Chemical Composition of Natural Hydrolates and Their Antimicrobial Activity on Arcobacter-Like Cells in Comparison with Other Microorganisms
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Composition of Hydrolates
2.1.1. Lavender Hydrolates
2.1.2. Bay Leaves Hydrolates
2.1.3. Fennel Hydrolates
2.1.4. Clove Hydrolates
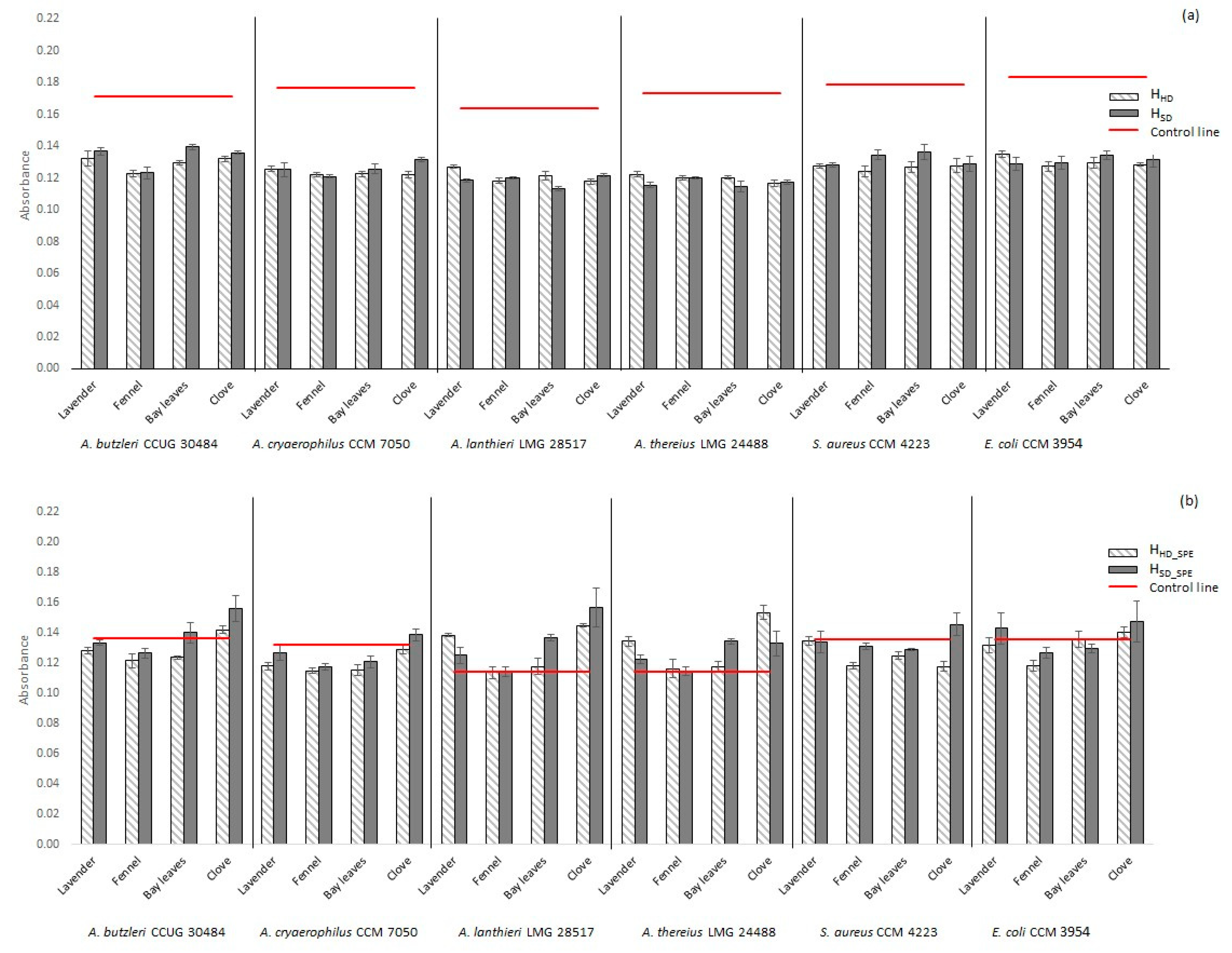
2.2. Antimicrobial Activity of Hydrolates
2.2.1. Non-Concentrated Hydrolate (HHD, HSD)
2.2.2. Concentrated Hydrolate (HHD_SPE, HSD_SPE)
2.3. Biofilm Activity of Selected Microorganisms in the Presence of Hydrolate
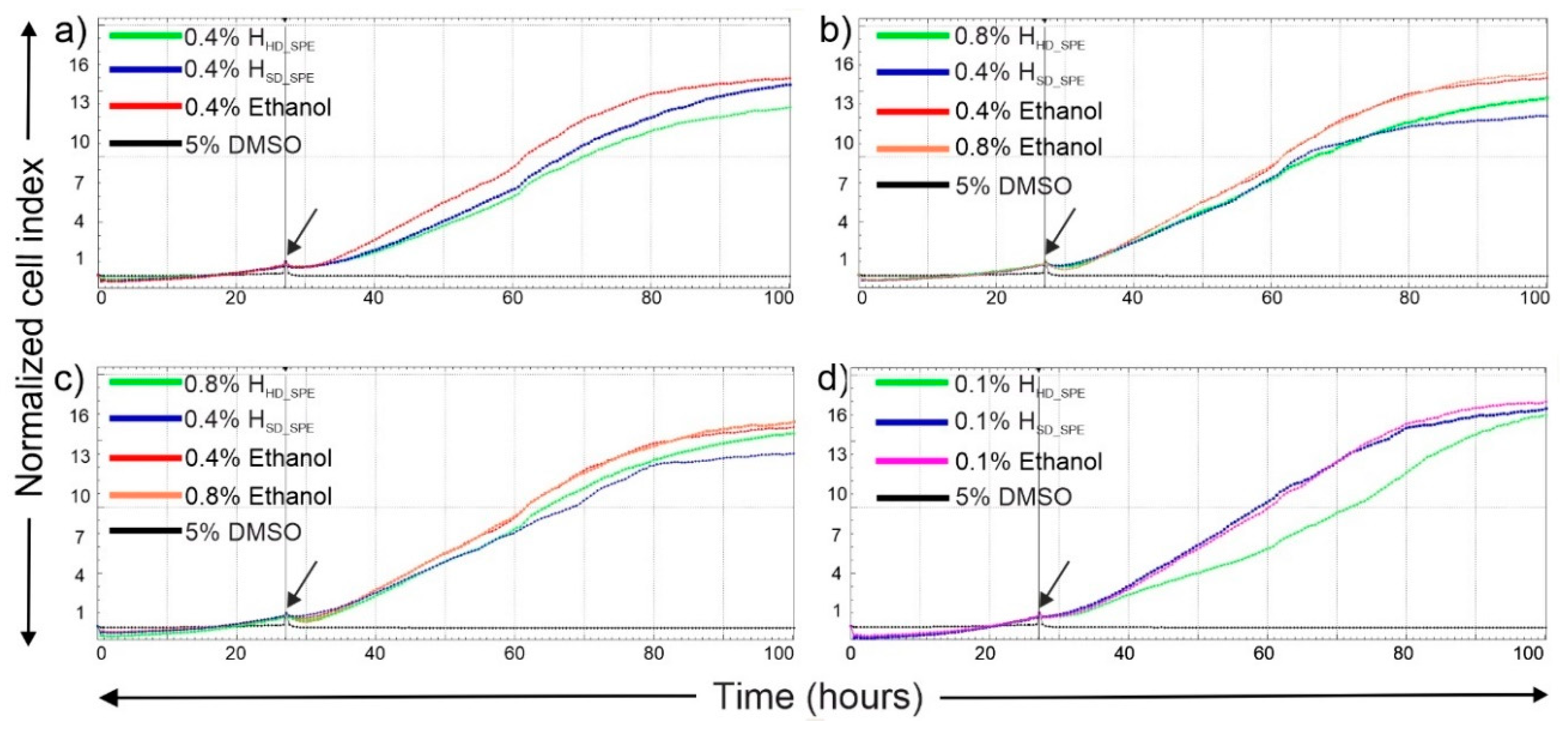
2.4. In Vitro Cytotoxicity of Hydrolates
3. Materials and Methods
3.1. Plant Materials and Sample Preparation
3.1.1. Steam Distillation
3.1.2. Hydrodistillation
3.2. Solid Phase Extraction (SPE)
3.3. Antimicrobial Testing
3.4. Biofilm Formation Determination
3.5. GC-MS Analysis
3.6. GC-FID Analysis
3.7. In Vitro Cytotoxicity Assay
3.7.1. Cell Lines
3.7.2. Real-Time Cytotoxicity Assay
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mohamed, A.; El-Sayed, M.A.; Hegazy, M.E.; Helaly, S.E.; Esmail, A.M.; Mohamed, N.S. Chemical Constituents and Biological Activities of Artemisia herba-alba. Rec. Nat. Prod. 2010, 4, 1–25. [Google Scholar]
- Sowndhararajan, K.; Deepa, P.; Kim, M.; Park, S.J.; Kim, S. A Review of the Composition of the Essential Oils and Biological Activities of Angelica Species. Sci. Pharm. 2017, 85, 33. [Google Scholar] [CrossRef] [PubMed]
- Pejin, B.; Vujisic, L.; Sabovljevic, M.; Tesevic, V.; Vajs, V. Preliminary data on essential oil composition of the moss Rhodobryum ontariense (Kindb.) Kindb. Cryptogam. Bryol. 2011, 32, 113–117. [Google Scholar] [CrossRef]
- Dadalioglu, I.; Evrendilek, G.A. Chemical compositions and antibacterial effects of essential oils of Turkish oregano (Origanum minutiflorum), bay laurel (Laurus nobilis), Spanish lavender (Lavandula stoechas L.), and fennel (Foeniculum vulgare) on common foodborne pathogens. J. Agric. Food Chem. 2004, 52, 8255–8260. [Google Scholar] [CrossRef]
- Diao, W.R.; Hu, Q.P.; Zhang, H.; Xu, J.G. Chemical composition, antibacterial activity and mechanism of action of essential oil from seeds of fennel (Foeniculum vulgare Mill.). Food Control 2014, 35, 109–116. [Google Scholar] [CrossRef]
- Hamedi, A.; Moheimani, S.M.; Sakhteman, A.; Etemadfard, H.; Moein, M. An overview on indications and chemical composition of aromatic waters (hydrosols) as functional beverages in Persian nutrition culture and folk medicine for hyperlipidemia and cardiovascular conditions. J. Evid. Based Complement. Altern. 2017, 22, 544–561. [Google Scholar] [CrossRef]
- Lu, H.; Li, H.; Li, X.L.; Zhou, A.G. Chemical composition of lavender essential oil and its antioxidant activity and inhibition against rhinitis-related bacteria. Afr. J. Microbiol. Res. 2010, 4, 309–313. [Google Scholar]
- Chaieb, K.; Hajlaoui, H.; Zmantar, T.; Kahla-Nakbi, A.B.; Rouabhia, M.; Mahdouani, K.; Bakhrouf, A. The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzigium aromaticum L. myrtaceae): A short review. Phytother. Res. 2007, 21, 501–506. [Google Scholar]
- Edris, A.E. Identification and absolute quantification of the major water-soluble aroma components isolated from the hydrosols of some aromatic plants. J. Essent. Oil Bear. Plants 2009, 12, 155–161. [Google Scholar] [CrossRef]
- Politi, M.; Menghini, L.; Conti, B.; Bedini, S.; Farina, P.; Cioni, P.L.; Braca, A.; De Leo, M. Reconsidering hydrosols as main products of aromatic plants manufactory: The lavandin (Lavandula × intermedia) case study in Tuscany. Molecules 2020, 25, 2225. [Google Scholar] [CrossRef]
- Smigielski, K.; Prusinowska, R.; Stobiecka, A.; Kunicka-Styczynska, A.; Gruska, R. Biological properties and chemical composition of essential oils from flowers and aerial parts of lavender (Lavandula angustifolia). J. Essent. Oil Bear. Plants 2018, 21, 1303–1314. [Google Scholar] [CrossRef]
