Effects of Citicoline, Homotaurine, and Vitamin E on Contrast Sensitivity and Visual-Related Quality of Life in Patients with Primary Open-Angle Glaucoma: A Preliminary Study
Abstract
1. Introduction
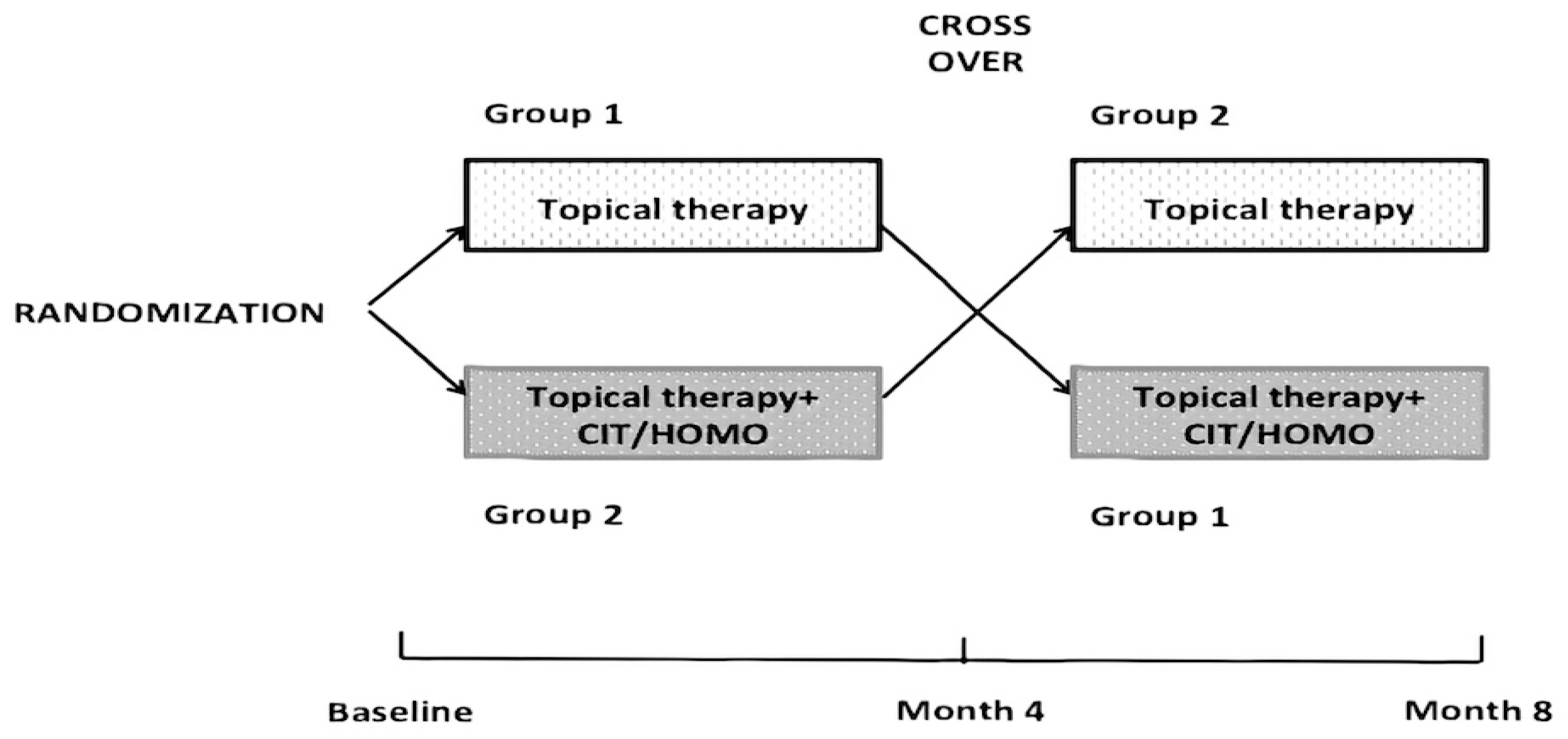
2. Materials and Methods
Statistical Analysis
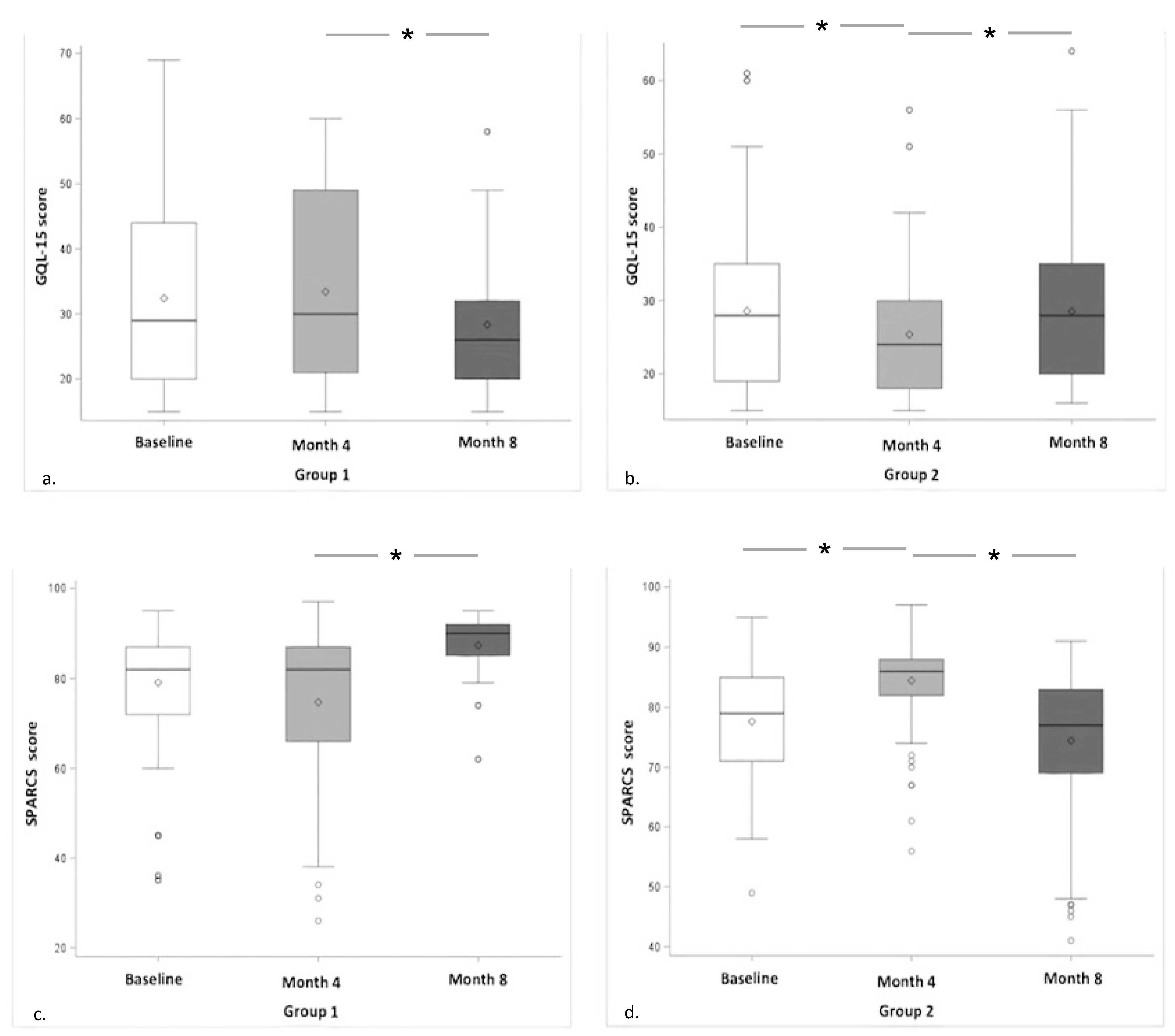
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Costagliola, C.; Dell’Omo, R.; Romano, M.R.; Rinaldi, M.; Zeppa, L.; Parmeggiani, F. Pharmacotherapy of intraocular pressure: Part I. Parasympathomimetic, sympathomimetic and sympatholytics. Expert Opin. Pharmacother. 2009, 10, 2663–2677. [Google Scholar] [CrossRef]
- Grzybowski, A.; Och, M.; Kanclerz, P.; Leffler, C.T.; De Moraes, C.G. Primary Open Angle Glaucoma and Vascular Risk Factors: A Review of Population Based Studies from 1990 to 2019. J. Clin. Med. 2020, 9, 761. [Google Scholar] [CrossRef]
- Quigley, H. Number of people with glaucoma worldwide. Am. J. Ophthalmol. 1996, 122, 460. [Google Scholar] [CrossRef][Green Version]
- Weinreb, R.N.; Khaw, P.T. Primary open-angle glaucoma. Lancet 2004, 363, 1711–1720. [Google Scholar] [CrossRef]
- Daga, F.B.; Gracitelli, C.P.B.; Diniz-Filho, A.; Medeiros, F.A. Is vision-related quality of life impaired in patients with preperimetric glaucoma? Br. J. Ophthalmol. 2018, 103, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Terminology and Guidelines for Glaucoma, 4th ed.; Publicomm: Savona, Italy, 2014; pp. 132–140.
- Montana, C.L.; Bhorade, A.M. Glaucoma and quality of life. Curr. Opin. Ophthalmol. 2018, 29, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Onal, S.; Yenice, O.; Cakir, S.; Temel, A. FACT contrast sensitivity as a diagnostic tool in glaucoma. Int. Ophthalmol. 2007, 28, 407–412. [Google Scholar] [CrossRef]
- Shoshani, Y.Z.; Harris, A.; Rusia, D.; Spaeth, G.L.; Siesky, B.; Pollack, A.; Wirostko, B. Contrast sensitivity, ocular blood flow and their potential role in assessing ischaemic retinal disease. Acta Ophthalmol. 2011, 89, 382–395. [Google Scholar] [CrossRef]
- Fatehi, N.; Nowroozizadeh, S.; Henry, S.; Coleman, A.L.; Caprioli, J.; Nouri-Mahdavi, K. Association of Structural and Functional Measures With Contrast Sensitivity in Glaucoma. Am. J. Ophthalmol. 2017, 178, 129–139. [Google Scholar] [CrossRef]
