Fiber-Coupled Quartz-Enhanced Photoacoustic Spectroscopy System for Methane and Ethane Monitoring in the Near-Infrared Spectral Range
Abstract
1. Introduction
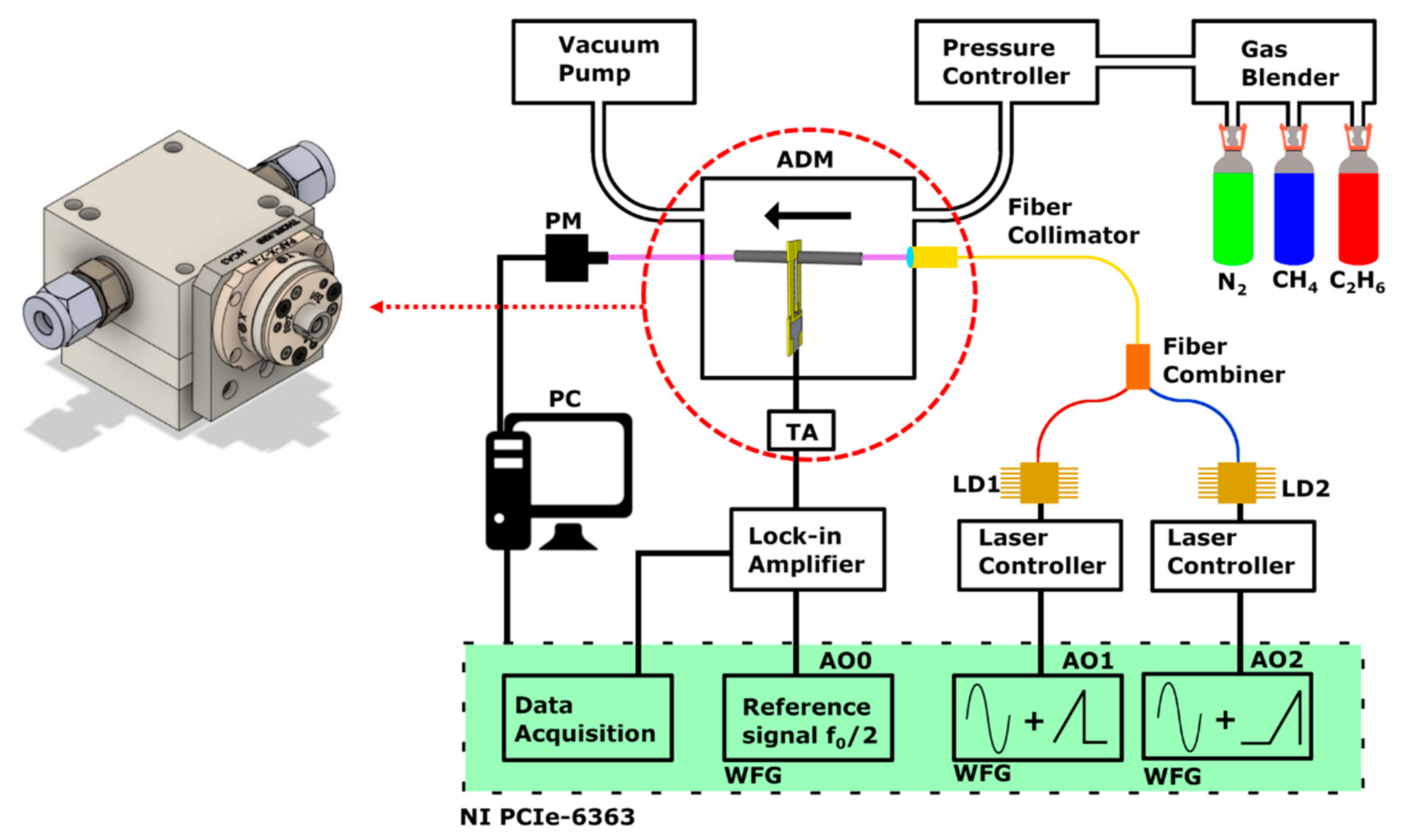
2. Fiber-Based, Quartz-Enhanced Photoacoustic Sensor Architecture
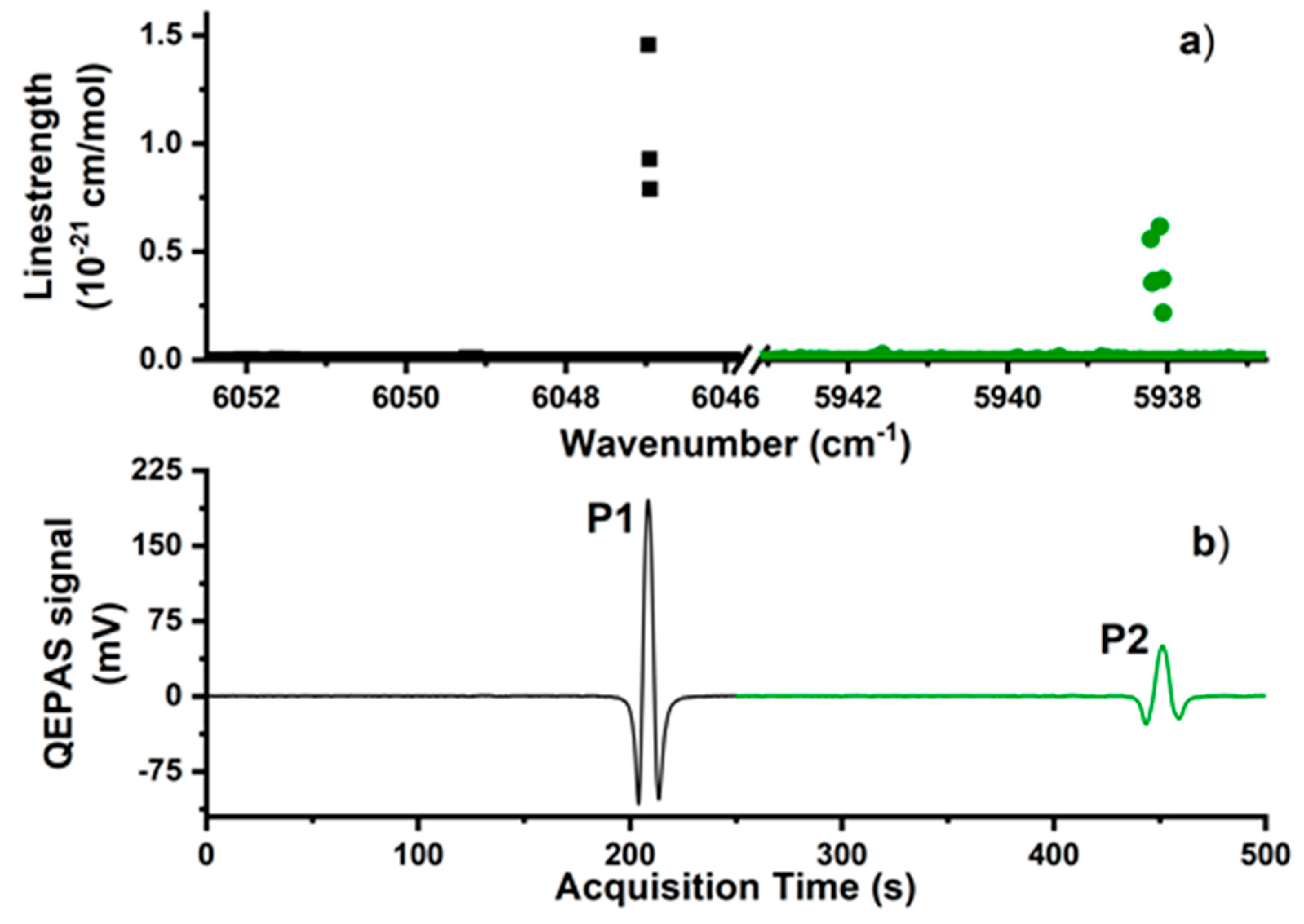
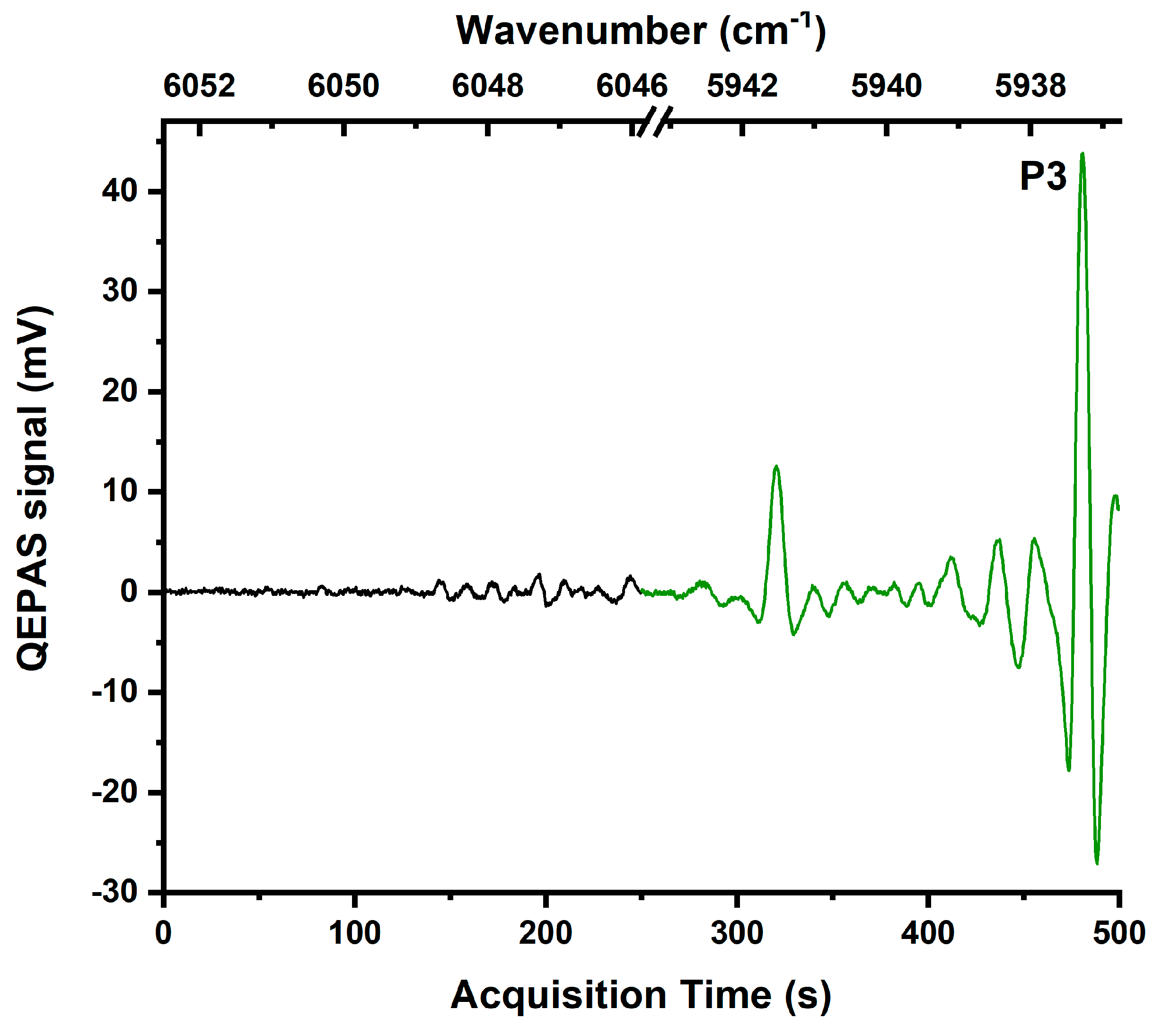
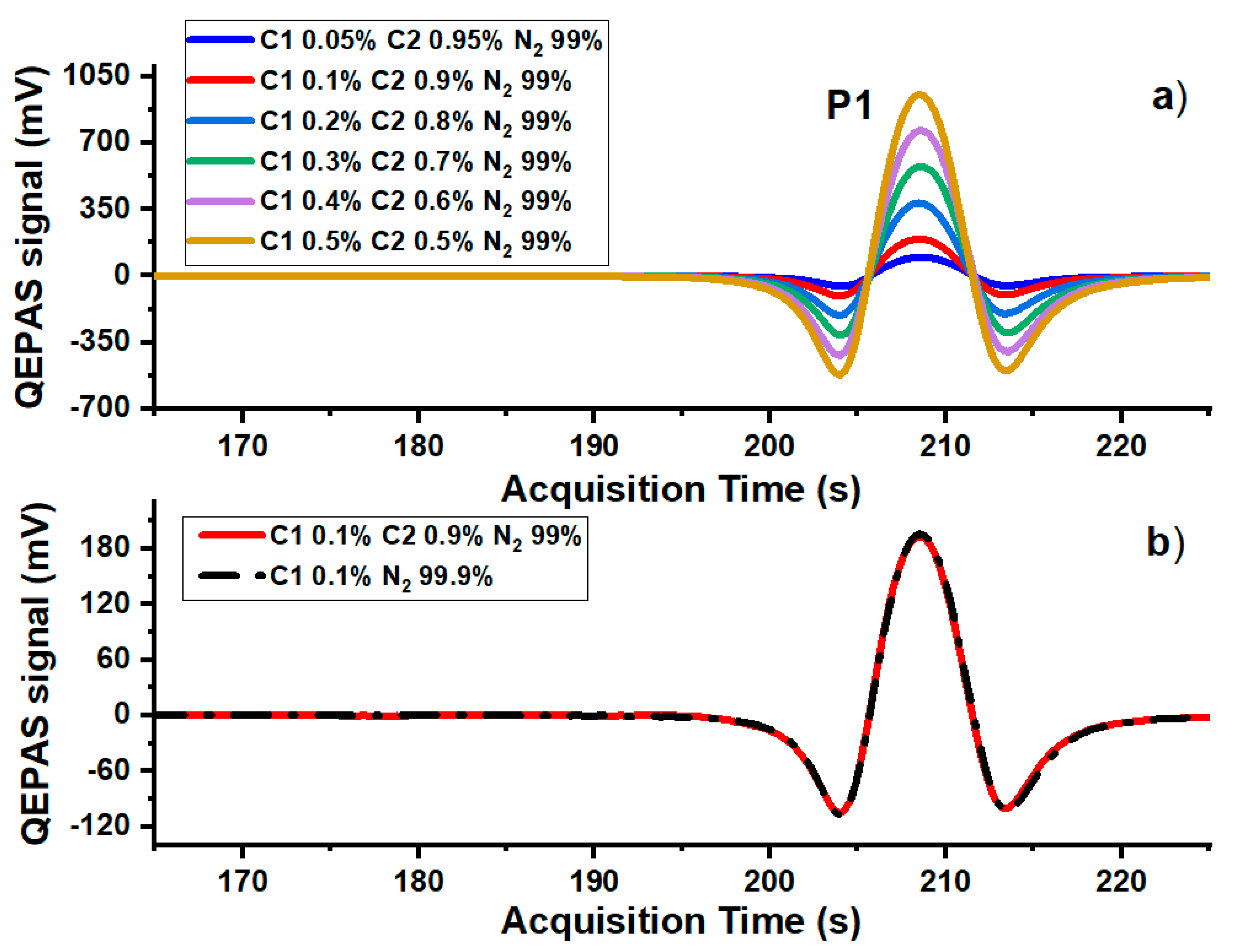
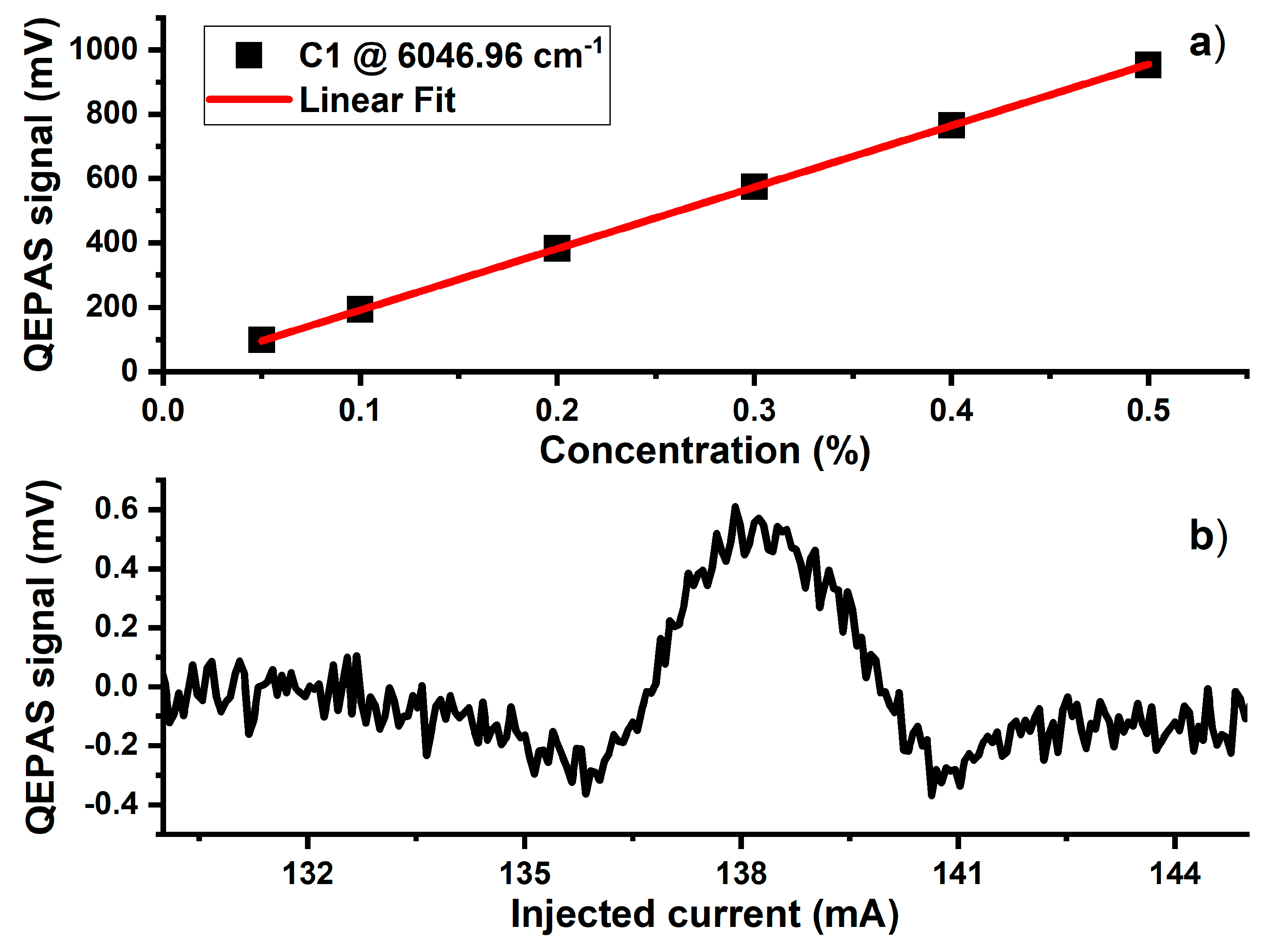
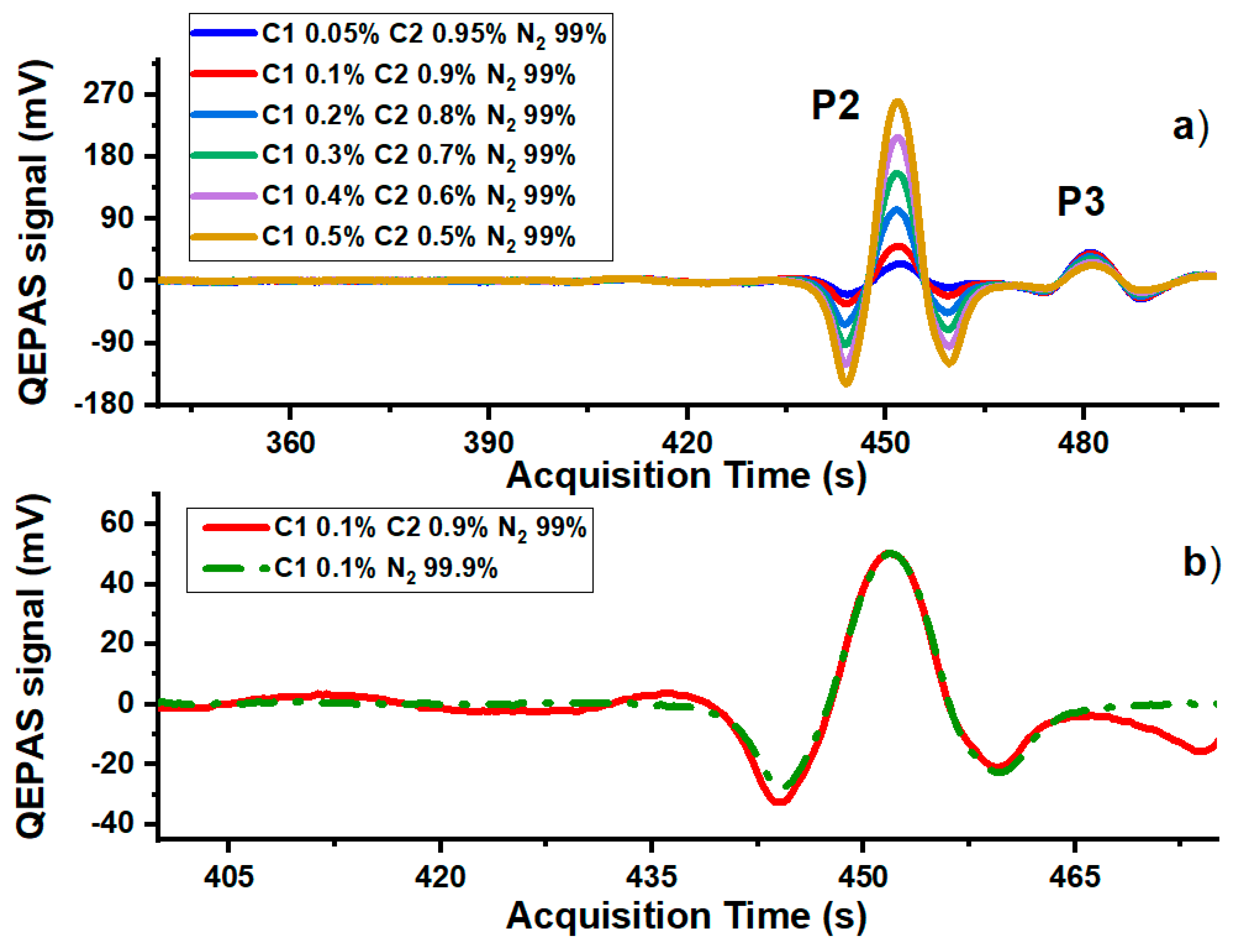
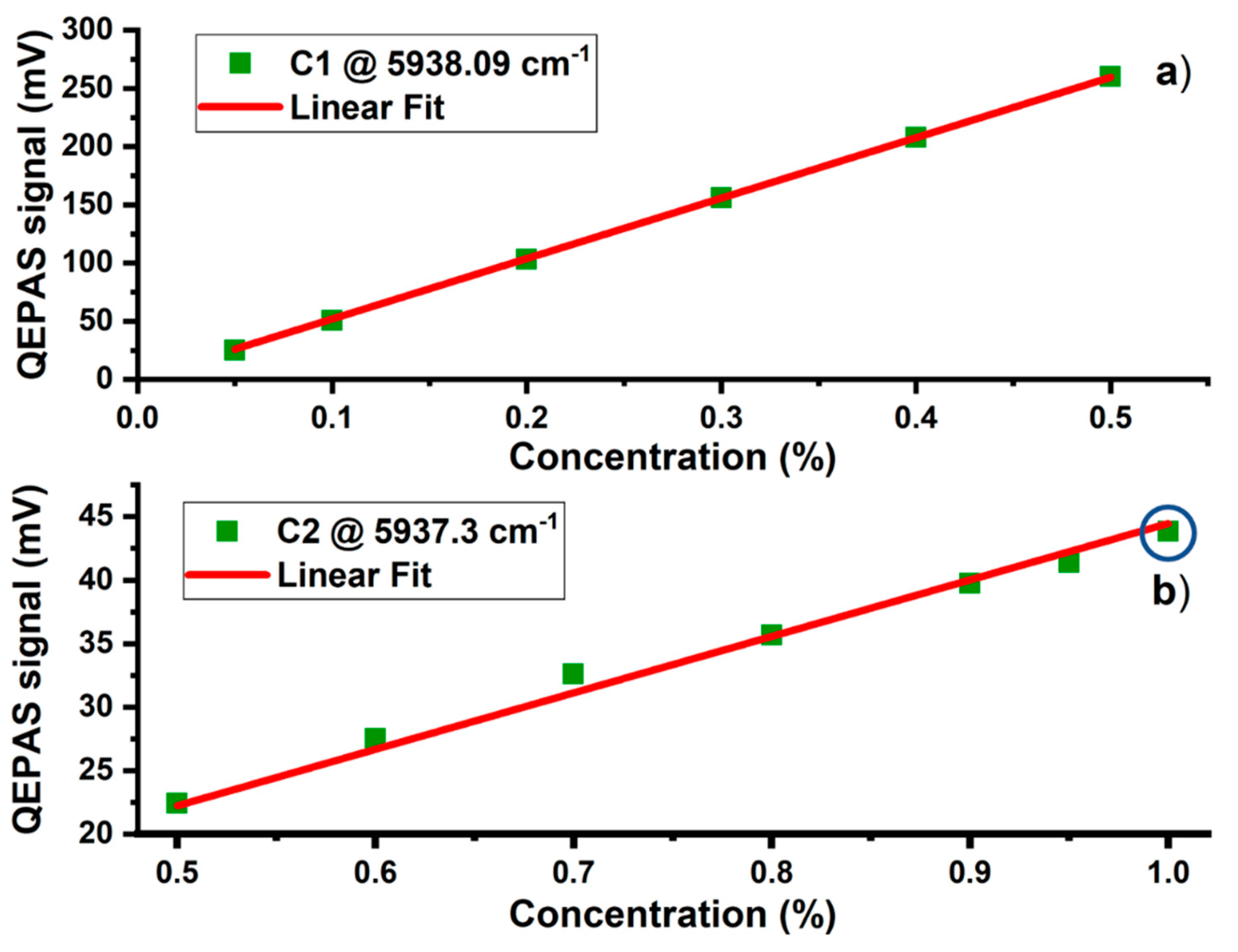
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADM | Acoustic Detection Module |
| C1 | Methane |
| C2 | Ethane |
| LD1 | Laser Diode 1 |
| LD2 | Laser Diode 2 |
| Near-IR | Near-InFrared |
| QEPAS | Quartz Enhanced PhotoAcoustic Spectroscopy |
| QTF | Quartz Tuning Fork |
| T-QTF | T-shaped QTF |
| UAV | Unmanned Aerial Vehicle |
References
- Moore, C.W.; Zielinska, B.; Pétron, G.; Jackson, R.B. Air impacts of increased natural gas acquisition, processing, and use: A critical review. Environ. Sci. Technol. 2014, 48, 8349–8359. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.T.; Torres, V.M.; Thomas, J.; Sullivan, D.W.; Harrison, M.; Hendler, A.; Herndon, S.C.; Kolb, C.E.; Fraser, M.P.; Hill, A.D.; et al. Measurements of methane emissions at natural gas production sites in the United States. Proc. Natl. Acad. Sci. USA 2013, 110, 17768–17773. [Google Scholar] [CrossRef] [PubMed]
