Preparation and Biological Evaluation of [99mTc]Tc-CNGU as a PSMA-Targeted Radiotracer for the Imaging of Prostate Cancer
Abstract
1. Introduction
2. Results
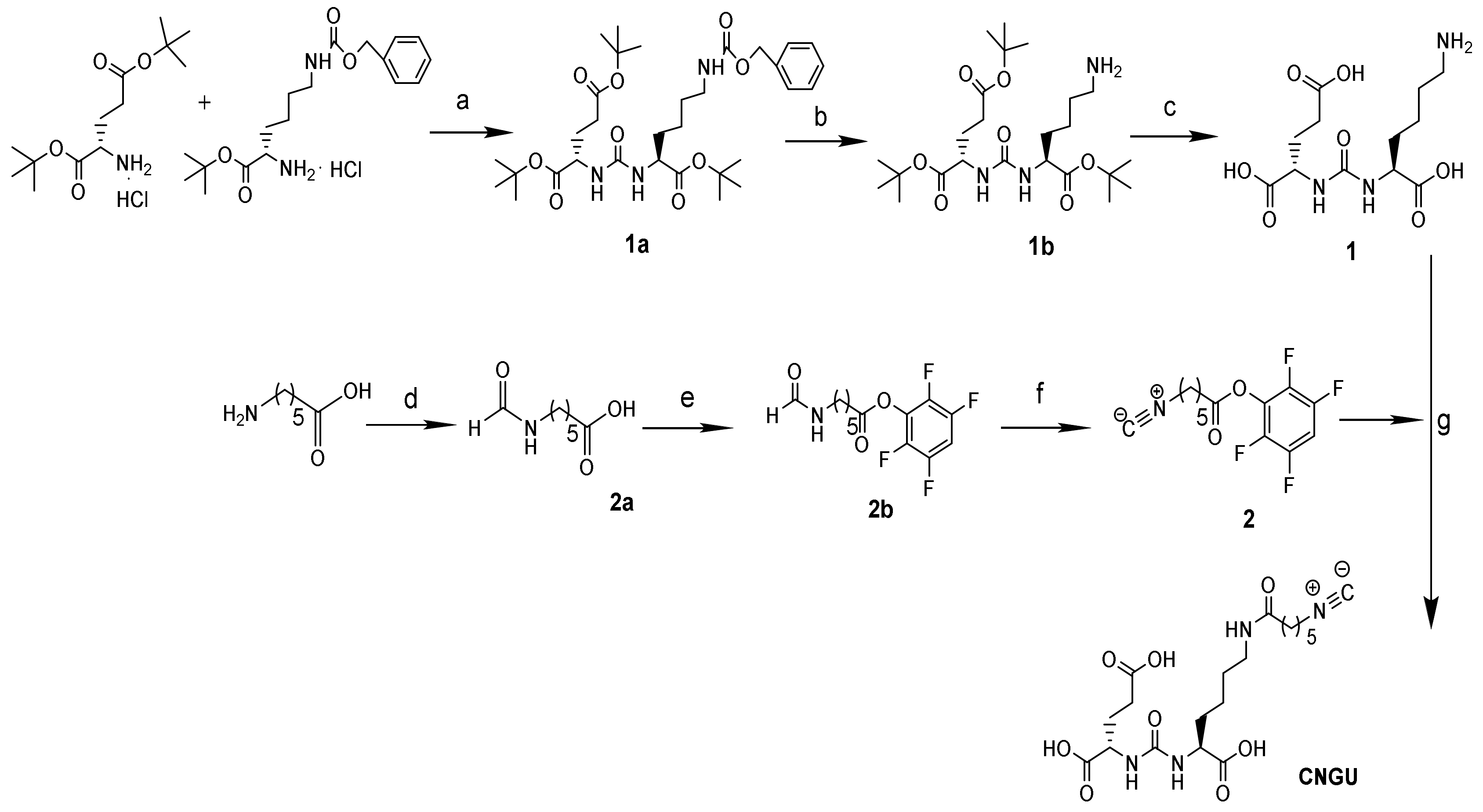
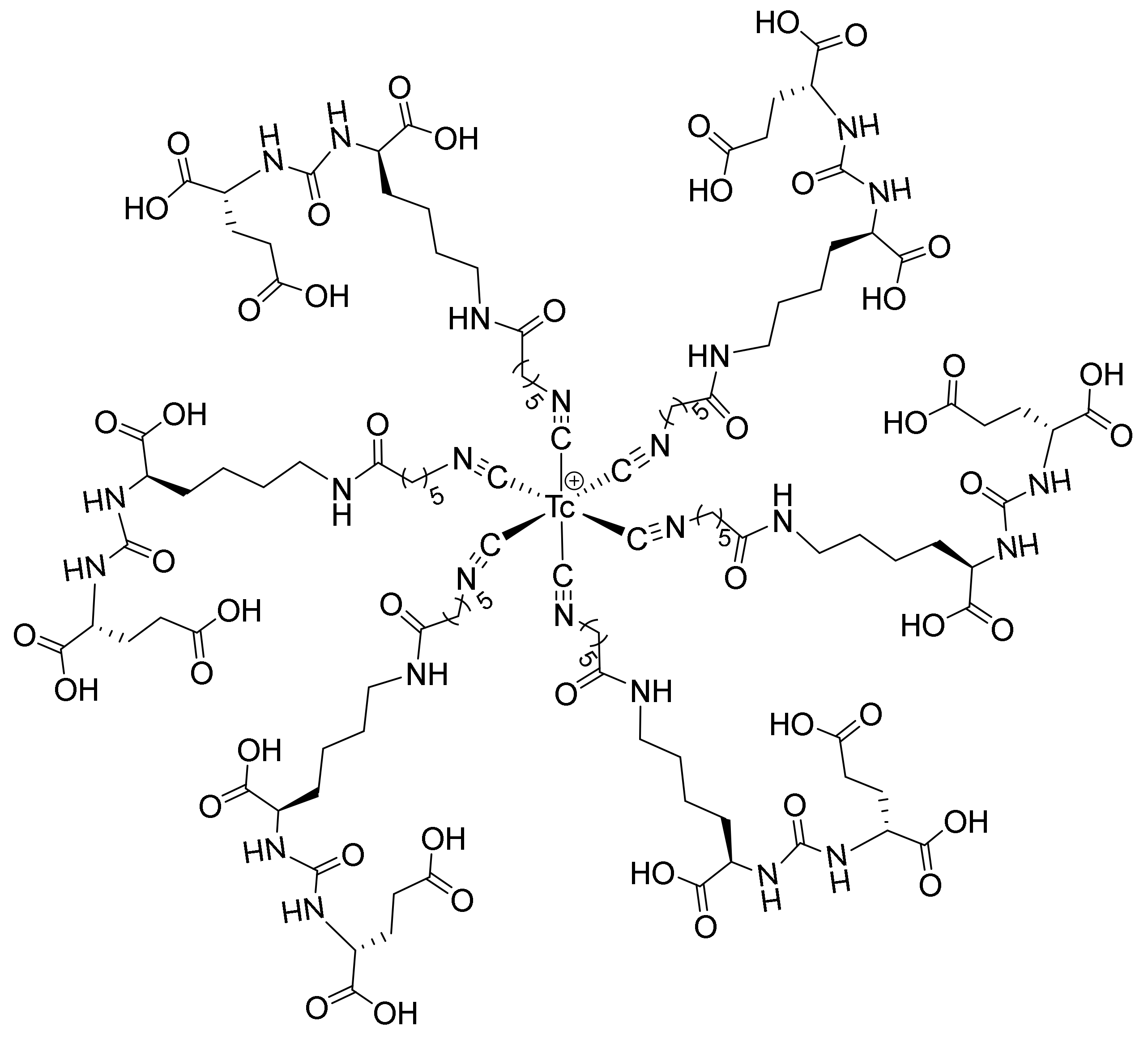
2.1. Chemistry and Radiolabeling
2.2. Stability and Partition Coefficients
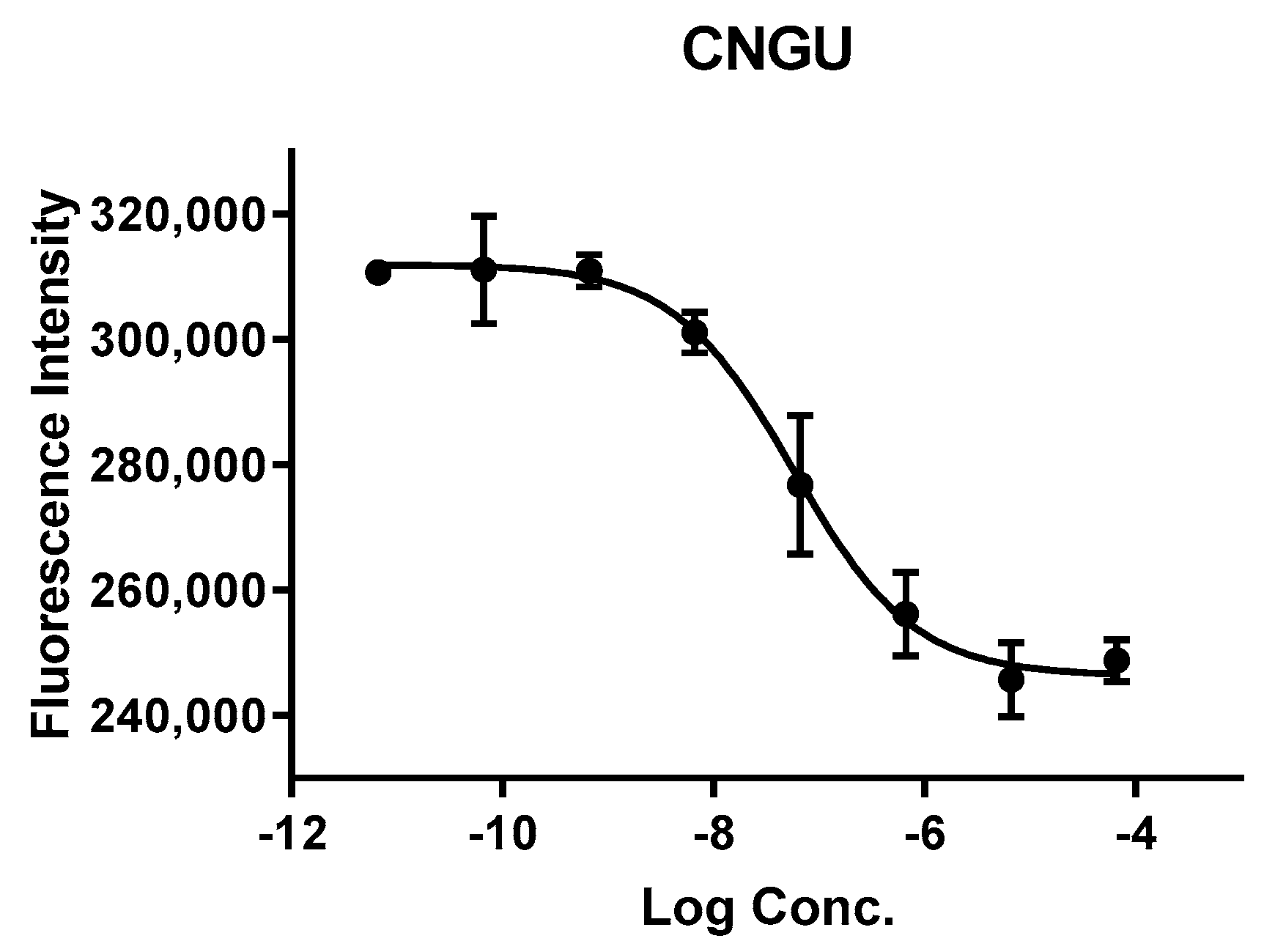
2.3. In Vitro Cell Experiments
2.4. Biodistribution Study
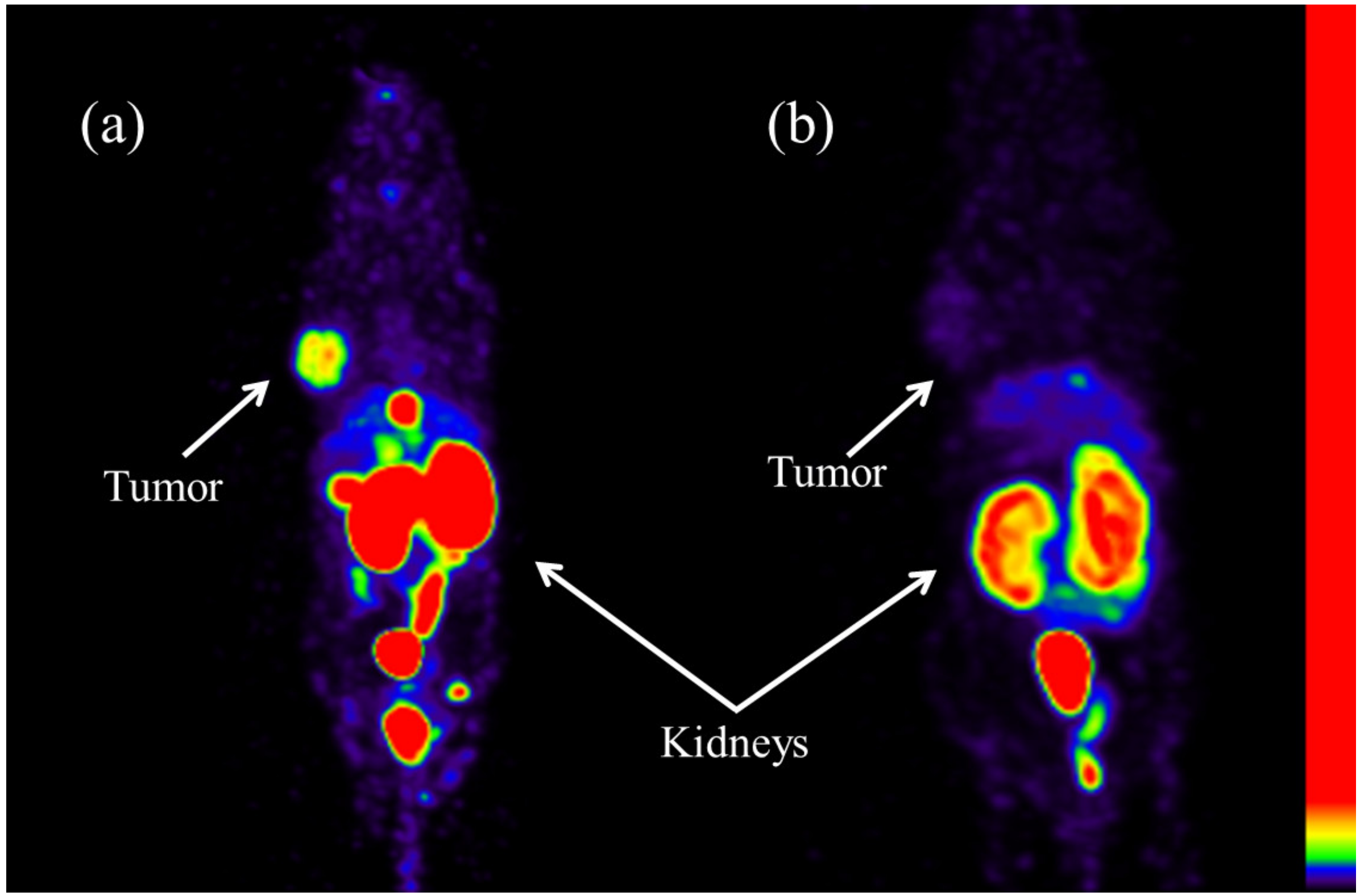
2.5. SPECT Imaging
3. Discussion
4. Materials and Methods
4.1. General Methods
4.2. Synthesis
4.3. Radiolabeling
4.4. Stability Studies
4.5. Determination of the Partition Coefficient
4.6. NAALADase Assay
4.7. Biodistribution Experiments
4.8. SPECT Imaging
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Vallabhajosula, S.; Nikolopoulou, A.; Babich, J.W.; Osborne, J.R.; Tagawa, S.T.; Lipai, I.; Solnes, L.; Maresca, K.P.; Armor, T.; Joyal, J.L.; et al. 99mTc-labeled small-molecule inhibitors of prostate-specific membrane antigen: Pharmacokinetics and biodistribution studies in healthy subjects and patients with metastatic prostate cancer. J. Nucl. Med. 2014, 55, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- Mosayebnia, M.; Hajimahdi, Z.; Beiki, D.; Rezaeianpourc, M.; Hajiramezanalid, M.; Geramifarb, P.; Sabzevarie, O.; Aminif, M.; Hatamabadia, D.; Shahhosseinig, S. Design, synthesis, radiolabeling and biological evaluation of new urea-based peptides targeting prostate specific membrane antigen. Bioorg. Chem. 2020, 99, 103743–103753. [Google Scholar] [CrossRef] [PubMed]
