Ethylene Induction of Non-Enzymatic Metabolic Antioxidants in Matricaria chamomilla
Abstract
1. Introduction
2. Results and Discussion
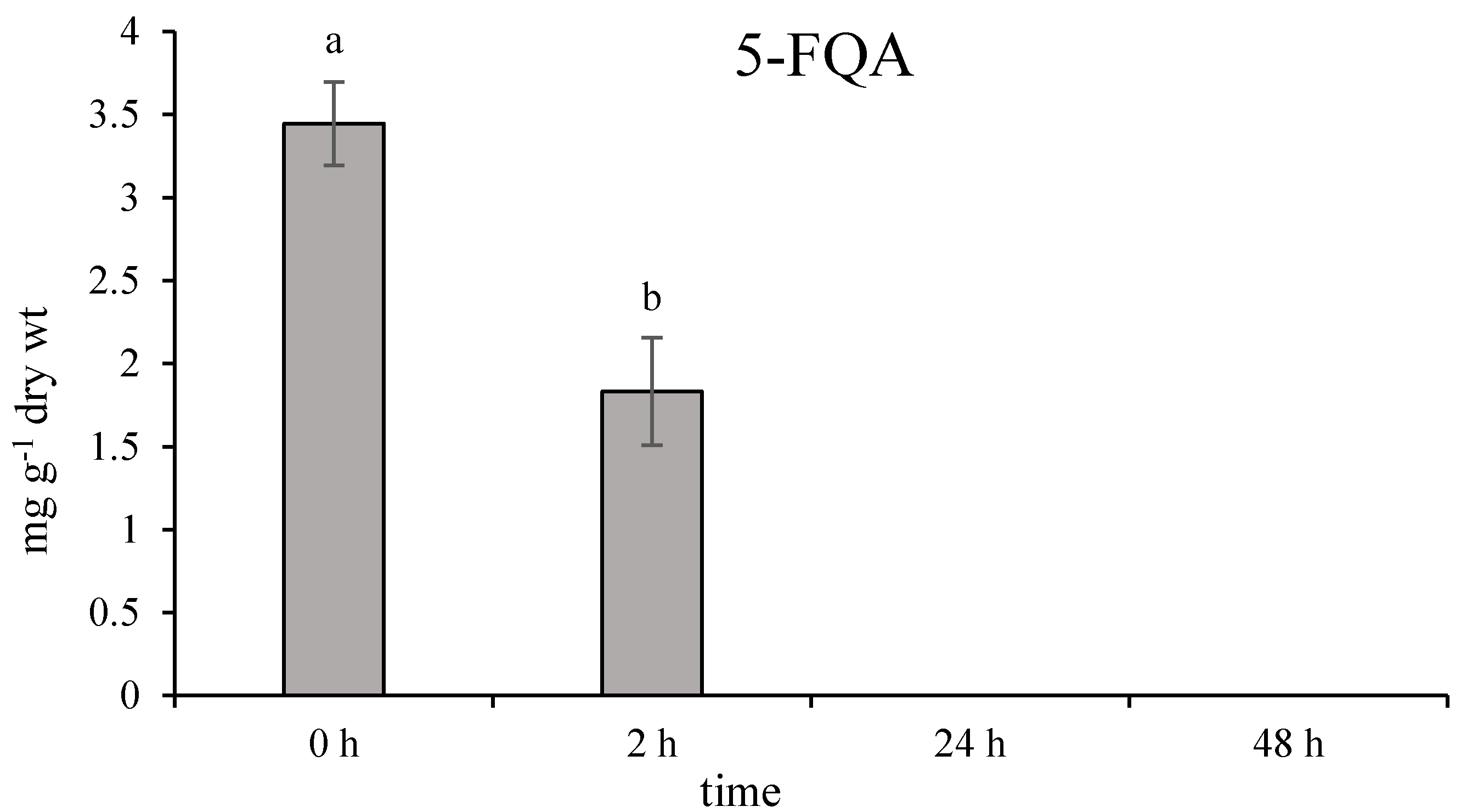
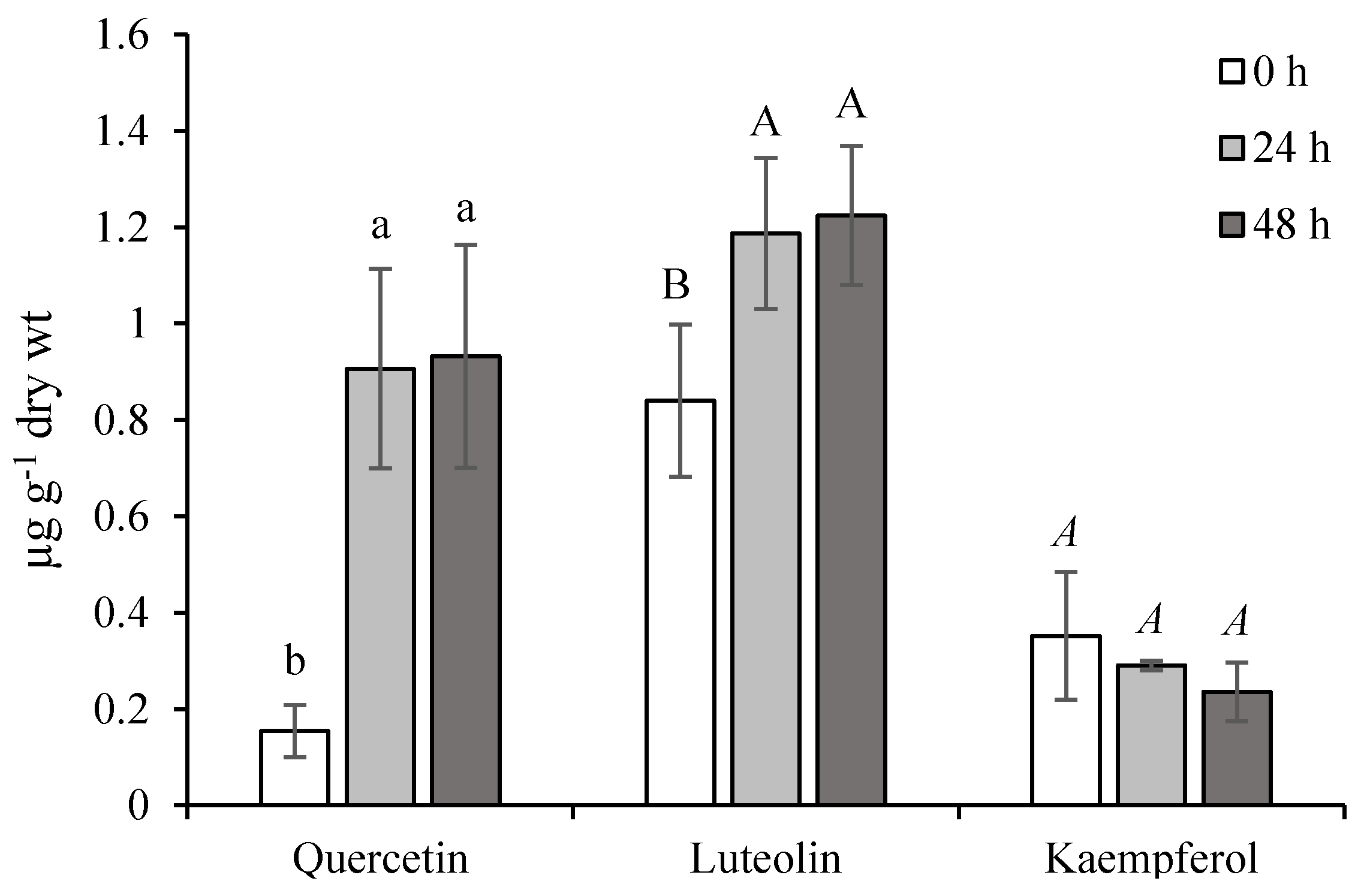
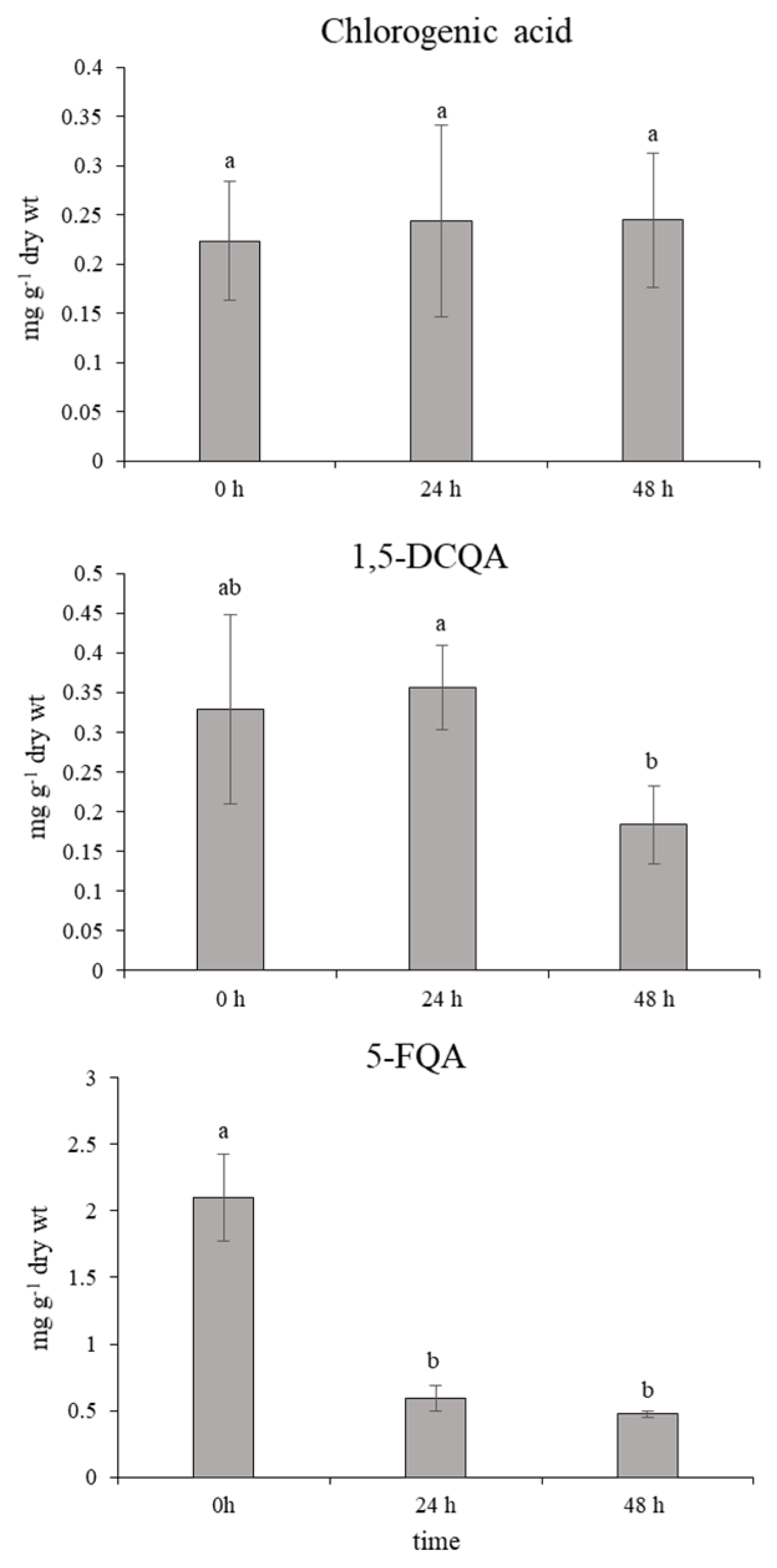
2.1. Effect of Ethephon on PAL Activity and Phenylpropanoids Accumulation
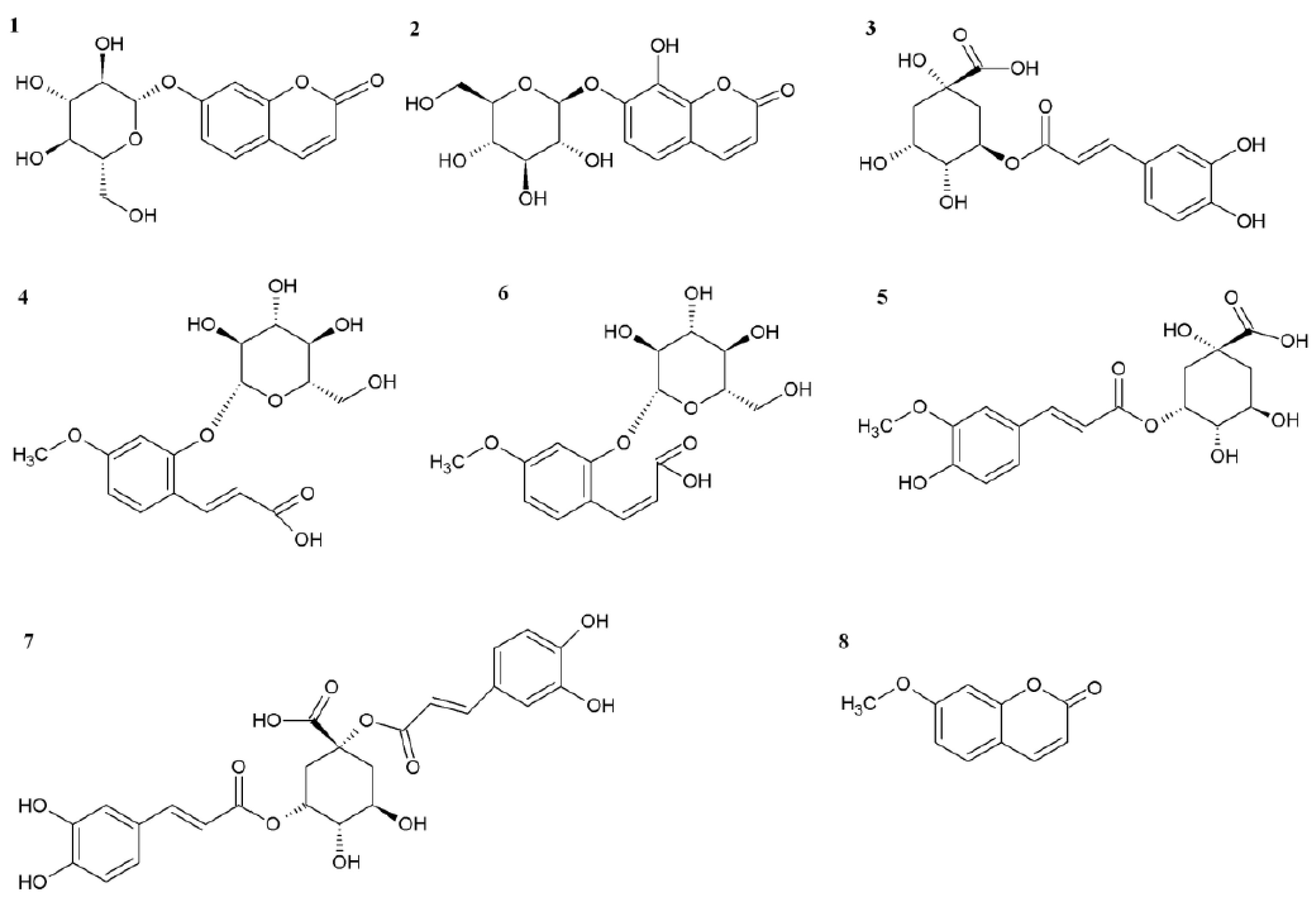
2.2. Role of Chlorogenic Acid Derivatives in Chamomile Plant Physiology and Their Antioxidant Potential
2.3. Molecular Modelling
3. Materials and Methods
3.1. Plant Material and Treatments
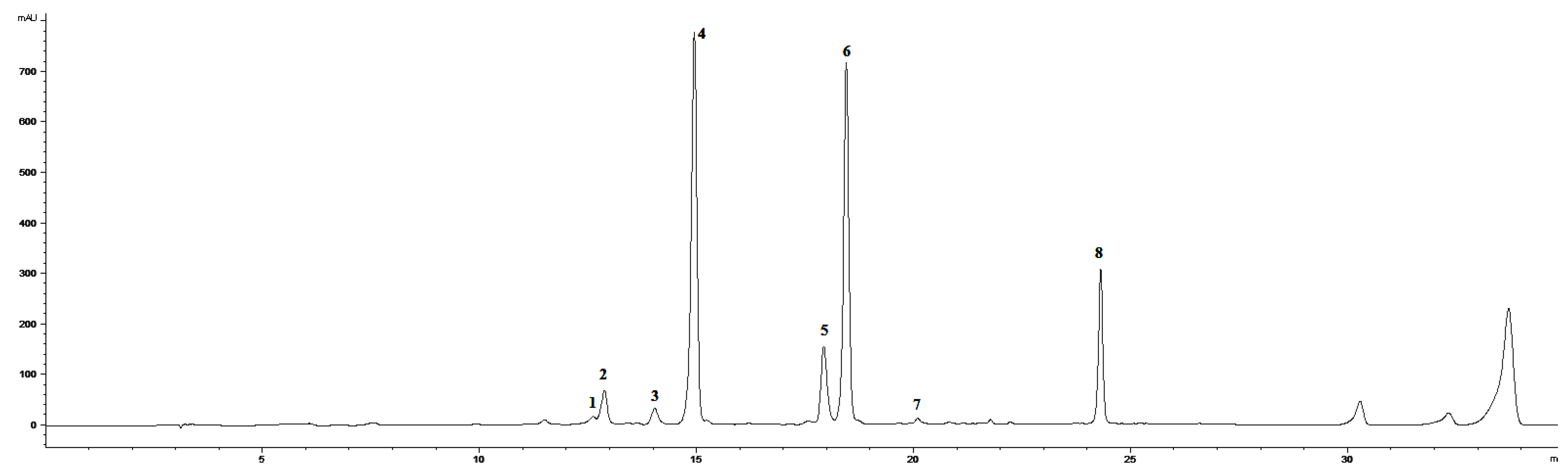
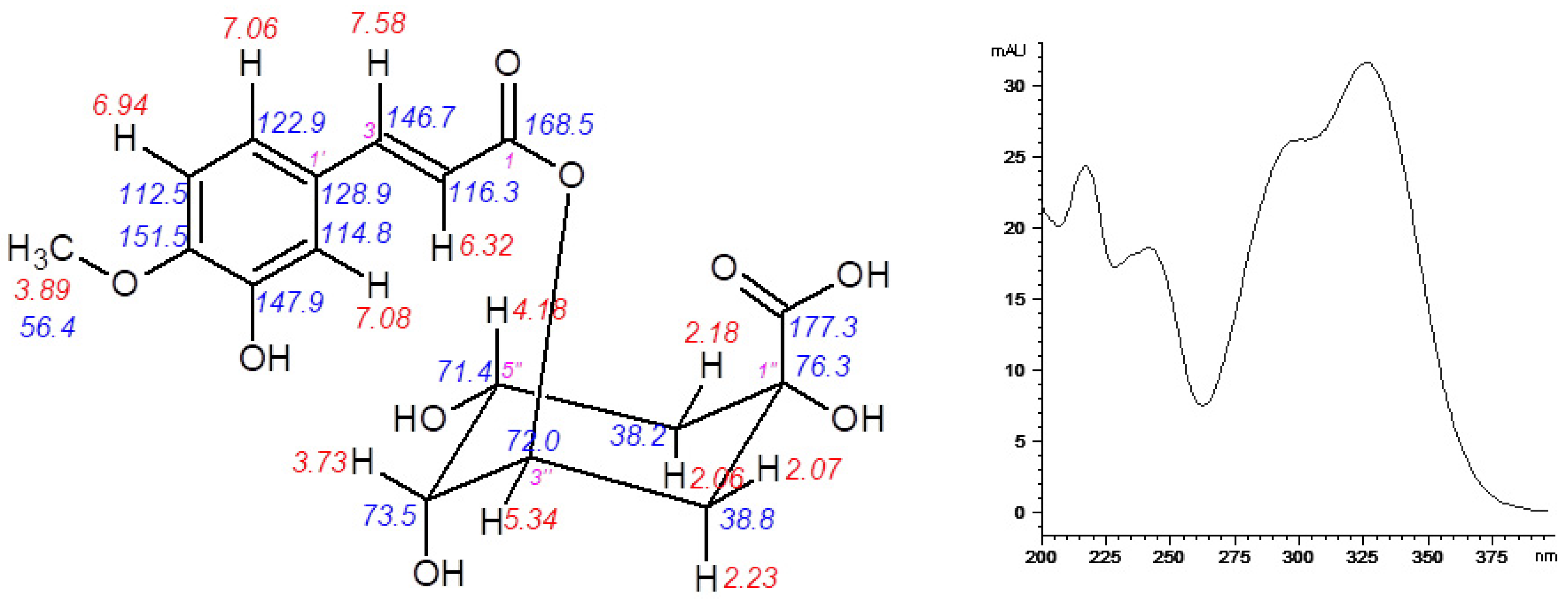
3.2. Isolation and Identification of 5-O-feruloylquinic Acid
3.3. Extraction and HPLC Analyses of Secondary Metabolites and PAL Activity
3.4. HPLC-MS Analysis of Phenolic Acids and Flavonoids
3.5. Antioxidant Capacity Assay
3.6. Molecular Modelling
3.7. Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

References
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H.B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Waskiewicz, A.; Besztedra, M.; Goliński, P. Nonenzymatic Antioxidants in Plants. In Oxidative Damage to Plants; Parvaiz, A., Ed.; Acdemic Press: San Diego, CA, USA, 2014; pp. 201–234. [Google Scholar] [CrossRef]
- Formisano, C.; Delfine, S.; Oliviero, F.; Tenore, G.C.; Rigano, D.; Senatore, F. Correlation among environmental factors, chemical composition and antioxidative properties of essential oil and extracts of chamomile (Matricaria chamomilla L.) collected in Molise (South-central Italy). Ind. Crop. Prod. 2015, 63, 256–263. [Google Scholar] [CrossRef]
