Flavonoid Preparations from Taraxacum officinale L. Fruits—A Phytochemical, Antioxidant and Hemostasis Studies
Abstract
1. Introduction
2. Results
2.1. Phytochemical Characteristics of Plant Extracts and Fractions
2.2. DPPH Free Radical Scavenging Activity
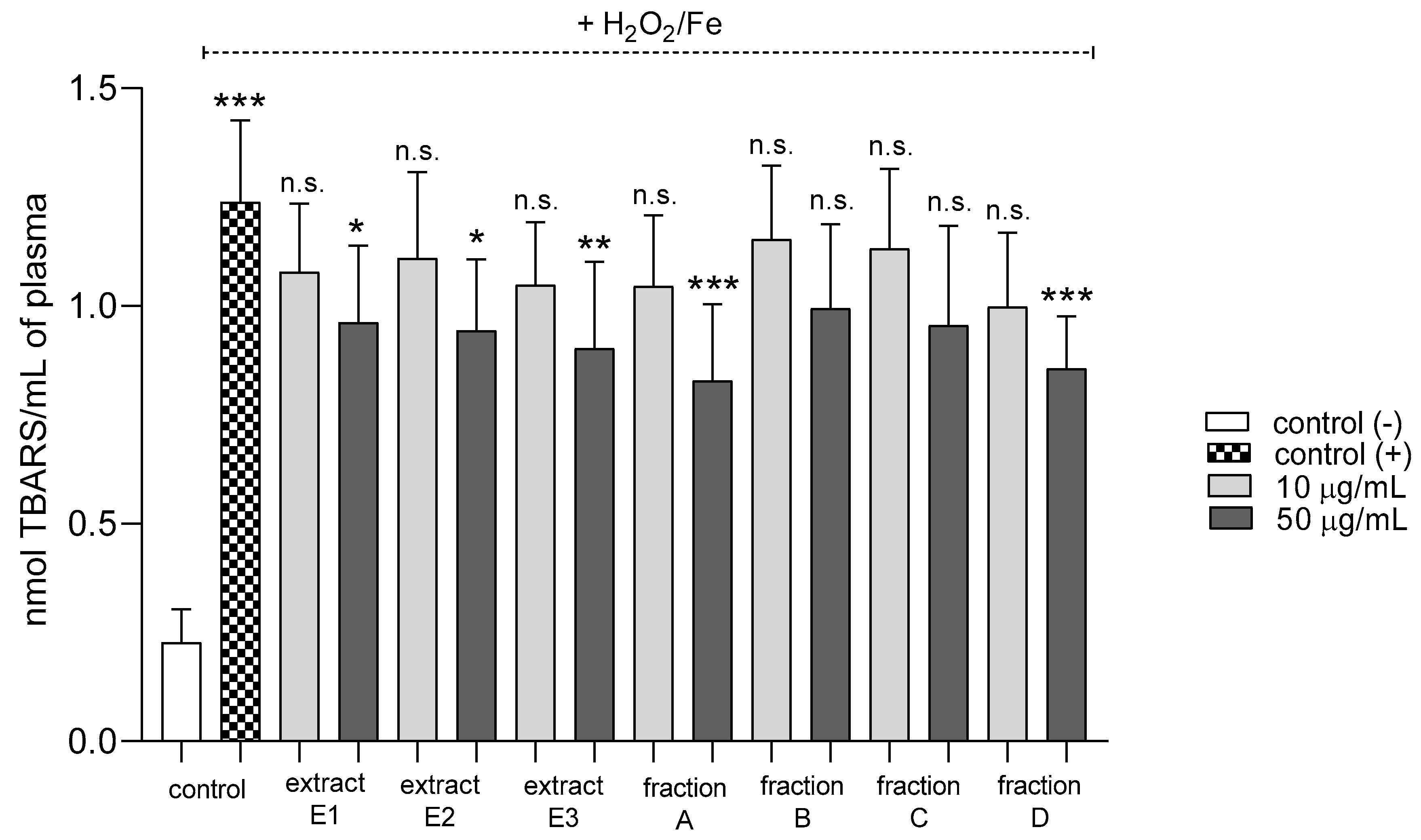
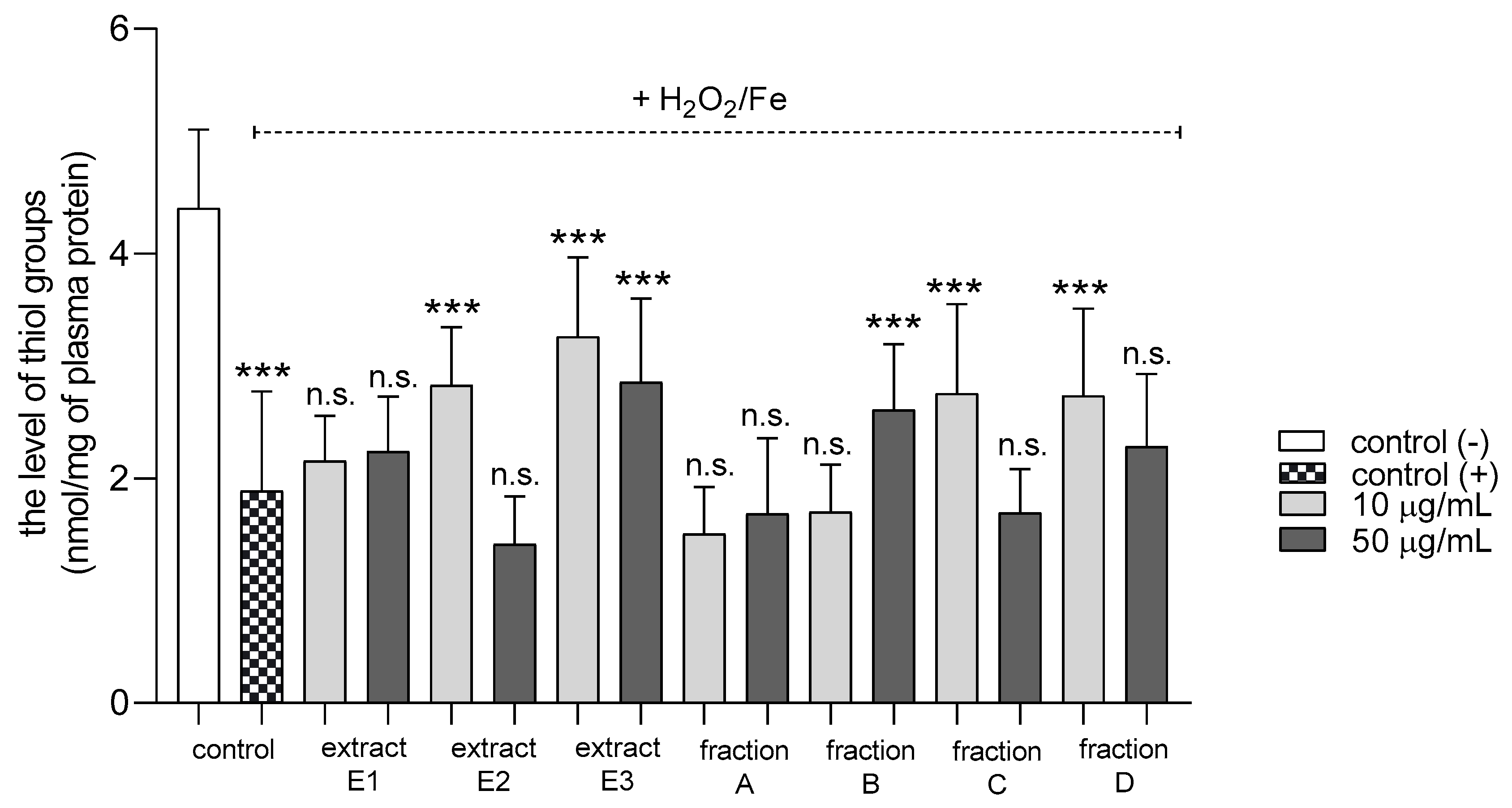
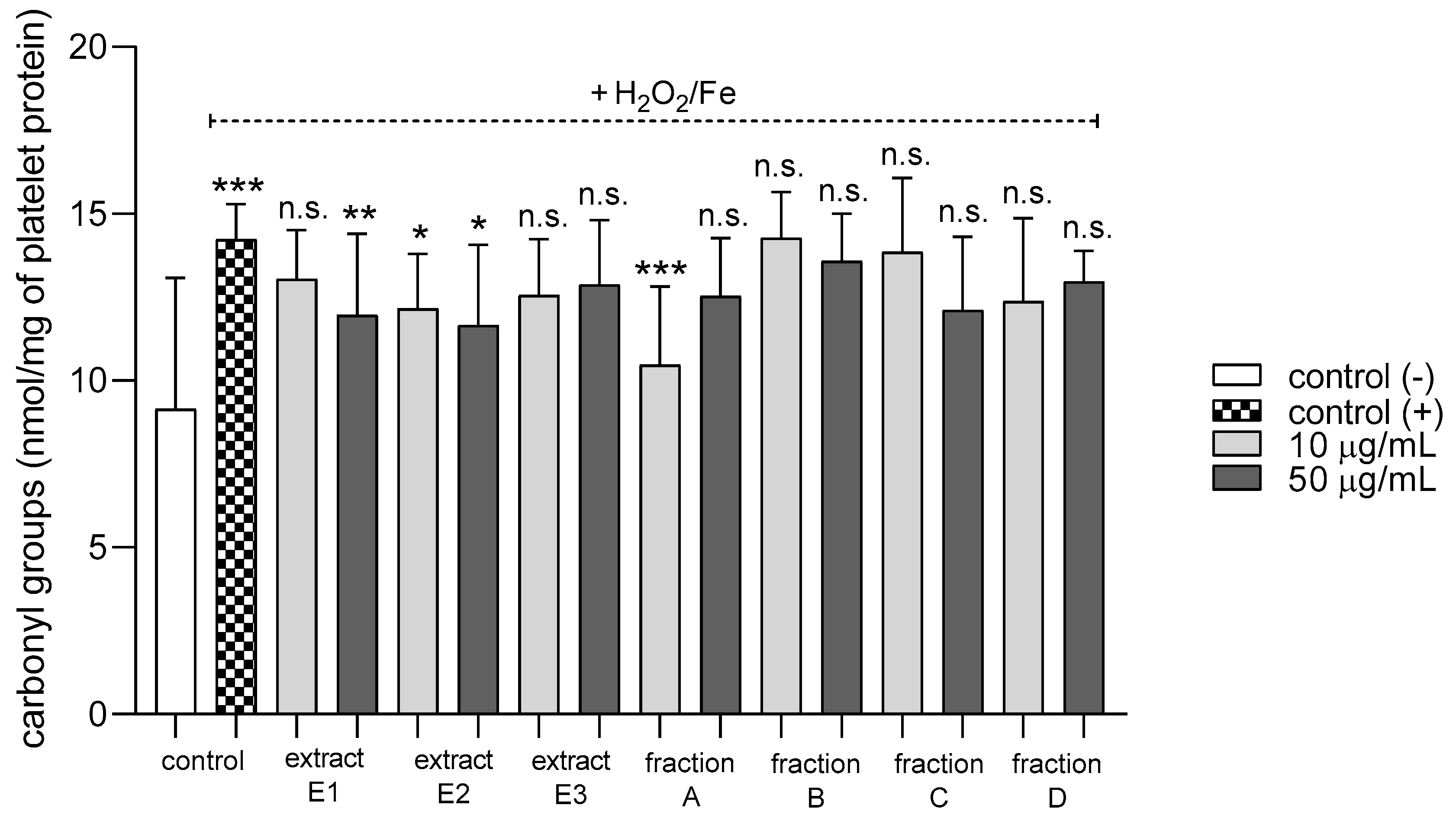
2.3. Biomarkers of Oxidative Stress in Plasma and Blood Platelets
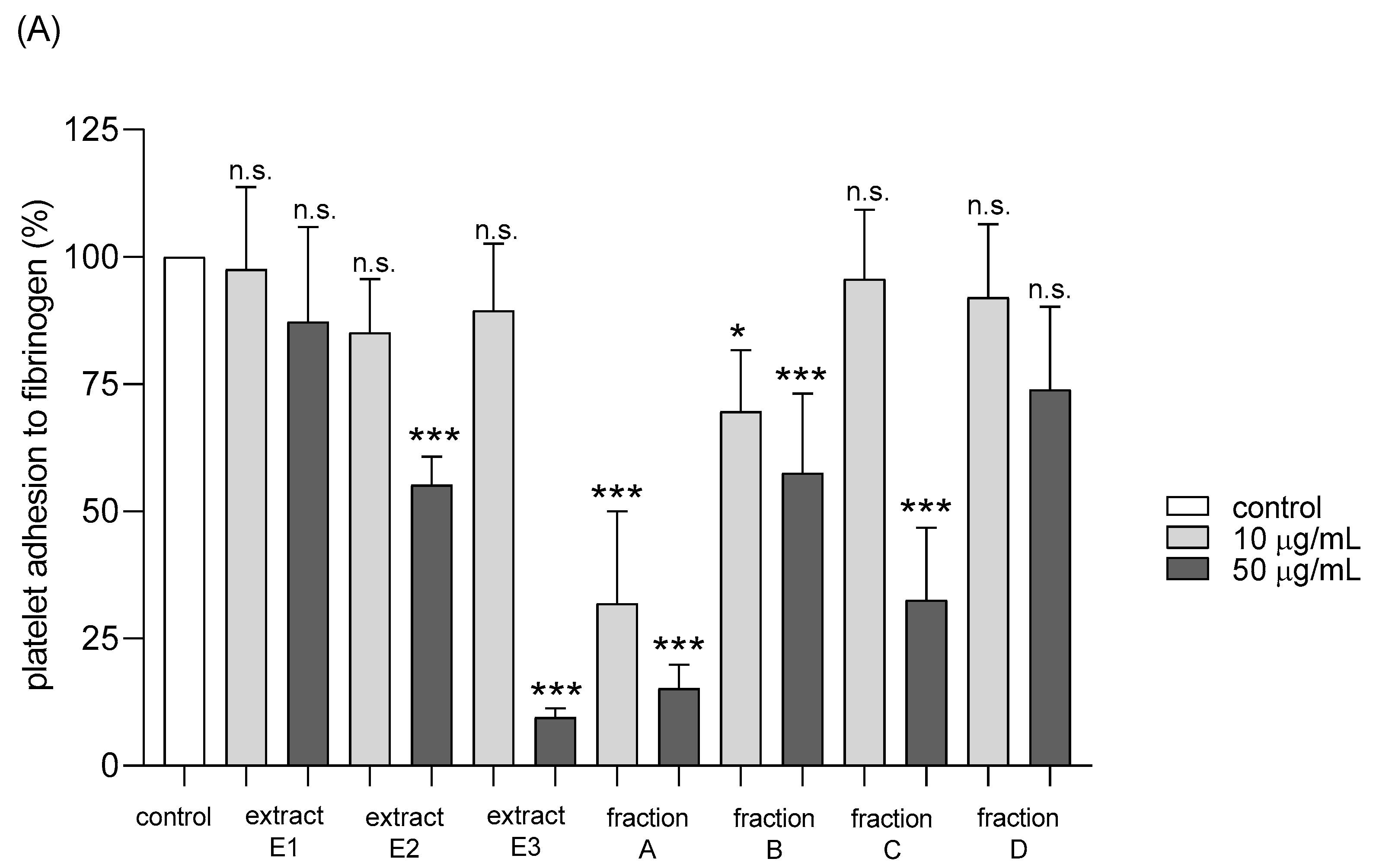
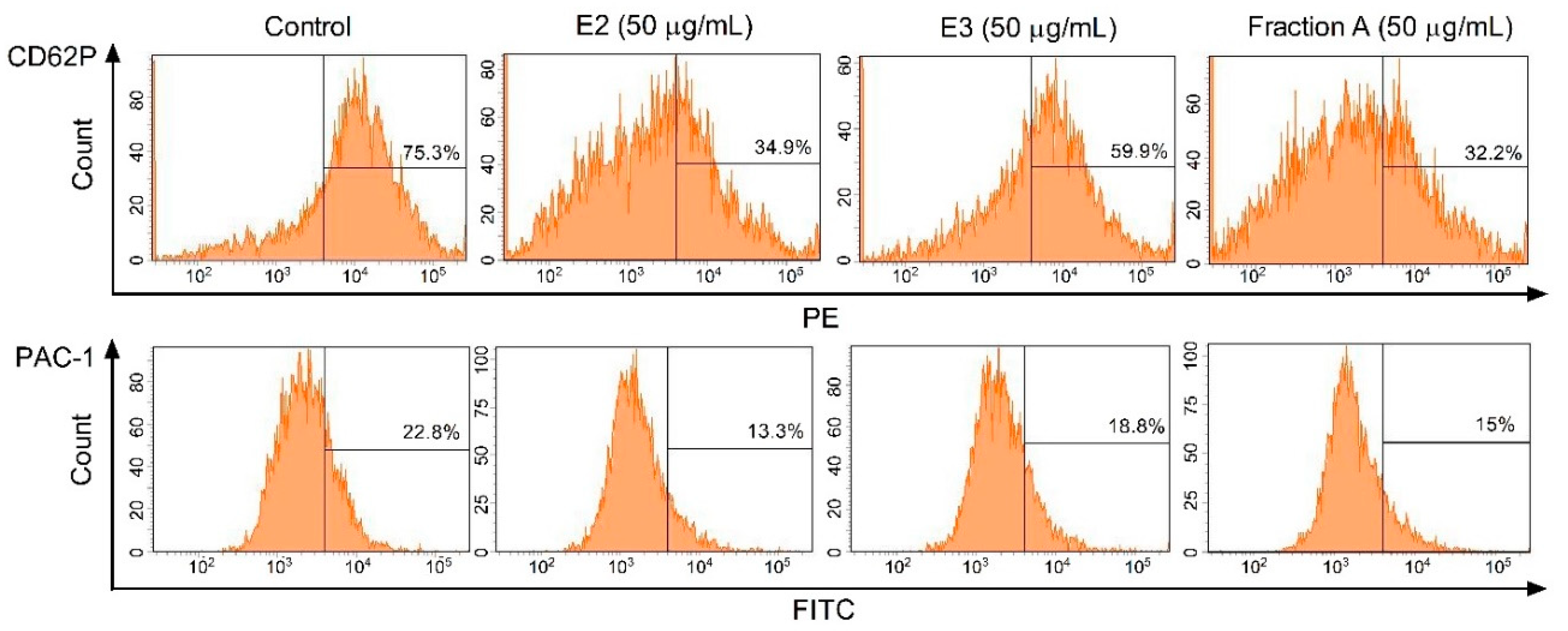
