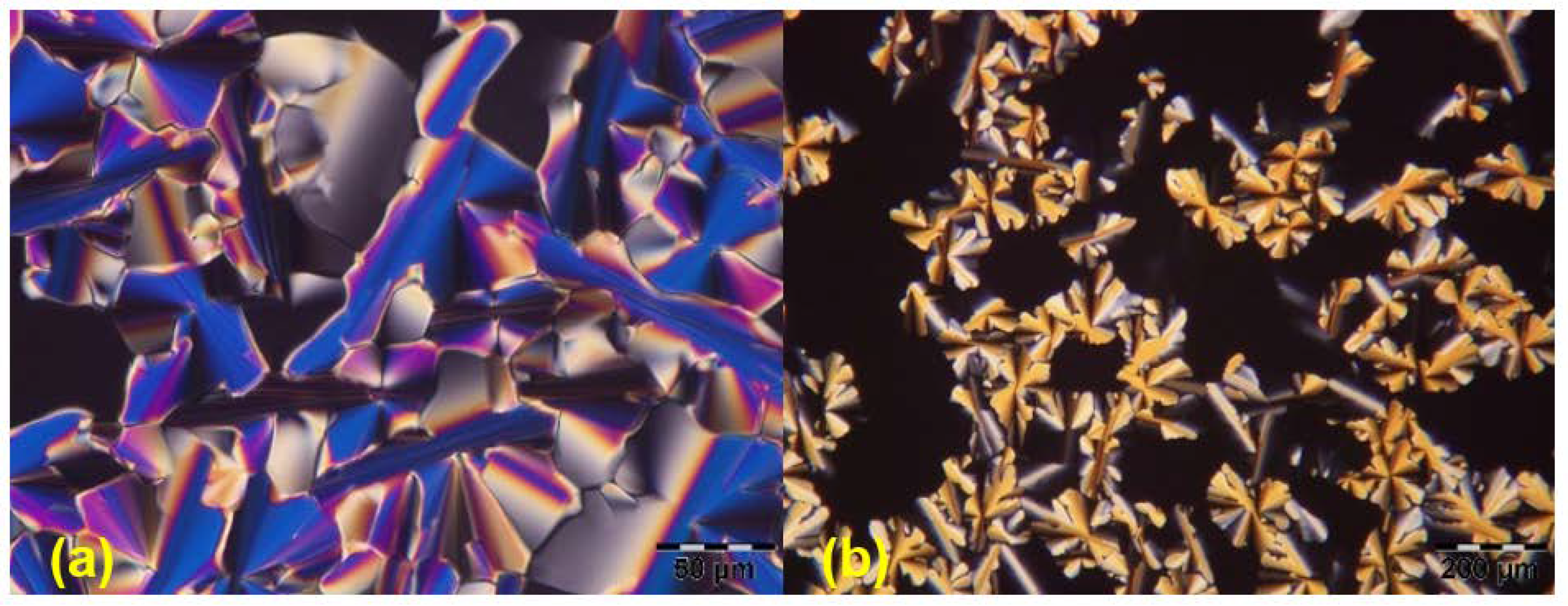
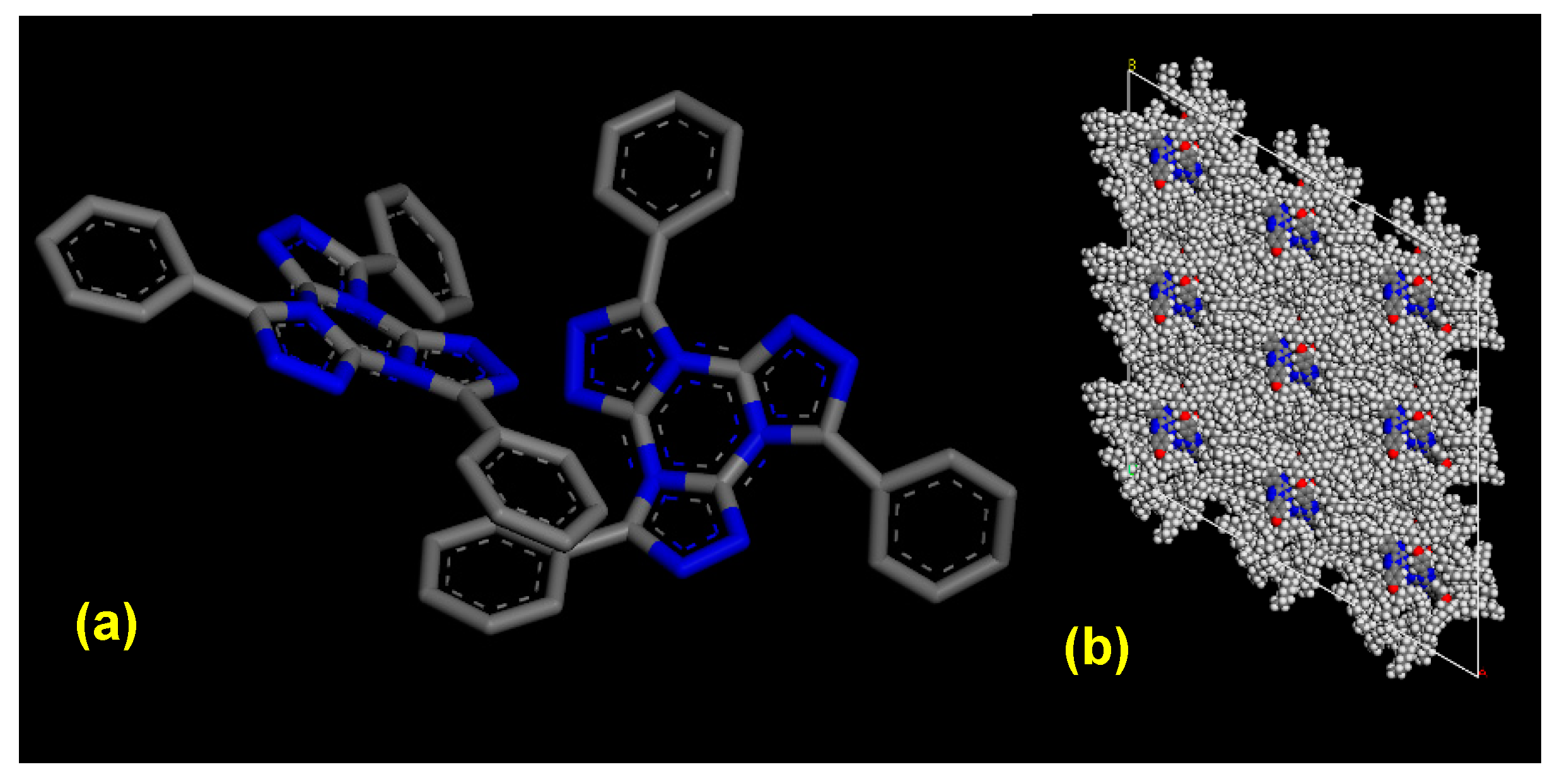
| Entry | Substitution Pattern | Transition/°C (Enthalpy/kJ/mol) | Ref |
|---|---|---|---|
| t-1 | Ri = H | Mp. 360 °C | [56] |
| t-2 | R4 = OC3H7 | Mp. > 275 | [42] |
| t-3 | R4 = OC6H13 | Cr 129.9 I; Tg = 55.4 | [37] |
| t-4 | R4 = OC8H17 | Cr 93.8 I; Tg = 46.7 | SI |
| t-5 | R4 = OC10H21 | Cr 76 I | SI |
| t-6 | R4 = OC12H25 | Cr 86.8 I; Tg = 36.7 | SI |
| t-7 | R4 = OC13H27 | Cr 78 I | SI |
| t-8 | R3 = OC10H21 | Cr 134 I | SI |
| t-9 | R2 = OC10H21 | Tg = −64 I | SI |
| t-10 | R2 = R3 = OC10H21 | Cr 69 I | SI |
| t-11 | R2 = R4 = OC10H21 | Cr 50 I | SI |
| t-12 | R2 = R5 = OC10H21 | Cr 89 I | SI |
| t-13 | R3 = R4 = OC4H9 | Cr 131 I | SI |
| t-14 | R3 = R4 = OC5H11 | Cr 102 M 201 I | SI |
| t-15 | R3 = R4 = OC6H13 | Cr 98 (21.0) M 232 (3.6) I | SI |
| t-16 | R3 = R4 = OC7H15 | Cr 100 (22.7) M225 (4.7) I | SI |
| t-17 | R3 = R4 = OC8H17 | Cr 100 (19.9) M 226 (4.5) I | [38] |
| t-18 | R3 = R4 = OC9H19 | Cr 94 (19.8) M 215 (5.7) I | SI |
| t-19 | R3 = R4 = OC10H21 | Cr 89 (14.3) Colhd 207 (6.7) I | [42] |
| t-20 | R3 = R4 = OC11H23 | Cr 81 (5.7) M 200 (5.2) I | SI |
| t-21 | R3 = R4 = OC12H25 | Cr 92.2 (19.3) Colh 207.6 (6.8) I | [42] |
| t-22 | R3 = R4 = OC13H27 | Cr 74 (12.4) M 175 (6.1) I | SI |
| t-23 | R3 = R4 = OC14H29 | Cr-11(46.3) M2 68 (9.4) M1 181 (5.4) I | [42] |
| t-24 | R3 = R4 = OC16H33 | Cr 19 (58.7) M2 68 (9.4) M1 171 (5.7) I | [42] |
| t-25 | R3 = R4 = OC18H37 | Cr 40 (96.9) M2 74 (9.1) M1 164 (7.5) I | SI |
| t-26 | R3 = R5 = OC6H13 | Cr 136 M 187 I | [60] |