- Song-Lin, H.; Li, X.-X.; Mian, Q.-H.; Lan, W.; Liu, Y. Comparison of antioxidant activity between two species of chamomiles produced in Xinjiang by TLC-bioautography. Zhongguo Zhong Yao Za Zhi 2013, 38. [Google Scholar] [CrossRef]
- Buono-Core, G.E.; Nuñez, M.V.; Lucero, A.; Vargas, R.; Jullian, C. Structural elucidation of bioactive principles in floral extracts of German chamomille (Matricaria recutita L.). J. Chil. Chem. Soc. 2011, 56, 549–553. [Google Scholar] [CrossRef]
- Eliašová, A.; Repčák, M.; Pastírová, A. Quantitative changes of secondary metabolites of Matricaria chamomilla by abiotic stress. Z. Naturforsch. 2004, 59, 543–548. [Google Scholar] [CrossRef]
- Guimarães, R.; Barros, L.; Dueñas, M.; Calhelha, R.C.; Carvalho, A.M.; Santos-Buelga, C.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Infusion and decoction of wild German chamomile: Bioactivity and characterization of organic acids and phenolic compounds. Food Chem. 2013, 136, 947–954. [Google Scholar] [CrossRef]
- Petrulova-Poracka, V.; Repcak, M.; Vilkova, M.; Imrich, J. Coumarins of Matricaria chamomilla L.: Aglycones and glycosides. Food Chem 2013, 141, 54–59. [Google Scholar] [CrossRef]
- Molnar, M.; Mendešević, N.; Šubarić, D.; Banjari, I.; Jokić, S. Comparison of various techniques for the extraction of umbelliferone and herniarin in Matricaria chamomilla processing fractions. Chem. Cent. J. 2017, 11, 78. [Google Scholar] [CrossRef] [PubMed]
- Cvetanović, A.; Švarc-Gajić, J.; Mašković, P.; Savić, S.; Nikolić, L. Antioxidant and biological activity of chamomile extracts obtained by different techniques: Perspective of using superheated water for isolation of biologically active compounds. Ind. Crops. Prod. 2015, 65, 582–591. [Google Scholar] [CrossRef]