- Czerwinska, M.; Bilewicz, A.; Kruszewski, M.; Wegierek-Ciuk, A.; Lankoff, A. Targeted radionuclide therapy of prostate cancer—from basic research to clinical perspectives. Molecules 2020, 25, 1743. [Google Scholar] [CrossRef] [PubMed]
- Gourni, E.; Henriksen, G. Metal-based PSMA radioligands. Molecules 2017, 22, 523. [Google Scholar] [CrossRef]
- Bendre, S.; Zhang, Z.; Kuo, H.T.; Rousseau, J.; Zhang, C.; Merkens, H.; Roxin, Á.; Bénard, F.; Lin, K.S. Evaluation of Met-Val-Lys as a renal brush border enzyme-cleavable linker to reduce kidney uptake of 68Ga-labeled DOTA-conjugated peptides and peptidomimetics. Molecules 2020, 25, 3854. [Google Scholar] [CrossRef]
- Weineisen, M.; Schottelius, M.; Simecek, J.; Baum, R.P.; Yildiz, A.; Beykan, S.; Kulkarni, H.R.; Lassmann, M.; Klette, I.; Eiber, M.; et al. 68Ga- and 177Lu-labeled PSMA I&T: Optimization of a PSMA-targeted theranostic concept and first proof-of-concept human studies. J. Nucl. Med. 2015, 56, 1169–1176. [Google Scholar]
- Deberle, L.M.; Tschan, V.J.; Borgna, F.; Sozzi-Guo, F.; Bernhardt, P.; Schibli, R.; Müller, C. Albumin-binding PSMA radioligands: Impact of minimal structural changes on the tissue distribution profile. Molecules 2020, 25, 2542. [Google Scholar] [CrossRef]
- Banerjee, S.R.; Foss, C.A.; Castanares, M.; Mease, R.C.; Byun, Y.; Fox, J.J.; Hilton, J.; Lupold, S.E.; Kozikowski, A.P.; Pomper, M.G. Synthesis and evaluation of technetium-99m and rhenium-labeled inhibitors of the prostate-specific membrane antigen (PSMA). J. Med. Chem. 2008, 51, 4504–4517. [Google Scholar] [CrossRef]