| t-27 | R3 = R5 = OC7H15 | Cr 122 Colh 179 I | [60] |
| t-28 | R3 = R5 = OC8H17 | Cr 92 M 152 I | [60] |
| t-29 | R3 = R5 = OC10H21 | Cr 73 M 147 I | [60] |
| t-30 | R3 = R5 = OC12H25 | Cr 74 (6.8) Colh 143 (5.8) I | [60] |
| t-31 | R3 = R5 = OC12H25, R4 = Br | Cr 58 (9.5) M 120 I | SI |
| t-32 | R3 = R5 = OC16H33 | Cr 70 (7.4) M 115 (6.0) I | SI |
| t-33 | R3 = R4 = R5 = OC6H13 | Cr 116 (19.9) Colhd 184 (4.6) I | [37] |
| t-34 | R3 = R4 = R5 = OC8H17 | Cr 126 (22.8) Colhd 184 (5.6) I | [42] |
| t-35 | R3 = R4 = R5 = OC10H21 | Cr 126 (18.6) Colhd 165 (3.7) I | [42] |
| t-36 | R3 = R4 = R5 = OC12H25 | Cr 128 (22.7) Colhd 153 (6.9) I | SI |
| t-37 | R3 = R4 = R5 = OC16H33 | Cr 124 (24.6) M 144 (9.4) I | SI |
| Entry | Substitution Pattern | Transition/°C (Enthalpy/kJ/mol) | Ref |
|---|---|---|---|
| t-38 | 6-OC10H21-Nap [a] | Cr 121 I | SI |
| t-39 | 5,6-OC8H17-Nap [a] | Cr 112 (1.3) Colh 197(2.6) I | [38] |
| t-40 | 5,6-OC10H21-Nap [a] | Cr 76 (7.6) M 210(2.3) I | SI |
| t-41 | 5,6-OC12H25-Nap [a] | Cr 59 (1.5) Colh 189(2.9) I | SI |
| t-42 | 3´,4´-OC8H17-Bip [a] | Cr 148 I | SI |
| t-43 | 3´,4´-OC10H21-Bip [a] | Cr 172 I | [38] |
| t-44 | 3´,4´-OC12H25-Bip [a] | Cr 89 (31.1) M 199 (2.6) I | SI |
| t-45 | 2’,5’-(OC12H25)2-Bip [a] | Cr 87 I | SI |
| t-46 | 4’-(H13C6O)2-St [a] | mp > 240 | [37] |
| t-47 | 3’,5’-(H13C6O)2-St [a] | m.p. 124, Tg 13 | [37] |
| t-48 | 3’,4’,5’-(H13C6O)2-St [a] | Cr 175 M 200 I; Tg 178 | [37] |
| t-49 | 4’-Hexo-PEP [a] | Cr 245 I | [4] |
| t-50 | 3,4,5-(H17C8O)3-PEP [a] | Cr 82 I | [4] |