- Petruľová, V.; Dučaiová, Z.; Repčák, M. Short-term UV-B dose stimulates production of protective metabolites in Matricaria chamomilla leaves. Photochem. Photobiol. 2014, 90, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Kovacik, J.; Klejdus, B. Induction of phenolic metabolites and physiological changes in chamomile plants in relation to nitrogen nutrition. Food Chem. 2004, 142, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenicacid and caffeicacid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Trivellini, A.; Masood, A.; Ferrante, A.; Khan, N.A. Current understanding on ethylene signaling in plants: The influence of nutrient availability. Plant Physiol. Biochem. 2013, 73, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Munné-Bosch, S. Ethylene response factors: A key regulatory hub in hormone and stress signaling. Plant Physiol. 2015, 169, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Abiri, R.; Shaharuddin, N.A.; Maziah, M.; Yusof, Z.N.B.; Atabaki, N.; Sahebi, M.; Valdiani, A.; Kalhori, N.; Azizi, P.; Hanafi, M.M. Role of ethylene and the APETALA 2/ethylene response factor superfamily in rice under various abiotic and biotic stress conditions. Environ. Exp. Bot. 2017, 134, 33–44. [Google Scholar] [CrossRef]
- Jakubowicz, M.; Nowak, W. 1-aminocyclopropane-1-carboxylate synthase, an enzyme of ethylene biosynthesis. In Comprehensive Natural Products II: Chemistry and Biology; Liu, H.W., Mander, L., Eds.; Elsevier: San Diego, CA, USA, 2010; pp. 91–120. [Google Scholar]