- Duan, X.; Liu, F.; Kwon, H.; Byun, Y.; Minn, I.; Cai, X.; Zhang, J.; Pomper, M.G.; Yang, Z.; Xi, Z.; et al. (S)-3-(Carboxyformamido)-2-(3-(carboxymethyl)ureido) propanoic acid as a novel PSMA targeting scaffold for prostate cancer imaging. J. Med. Chem. 2020, 63, 3563–3576. [Google Scholar] [CrossRef]
- Frei, A.; Fischer, E.; Childs, B.C.; Holland, J.P.; Alberto, R. Two is better than one: Difunctional high-affinity PSMA probes based on a [CpM(CO)3] (M = Re/99mTc) scaffold. Dalton Trans. 2019, 48, 14600–14605. [Google Scholar] [CrossRef] [PubMed]
- Robu, S.; Schottelius, M.; Eiber, M.; Maurer, T.; Gschwend, J.; Schwaiger, M.; Wester, H.J. Preclinical evaluation and first patient application of Tc-99m-PSMA-I&S for SPECT imaging and radioguided surgery in prostate cancer. J. Nucl. Med. 2017, 58, 235–242. [Google Scholar] [PubMed]
- Vats, K.; Agrawal, K.; Sharma, R.; Sarma, H.D.; Satpati, D.; Dash, A. Preparation and clinical translation of 99mTc-PSMA-11 for SPECT imaging of prostate cancer. Med. Chem. Comm. 2019, 10, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Zia, N.A.; Cullinane, C.; Zuylekom, J.K.V.; McInnes, L.E.; Buncic, G.; Haskali, M.B.; Roselt, P.D.; Hicks, R.J.; Donnelly, P.S. A bivalent inhibitor of prostate specific membrane antigen radiolabeled with copper-64 with high tumor uptake and retention. Angew. Chem. Int. Ed. 2019, 58, 1–5. [Google Scholar] [CrossRef]
- Rahbar, K.; Bögeman, M.; Yordanova, A.; Eveslage, M.; Schäfers, M.; Essler, M.; Ahmadzadehfar, H. Delayed response after repeated 177Lu-PSMA-617 radioligand therapy in patients with metastatic castration resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 243–246. [Google Scholar] [CrossRef]