- Hay, Y.O.; Abril-Sierra, M.A.; Sequeda-Castaneda, L.G.; Bonnafous, C.; Raynaud, C. Evaluation of combinations of essential oils and essential oils with hydrosols on antimicrobial and antioxidant activities. J. Pharm. Pharmacogn. Res. 2018, 6, 216–230. [Google Scholar]
- Lira, P.D.; Reeta, D.; Tkacik, E.; Ringuelet, J.; Coussio, J.D.; van Baren, C.; Bandoni, A.L. Essential oil and by-products of distillation of bay leaves (Laurus nobilis L.) from Argentina. Ind. Crop. Prod. 2009, 30, 259–264. [Google Scholar] [CrossRef]
- Baydar, H.; Sagdic, O.; Ozkan, G.; Karadogan, T. Antibacterial activity and composition of essential oils from Origanum, Thymbra and Satureja species with commercial importance in Turkey. Food Control 2004, 15, 169–172. [Google Scholar] [CrossRef]
- Rai, M.; Paralikar, P.; Jogee, P.; Agarkar, G.; Ingle, A.P.; Derita, M.; Zacchino, S. Synergistic antimicrobial potential of essential oils in combination with nanoparticles: Emerging trends and future perspectives. Int. J. Pharm. 2017, 519, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Delaquis, P.J.; Stanich, K.; Girard, B.; Mazza, G. Antimicrobial activity of individual and mixed fractions of dill, cilantro, coriander and eucalyptus essential oils. Int. J. Food Microbiol. 2002, 74, 101–109. [Google Scholar] [CrossRef]
- Sagdic, O.; Ozcan, M. Antibacterial activity of Turkish spice hydrosols. Food Control 2003, 14, 141–143. [Google Scholar] [CrossRef]
- Brenes, M.; Medina, E.; Romero, C.; De Castro, A. Antimicrobial activity of olive oil. Agro Food Ind. Hi Tech 2007, 18, 6–8. [Google Scholar]
- Medina, E.; Romero, C.; Brenes, M.; de Castro, A. Antimicrobial activity of olive oil, vinegar, and various beverages against foodborne pathogens. J. Food Prot. 2007, 70, 1194–1199. [Google Scholar] [CrossRef]
- Chieffi, D.; Fanelli, F.; Fusco, V. Arcobacter butzleri: Up-to-date taxonomy, ecology, and pathogenicity of an emerging pathogen. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2071–2109. [Google Scholar] [CrossRef]
- Collado, L.; Guarro, J.; Figueras, M.J. Prevalence of Arcobacter in meat and shellfish. J. Food Prot. 2009, 72, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Van den Abeele, A.-M.; Vogelaers, D.; Van Hende, J.; Houf, K. Prevalence of Arcobacter species among humans, Belgium, 2008–2013. Emerg Infect Dis. 2014, 20, 1731–1734. [Google Scholar] [CrossRef] [PubMed]
- Reuter, M.; Mallett, A.; Pearson, B.M.; van Vliet, A.H.M. Biofilm formation by Campylobacter jejuni is increased under aerobic conditions. Appl. Environ. Microbiol. 2010, 76, 2122–2128. [Google Scholar] [CrossRef] [PubMed]
- Elmali, M.; Can, H.Y. Occurence and antimicrobial resistance of Arcobacter species in food and slaughterhouse samples. Food Sci. Technol. 2017, 37, 280–285. [Google Scholar] [CrossRef]
- Levican, A.; Collado, L.; Figueras, M.J. Arcobacter cloacae sp nov and Arcobacter suis sp nov., two new species isolated from food and sewage. Syst. Appl. Microbiol. 2013, 36, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.H.; Saleha, A.A.; Zunita, Z.; Murugaiyah, M. Arcobacter—An emerging threat to animals and animal origin food products? Trends Food Sci. Technol. 2011, 22, 225–236. [Google Scholar] [CrossRef]
- Abay, S.; Kayman, T.; Hizlisoy, H.; Aydin, F. In vitro antibacterial susceptibility of Arcobacter butzleri isolated from different sources. J. Vet. Med. Sci. 2012, 74, 613–616. [Google Scholar] [CrossRef][Green Version]
- Fera, M.T.; Maugeri, T.L.; Giannone, M.; Gugliandolo, C.; La Camera, E.; Blandino, G.; Carbone, M. In vitro susceptibility of Arcobacter butzleri and Arcobacter cryaerophilus to different antimicrobial agents. Int. J. Antimicrob. Agents 2003, 21, 488–491. [Google Scholar] [CrossRef]
- Kabeya, H.; Maruyama, S.; Morita, Y.; Ohsuga, T.; Ozawa, S.; Kobayashi, Y.; Abe, M.; Katsube, Y.; Mikami, T. Prevalence of Arcobacter species in retail meats and antimicrobial susceptibility of the isolates in Japan. Int. J. Food Microbiol. 2004, 90, 303–308. [Google Scholar] [CrossRef]
