Biogas Potential of the Side Streams Obtained in a Novel Phenolic Extraction System from Olive Mill Solid Waste
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterisation of the Side Streams Obtained
2.2. Biogas Potential of the Side Streams Obtained
2.2.1. Biogas Potential and Kinetic Study
2.2.2. Effluent Characterisation
2.3. Potential Energy and Valuable Compounds Recovery
3. Materials and Methods
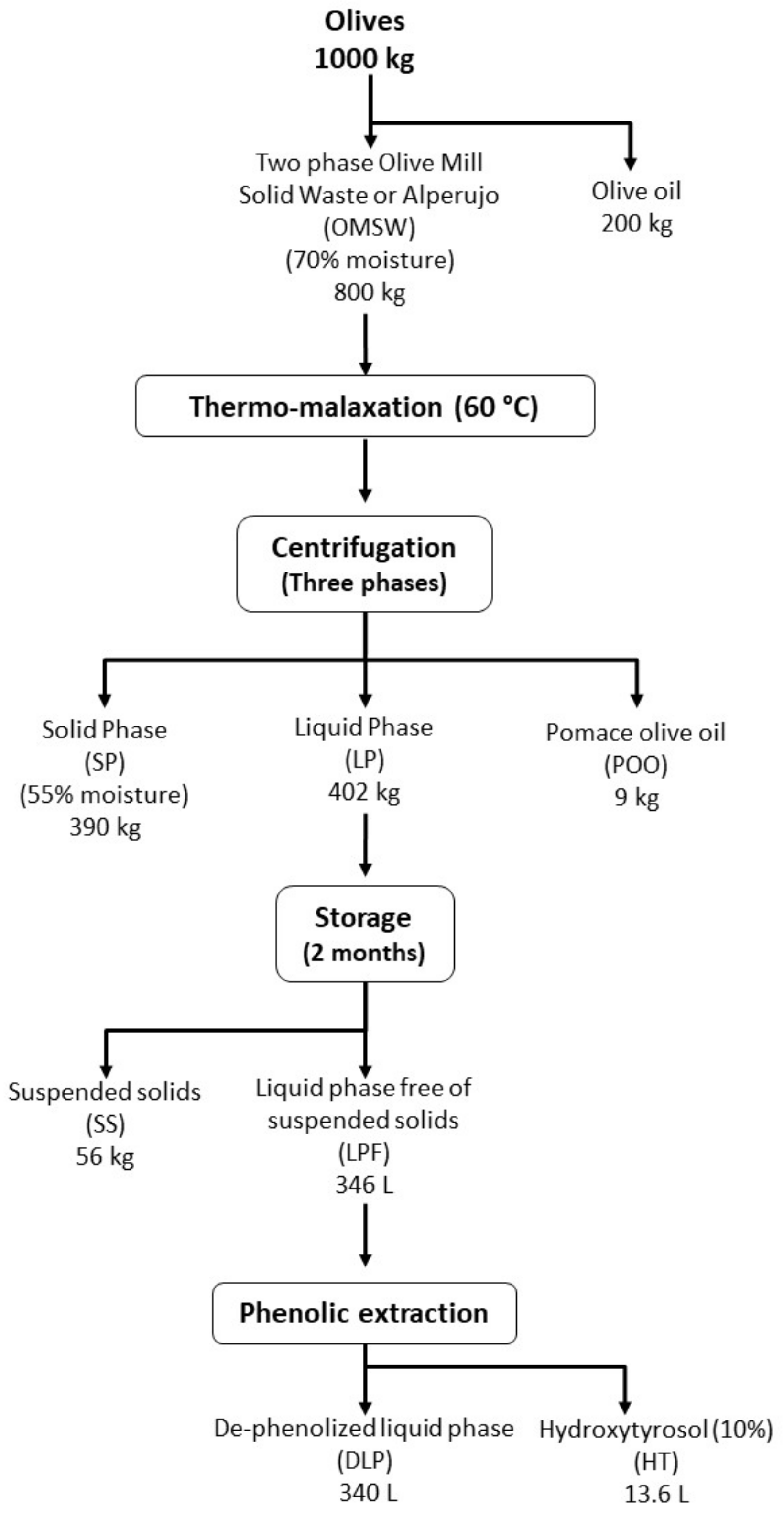
3.1. Olive Mill Solid Waste Obtained from a Two-Phase Olive Oil Extraction System or Alperujo
3.2. Process Scheme of the Biorefinery Approach
3.3. Anaerobic Digestion Experimental Procedure
3.4. Kinetic Study
3.5. Chemical Analyses
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Uceda, M.; Jiménez, A.; Beltrán, G. Olive oil extraction and quality. Grasas Aceites 2006, 57, 25–31. [Google Scholar] [CrossRef]
- Fermoso, F.G.; Serrano, A.; Alonso-Fariñas, B.; Fernández-Bolaños, J.; Borja, R.; Rodríguez-Gutiérrez, G. Valuable Compound Extraction, Anaerobic Digestion, and Composting: A Leading Biorefinery Approach for Agricultural Wastes. J. Agric. Food Chem. 2018, 66, 8451–8468. [Google Scholar] [CrossRef] [PubMed]
- Alburquerque, J.A.; Gonzálvez, J.; García, D.; Cegarra, J. Agrochemical characterisation of “alperujo”, a solid by-product of the two-phase centrifugation method for olive oil extraction. Bioresour. Technol. 2004, 91, 195–200. [Google Scholar] [CrossRef]
- Hendriks, A.T.W.M.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef]
- Rubio-Senent, F.; Rodríguez-Gutiérrez, G.; Lama-Muñoz, A.; Fernández-Bolaños, J. Phenolic extract obtained from steam-treated olive oil waste: Characterization and antioxidant activity. LWT Food Sci. Technol. 2013, 54, 114–124. [Google Scholar] [CrossRef]
- Serrano, A.; Fermoso, F.G.; Rodríguez-Gutierrez, G.; Fernandez-Bolaños, J.; Borja, R. Biomethanization of olive mill solid waste after phenols recovery through low-temperature thermal pre-treatment. Waste Manag. 2017, 61, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Serrano, A.; Fermoso, F.G.; Alonso-Fariñas, B.; Rodríguez-Gutierrez, G.; Fernandez-Bolaños, J.; Borja, R. Phenols recovery after steam explosion of Olive Mill Solid Waste and its influence on a subsequent biomethanization process. Bioresour. Technol. 2017, 243, 169–178. [Google Scholar] [CrossRef]