- Pedersen, K.S.; Baun, C.; Nielsen, K.M.; Thisgaard, H.; Jensen, A.I.; Zhuravlev, F. Design, synthesis, computational, and preclinical evaluation of natTi/45Ti-labeled urea-based glutamate PSMA ligand. Molecules 2020, 25, 1104. [Google Scholar] [CrossRef]
- Rauscher, I.; Maurer, T.; Souvatzoglou, M.; Beer, A.J.; Vag, T.; Wirtz, M.; Weirich, G.; Wester, H.; Gschwend, J.E.; Schwaiger, M.; et al. Intrapatient comparison of 111In-PSMA I&T SPECT/CT and hybrid 68Ga-HBED-CC PSMA PET in patients with early recurrent prostate cancer. Clin. Nucl. Med. 2016, 41, 397–402. [Google Scholar]
- Cardinale, J.; Roscher, M.; Schafer, M.; Geerlings, M.; Benesova, M.; Bauder-Wust, U.; Remde, Y.; Eder, M.; Novakova, Z.; Motlova, L.; et al. Development of PSMA-1007-related series of 18F-labeled GluUreido-type PSMA inhibitors. J. Med. Chem. 2020, 63, 10897–10907. [Google Scholar] [CrossRef]
- Liu, T.; Liu, C.; Xu, X.; Liu, F.; Guo, X.; Li, N.; Wang, X.; Yang, J.; Yang, X.; Zhu, H.; et al. Preclinical evaluation and pilot clinical study of Al18F-PSMA-BCH for prostate cancer imaging. J Nucl Med. 2019, 60, 1284–1292. [Google Scholar] [CrossRef]
- Lawal, I.O.; Mokoala, K.M.G.; Mahapane, J.; Kleyhans, J.; Meckel, M.; Vorster, M.; Ebenhan, T.; Rösch, F.; Sathekge, M.M. A prospective intra-individual comparison of [68Ga]Ga-PSMA-11 PET/CT, [68Ga]Ga-NODAGA ZOL PET/CT, and [99mTc]Tc-MDP bone scintigraphy for radionuclide imaging of prostate cancer skeletal metastases. Eur. J. Nucl. Med. Mol. Imaging 2020. [Google Scholar] [CrossRef]