- Shah, A.H.; Saleha, A.A.; Zunita, Z.; Murugaiyah, M.; Aliyu, A.B. Antimicrobial susceptibility of an emergent zoonotic pathogen, Arcobacter butzleri. Int. J. Antimicrob. Agents 2012, 40, 569–570. [Google Scholar] [CrossRef]
- Son, I.; Englen, M.D.; Berrang, M.E.; Fedorka-Cray, P.J.; Harrison, M.A. Antimicrobial resistance of Arcobacter and Campylobacter from broiler carcasses. Int. J. Antimicrob. Agents 2007, 29, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Turek, C.; Stintzing, F.C. Stability of Essential Oils: A Review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Chlodwig, F.; Johannes, N. Production of Essential Oils. In Handbook of Essential Oils; CRC Press: Boca Raton, FL, USA, 2015; pp. 43–86. [Google Scholar]
- Diaz-Maroto, M.C.; Perez-Coello, M.S.; Cabezudo, M.D. Effect of drying method on the volatiles in bay leaf (Laurus nobilis L.). J. Agric. Food Chem. 2002, 50, 4520–4524. [Google Scholar] [CrossRef] [PubMed]
- Mota, A.S.; Martins, M.R.; Arantes, S.; Lopes, V.R.; Bettencourt, E.; Pombal, S.; Gomes, A.C.; Silva, L.A. Antimicrobial activity and chemical composition of the essential oils of Portuguese Foeniculum vulgare Fruits. Nat. Prod. Commun. 2015, 10, 673–676. [Google Scholar] [CrossRef]
- Pouryousef, M. Variation in the essential oil constituents in indigenous populations of Foeniculum vulgare var. vulgare from different locations of Iran. J. Essent. Oil Res. 2014, 26, 441–445. [Google Scholar] [CrossRef]
- Gonzalez-Rivera, J.; Duce, C.; Falconieri, D.; Ferrari, C.; Ghezzi, L.; Piras, A.; Tine, M.R. Coaxial microwave assisted hydrodistillation of essential oils from five different herbs (lavender, rosemary, sage, fennel seeds and clove buds): Chemical composition and thermal analysis. Innovative Food Sci. Emerg. Technol. 2016, 33, 308–318. [Google Scholar] [CrossRef]
- Xiao, Z.B.; Chen, J.Y.; Niu, Y.W.; Chen, F. Characterization of the key odorants of fennel essential oils of different regions using GC-MS and GC-O combined with partial least squares regression. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1063, 226–234. [Google Scholar] [CrossRef]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Mnichowska-Polanowska, M.; Pruss, A.; Masiuk, H.; Dzieciol, M.; Giedrys-Kalemba, S.; Sienkiewicz, M. The effect of fennel essential oil in combination with antibiotics on Staphylococcus aureus strains isolated from carriers. Burns 2017, 43, 1544–1551. [Google Scholar] [CrossRef]
- Roby, M.H.H.; Sarhan, M.A.; Selim, K.A.H.; Khalel, K.I. Antioxidant and antimicrobial activities of essential oil and extracts of fennel (Foeniculum vulgare L.) and chamomile (Matricaria chamomilla L.). Ind. Crop. Prod. 2013, 44, 437–445. [Google Scholar] [CrossRef]
- De Oliveira, S.P.; Cunha, G.S.P.; Prates, J.P.B.; Fonseca, F.S.A.; de Souza, K.S.S.; Azevedo, A.M.; Xavier, A.; Santos, E.M.S.; Santos, H.O.; de Almeida, A.C. Antimicrobial activity of essential oils extracted from clove and lemongrass against pathogenic bacteria isolated from bovine, swine and poultry feces. Semin. Cienc. Agrar. 2019, 40, 1937–1950. [Google Scholar] [CrossRef]
- Carson, C.F.; Riley, T.V. Antimicrobial activity of the major components of the essential oil of Melalauca-alternifolia. J. Appl. Bacteriol. 1995, 78, 264–269. [Google Scholar] [CrossRef] [PubMed]
- De Souza, S.M.; Delle Monache, F.; Smania, A., Jr. Antibacterial activity of coumarins. Z. Naturforsch. C 2005, 60, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Kayser, O.; Kolodziej, H. Antibacterial activity of simple coumarins: Structural requirements for biological activity. Z. Naturforsch. C J. Biosci. 1999, 54, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.; Tambor, K. Linalool affects the antimicrobial efficacy of essential oils. Curr. Microbiol. 2016, 72, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, S.; Vaseeharan, B.; Malaikozhundan, B.; Shobiya, M. Laurus nobilis leaf extract mediated green synthesis of ZnO nanoparticles: Characterization and biomedical applications. Biomed. Pharmacother. 2016, 84, 1213–1222. [Google Scholar] [CrossRef]