- Watanabe, T.; Sakai, S. Effects of active oxygen species and methyl jasmonate on expression of the gene for a wound-inducible 1-aminocyclopropane-1-carboxylatesynthase in winter squash (Cucurbita maxima). Planta 1998, 206, 570–576. [Google Scholar] [CrossRef]
- Sajko, M.; Kovalíková-Dučaiová, Z.; Paľove-Balang, P.; Repčák, M. Physiological responses of Matricaria chamomilla to potassium nitrate supply and foliar application of ethephon. J. Plant Growth Regul. 2017, 37, 360–369. [Google Scholar] [CrossRef]
- Dixon, R.A.; Paiva, N.L. Stress-induced phenylpropanoid metabolism. Plant Cell 1995, 7, 1085–1087. [Google Scholar] [CrossRef]
- Heredia, J.B.; Cisneros-Zevallos, L. The effects of exogenous ethylene and methyl jasmonate on the accumulation of phenolic antioxidants in selected whole and wounded fresh produce. Food Chem. 2009, 115, 1500–1508. [Google Scholar] [CrossRef]
- Guzman, J.D. Natural Cinnamic Acids, Synthetic Derivatives and Hybrids with Antimicrobial Activity. Molecules 2014, 19, 19292–19349. [Google Scholar] [CrossRef]
- Srinivasulu, C.; Ramgopal, M.; Ramanjaneyulu, G.; Anuradha, C.M.; Suresh Kumar, C. Syringic acid (SA) ‒ A review of its occurrence, biosynthesis, pharmacological and industrial importance. Biomed. Pharmacother. 2018, 108, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolicacids. Free Radic. Biol. Med. 1996, 20, 933956. [Google Scholar] [CrossRef]
- Kurepa, J.; Shull, T.E.; Smalle, J.A. Quercetin feeding protects plants against oxidative stress. F1000Research 2016, 5, 2430–2439. [Google Scholar] [CrossRef]
- Di Ferdinando, M.; Brunetti, C.; Fini, A.; Tattini, M. Flavonoids as antioxidants in plants under abiotic stresses. In Abiotic Stress Responses in Plants: Metabolism, Productivity and Sustainability; Ahmad, P., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2012; pp. 159–179. [Google Scholar]