- Wustemann, T.; Haberkorn, U.; Babich, J.; Mier, W. Targeting prostate cancer: Prostate-specific membrane antigen based diagnosis and therapy. Med. Res. Rev. 2019, 39, 40–69. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, J.; Hu, S.; He, S.; Bao, X.; Ma, G.; Luo, J.; Cheng, J.; Zhang, Y. 99mTc-labeling and evaluation of a HYNIC modified small-molecular inhibitor of prostate-specific membrane antigen. Nucl. Med. Biol. 2017, 48, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, J.; Xu, X.; Lu, L.; Hu, S.; Liu, C.; Cheng, J.; Song, S.; Zhang, Y.; Shi, L.Q. Evaluation of radiation dosimetry of 99mTc-HYNIC-PSMA and imaging in prostate cancer. Sci. Rep. 2020, 10, 4179–4187. [Google Scholar] [CrossRef]
- Ferro-Flores, G.; Luna-Gutiérrez, M.; Ocampo-García, B.; Santos-Cuevas, C.; Azorín-Vega, E.; Jiménez-Mancilla, N.; Orocio-Rodríguez, E.; Davanzo, J.; García-Pérez, F.O. Clinical translation of a PSMA inhibitor for 99mTc-based SPECT. Nucl. Med. Biol. 2017, 48, 36–44. [Google Scholar] [CrossRef]
- Santos-Cuevas, C.; Davanzo, J.; Ferro-Flores, G.; García-Pérez, F.O.; Ocampo-García, B.; Ignacio-Alvarez, E.; Gómez-Argumosa, E.; Pedraza-López, M. 99mTc-labeled PSMA inhibitor: Biokinetics and radiation dosimetry in healthy subjects and imaging of prostate cancer tumors in patients. Nucl. Med. Biol. 2017, 52, 1–6. [Google Scholar] [CrossRef]