- Kunicka-Styczynska, A.; Smigielski, K.; Prusinowska, R.; Rajkowska, K.; Kusmider, B.; Sikora, M. Preservative activity of lavender hydrosols in moisturizing body gels. Lett. Appl. Microbiol. 2015, 60, 27–32. [Google Scholar] [CrossRef]
- Bajer, T.; Silha, D.; Ventura, K.; Bajerova, P. Composition and antimicrobial activity of the essential oil, distilled aromatic water and herbal infusion from Epilobium parviflorum Schreb. Ind. Crop. Prod. 2017, 100, 95–105. [Google Scholar] [CrossRef]
- Cai, L.N.; Wu, C.D. Compounds from Syzygium aromaticum possessing growth inhibitory activity against oral pathogens. J. Nat. Prod. 1996, 59, 987–990. [Google Scholar] [CrossRef]
- Akhondzadeh, S.; Kashani, L.; Fotouhi, A.; Jarvandi, S.; Mobaseri, M.; Moin, M.; Khani, M.; Jamshidi, A.H.; Baghalian, K.; Taghizadeh, M. Comparison of Lavandula angustifolia Mill. tincture and imipramine in the treatment of mild to moderate depression: A double-blind, randomized trial. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2003, 27, 123–127. [Google Scholar] [CrossRef]
- Verma, R.S.; Rahman, L.U.; Chanotiya, C.S.; Verma, R.K.; Chauhan, A.; Yadav, A.; Singh, A.; Yadav, A.K. Essential oil composition of Lavandula angustifolia Mill. cultivated in the mid hills of Uttarakhand, India. J. Serb. Chem. Soc. 2010, 75, 343–348. [Google Scholar] [CrossRef]
- Vanin, A.B.; Orlando, T.; Piazza, S.P.; Puton, B.M.S.; Cansian, R.L.; Oliveira, D.; Paroul, N. Antimicrobial and antioxidant activities of clove essential oil and eugenyl acetate produced by enzymatic esterification. Appl. Biochem. Biotechnol. 2014, 174, 1286–1298. [Google Scholar] [CrossRef] [PubMed]
- Sahan, Y. Effect of Prunus laurocerasus L. (Cherry laurel) leaf extracts on growth of bread spoilage fungi. Bulg. J. Agric. Sci. 2011, 17, 83–92. [Google Scholar]
- Ferreira, F.M.; Delmonte, C.C.; Novato, T.L.P.; Monteiro, C.M.O.; Daemon, E.; Vilela, F.M.P.; Amaral, M.P.H. Acaricidal activity of essential oil of Syzygium aromaticum, hydrolate and eugenol formulated or free on larvae and engorged females of Rhipicephalus microplus. Med. Vet. Entomol. 2018, 32, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.G.; Shibamoto, T. Antioxidant property of aroma extract isolated from clove buds Syzygium aromaticum (L.) Merr. et Perry. Food Chem. 2001, 74, 443–448. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
- Nikolic, M.; Markovic, T.; Mojovic, M.; Pejin, B.; Savic, A.; Peric, T.; Markovic, D.; Stevic, T.; Sokovic, M. Chemical composition and biological activity of Gaultheria procumbens L. essential oil. Ind. Crop. Prod. 2013, 49, 561–567. [Google Scholar] [CrossRef]
- Jabra-Rizk, M.A.; Meiller, T.F.; James, C.E.; Shirtliff, M.E. Effect of farnesol on Staphylococcus aureus biofilm formation and antimicrobial susceptibility. Antimicrob. Agents Chemother. 2006, 50, 1463–1469. [Google Scholar] [CrossRef]
- Quave, C.L.; Plano, L.R.W.; Pantuso, T.; Bennett, B.C. Effects of extracts from Italian medicinal plants on planktonic growth, biofilm formation and adherence of methicillin-resistant Staphylococcus aureus. J. Ethnopharmacol. 2008, 118, 418–428. [Google Scholar] [CrossRef]
- Rasmussen, T.B.; Givskov, M. Quorum sensing inhibitors: A bargain of effects. Microbiology 2006, 152, 895–904. [Google Scholar] [CrossRef]
- Gomes, F.I.A.; Teixeira, P.; Azeredo, J.; Oliveira, R. Effect of Farnesol on Planktonic and Biofilm Cells of Staphylococcus epidermidis. Curr. Microbiol. 2009, 59, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Bazargani, M.M.; Rohloff, J. Antibiofilm activity of essential oils and plant extracts against Staphylococcus aureus and Escherichia coli biofilms. Food Control 2016, 61, 156–164. [Google Scholar] [CrossRef]