- Lee, S.S.-Y.; Wood, J.M.; Black, A.A. Impact of glaucoma on executive function and visual search. Ophthalmic Physiol. Opt. 2020, 40, 333–342. [Google Scholar] [CrossRef]
- Nucci, C.; Martucci, A.; Giannini, C.; Morrone, L.A.; Bagetta, G.; Mancino, R. Neuroprotective agents in the management of glaucoma. Eye 2018, 32, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, S.; Marchini, G.; Caporossi, A.; Scuderi, G.; Tomasso, L.; Brunoro, A. Cytidine 5′-Diphosphocholine (Citicoline): Evidence for a Neuroprotective Role in Glaucoma. Nutrients 2020, 12, 793. [Google Scholar] [CrossRef] [PubMed]
- D’Orlando, K.J.; Sandage, B.W. Citicoline (CDP-Choline): Mechanisms of action and effects in ischemic brain injury. Neurol. Res. 1995, 17, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Porciatti, V.; Schiavi, C.; Benedetti, P.; Baldi, A.; Campos, E.C. Cytidine-5’-diphosphocholine improves visual acuity, contrast sensitivity and visually-evoked potentials of amblyopic subjects. Curr. Eye Res. 1998, 17, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Roberti, G.; Tanga, L.; Michelessi, M.; Quaranta, L.; Parisi, V.; Manni, G.; Oddone, F. Cytidine 5′-Diphosphocholine (Citicoline) in Glaucoma: Rationale of Its Use, Current Evidence and Future Perspectives. Int. J. Mol. Sci. 2015, 16, 28401–28417. [Google Scholar] [CrossRef] [PubMed]
- Frontiers in Aging Neuroscience. Front. Aging Neurosci. 2018, 8, 73. [CrossRef]
- Lanza, M.; Carnevale, U.A.G.; Mele, L.; Sconocchia, M.B.; Bartollino, S.; Costagliola, C. Morphological and Functional Evaluation of Oral Citicoline Therapy in Chronic Open-Angle Glaucoma Patients: A Pilot Study With a 2-Year Follow-Up. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef]
- Caltagirone, C.; Ferrannini, L.; Marchionni, N.; Nappi, G.; Scapagnini, G.; Trabucchi, M. The potential protective effect of tramiprosate (homotaurine) against Alzheimer’s disease: A review. Aging Clin. Exp. Res. 2012, 24. [Google Scholar] [CrossRef]
- Bossù, P.; Salani, F.; Ciaramella, A.; Sacchinelli, E.; Mosca, A.; Banaj, N.; Assogna, F.; Orfei, M.D.; Caltagirone, C.; Gianni, W.; et al. Anti-inflammatory Effects of Homotaurine in Patients With Amnestic Mild Cognitive Impairment. Front. Aging Neurosci. 2018, 10, 285. [Google Scholar] [CrossRef]