- Repčák, M.; Suvák, M. Methyl jasmonate and Echinothrips americanus regulate coumarin accumulation in leaves of Matricaria chamomilla. Biochem. Syst. Ecol. 2013, 47, 38–41. [Google Scholar] [CrossRef]
- Bourgaud, F.; Hehn, A.; Larbat, R.; Doerper, S.; Gontier, E.; Kellner, S.; Matern, U. Biosynthesis of coumarins in plants: A major pathway still to be unraveled for cytochrome P450 enzymes. Phytochem. Review. 2006, 5, 293–308. [Google Scholar] [CrossRef]
- Chu, L.L.; Pandey, R.P.; Lim, H.N.; Jung, H.J.; Thuan, N.H.; Kim, T.-S.; Sohng, J.K. Synthesis of umbelliferone derivatives in Escherichia coli and their biological activities. J. Biol. Eng. 2017, 11, 15. [Google Scholar] [CrossRef]
- Witaicenis, A.; Seito, L.N.; da Silveira Chagas, A.; de Almeida Jr, L.D.; Luchini, A.C.; Rodrigues-Orsi, P.; Cestari, S.H.; Di Stasi, L.C. Antioxidant and intestinal anti-inflammatory effects of plant-derived coumarin derivatives. Phytomedicine 2014, 21, 240–246. [Google Scholar] [CrossRef]
- Avena-Bustillos, R.; Du, W.-X.; Woods, R.; Olson, D.; Breksa, A.P.; McHugh, T.H. Ultraviolet-B light treatment increases antioxidant capacity of carrot products. J. Sci. Food Agric. 2012, 92, 2341–2348. [Google Scholar] [CrossRef]
- Kono, Y.; Kashine, S.; Yoneyama, T.; Sakamoto, Y.; Matsui, Y.; Shibata, H. Iron chelation by chlorogenic acid as a natural antioxidant. Biosci. Biotechnol. Biochem. 1998, 62, 22–27. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Lim, Y.Y.; Ling, S.K.; Tan, S.P.; Lim, K.K.; Khoo, M.G.H. Caffeoylquinic acids from leaves of Etlingera species (Zingiberaceae). Food Sci. Technol. 2009, 42, 1026–1030. [Google Scholar] [CrossRef]