- Oliveira, N.M.; Martinez-Garcia, E.; Xavier, J.; Durham, W.M.; Kolter, R.; Kim, W.; Foster, K.R. Biofilm formation as a response to ecological competition. PLoS Biol. 2015, 13, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-G.; Lee, J.-H.; Gwon, G.; Kim, S.-I.; Park, J.G.; Lee, J. Essential oils and eugenols inhibit biofilm formation and the virulence of Escherichia coli O157:H7. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sandasi, M.; Leonard, C.M.; Viljoen, A.M. The effect of five common essential oil components on Listeria monocytogenes biofilms. Food Control 2008, 19, 1070–1075. [Google Scholar] [CrossRef]
- D’Amato, S.; Serio, A.; Lopez, C.C.; Paparella, A. Hydrosols: Biological activity and potential as antimicrobials for food applications. Food Control 2018, 86, 126–137. [Google Scholar] [CrossRef]
- Palombo, E.A. Traditional medicinal plant extracts and natural products with activity against oral bacteria: Potential application in the prevention and treatment of oral diseases. Evid. Based Complement. Altern. Med. 2011, 2011, 1–15. [Google Scholar] [CrossRef]
- Duarte, A.; Luis, A.; Oleastro, M.; Domingues, F.C. Antioxidant properties of coriander essential oil and linalool and their potential to control Campylobacter spp. Food Control 2016, 61, 115–122. [Google Scholar] [CrossRef]
- Packiavathy, I.; Priya, S.; Pandian, S.K.; Ravi, A.V. Inhibition of biofilm development of uropathogens by curcumin—An anti-quorum sensing agent from Curcuma longa. Food Chem. 2014, 148, 453–460. [Google Scholar] [CrossRef]
- Morobe, I.; Mthethwa, S.; Bisi-Johnson, M.; Vasaikar, S.; Obi, C.; Oyedeji, A.; Kambizi, L.; Eloff, J.; Hattori, T. Cytotoxic effects and safety profiles of extracts of active medicinal plants from South Africa. J. Microbiol. Res. 2012, 2, 176–182. [Google Scholar]
- De Oliveira, P.F.; Alves, J.M.; Damasceno, J.L.; Oliveira, R.A.M.; Dias, H.J.; Crotti, A.E.M.; Tavares, D.C. Cytotoxicity screening of essential oils in cancer cell lines. Rev. Bras. Farmacogn. 2015, 25, 183–188. [Google Scholar] [CrossRef]
- Prashar, A.; Locke, I.C.; Evans, C.S. Cytotoxicity of lavender oil and its major components to human skin cells. Cell Prolif. 2004, 37, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Rizwana, H.; Al Kubaisi, N.; Al-Meghailaith, N.N.; Moubayed, N.M.S.; Albasher, G. Evaluation of chemical composition, antibacterial, antifungal, and cytotoxic activity of Laurus nobilis L. Grown in Saudi Arabia. J. Pure Appl. Microbiol. 2019, 13, 2073–2085. [Google Scholar] [CrossRef]
- Silhova-Hruskova, L.; Mot’kova, P.; Silha, D.; Vytrasova, J. Detection of biofilm formation by selected pathogens relevant to the food industry. Epidemiol. Mikrobiol. Imunol. 2015, 64, 169–175. [Google Scholar]
- Xing, J.Z.; Zhu, L.; Jackson, J.A.; Gabos, S.; Sun, X.-J.; Wang, X.-B.; Xu, X. Dynamic monitoring of cytotoxicity on microelectronic sensors. Chem. Res. Toxicol. 2005, 18, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.Z.; Zhu, L.; Gabos, S.; Xie, L. Microelectronic cell sensor assay for detection of cytotoxicity and prediction of acute toxicity. Toxicol. In Vitro 2006, 20, 995–1004. [Google Scholar] [CrossRef]
| Hydrolate from | Peaks in Chromatograms | Identified Compounds (Total Rel. Content) | Main Compounds (Rel. Content) | |||
|---|---|---|---|---|---|---|
| HSD_SPE | HHD_SPE | HSD_SPE | HHD_SPE | HSD_SPE | HHD_SPE | |
| Lavender | 186 | 172 | 48 (90.0%) | 48 (93.2%) | 1,8-Cineole (20.6%) | Linalool (23.2%) |
| (Z)-Linalool furanoxide (11.9%) | 1,8-Cineole (19.5%) | |||||
| α-Terpineol (10.4%) | α-Terpineol (13.0%) | |||||
| Bay leaves | 227 | 166 | 33 (78.0%) | 33 (79.4%) | 1,8-Cineol (56.4%) | 1,8-Cineol (54.1%) |
| 4-Terpineol (6.0%) | 4-Terpineol (7.1%) | |||||
| α-Terpineol (5.0%) | α-Terpineol (6.4%) | |||||
| Fennel | 87 | 68 | 13 (84.1%) | 11 (85.1%) | Estragole (37.4%) | Estragole (33.0%) |
| Fenchone 22.5%) | Fenchone (26.5%) | |||||
| p-Methoxy Cinnamaldehyde (7.0%) | Eugenol (5.6%) | |||||
| Clove | 98 | 80 | 9 (99.3%) | 9 (99.3%) | Eugenol (89.1%) | Eugenol (92.7%) |
| Eugenyl acetate (9.3%) | Eugenyl acetate (5.6%) | |||||
| Chavicol (0.4%) | Chavicol (0.4%) | |||||
| CAS | Compound | RI | % of Total Peak Area | |
|---|---|---|---|---|
| HSD_SPE | HHD_SPE | |||