- Russo, R.; Adornetto, A.; Cavaliere, F.; Varano, G.P.; Rusciano, D.; Morrone, L.A.; Corasaniti, M.T.; Bagetta, G.; Nucci, C. Intravitreal injection of forskolin, homotaurine, and L-carnosine affords neuroprotection to retinal ganglion cells following retinal ischemic injury. Mol. Vis. 2015, 21, 718–729. [Google Scholar]
- Rolle, T.; Dallorto, L.; Rossatto, S.; Curto, D.; Nuzzi, R. Assessing the Performance of Daily Intake of a Homotaurine, Carnosine, Forskolin, Vitamin B2, Vitamin B6, and Magnesium Based Food Supplement for the Maintenance of Visual Function in Patients with Primary Open Angle Glaucoma. J. Ophthalmol. 2020, 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Acar, S.E.; Sarıcaoğlu, M.S.; Çolak, A.; Aktaş, Z.; Dinçel, A.S. Neuroprotective effects of topical coenzyme Q10 + vitamin E in mechanic optic nerve injury model. Eur. J. Ophthalmol 2019, 30, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Ko, M.-L.; Peng, P.-H.; Hsu, S.-Y.; Chen, C.-F. Dietary Deficiency of Vitamin E Aggravates Retinal Ganglion Cell Death in Experimental Glaucoma of Rats. Curr. Eye Res. 2010, 35, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Cellini, M.; Caramazza, N.; Mangiafico, P.; Possati, G.L.; Caramazza, R. Fatty acid use in glaucomatous optic neuropathy treatment. Acta Ophthalmol. Scand. Suppl. 1998, 76, 41–42. [Google Scholar] [CrossRef]
- Costagliola, C.; Libondi, T.; Menzione, M.; Rinaldi, E.; Auricchio, G. Vitamin E and red blood cell glutathione. Metabolism 1985, 34, 712–714. [Google Scholar] [CrossRef]
- Bartollino, S.; Palazzo, M.; Semeraro, F.; Parolini, B.; Caruso, C.; Merolla, F.; Guerra, G.; Costagliola, C. Effects of an antioxidant protective topical formulation on retinal tissue of UV-exposed rabbits. Int. Ophthalmol. 2020, 40, 925–933. [Google Scholar] [CrossRef]
- Sun, Y.; Erdem, E.; Lyu, A.; Zangalli, C.; Wizov, S.S.; Lo, D.; E Spaeth, E.; Richman, J.; Spaeth, G.L. The SPARCS: A novel assessment of contrast sensitivity and its reliability in patients with corrected refractive error. Br. J. Ophthalmol. 2016, 100, 1421–1426. [Google Scholar] [CrossRef]