- Mathew, S.; Abraham, T.E.; Zakaria, Z.A. Reactivity of phenolic compounds towards free radicals under in vitro conditions. J. Food Sci. Technol. 2015, 52, 5790–5798. [Google Scholar] [CrossRef] [PubMed]
- Molyneux, P. The use of the stable free radical Diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Yang, Y.J.; Liu, X.; Wu, H.R.; He, X.F.; Bi, Y.R.; Zhu, Y.; Liu, Z.L. Radical scavenging activity and cytotoxicity of active quinic acid derivatives from Scorzonera divaricata roots. Food Chem. 2013, 138, 2057–2063. [Google Scholar] [CrossRef] [PubMed]
- Teles Fujishima, M.A.; Silva, N.D.S.R.; Ramos, R.D.S.; Batista Ferreira, E.F.; Santos, K.L.B.; Silva, C.H.T.P.; Silva, J.O.; Campos Rosa, J.M.; Santos, C.B.R. An Antioxidant Potential, Quantum-Chemical and Molecular Docking Study of the Major Chemical Constituents Present in the Leaves of Curatella americana Linn. Pharmaceuticals 2018, 11, 72. [Google Scholar] [CrossRef]
- Urbaniak, A.; Kujawski, J.; Czaja, K.; Szelag, M. Antioxidant properties of several caffeic acid derivatives: A theoretical study. C. R. Chim. 2017, 20, 1072–1082. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Silva, A.B.F.; Marinho, M.M.; Da Silva Mendes, F.R. In Silico Study of Phytochemical Chlorogenic Acid: A Semi-Empirical Quantum Study and Adme. Int. J. Curr. Res. Rev. 2019, 12, 34–39. [Google Scholar]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacoth. 2018, 97, 6–74. [Google Scholar] [CrossRef]