- Yacovitch, T.I.; Herndon, S.C.; Roscioli, J.R.; Floerchinger, C.; McGovern, R.M.; Agnese, M.; Pétron, G.; Kofler, J.; Sweeney, C.; Karion, A.; et al. Demonstration of an ethane spectrometer for methane source identification. Environ. Sci. Technol. 2014, 48, 8028–8034. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.L.; Kort, E.A.; Karion, A.; Sweeney, C.; Herndon, S.C.; Yacovitch, T.I. Airborne Ethane Observations in the Barnett Shale: Quantification of Ethane Flux and Attribution of Methane Emissions. Environ. Sci. Technol. 2015, 49, 8158–8166. [Google Scholar] [CrossRef]
- Faramawy, S.; Zaki, T.; Sakr, A.A.-E. Natural gas origin, composition, and processing: A review. J. Nat. Gas. Sci. Eng. 2016, 34, 34–54. [Google Scholar] [CrossRef]
- Schwietzke, S.; Griffin, W.M.; Matthews, H.S.; Bruhwiler, L.M.P. Natural gas fugitive emissions rates constrained by global atmospheric methane and ethane. Environ. Sci. Technol. 2014, 48, 7714–7722. [Google Scholar] [CrossRef]
- Bertschi, I.; Yokelson, R.J.; Ward, D.E.; Babbitt, R.E.; Susott, R.A.; Goode, J.G.; Hao, W.M. Trace gas and particle emissions from fires in large diameter and belowground biomass fuels. J. Geophys. Res. Atmos. 2003, 108, 8472. [Google Scholar] [CrossRef]
- Simpson, I.J.; Rowland, F.S.; Meinardi, S.; Blake, D.R. Influence of biomass burning during recent fluctuations in the slow growth of global tropospheric methane. Geophys. Res. Lett. 2006, 33, 1–5. [Google Scholar] [CrossRef]