- Hillier, S.M.; Maresca, K.P.; Lu, G.; Merkin, R.D.; Marquis, J.C.; Zimmerman, C.N.; Eckelman, W.C.; Joyal, J.L.; Babich, J.W. 99mTc-labeled small-molecule inhibitors of prostate-Specific membrane antigen for molecular imaging of prostate cancer. J. Nucl. Med. 2013, 54, 1369–1376. [Google Scholar] [CrossRef]
- Schmidkonz, C.; Hollweg, C.; Beck, M.; Reinfelder, J.; Goetz, T.I.; Sanders, J.C.; Schmidt, D.; Prante, O.; Bäuerle, T.; Cavallaro, A.; et al. 99mTc-MIP-1404-SPECT/CT for the detection of PSMA-positive lesions in 225 patients with biochemical recurrence of prostate cancer. Prostate 2018, 78, 54–63. [Google Scholar] [CrossRef]
- Mizuno, Y.; Uehara, T.; Hanaoka, H.; Endo, Y.; Jen, C.; Arano, Y. Purification-free method for preparing technetium-99m-labeled multivalent probes for enhanced in vivo imaging of saturable systems. J. Med. Chem. 2016, 59, 3331–3339. [Google Scholar] [CrossRef]
- Zhang, X.; Ruan, Q.; Duan, X.; Gan, Q.; Song, X.; Fang, S.; Lin, X.; Du, J.; Zhang, J. Novel 99mTc-labeled glucose derivative for single photon emission computed tomography: A promising tumor imaging agent. Mol. Pharmaceutics 2018, 15, 3417–3424. [Google Scholar] [CrossRef]
- Lodhi, N.A.; Park, J.Y.; Kim, K.; Hong, M.K.; Kim, Y.J.; Lee, Y.; Cheon, G.J.; Kang, K.W.; Jeong, J.M. Synthesis and evaluation of 99mTc-tricabonyl labeled isonitrile conjugates for prostate-specific membrane antigen (PSMA) image. Inorganics 2020, 8, 5–23. [Google Scholar] [CrossRef]
- Ruan, Q.; Zhang, X.; Zhang, J. Radiosynthesis and evaluation of novel [Tc-99m(I)]+ and [Tc-99m(I)(CO)3]+ complexes with a 4-nitroimidazole isocyanide for imaging tumor hypoxia. Appl. Organomet. Chem. 2020, 34, e5798. [Google Scholar] [CrossRef]