- Repčák, M.; Pastírová, A.; Imrich, J.; Švehlíková, V.; Mártonfi, P. The variability of (Z)-and (E)-2-β-D-glucopyranosyloxy-4-methoxycinnamic acids and apigenin glucosides in diploid and tetraploid Chamomilla recutita. Plant Breeding 2001, 120, 188–190. [Google Scholar] [CrossRef]
- dos Santos, W.D.; Ferrarese, M.L.L.; Finger, A.; Teixeira, A.C.N.; Ferrarese-Filho, O. Lignification and related enzymes in glycine max root growth-inhibition by ferulic acid. J. Chem. Ecol. 2004, 30, 1203–1212. [Google Scholar] [CrossRef]
- Kovalikova, Z.; Kubes, J.; Skalicky, M.; Kuchtickova, N.; Maskova, L.; Tuma, J.; Vachova, P.; Hejnak, V. Changes in content of polyphenols and ascorbic acid in leaves of white cabbage after pest infestation. Molecules 2019, 24, 2622. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, A.; Mingozzi, M.; Madeo, M.; Speranza, G.; Cocucci, M. Effect of nitrogen starvation on the phenolic metabolism and antioxidant properties of yarrow (Achillea collina Becker ex Rchb.). Food Chem. 2009, 114, 204–211. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.A.; Millam, J.M. GAUSSVIEW 6.0; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R. GAUSSIAN 16, Revision, C.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminformatics 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available. |



| Ethephon Treatment | |||
|---|---|---|---|
| 0 h | 24 h | 48 h | |
| Hydroxycinnamic and hydroxybenzoic acid derivatives (µg g−1 dry wt) | |||
| Caffeic acid | 7.445 ± 0.595 b | 10.047 ± 0.964 b | 14.149 ± 2.399 a |
| Vanillic acid | 41.368 ± 6.236 b | 62.463 ± 6.877 a | 29.276 ± 6.914 b |
| Syringic acid | 1.026 ± 0.601 bc | 2.834 ± 0.5633 ab | 4.780 ± 1.372 ab |