- Chen, X.; Tang, J.; Lao, S. Review of unmanned aerial vehicle swarm communication architectures and routing protocols. Appl. Sci. 2020, 10, 3661. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, S.; Liu, H.; Hu, S.; Zhang, D.; Ning, H. A survey on gas sensing technology. Sensors 2012, 12, 9635–9665. [Google Scholar] [CrossRef]
- Hodgkinson, J.; Tatam, R.P. Optical gas sensing: A review. Meas. Sci. Technol. 2012, 24, 012004. [Google Scholar] [CrossRef]
- Patimisco, P.; Sampaolo, A.; Dong, L.; Tittel, F.K.; Spagnolo, V. Recent advances in quartz enhanced photoacoustic sensing. Appl. Phys. Rev. 2018, 5, 011106. [Google Scholar] [CrossRef]
- Starecki, T.; Wieczorek, P.Z. A high sensitivity preamplifier for quartz tuning forks in QEPAS (Quartz enhanced photoacoustic spectroscopy) applications. Sensors 2017, 17, 2528. [Google Scholar] [CrossRef] [PubMed]
- Winkowski, M.; Stacewicz, T. Low noise, open-source QEPAS system with instrumentation amplifier. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Menduni, G.; Sampaolo, A.; Patimisco, P.; Giglio, M.; Dello Russo, S.; Zifarelli, A.; Elefante, A.; Wieczorek, P.Z.; Starecki, T.; Passaro, V.M.N.; et al. Front-end amplifiers for tuning forks in quartz enhanced photoacoustic spectroscopy. Appl. Sci. 2020, 10, 2947. [Google Scholar] [CrossRef]