- Gupta, L.; Cvintal, V.; Delvadia, R.; Sun, Y.; Erdem, E.; Zangalli, C.; Lu, L.; Wizov, S.S.; Richman, J.; Spaeth, E.; et al. SPARCS and Pelli–Robson contrast sensitivity testing in normal controls and patients with cataract. Eye 2017, 31, 753–761. [Google Scholar] [CrossRef]
- Richman, J.; Spaeth, G.L.; Wirostko, B. Contrast sensitivity basics and a critique of currently available tests. J. Cataract. Refract. Surg. 2013, 39, 1100–1106. [Google Scholar] [CrossRef]
- Richman, J.; Zangalli, C.; Lu, L.; Wizov, S.S.; Spaeth, E.; Spaeth, G.L. The Spaeth/Richman contrast sensitivity test (SPARCS): Design, reproducibility and ability to identify patients with glaucoma. Br. J. Ophthalmol. 2015, 99, 16–20. [Google Scholar] [CrossRef]
- Nelson, P.; Aspinall, P.; Papasouliotis, O.; Worton, B.; O’Brien, C. Quality of Life in Glaucoma and Its Relationship with Visual Function. J. Glaucoma 2003, 12, 139–150. [Google Scholar] [CrossRef]
- Rossi, G.C.M. Age and Gender Influence Reaction to Glaucoma Diagnosis. Eur. Ophthalmic Rev. 2018, 12, 85–87. [Google Scholar] [CrossRef]
- Floriani, I.; Quaranta, L.; Rulli, E.; Katsanos, A.; Varano, L.; Frezzotti, P.; Rossi, G.C.M.; Carmassi, L.; Rolle, T.; Ratiglia, R.; et al. Health-related quality of life in patients with primary open-angle glaucoma. An Italian multicentre observational study. Acta Ophthalmol. 2016, 94, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Ang, G.S.; Fenwick, E.K.; Constantinou, M.; Gan, A.T.L.; Man, R.E.K.; Casson, R.J.; A Finkelstein, E.; Goldberg, I.; Healey, P.R.; Pesudovs, K.; et al. Selective laser trabeculoplasty versus topical medication as initial glaucoma treatment: The glaucoma initial treatment study randomised clinical trial. Br. J. Ophthalmol. 2020, 104, 813–821. [Google Scholar] [CrossRef] [PubMed]
- King, A.J.; Fernie, G.; Azuara-Blanco, A.; Burr, J.M.; Garway-Heath, T.; Sparrow, J.M.; Vale, L.; Hudson, J.; MacLennan, G.; McDonald, A.M.; et al. Treatment of Advanced Glaucoma Study: A multicentre randomised controlled trial comparing primary medical treatment with primary trabeculectomy for people with newly diagnosed advanced glaucoma—study protocol. Br. J. Ophthalmol. 2018, 102, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Stewart, W.C.; Stewart, J.A.; Nelson, L.A. Ocular Surface Disease in Patients with Ocular Hypertension and Glaucoma. Curr. Eye Res. 2011, 36, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.C.M.; Pasinetti, G.M.; Scudeller, L.; Bianchi, P.E. Ocular Surface Disease and Glaucoma: How to Evaluate Impact on Quality of Life. J. Ocul. Pharmacol. Ther. 2013, 29, 390–394. [Google Scholar] [CrossRef]