| t-51 | 3,4,5-(H25C12O)3-PEP [a] | Cr 80 I | SI |
| t-52 | 3,4,5-(H17C8O)3-PEPEP [a] | Cr 159 M 204 I | [4] |
| Entry | Substitution Pattern | Transition/°C (Enthalpy/kJ/mol) | Ref |
|---|---|---|---|
| t-53 | R4 = O(CH2)2CH(CH3)(CH2)3CH(CH3)2 | I | SI |
| t-54 | R4 = neomenthyloxy | Cr 185 I | [41] |
| t-55 | R3 = R4 = O-CH(C2H5)C5H11 | Colh 158 I | [1] |
| t-56 | R3 = R4 = O-CH2CH(C2H5)C4H9 | Cr −9 M 157 I | SI |
| t-57 | R3 = R4 = O-(CH2)3CH(C2H5)C4H9 | Cr72 (15.8) M 182 (5.7) | SI |
| t-58 | R3 = R4 = O(CH2)2CH(CH3) (CH2)3CH(CH3)2 | Cr (51) M 182 (5.8) I | SI |
| t-59 | R3 = R4 = (R)-O(CH2)2CH(CH3)- (CH2)2CH=C(CH3)2 | Cr 41 Colh 167 I | [1] |
| t-60 | R3 = R4 = O(CH2)2C(CH3)2 C5H11 | Cr 56 (17.4) Colh 154 (4.6) I (Tg 8) | SI |
| t-61 | R3 = R4 = R5 = O-CH2-CH(C2H5)C4H9 | Cr 160 I [a] | SI |
| t-62 | R3 = R4 = R5 = O-(CH2)3CH(C2H5)C4H9 | Cr 124 I | [42] |
| t-63 | R3 = OCH2CH(C8H17)2 | −64 (Tg) I | SI |
| t-64 | R4 = OCH2CH(C6H13)2 | 14 (Tg) Colh 65 I | SI |
| t-65 | R4 = OCH2CH(C8H17)2 | Cr −15 (2.1) M 101 (2.1) I | SI |
| t-66 | R4 = OCH2CH(C10H21)2 | Cr −70 Tg 9 Tg Colh 85 (2,0) I | SI |
| t-67 | R4 = OCH2CH(C12H25)2 | Cr −54 (19.5) Colh 82 (2.4) I | SI |
| t-68 | R4 = OCH(C6H13) | Cr 8 (1.6) I | SI |
| t-69 | R4 = O(CH2)2CH(C6H13) | Cr −71 I | SI |
| t-70 | R4 = OCH2CH((CH2)2CH(CH3)2)2 | Tg = 42 Tm = 47 | SI |
| t-71 | R4 = O-CH2CH(CH2CH(C2H5)C4H9)2 | Cr 95 (7.1) M 140 I [b] POM: 80–120 | SI |
| t-72 | R3 = R4 = OCH2CH(C6H13)2 | Cr 86 I | SI |
| t-73 | R3 = OC4H9, R5 = OC16H33 | 47 (Tg) M 91 (2.1) I | SI |
| t-74 | R3 = OC6H13, R5 = OC14H29 | Cr 59 M 132 (4.5) I | SI |