| Oxidized Monoterpenes | ||||
| 7392-19-0 | Bois de Rose oxide/Linaloyl oxide | 968 | 0.2 | 0.1 |
| 54750-70-8 | (E)-Dehydroxy linalool oxide | 987 | 0.1 | 0.1 |
| 54750-69-5 | (Z)-Dehydroxy linalool oxide | 1004 | 0.1 | <0.1 |
| 470-67-7 | 1,4-Cineol | 1014 | <0.1 | <0.1 |
| 470-82-6 | 1,8-Cineole | 1030 | 20.6 | 19.5 |
| 5989-33-3 | (Z)-Linalool furanoxide | 1070 | 11.9 | 7.9 |
| 34995-77-2 | (E)-Linalool furanoxide | 1086 | 9.1 | 5. 9 |
| 78-70-6 | Linalool | 1101 | 7.9 | 23.2 |
| 29957-43-5 | Hotrienol | 1103 | 0.8 | 0.5 |
| 471-16-9 | Sabinol | 1139 | 0.1 | 0.2 |
| 76-22-2 | Camphor | 1144 | 0.4 | 0.5 |
| --- | Lilac aldehyde isomer (B or C) | 1148 | <0.1 | <0.1 |
| 1786-08-9 | Nerol oxide | 1151 | 0.4 | 0.2 |
| 5986-38-9 | (E)-Ocimenol | 1154 | <0.1 | <0.1 |
| 513-20-2 | Sabina ketone | 1158 | <0.1 | <0.1 |
| 30460-92-5 | Pinocarvone | 1160 | <0.1 | <0.1 |
| 53447-47-5 | Lilac aldehyde D | 1163 | <0.1 | <0.1 |
| 14009-71-3 | (Z)-Linalool pyranoxide | 1169 | 1.3 | 1.1 |
| 39028-58-5 | (E)-Linalool pyranoxide | 1174 | 1.0 | 0.8 |
| 562-74-3 | 4-Terpineol | 1179 | 1.1 | 1.2 |
| 500-02-7 | Cryptone | 1185 | 0.9 | 0.8 |
| 13741-21-4 | 2,6-Dimethyl-3,7-octadiene-2,6-diol | 1190 | 5.9 | 2.2 |
| 98-55-5 | α-Terpineol | 1194 | 10.4 | 13.0 |
| 80-57-9 | Verbenone | 1207 | 0.2 | 0.2 |
| 1197-07-5 | (E)-Carveol | 1219 | <0.1 | <0.1 |
| 106-25-2 | Nerol | 1225 | 0.7 | 0.9 |
| 18675-34-8 | Neodihydrocarveol | 1231 | 5.9 | 4.9 |
| 122-03-2 | Cuminaldehyde | 1240 | 0.1 | 0.1 |
| 106-24-1 | Geraniol | 1252 | 1.0 | 2.3 |
| 51276-33-6 | 2,6-Dimethyl-1,7-octadien-3,6-diol | 1273 | 1.1 | 0.3 |
| 536-60-7 | Cumin alcohol | 1291 | 0.4 | 0.3 |
| 39725-34-3 | 4-Hydroxy-cryptone | 1322 | 0.2 | <0.1 |
| 160152-34-1 | 3-Oxo-p-menth-1-en-7-al | 1336 | 0.1 | <0.1 |
| 7712-46-1 | 8-Hydroxycarvotanacetone | 1427 | <0.1 | <0.1 |
| 26184-88-3 | α-Bisabolol oxide B | 1653 | 0.1 | 0.1 |
| 3790-71-4 | (2Z,6E)-Farnesol | 1684 | 0.2 | 0.2 |
| Others | ||||
| 111-27-3 | Hexyl alcohol | 872 | 0.2 | 0.2 |
| 3391-86-4 | 1-Octen-3-ol | 981 | 0.2 | 0.2 |
| 589-98-0 | 3-Octanol | 999 | 0.1 | 0.1 |
| 106-68-3 | 3-Octanone | 985 | 0.8 | 0.9 |
| --- | Cymene isomer | 1022 | <0.1 | <0.1 |
| 1073-11-6 | Lavender lactone | 1035 | 0.8 | 2.0 |
| 1604-28-0 | 6-Methyl-3,5-heptadien-2-one | 1103 | 0.6 | 0.7 |
| 24903-95-5 | Nopinone | 1137 | 0.2 | 0.1 |
| 97-53-0 | Eugenol | 1351 | 0.5 | 1.3 |
| 91-64-5 | Coumarin | 1432 | 3.6 | 0.8 |
| 17092-92-1 | Dihydroactinidiolide | 1524 | <0.1 | <0.1 |
| 531-59-9 | 7-Methoxycoumarin (Hernianin) | 1720 | 0.2 | <0.1 |
| CAS | Compound | RI | % of Total Peak Area | |
|---|---|---|---|---|
| HSD_SPE | HHD_SPE | |||
| Oxidized Monoterpenes | ||||
| 470-67-7 | 1,4-Cineol | 1014 | <0.1 | <0.1 |
| 470-82-6 | 1,8-Cineol | 1030 | 56.4 | 54.0 |
| 78-70-6 | Linalool | 1099 | 0.3 | 0.5 |
| 29957-43-5 | Hotrienol | 1101 | <0.1 | <0.1 |
| 36262-12-1 | Dehydrosabina ketone | 1120 | 0.1 | 0.1 |
| 471-16-9 | Sabinol | 1136 | <0.1 | <0.1 |
| 1786-08-9 | Nerol oxide | 1151 | <0.1 | <0.1 |
| 513-20-2 | Sabina ketone | 1157 | 0.5 | 0.6 |
| 30460-92-5 | Pinocarvone | 1160 | 0.4 | 0.3 |
| 562-74-3 | 4-Terpineol | 1178 | 6.0 | 7.1 |
| 13741-21-4 | 2,6-Dimethyl-3,7-octadiene-2,6-diol | 1189 | 0.7 | 0.4 |
| 98-55-5 | α-Terpineol | 1193 | 5.0 | 6.4 |
| 80-57-9 | Verbenone | 1207 | <0.1 | <0.1 |
| 99-48-9 | Carveol | 1218 | 0.2 | 0.4 |
| 18679-48-6 | 2-Hydroxy-1,8-cineole | 1225 | 0.4 | 0.5 |
| 22626-43-3 | cis-p-Mentha-1(7),8-diene-2-ol | 1228 | 0.5 | 0.8 |
| 494-99-5 | Homoveratrole | 1236 | <0.1 | <0.1 |
| 122-03-2 | Cuminaldehyde | 1240 | <0.1 | <0.1 |
| 51276-33-6 | 2,6-Dimethyl-1,7-octadien-3,6-diol | 1272 | 0.4 | 0.2 |
| 536-60-7 | Cumin alcohol | 1288 | 0.7 | 1.3 |
| 89-83-8 | Thymol | 1298 | 0.2 | 0.1 |
| 22539-72-6 | p-Mentha-1,4-dien-7-ol | 1328 | 0.2 | 0.2 |
| Others | ||||
| 100-52-7 | Benzaldehyde | 960 | <0.1 | <0.1 |
| 110-93-0 | 6-Methyl-5-hepten-2-one | 985 | <0.1 | 0.2 |
| 1073-11-6 | Lavender lactone | 1037 | <0.1 | 0.1 |
| 6090-09-1 | Limona ketone | 1130 | <0.1 | <0.1 |