- Sampaolo, A.; Csutak, S.; Patimisco, P.; Giglio, M.; Menduni, G.; Passaro, V.; Tittel, F.K.; Deffenbaugh, M.; Spagnolo, V. Methane, ethane and propane detection using a compact quartz enhanced photoacoustic sensor and a single interband cascade laser. Sens. Actuators B Chem. 2019, 282, 952–960. [Google Scholar] [CrossRef]
- Sampaolo, A.; Patimisco, P.; Giglio, M.; Chieco, L.; Scamarcio, G.; Tittel, F.K.; Spagnolo, V. Highly sensitive gas leak detector based on a quartz-enhanced photoacoustic SF6 sensor. Opt. Express 2016, 24, 15872. [Google Scholar] [CrossRef]
- Loh, A.; Wolff, M. Multivariate analysis of photoacoustic spectra for the detection of short-chained hydrocarbon isotopologues. Molecules 2020, 25, 2266. [Google Scholar] [CrossRef]
- Sampaolo, A.; Menduni, G.; Patimisco, P.; Giglio, M.; Passaro, V.M.N.; Dong, L.; Wu, H.; Tittel, F.K.; Spagnolo, V. Quartz-enhanced photoacoustic spectroscopy for hydrocarbon trace gas detection and petroleum exploration. Fuel 2020, 277, 118118. [Google Scholar] [CrossRef]
- Jones, C.M.; Freese, R.; Perkins, D.; Dai, B. Multivariate optical computing enables accurate harsh-environment sensing for the oil and gas industry. Laser Focus World 2014, 50, 27–31. [Google Scholar]
- Zifarelli, A.; Giglio, M.; Menduni, G.; Sampaolo, A.; Patimisco, P.; Passaro, V.M.N.; Wu, H.; Dong, L.; Spagnolo, V. Partial Least-Squares Regression as a Tool to Retrieve Gas Concentrations in Mixtures Detected Using Quartz-Enhanced Photoacoustic Spectroscopy. Anal. Chem. 2020, 92, 11035–11043. [Google Scholar] [CrossRef] [PubMed]