| t-75 | R3 = OC16H33, R4 = OCH3 | Cr 98 I | SI |
| t-76 | R3 = OC14H29, R4 = OC6H13 | Cr 103 (23.0) M 183 (4.0) I | SI |
| t-77 | R3 = COOC10H21, R4 = OC10H21 | Cr1 61 (6.0) Cr2 89 (5.3) I | SI |
| t-78 | R3 = R4 = OC6H13, R5 = OC12H25 | Cr 127 (21.4) M 158 (4.9) I | SI |
| t-79 | R3 = R4 = OC6H13, R5 = O-(CH2)3CH(C2H5)C4H9 | Cr 80 (23.8) Colh 136 (1.5) I | SI |
| Entry | Substitution Pattern | Transition/°C (Enthalpy/kJ/mol) | Ref |
|---|---|---|---|
| r-1 | Ri = H | Cr > 390 | [58] |
| r-2 | R4 = OC3H7 | Cr > 350 | [42] |
| r-3 | R4 = OC6H13 | Cr 178 I | SI |
| r-5 | R4 = OC10H21 | Cr 142 I | SI |
| r-15 | R3 = R4 = OC6H13 | Cr 146 I | SI |
| r-19 | R3 = R4 = OC10H21 | Cr 136 I | [42] |
| r-21 | R3 = R4 = OC12H25 | Cr1 90 Cr2 131(25.2) I | [42] |
| r-22 | R3 = R4 = OC13H27 | Cr 94 (16.8) M 119 (9.9) I | SI |
| r-23 | R3 = R4 = OC14H29 | Cr 128 (25.4) Col 136 (1.6) I | [42] |
| r-24 | R3 = R4 = OC16H33 | Cr 36 M1 97 M2 125 (23.4) Colh 132 (1.4) I | [42] |
| r-30 | R3 = R5 = OC12H25 | Cr 127 I | SI |
| r-33 | R3 = R4 = R5 = OC6H13 | Cr 13 (8.9) Colh180 (3.0) I | SI |
| r-34 | R3 = R4 = R5 = OC8H17 | Cr 35 (16.9) [a] Col 205 (2.7) I | SI |
| r-35 | R3 = R4 = R5 = OC10H21 | Cr 121 Colh 181 (3.0) I [a] | SI |
| r-61 | R3 = R4 = R5 = O-CH2CH(C2H5)C4H9 | Cr 241 I [b] | SI |
| r-62 | R3 = R4 = R5 = O-(CH2)3CH(C2H5)C4H9 | Cr 75 (2.8) Col 207(4.8) I | [42] |
| r-63 | R3 = OCH2CH(C8H17)2 | Cr 75 I | SI |
| r-66 | R4 = OCH2CH(C10H21)2 | Cr 6 (0.7) I | SI |
| r-71 | R4 = O-CH2CH(CH2CH(C2H5)C4H9)2 | Cr 136 I | SI |