| p-Coumaric acid | 1.064 ± 0.267 ab | 0.592 ± 0.107 b | 1.596 ± 0.526 a |
| Ferulic acid | 0.485 ± 0.070 a | 0.504 ± 0.1771 a | 0.692 ± 0.281 a |
| Coumarin-related compounds (mg g−1 dry wt) | |||
| Daphnin | 0.950 ± 0.193 b | 1.626 ± 0.113 a | 1.125 ± 0.146 b |
| Z-GMCA | 6.602 ± 0.849 a | 1.225 ± 0.390 b | 6.032 ± 0.539 a |
| E-GMCA | 9.626 ± 0.71 a | 6.867 ± 0.686 b | 7.455 ± 1.307 ab |
| Herniarin | 0.107 ± 0.0381 b | 0.236 ± 0.064 a | 0.259 ± 0.023 a |
| Parameters | Value |
|---|---|
| Gas temperature | 300 °C |
| Gas flow | 9 L min−1 |
| Nebulizer | 35 psi |
| Sheat gas temperature | 380 °C |
| Sheat gas flow | 12 L min−1 |
| Capillary voltage | 5000 V |
| Nozzle voltage | 300 V |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrulova, V.; Vilkova, M.; Kovalikova, Z.; Sajko, M.; Repcak, M. Ethylene Induction of Non-Enzymatic Metabolic Antioxidants in Matricaria chamomilla. Molecules 2020, 25, 5720. https://doi.org/10.3390/molecules25235720
Petrulova V, Vilkova M, Kovalikova Z, Sajko M, Repcak M. Ethylene Induction of Non-Enzymatic Metabolic Antioxidants in Matricaria chamomilla. Molecules. 2020; 25(23):5720. https://doi.org/10.3390/molecules25235720
Chicago/Turabian StylePetrulova, Veronika, Maria Vilkova, Zuzana Kovalikova, Matus Sajko, and Miroslav Repcak. 2020. "Ethylene Induction of Non-Enzymatic Metabolic Antioxidants in Matricaria chamomilla" Molecules 25, no. 23: 5720. https://doi.org/10.3390/molecules25235720
APA StylePetrulova, V., Vilkova, M., Kovalikova, Z., Sajko, M., & Repcak, M. (2020). Ethylene Induction of Non-Enzymatic Metabolic Antioxidants in Matricaria chamomilla. Molecules, 25(23), 5720. https://doi.org/10.3390/molecules25235720