- Pathak, R.K.; Basu, U.; Ahmadd, A.; Sarkar, S.; Kumar, A.; Surnar, B.; Ansari, S.; Wilczek, K.; Ivan, M.E.; Marples, B.; et al. A designer bow-tie combination therapeutic platform: An approach to resistant cancer treatment by simultaneous delivery of cytotoxic and anti-inflammatory agents and radiation. Biomaterials 2018, 187, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Lindner, T.; Altmann, A.; Kräme, S.; Kleist, C.; Loktev, A.; Kratochwil, C.; Giesel, F.; Mier, W.; Marme, F.; Debus, J.; et al. Design and development of 99mTc-labeled FAPI-tracers for SPECT-imaging and 188Re therapy. J. Nucl. Med. 2020, 61, 1507–1513. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of all the compounds are available from the authors. |
| Complex | [99mTc]Tc- CNGU | 99mTc-MIP-1404 | 99mTc-EDDA/HYNIC- iPSMA | 99mTc-HYNIC- ALUG | [99mTc]Tc-16 |
|---|---|---|---|---|---|
| Cell | LNCaP | LNCaP | LNCaP | LNCaP | 22Rv1 |
| Animal | BALB/c | NCr nude | Athymic mice | SCID | BALB/c |
| Tumor | 4.86 ± 1.19 | 10.3 ± 2.5 | 10.22 ± 2.96 | 14.13 ± 2.95 | 1.87 ± 0.11 |
| Kidneys | 70.95 ± 12.28 | 105 ± 37 | 23.63 ± 3.56 | 197.50 ± 7.1 | 24.66 ± 2.17 |
| T/B | 2.89 | 79 | 62.33 | 11.78 | 4.43 ± 0.39 |
| T/M | 12.46 | 57 | 68.13 | 19.45 | 14.05 ± 1.78 |
| log P | −1.97 ± 0.03 | -- | -- | −2.68 ± 0.12 | −2.10 ± 0.03 |
| Reference | This study | [26] | [24] | [22] | [30] |
| Organs | 1 h | 1 h Blockade |
|---|---|---|
| Heart | 0.74 ± 0.15 | 0.46 ± 0.04 |
| Liver | 2.46 ± 0.72 | 1.48 ± 0.16 |
| Spleen | 5.84 ± 1.51 | 0.78 ± 0.19 * |