| 76-49-3 | Bornyl acetate | 1283 | <0.1 | <0.1 |
| 81781-24-0 | 1,3,3-Trimethyl-2-oxabicyclo[2.2.2]octan-5-yl acetate | 1337 | 1.0 | 1.2 |
| 97-53-0 | Eugenol | 1350 | 2.2 | 2.4 |
| 57709-95-2 | 1,3,3-Trimethyl-2-oxabicyclo[2.2.2]octan-6-yl acetate | 1357 | 0.1 | 0.1 |
| 121-33-5 | Vanilin | 1393 | 0.5 | 0.3 |
| 93-15-2 | Methyleugenol | 1399 | 1.8 | 1.7 |
| 17092-92-1 | Dihydroactinidiolide | 1524 | 0.2 | <0.1 |
| % of Total Peak Area | ||||
|---|---|---|---|---|
| CAS | Compound | RI | HSD_SPE | HHD_SPE |
| 142-62-1 | Capronic acid | 982 | 0.2 | n.i. |
| 470-82-6 | 1,8-Cineol | 1030 | 2.8 | 3.8 |
| 122-78-1 | Phenylacetaldehyde | 1042 | 0.1 | n.i. |
| 1195-79-5 | Fenchone | 1086 | 22.5 | 26.5 |
| 78-70-6 | Linalool | 1099 | 0.3 | 0.2 |
| 76-22-2 | Camphor | 1144 | 0.7 | 0.9 |
| 140-67-0 | Estragole | 1196 | 37.4 | 33.0 |
| 99-48-9 | Carveol | 1218 | 2.6 | 2.3 |
| 99-49-0 | Carvone | 1242 | 2.2 | 2.3 |
| 123-11-5 | p-Anisaldehyde | 1253 | 5.2 | 5.5 |
| 97-53-0 | Eugenol | 1350 | 2.5 | 5.6 |
| 93-28-7 | Eugenyl acetate | 1513 | 0.8 | 1.0 |
| 1963-36-6 | p-Methoxy Cinnamaldehyde | 1567 | 7.1 | 4.0 |
| CAS | Compound | RI | % of Total Peak Area | |
|---|---|---|---|---|
| HSD_SPE | HHD_SPE | |||
| 97-53-0 | Eugenol | 1360 | 89.1 | 92.7 |
| 93-28-7 | Eugenyl acetate | 1516 | 9.4 | 5.6 |
| 501-92-8 | Chavicol | 1253 | 0.4 | 0.4 |
| 121-33-5 | Vanilin | 1393 | 0.3 | 0.4 |
| 458-36-6 | Coniferyl aldehyde | 1727 | 0.2 | 0.2 |
| 119-36-8 | Methyl salicylate | 1189 | <0.1 | <0.1 |
| 87-44-5 | (E)-β-Caryophyllene | 1417 | <0.1 | <0.1 |
| 6753-98-6 | α-Caryophyllene | 1451 | <0.1 | <0.1 |
| 120-51-4 | Benzyl benzoate | 1764 | <0.1 | <0.1 |
| Ab LMG 10828 | Ab CCUG 30484 | Ab UPa 2012/3 | Ac CCM 7050 | Ac UPa 2013/13 | Al LMG 28517 | As LMG 6621 | At LMG 24488 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Lavender | IZ | HHD_SPE | 9.5 ± 0.3 | 8.8 ± 0.3 | 12.8 ± 0.3 | 8.3 ± 0.5 | 8.0 ± 0 | 11.3 ± 1.0 | 9.0 ± 0.8 | 11.5 ± 0.3 |
| HSD_SPE | 10.8 ± 0.3 | 13.5 ± 0.6 | 10.5 ± 0.3 | 9.5 ± 0.9 | 11.5 ± 0.1 | 11.8 ± 0.3 | 10.3 ± 0.5 | 13.3 ± 0.9 | ||
| MIC/ MBC | HHD_SPE | 0.4/0.8 | 0.8/0.8 | 0.8/1.6 | 0.8/0.8 | 0.8/0.8 | 0.8/0.8 | 0.4/0.4 | 0.8/1.6 | |
| HSD_SPE | 0.4/0.4 | 0.4/0.8 | 1.6/1.6 | 0.4/0.8 | 0.4/0.4 | 0.4/0.8 | 0.4/0.4 | 0.4/0.8 | ||
| Fennel | IZ | HHD_SPE | 7.8 ± 0.2 | 9.0 ± 0.8 | 10.5 ± 0.9 | 9.8 ± 0 | 8.5 ± 1.3 | 10.8 ± 0.5 | 7.8 ± 0.5 | 12.0 ± 0.1 |
| HSD_SPE | 10.8 ± 0 | 10.8 ± 0.5 | 10.8 ± 0.9 | 10.0 ± 0.2 | 10.0 ± 0.8 | 11.8 ± 0 | 10.3 ± 0.3 | 11.3 ± 0.5 | ||
| MIC/ MBC | HHD_SPE | 1.6/1.6 | 3.1/3.1 | 1.6/3.1 | 0.8/0.8 | 1.6/1.6 | 1.6/1.6 | 0.8/0.8 | 1.6/1.6 | |
| HSD_SPE | 0.8/1.6 | 1.6/3.1 | 1.6/1.6 | 0.4/0.8 | 0.8/0.8 | 0.8/1.6 | 0.4/0.4 | 0.8/0.8 | ||
| Bay leaves | IZ | HHD_SPE | 8.0 ± 0.2 | 9.0 ± 0.8 | 9.0 ± 0 | 9.5 ± 0.3 | 9.5 ± 0.3 | 11.3 ± 0.3 | 9.0 ± 0.3 | 10.8 ± 0 |
| HSD_SPE | 10.5 ± 0.3 | 9.5 ± 0.6 | 14.0 ± 0.8 | 10.3 ± 0.3 | 10.0 ± 0.3 | 10.5 ± 0.6 | 12.5 ± 0.7 | 13.3 ± 0.6 | ||
| MIC/ MBC | HHD_SPE | 1.6/1.6 | 1.6/1.6 | 1.6/3.1 | 0.8/1.6 | 0.8/1.6 | 1.6/3.1 | 1.6/3.1 | 1.6/1.6 | |
| HSD_SPE | 0.8/1.6 | 0.8/1.6 | 0.8/1.6 | 0.8/1.6 | 0.8/1.6 | 1.6/3.1 | 1.6/1.6 | 0.8/0.8 | ||
| Clove | IZ | HHD_SPE | 10.5 ± 0.3 | 12.0 ± 0.9 | 13.0 ± 0.6 | 10.5 ± 0.3 | 14.3 ± 0.5 | 12.0 ± 0.6 | 12.0 ± 0.9 | 14.8 ± 0.5 |
| HSD_SPE | 12.5 ± 0.7 | 13.5 ± 0.4 | 14.5 ± 0.3 | 12.5 ± 0.3 | 16.5 ± 0.3 | 12.8 ± 0.7 | 11.0 ± 0.3 | 15.5 ± 0.7 | ||
| MIC/ MBC | HHD_SPE | 0.1/0.1 | 0.2/0.2 | 0.8/0.8 | 0.2/0.2 | 0.4/0.4 | 0.4/0.4 | 0.2/0.2 | 0.4/0.4 | |
| HSD_SPE | 0.1/0.1 | 0.1/0.1 | 0.8/0.8 | 0.1/0.1 | 0.1/0.1 | 0.4/0.4 | 0.1/0.1 | 0.2/0.4 | ||
| Control | IZ | 8.0 ± 0.8 | 7.7 ± 0.5 | 7.7 ± 0.5 | 8.0 ± 0.8 | 7.0 ± 0.8 | 6.7 ± 0.5 | 8.3 ± 0.5 | 8.0 ± 0.8 | |
| MIC/MBC | 3.1/6.3 | 3.1/6.3 | 3.1/6.3 | 1.6/3.1 | 3.1/3.1 | 3.1/3.1 | 1.6/3.1 | 3.1/3.1 | ||
| Sa CCM 4223 | Ef CCM 4224 | Pa CCM 3955 | Ec CCM 3954 | Ca CCM 8186 | |||
|---|---|---|---|---|---|---|---|
| Lavender | IZ | HHD_SPE | 8.5 ± 0.7 | 7.5 ± 0.6 | 8.5 ± 0.4 | 8.8 ± 0.5 | 6.0 ± 0 |