- Borja, R.; Alba, J.; Banks, C.J. Impact of the main phenolic compounds of olive mill wastewater (OMW) on the kinetics of acetoclastic methanogenesis. Process Biochem. 1997, 32, 121–133. [Google Scholar] [CrossRef]
- Marks, E.A.N.; Kinigopoulou, V.; Akrout, H.; Azzaz, A.A.; Doulgeris, C.; Jellali, S.; Rad, C.; Zulueta, P.S.; Tziritis, E.; El-Bassi, L.; et al. Potential for production of biochar-based fertilizers from olive millwaste in mediterranean basin countries: An initial assessment for Spain, Tunisia, and Greece. Sustainability 2020, 12, 81. [Google Scholar] [CrossRef]
- Ortega, L.; Husser, C.; Barrington, S.; Guiot, S.R. Evaluating limiting steps of anaerobic degradation of food waste based on methane production tests. Water Sci. Technol. 2008, 57, 419–422. [Google Scholar] [CrossRef]
- Borja, R.; Banks, C.J.; Maestro-Durán, R.; Alba, J. The effects of the most important phenolic constituents of Olive Mill Wastewater on batch anaerobic methanogenesis. Environ. Technol. 1996, 17, 167–174. [Google Scholar] [CrossRef]
- Lü, F.; Xu, X.; Shao, L.; He, P. Importance of storage time in mesophilic anaerobic digestion of food waste. J. Environ. Sci. 2015, 45, 76–83. [Google Scholar] [CrossRef]
- Cubero-Cardoso, J.; Trujillo-Reyes, A.; Serrano, A.; Rodríguez-Gutiérrez, G.; Borja, R.; Fermoso, F.G. High-value-added compound recovery with high-temperature hydrothermal treatment and steam explosion, and subsequent biomethanization of residual strawberry extrudate. Foods 2020, 9, 182. [Google Scholar] [CrossRef]
- Wheatley, A. Anaerobic Digestion: A Waste Treatment Technology; Elsevier: New York, NY, USA, 1990. [Google Scholar]
- Serrano, A.; Fermoso, F.G.; Alonso-Fariñas, B.; Rodríguez-Gutiérrez, G.; López, S.; Fernandez-Bolaños, J.; Borja, R. Performance evaluation of mesophilic semi-continuous anaerobic digestion of high-temperature thermally pre-treated olive mill solid waste. Waste Manag. 2019, 87, 250–257. [Google Scholar] [CrossRef] [PubMed]
- POOLred. Available online: http://www.poolred.com/ (accessed on 26 October 2020).