- Giglio, M.; Zifarelli, A.; Sampaolo, A.; Menduni, G.; Elefante, A.; Blanchard, R.; Pfluegl, C.; Witinski, M.F.; Vakhshoori, D.; Wu, H.; et al. Broadband detection of methane and nitrous oxide using a distributed-feedback quantum cascade laser array and quartz-enhanced photoacoustic sensing. Photoacoustics 2020, 17, 100159. [Google Scholar] [CrossRef] [PubMed]
- Bruhns, H.; Wolff, M.; Saalberg, Y.; Spohr, K.M. Quantitative Evaluation of Broadband Photoacoustic Spectroscopy in the Infrared with an Optical Parametric Oscillator. Sensors 2018, 18, 3971. [Google Scholar] [CrossRef] [PubMed]
- Giglio, M.; Elefante, A.; Patimisco, P.; Sampaolo, A.; Sgobba, F.; Rossmadl, H.; Mackowiak, V.; Wu, H.; Tittel, F.K.; Dong, L.; et al. Quartz-enhanced photoacoustic sensor for ethylene detection implementing optimized custom tuning fork-based spectrophone. Opt. Express 2019, 27, 4271–4280. [Google Scholar] [CrossRef] [PubMed]
- Rothman, L.S.; Gordon, I.E.; Babikov, Y.; Barbe, A.; Benner, D.C.; Bernath, P.F.; Birk, M.; Bizzocchi, L.; Boudon, V.; Brown, L.R.; et al. The HITRAN2012 molecular spectroscopic database. J. Quant. Spectrosc. Radiat. Transf. 2013, 130, 4–50. [Google Scholar] [CrossRef]