| HSD_SPE | 8.3 ± 0.5 | 9.5 ± 0.3 | 10.5 ± 0.3 | 9.0 ± 0.3 | 6.0 ± 0 | ||
| MIC/ MBC | HHD_SPE | 1.6/3.1 | 3.1/3.1 | 1.6/3.1 | 1.6/3.1 | 6.3/6.3 | |
| HSD_SPE | 1.6/3.1 | 1.6/3.1 | 1.6/1.6 | 1.6/3.1 | 6.3/6.3 | ||
| Fennel | IZ | HHD_SPE | 10.8 ± 0.9 | 9.3 ± 0.9 | 11.0 ± 0.8 | 11.3 ± 0.5 | 10.5 ± 0.6 |
| HSD_SPE | 11.3 ± 0.7 | 15.0 ± 0.1 | 11.5 ± 0.8 | 11.5 ± 0.3 | 11.8 ± 0.7 | ||
| MIC/ MBC | HHD_SPE | 3.1/6.3 | 3.1/6.3 | 3.1/3.1 | 1.6/3.1 | 1.6/3.1 | |
| HSD_SPE | 3.1/6.3 | 1.6/3.2 | 1.6/3.1 | 1.6/3.1 | 1.6/3.1 | ||
| Bay leaves | IZ | HHD_SPE | 9.5 ± 0.3 | 11.0 ± 0.8 | 10.3 ± 0.7 | 10.3 ± 0.7 | 9.8 ± 0.9 |
| HSD_SPE | 9.5 ± 0.6 | 12.3 ± 0.5 | 11.5 ± 0.9 | 10.5 ± 0.9 | 13.3 ± 0.9 | ||
| MIC/ MBC | HHD_SPE | 1.6/1.6 | 6.3/12.5 | 1.6/3.1 | 3.1/6.3 | 1.6/3.1 | |
| HSD_SPE | 1.6/1.6 | 6.3/6.3 | 3.1/6.3 | 1.6/3.1 | 0.4/0.8 | ||
| Clove | IZ | HHD_SPE | 15.8 ± 0.7 | 15.3 ± 0.3 | 11.3 ± 0.1 | 13.5 ± 0.7 | 14.8 ± 0.4 |
| HSD_SPE | 15.5 ± 0.9 | 18.8 ± 0.9 | 12.8 ± 0.7 | 14.0 ± 0.8 | 23.5 ± 0.7 | ||
| MIC/ MBC | HHD_SPE | 0.4/0.4 | 0.8/0.8 | 0.8/1.6 | 3.1/6.3 | 6.3/6.3 | |
| HSD_SPE | 0.4/0.8 | 0.4/0.8 | 0.4/0.8 | 1.6/3.1 | 1.6/3.1 | ||
| Control | IZ | 7.3 ± 0.5 | 9.0 ± 0.8 | 8.7 ± 0.5 | 8.0 ± 0 | 6.3 ± 0.5 | |
| MIC/MBC | 12.5/12.5 | 12.5/12.5 | 3.1/6.3 | 6.3/12.5 | 6.3/12.5 | ||
| AMP | CIP | DA | E | TE | FCA | |
|---|---|---|---|---|---|---|
| A. butzleri CCUG 30484 | 6.0 ± 0 | 43.5 ± 2.1 | 7.5 ± 0.7 | 37.5 ± 0.7 | 31.5 ± 2.1 | n.t. |
| A. butzleri LMG 10828 | 6.0 ± 0 | 34.5 ± 0.7 | 6.0 ± 0 | 23.0 ± 0 | 16.0 ± 0 | n.t. |
| A. butzleri UPa 2012/3 | 6.0 ± 0 | 39.0 ± 1.4 | 6.0 ± 0 | 29.0 ± 1.4 | 24.0 ± 1.4 | n.t. |
| A. cryaerophilus CCM 7050 | 6.0 ± 0 | 25.0 ± 0 | 6.0 ± 0 | 31.5 ± 0.7 | 27.5 ± 0.7 | n.t. |
| A. cryaerophilus UPa 2013/13 | 6.0 ± 0 | 36.5 ± 0.7 | 6.0 ± 0 | 30.5 ± 0.7 | 29.0 ± 1.4 | n.t. |
| A. lanthieri LMG 28517 | 6.0 ± 0 | 37.0 ± 1.4 | 6.0 ± 0 | 22.0 ± 2.8 | 17.5 ± 0.7 | n.t. |
| A. skirrowii LMG 6621 | 6.0 ± 0 | 41.0 ± 1.4 | 23.0 ± 2.8 | 30.0 ± 0 | 34.0 ± 2.8 | n.t. |
| A. thereius LMG 24488 | 6.0 ± 0 | 32.5 ± 0.7 | 35.5 ± 0.7 | 11.0 ± 0 | 35.0 ± 0 | n.t. |
| S. aureus CCM 4232 | 27.0 ± 0 | 25.5 ± 0.5 | 27.0 ± 1.4 | 28.0 ± 2.8 | 14.5 ± 0.7 | n.t. |
| E. faecalis CCM 4224 | 13.0 ± 0 | 22.0 ± 0 | 7.0 ± 0 | 17.5 ± 0.7 | 29.0 ± 1.4 | n.t. |
| P. aeruginosa CCM 3955 | 6.0 ± 0 | 34.0 ± 1.4 | 6.0 ± 0 | 8.5 ± 0.7 | 13.5 ± 0.7 | n.t. |
| E. coli CCM 3954 | 6.0 ± 0 | 31.0 ± 1.4 | 6.0 ± 0 | 9.5 ± 0.7 | 20.5 ± 0.7 | n.t. |
| C. albicans CCM 8186 | n.t. | n.t. | n.t. | n.t. | n.t. | 16.0 ± 1.4 |
Sample Availability: Samples of the compounds are not available from the authors. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šilha, D.; Švarcová, K.; Bajer, T.; Královec, K.; Tesařová, E.; Moučková, K.; Pejchalová, M.; Bajerová, P. Chemical Composition of Natural Hydrolates and Their Antimicrobial Activity on Arcobacter-Like Cells in Comparison with Other Microorganisms. Molecules 2020, 25, 5654. https://doi.org/10.3390/molecules25235654
Šilha D, Švarcová K, Bajer T, Královec K, Tesařová E, Moučková K, Pejchalová M, Bajerová P. Chemical Composition of Natural Hydrolates and Their Antimicrobial Activity on Arcobacter-Like Cells in Comparison with Other Microorganisms. Molecules. 2020; 25(23):5654. https://doi.org/10.3390/molecules25235654
Chicago/Turabian StyleŠilha, David, Karolína Švarcová, Tomáš Bajer, Karel Královec, Eliška Tesařová, Kristýna Moučková, Marcela Pejchalová, and Petra Bajerová. 2020. "Chemical Composition of Natural Hydrolates and Their Antimicrobial Activity on Arcobacter-Like Cells in Comparison with Other Microorganisms" Molecules 25, no. 23: 5654. https://doi.org/10.3390/molecules25235654
APA StyleŠilha, D., Švarcová, K., Bajer, T., Královec, K., Tesařová, E., Moučková, K., Pejchalová, M., & Bajerová, P. (2020). Chemical Composition of Natural Hydrolates and Their Antimicrobial Activity on Arcobacter-Like Cells in Comparison with Other Microorganisms. Molecules, 25(23), 5654. https://doi.org/10.3390/molecules25235654