- Ekici, F.; Loh, R.; Waisbourd, M.; Sun, Y.; Martinez, P.; Nayak, N.; Wizov, S.S.; Hegarty, S.; Hark, L.A.; Spaeth, G.L. Relationships Between Measures of the Ability to Perform Vision-Related Activities, Vision-Related Quality of Life, and Clinical Findings in Patients With Glaucoma. JAMA Ophthalmol. 2015, 133, 1377–1385. [Google Scholar] [CrossRef]
- Fresina, M.; Dickmann, A.; Salerni, A.; De Gregorio, F.; Campos, E.C. Effect of oral CDP-choline on visual function in young amblyopic patients. Graefe’s Arch. Clin. Exp. Ophthalmol. 2007, 246, 143–150. [Google Scholar] [CrossRef]
- Rejdak, R.; Toczołowski, J.; Solski, J.; Duma, D.; Grieb, P. Citicoline Treatment Increases Retinal Dopamine Content in Rabbits. Ophthalmic Res. 2002, 34, 146–149. [Google Scholar] [CrossRef]
- Spalletta, G.; Cravello, L.; Gianni, W.; Piras, F.; Eiorio, M.; Ecacciari, C.; Casini, A.R.; Echiapponi, C.; Sancesario, G.; Fratangeli, C.; et al. Homotaurine Effects on Hippocampal Volume Loss and Episodic Memory in Amnestic Mild Cognitive Impairment. J. Alzheimer’s Dis. 2016, 50, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Chiosi, F.; Di Marco, R.; Costagliola, C.; Scapagnini, G. Cytoprotective Effects of Citicoline and Homotaurine against Glutamate and High Glucose Neurotoxicity in Primary Cultured Retinal Cells. Oxidative Med. Cell. Longev. 2017, 2017, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Srivastava, A.; Sihota, R.; Kaur, J. Evaluation of Oxidative Stress Markers in Aqueous Humor of Primary Open Angle Glaucoma and Primary Angle Closure Glaucoma Patients. Curr. Eye Res. 2014, 39, 823–829. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| Parameter | n (%) |
|---|---|
| Sex | |
| Female | 57 (52.29) |
| Male | 52 (47.61) |
| Years since diagnosis of glaucoma * (years) | 7 (1 to 19) |
| Current topical therapy | |
| Monotherapy | 72 (66.06) |
| Multitherapy | 37 (33.94) |
| Number of Intraocular Pressure (IOP) lowering eye-drop bottles per day | |