- Hepp, M.; Herman, M. Vibration-rotation bands in ethane. Mol. Phys. 2000, 98, 57–61. [Google Scholar] [CrossRef]
- Smith, L.G. The infra-red spectrum of C2H6. J. Chem. Phys. 1949, 17, 139–167. [Google Scholar] [CrossRef]
- Tian, X.; Cao, Y.; Chen, J.; Liu, K.; Wang, G.; Tan, T.; Mei, J.; Chen, W.; Gao, X. Dual-gas sensor of CH4 /C2H6 based on wavelength modulation spectroscopy coupled to a home-made compact dense-pattern multipass cell. Sensors 2019, 19, 820. [Google Scholar] [CrossRef]
- Sgobba, F.; Menduni, G.; Dello Russo, S.; Sampaolo, A.; Patimisco, P.; Giglio, M.; Ranieri, E.; Passaro, V.M.N.; Tittel, F.K.; Spagnolo, V. Quartz-enhanced photoacoustic detection of ethane in the near-IR exploiting a highly performant spectrophone. Appl. Sci. 2020, 10, 2447. [Google Scholar] [CrossRef]
- Patimisco, P.; Sampaolo, A.; Giglio, M.; Dello Russo, S.; Mackowiak, V.; Rossmadl, H.; Cable, A.; Tittel, F.K.; Spagnolo, V. Tuning forks with optimized geometries for quartz-enhanced photoacoustic spectroscopy. Opt. Express 2019, 27, 1401–1415. [Google Scholar] [CrossRef]
- Schilt, S.; Besson, J.-P.; Thévenaz, L. Near-infrared laser photoacoustic detection of methane: The impact of molecular relaxation. Appl. Phys. B Lasers Opt. 2006, 82, 319–328. [Google Scholar] [CrossRef]