| Lung | 1.89 ± 0.30 | 1.47 ± 0.19 |
| Kidneys | 70.95 ± 12.28 | 19.85 ± 1.30 * |
| Stomach | 0.64 ± 0.21 | 0.44 ± 0.14 |
| Bone | 0.43 ± 0.11 | 0.40 ± 0.08 |
| Intestine | 2.16 ± 0.34 | 2.19 ± 0.37 |
| Pancreas | 0.86 ± 0.16 | 0.34 ± 0.04 |
| Muscle | 0.39 ± 0.06 | 0.24 ± 0.01 |
| Tumor | 4.86 ±1.19 | 1.74 ± 0.90 * |
| Blood | 1.68 ± 0.33 | 1.30 ± 0.10 |
| Thyroid (% ID) | 0.01 ± 0.00 | 0.01 ± 0.00 |
| Tumor/Blood | 2.89 | 1.34 |
| Tumor/Muscle | 12.46 | 7.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, D.; Duan, X.; Gan, Q.; Zhang, X.; Zhang, J. Preparation and Biological Evaluation of [99mTc]Tc-CNGU as a PSMA-Targeted Radiotracer for the Imaging of Prostate Cancer. Molecules 2020, 25, 5548. https://doi.org/10.3390/molecules25235548
Xiao D, Duan X, Gan Q, Zhang X, Zhang J. Preparation and Biological Evaluation of [99mTc]Tc-CNGU as a PSMA-Targeted Radiotracer for the Imaging of Prostate Cancer. Molecules. 2020; 25(23):5548. https://doi.org/10.3390/molecules25235548
Chicago/Turabian StyleXiao, Di, Xiaojiang Duan, Qianqian Gan, Xuran Zhang, and Junbo Zhang. 2020. "Preparation and Biological Evaluation of [99mTc]Tc-CNGU as a PSMA-Targeted Radiotracer for the Imaging of Prostate Cancer" Molecules 25, no. 23: 5548. https://doi.org/10.3390/molecules25235548
APA StyleXiao, D., Duan, X., Gan, Q., Zhang, X., & Zhang, J. (2020). Preparation and Biological Evaluation of [99mTc]Tc-CNGU as a PSMA-Targeted Radiotracer for the Imaging of Prostate Cancer. Molecules, 25(23), 5548. https://doi.org/10.3390/molecules25235548