| One bottle | 88 (80.73) |
| Two bottles | 20 (18.35) |
| Three bottles | 1 (0.92) |
| Type of current therapy | |
| Timolol | 31 (28.44) |
| Carbonic Anhydrase Inhibitors (CAI) | 3 (2.75) |
| Prostaglandin (PG) | 36 (33.02) |
| Timolol + PG | 21 (19.26) |
| Timolol + CAI | 5 (4,59) |
| CAI + PG | 3 (2.75) |
| Timolol + CAI + PG | 10 (9.17) |
| Contrast sensitivity (SPARCS) * (score) | 80 (35 to 95) |
| Quality of life (GQL-15) * (score) | 28 (15 to 69) |
| Best Corrected Visual Acuity (BCVA) * (decimals) | 1.0 (0.5 to 1.0) |
| Intraocular pressure (IOP) * (mmHg) | 16 (10 to 25) |
| Visual field * (mean deviation) | −1.72 (−19.00 to 3.18) |
| Parameter | Group 1 | Group 2 | p |
|---|---|---|---|
| Age (years) | 66.5 (45 to 79) | 68.0 (48 to 85) | 0.57 |
| Sex | 0.051 | ||
| Female | 18 (40.91) | 39 (60.00) | |
| Male | 26 (59.09) | 26 (40.00) | |
| Years since diagnosis of glaucoma (years) | 5.5 (1 to 19) | 7.0 (1 to 19) | 0.41 |
| Current topical therapy | 0.74 | ||
| Monotherapy | 28 (63.64) | 43 (66.15) | |
| Multitherapy | 16 (36.36) | 22 (33.85) | |
| Number of IOP lowering eye-drop bottles per day | 1.00 | ||
| One bottle | 37 (84.09) | 52 (80.00) | |
| Two bottles | 7 (15.90) | 12 (18.46) | |
| Three bottles | 0 (0.00) | 1 (1.54) | |
| Type of current therapy | 0.99 | ||
| Timolol | 12 (27.27) | 19 (29.23) | |
| Carbonic Anhydrase Inhibitors (CAI) | 1 (2.27) | 2 (3.08) | |
| Prostaglandin (PG) | 15 (34.09) | 22 (33.84) | |
| Timolol + CAI | 2 (4.55) | 3 (4.61) | |
| Timolol + PG | 9 (20.45) | 11 (16.92) | |
| CAI + PG | 1 (2.27) | 2 (3.08) | |
| Timolol + CAI + PG | 4 (9.09) | 6 (9.22) | |
| Contrast sensitivity (SPARCS) (score) | 82 (35 to 95) | 79 (49 to 95) | 0.06 |
| Quality of life (GQL-15) (score) | 29 (15 to 69) | 28 (15 to 61) | 0.17 |
| Best Corrected Visual Acuity (BCVA) (decimals) | 1.0 (0.6 to 1.0) | 1.0 (0.5 to 1.0) | 0.21 |
| Intraocular pressure (IOP) (mmHg) | 16 (10 to 25) | 16 (11 to 22) | 0.41 |
| Visual field (mean deviation) | −1.53 (−13.96 to 3.18) | −1.84 (−19.00 to 1.94) | 0.15 |
| Parameter | Beta ± SE | Beta ± SE | p |
|---|---|---|---|
| Quality of life (GQL-15) (score) | |||
| Group 1 vs. Group 2 | 30.75 ± 0.94 | 28.62 ± 0.94 | <0.0001 |
| Group 1/Group 2 vs. Group 2/Group 1 | 30.64 ± 1.46 | 28.72 ± 1.08 | 0.29 |