- Elefante, A.; Menduni, G.; Rossmadl, H.; Mackowiak, V.; Giglio, M.; Sampaolo, A.; Patimisco, P.; Passaro, V.M.N.; Spagnolo, V. Environmental monitoring of methane with quartz-enhanced photoacoustic spectroscopy exploiting an electronic hygrometer to compensate the H2O influence on the sensor signal. Sensors 2020, 20, 2935. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Dong, L.; Yin, X.; Sampaolo, A.; Patimisco, P.; Ma, W.; Zhang, L.; Yin, W.; Xiao, L.; Spagnolo, V.; et al. Atmospheric CH4 measurement near a landfill using an ICL-based QEPAS sensor with V-T relaxation self-calibration. Sens. Actuators B Chem. 2019, 297, 126753. [Google Scholar] [CrossRef]
- Kosterev, A.A.; Bakhirkin, Y.A.; Tittel, F.K.; McWhorter, S.; Ashcraft, B. QEPAS methane sensor performance for humidified gases. Appl. Phys. B. 2008, 92, 103–109. [Google Scholar] [CrossRef]
- De Carlo, M.; Menduni, G.; Sampaolo, A.; De Leonardis, F.; Spagnolo, V.; Passaro, V.M.N. Modeling and Design of a Semi-Integrated QEPAS Sensor. J. Light. Technol. 2020. [Google Scholar] [CrossRef]
| Reaction | 1/τM-Mi (s−1) | Reference |
|---|---|---|
| CH4*(nν4) + CH4 → CH4*[(n−1)ν4] + CH4 | 8 × 105 | [31] |
| CH4*(nν4) + N2 → CH4*[(n−1)ν4] + N2 | 8 × 104 | [31] |
| CH4*(nν4) + O2 → CH4*[(n−1)ν4] + O2 | 1.3 × 105 | [31] |
| CH4*(nν4) + H2O → CH4*[(n−1)ν4] + H2O | 8.2 × 107 | [34] |