- Ciriminna, R.; Meneguzzo, F.; Fidalgo, A.; Ilharco, L.M.; Pagliaro, M. Extraction, benefits and valorization of olive polyphenols. Eur. J. Lipid Sci. Technol. 2016, 118, 503–511. [Google Scholar] [CrossRef]
- Rodríguez, G.; Rodríguez, R.; Fernández-Bolaños, J.; Guillén, R.; Jiménez, A. Antioxidant activity of effluents during the purification of hydroxytyrosol and 3,4-dihydroxyphenyl glycol from olive oil waste. Eur. Food Res. Technol. 2007, 224, 733–741. [Google Scholar] [CrossRef]
- Baena-Moreno, F.M.; Zhang, Z.; Zhang, X.P.; Reina, T.R. Profitability analysis of a novel configuration to synergize biogas upgrading and Power-to-Gas. Energy Convers. Manag. 2020, 224. [Google Scholar] [CrossRef]
- Lama-Muñoz, A.; Rubio-Senent, F.; Bermúdez-Oria, A.; Fernández-Bolaños, J.; Prior, Á.F.; Rodríguez-Gutiérrez, G. The use of industrial thermal techniques to improve the bioactive compounds extraction and the olive oil solid waste utilization. Innov. Food Sci. Emerg. Technol. 2019, 55, 11–17. [Google Scholar] [CrossRef]
- Agalias, A.; Magiatis, P.; Skaltsounis, A.L.; Mikros, E.; Tsarbopoulos, A.; Gikas, E.; Spanos, I.; Manios, T. A new process for the management of olive oil mill waste water and recovery of natural antioxidants. J. Agric. Food Chem. 2007, 55, 2671–2676. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Reyes, Á.; Cubero-Cardoso, J.; Rodríguez-Gutiérrez, G.; García-Martín, J.F.; Rodríguez-Galán, M.; Borja, R.; Serrano, A.; Fermoso, F.G. Extraction of phenolic compounds and production of biomethane from strawberry and raspberry extrudates. Biochem. Eng. J. 2019, 147, 11–19. [Google Scholar] [CrossRef]
- Da Silva, C.; Astals, S.; Peces, M.; Campos, J.L.; Guerrero, L. Biochemical methane potential (BMP) tests: Reducing test time by early parameter estimation. Waste Manag. 2018, 71, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Angelidaki, I.; Alves, M.; Bolzonella, D.; Borzacconi, L.; Campos, J.L.; Guwy, A.J.; Kalyuzhnyi, S.; Jenicek, P.; Van Lier, J.B. Defining the biomethane potential (BMP) of solid organic wastes and energy crops: A proposed protocol for batch assays. Water Sci. Technol. 2009, 59, 927–934. [Google Scholar] [CrossRef]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Mokrasch, L.C. Analysis of hexose phosphates and sugar mixtures with the anthrone reagent. J. Biol. Chem. 1954, 208, 55–59. [Google Scholar] [PubMed]
- Scaramboni, C.; Urban, R.C.; Lima-Souza, M.; Nogueira, R.F.P.; Cardoso, A.A.; Allen, A.G.; Campos, M.L.A.M. Total sugars in atmospheric aerosols: An alternative tracer for biomass burning. Atmos. Environ. 2015, 100, 185–192. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]

| LPF | DLP | SP | SS | ||
|---|---|---|---|---|---|