| Contrast sensitivity (SPARCS) (score) | |||
| Group 1 vs. Group 2 | 79.14 ± 0.78 | 83.05 ± 0.79 | 0.0004 |
| Group 1/Group 2 vs. Group 2/Group 1 | 80.58 ± 0.68 | 81.61 ± 0.93 | 0.19 |
| Visual field (mean deviation) | |||
| Group 1 vs. Group 2 | −2.59 ± 0.42 | −2.72 ± 0.42 | 0.55 |
| Group 1/Group 2 vs. Group 2/Group 1 | −2.18 ± 0.66 | −3.13 ± 0.48 | 0.24 |
| Side Effects | n * (%) | n ** (%) |
|---|---|---|
| Anxiety | 3 (15.79) | 3 (2.75) |
| Headache | 2 (10.53) | 2 (1.83) |
| Sleep disorders | 6 (31.58) | 6 (5.50) |
| Irritability | 4 (21.05) | 4 (3.67) |
| Slight weight gain | 1 (5.26) | 1 (0.92) |
| Nausea | 2 (10.53) | 2 (1.83) |
| Tachycardia | 1 (5.26) | 1 (0.92) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marino, P.F.; Rossi, G.C.M.; Campagna, G.; Capobianco, D.; Costagliola, C.; on behalf of QUALICOS Study Group. Effects of Citicoline, Homotaurine, and Vitamin E on Contrast Sensitivity and Visual-Related Quality of Life in Patients with Primary Open-Angle Glaucoma: A Preliminary Study. Molecules 2020, 25, 5614. https://doi.org/10.3390/molecules25235614
Marino PF, Rossi GCM, Campagna G, Capobianco D, Costagliola C, on behalf of QUALICOS Study Group. Effects of Citicoline, Homotaurine, and Vitamin E on Contrast Sensitivity and Visual-Related Quality of Life in Patients with Primary Open-Angle Glaucoma: A Preliminary Study. Molecules. 2020; 25(23):5614. https://doi.org/10.3390/molecules25235614
Chicago/Turabian StyleMarino, Pier Franco, Gemma Caterina Maria Rossi, Giuseppe Campagna, Decio Capobianco, Ciro Costagliola, and on behalf of QUALICOS Study Group. 2020. "Effects of Citicoline, Homotaurine, and Vitamin E on Contrast Sensitivity and Visual-Related Quality of Life in Patients with Primary Open-Angle Glaucoma: A Preliminary Study" Molecules 25, no. 23: 5614. https://doi.org/10.3390/molecules25235614
APA StyleMarino, P. F., Rossi, G. C. M., Campagna, G., Capobianco, D., Costagliola, C., & on behalf of QUALICOS Study Group. (2020). Effects of Citicoline, Homotaurine, and Vitamin E on Contrast Sensitivity and Visual-Related Quality of Life in Patients with Primary Open-Angle Glaucoma: A Preliminary Study. Molecules, 25(23), 5614. https://doi.org/10.3390/molecules25235614