Sample Availability: Not available. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menduni, G.; Sgobba, F.; Russo, S.D.; Ranieri, A.C.; Sampaolo, A.; Patimisco, P.; Giglio, M.; Passaro, V.M.N.; Csutak, S.; Assante, D.; et al. Fiber-Coupled Quartz-Enhanced Photoacoustic Spectroscopy System for Methane and Ethane Monitoring in the Near-Infrared Spectral Range. Molecules 2020, 25, 5607. https://doi.org/10.3390/molecules25235607
Menduni G, Sgobba F, Russo SD, Ranieri AC, Sampaolo A, Patimisco P, Giglio M, Passaro VMN, Csutak S, Assante D, et al. Fiber-Coupled Quartz-Enhanced Photoacoustic Spectroscopy System for Methane and Ethane Monitoring in the Near-Infrared Spectral Range. Molecules. 2020; 25(23):5607. https://doi.org/10.3390/molecules25235607
Chicago/Turabian StyleMenduni, Giansergio, Fabrizio Sgobba, Stefano Dello Russo, Ada Cristina Ranieri, Angelo Sampaolo, Pietro Patimisco, Marilena Giglio, Vittorio M.N. Passaro, Sebastian Csutak, Dario Assante, and et al. 2020. "Fiber-Coupled Quartz-Enhanced Photoacoustic Spectroscopy System for Methane and Ethane Monitoring in the Near-Infrared Spectral Range" Molecules 25, no. 23: 5607. https://doi.org/10.3390/molecules25235607
APA StyleMenduni, G., Sgobba, F., Russo, S. D., Ranieri, A. C., Sampaolo, A., Patimisco, P., Giglio, M., Passaro, V. M. N., Csutak, S., Assante, D., Ranieri, E., Geoffrion, E., & Spagnolo, V. (2020). Fiber-Coupled Quartz-Enhanced Photoacoustic Spectroscopy System for Methane and Ethane Monitoring in the Near-Infrared Spectral Range. Molecules, 25(23), 5607. https://doi.org/10.3390/molecules25235607