| TS | mg/L | 83,790 ± 470 | 10,740 ± 220 | 436,520 ± 7565 | 235,200 ± 2100 |
| MS | mg/L | 13,050 ± 220 | 2925 ± 95 | 20,685 ± 625 a | 22,085 ± 490 a |
| VS | mg/L | 70,735 ± 425 | 7815 ± 310 | 415,835 ± 6 995 | 213,120 ± 2340 |
| VS/TS | 0.84 ± 0.01 | 0.73 ± 0.03 | 0.95 ± 0.02 | 0.91 ± 0.01 | |
| sCOD | mg O2/L | 111,791 ± 899 a | 10,868 ± 130 | 61,653 ± 518 | 100,938 ± 259 a |
| pH | 4.9 ± 0.1 a | 7.3 ± 0.1 | 4.6 ± 0.1 a | 4.9 ± 0.1 a | |
| Total sugars | mg/L | 23,224 ± 825 a | 725 ± 32 | 5449 ± 353 | 24,204 ± 438 a |
| Total phenols | % | 0.39 ± 0.03 a | 0.03 ± 0.00 | 0.20 ± 0.01a | 0.73 ± 0.08 |
| Gmax (mL CH4/g VS) | Rmax (mL CH4/(g VS·d)) | K′ (d−1) | R2 | Error (%) | |
|---|---|---|---|---|---|
| LPF | 358 ± 6 | 100 | 0.279 ± 0.018 | 0.9668 | −4.239 |
| DLP | 267 ± 4 | 190 | 0.711 ± 0.059 | 0.9576 | −5.676 |
| SP | 150 ± 1 | 158 | 1.056 ± 0.052 | 0.9808 | 9.002 |
| SS | 366 ± 7 | 90 | 0.245 ± 0.015 | 0.9754 | 11.888 |
| LPF | DLP | SP | SS | ||
|---|---|---|---|---|---|
| pH | 7.10 ± 0.02 | 7.16 ± 0.02 | 7.37 ± 0.08 | 7.58 ± 0.06 | |
| Alkalinity | mg CaCO3/L | 5523 ± 52 | 6200 ± 247 | 5819 ± 57 | 6035 ± 271 |
| TS | g/L | 20.8 ± 0.2 | 20.2 ± 0.6 | 17.7 ± 0.3 | 18.2 ± 0.2 |
| MS | g/L | 9.3 ± 0.1 | 10.1 ± 0.1 | 8.5 ± 0.2 | 8.4 ± 0.1 |
| VS | g/L | 11.5 ± 0.1 | 10.2 ± 0.8 | 9.1 ± 0.4 | 9.8 ± 0.1 |
| Biodegradability | % | 68 | 46 | 22 | 42 |
| sCOD | mg O2/L | 1045 ± 20 | 855 ± 10 | 480 ± 5 | 885 ± 5 |
Sample Availability: Samples of the compounds are available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Prior, Á.; Trujillo-Reyes, Á.; Serrano, A.; Rodríguez-Gutiérrez, G.; Reinhard, C.; Fermoso, F.G. Biogas Potential of the Side Streams Obtained in a Novel Phenolic Extraction System from Olive Mill Solid Waste. Molecules 2020, 25, 5438. https://doi.org/10.3390/molecules25225438
Fernández-Prior Á, Trujillo-Reyes Á, Serrano A, Rodríguez-Gutiérrez G, Reinhard C, Fermoso FG. Biogas Potential of the Side Streams Obtained in a Novel Phenolic Extraction System from Olive Mill Solid Waste. Molecules. 2020; 25(22):5438. https://doi.org/10.3390/molecules25225438
Chicago/Turabian StyleFernández-Prior, África, Ángeles Trujillo-Reyes, Antonio Serrano, Guillermo Rodríguez-Gutiérrez, Claudio Reinhard, and Fernando G. Fermoso. 2020. "Biogas Potential of the Side Streams Obtained in a Novel Phenolic Extraction System from Olive Mill Solid Waste" Molecules 25, no. 22: 5438. https://doi.org/10.3390/molecules25225438
APA StyleFernández-Prior, Á., Trujillo-Reyes, Á., Serrano, A., Rodríguez-Gutiérrez, G., Reinhard, C., & Fermoso, F. G. (2020). Biogas Potential of the Side Streams Obtained in a Novel Phenolic Extraction System from Olive Mill Solid Waste. Molecules, 25(22), 5438. https://doi.org/10.3390/molecules25225438