| r-73 | R3 = OC4H9, R5 = OC16H33 | Cr 99 (6.5) I | SI |
| r-74 | R3 = OC6H13, R5 = OC14H29 | Cr 13 (4.4) M 70 (3.4) I | SI |
| r-80 | R3 = Cl, R4 = OC6H13 | Cr 178 I | SI |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rieth, T.; Tober, N.; Limbach, D.; Haspel, T.; Sperner, M.; Schupp, N.; Wicker, P.; Glang, S.; Lehmann, M.; Detert, H. Impact of Substitution Pattern and Chain Length on the Thermotropic Properties of Alkoxy-Substituted Triphenyl-Tristriazolotriazines. Molecules 2020, 25, 5761. https://doi.org/10.3390/molecules25235761
Rieth T, Tober N, Limbach D, Haspel T, Sperner M, Schupp N, Wicker P, Glang S, Lehmann M, Detert H. Impact of Substitution Pattern and Chain Length on the Thermotropic Properties of Alkoxy-Substituted Triphenyl-Tristriazolotriazines. Molecules. 2020; 25(23):5761. https://doi.org/10.3390/molecules25235761
Chicago/Turabian StyleRieth, Thorsten, Natalie Tober, Daniel Limbach, Tobias Haspel, Marcel Sperner, Niklas Schupp, Philipp Wicker, Stefan Glang, Matthias Lehmann, and Heiner Detert. 2020. "Impact of Substitution Pattern and Chain Length on the Thermotropic Properties of Alkoxy-Substituted Triphenyl-Tristriazolotriazines" Molecules 25, no. 23: 5761. https://doi.org/10.3390/molecules25235761
APA StyleRieth, T., Tober, N., Limbach, D., Haspel, T., Sperner, M., Schupp, N., Wicker, P., Glang, S., Lehmann, M., & Detert, H. (2020). Impact of Substitution Pattern and Chain Length on the Thermotropic Properties of Alkoxy-Substituted Triphenyl-Tristriazolotriazines. Molecules, 25(23), 5761. https://doi.org/10.3390/molecules25235761