2.4. Hemostatic Parameters of Blood Platelets and Plasma
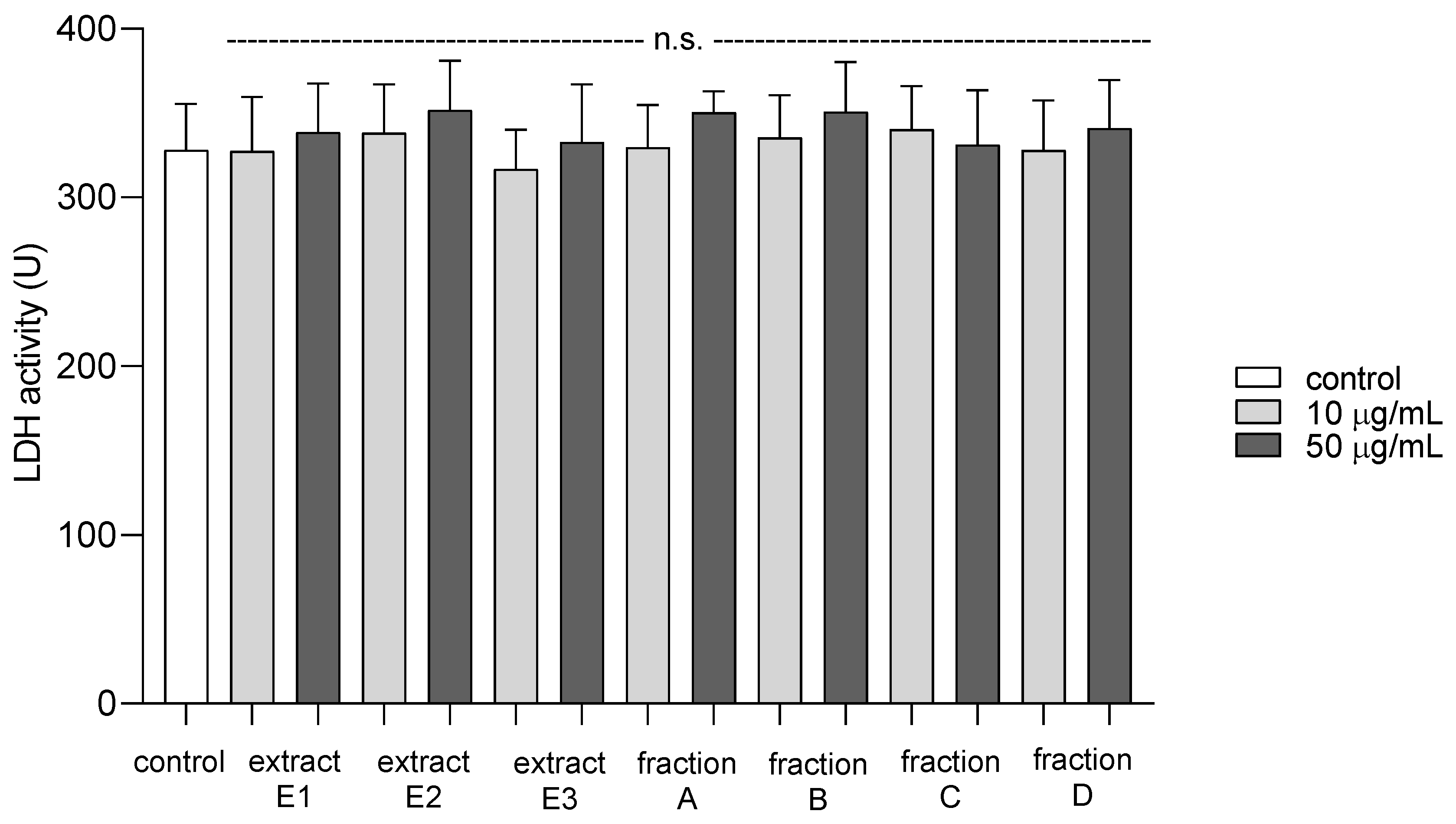
2.5. Cytotoxicity against Blood Platelets
3. Discussion
4. Materials and Methods
4.1. Chemical Reagents
4.2. Plant Material
4.3. Preparation of Phenolic Extracts and Fractions from Dandelion Fruits
4.4. Phytochemical Profiling
4.4.1. Qualitative High-Resolution LC-MS Analysis
4.4.2. Quantitative LC-UV Analysis of Flavonoids and Phenolic Acids
4.5. Determination of Total Phenolic Content (TPC)
4.6. DPPH Free Radical Scavenging Activity
4.7. Blood Platelets and Plasma Isolation
4.8. Incubation of Plasma, Blood Platelets, and Whole Blood with Plant Extracts and Fractions
4.9. Parameters of Blood Platelet Activation
4.9.1. Platelet Adhesion to Fibrinogen
4.9.2. Flow Cytometry
4.10. Parameters of the Coagulation Process
4.10.1. Thrombin Time (TT) Measurement
4.10.2. Prothrombin Time (PT) Measurement
4.10.3. Activated Partial Thromboplastin Time (APTT) Measurement
4.10.4. Total Thrombus Formation Analysis System (T-TAS®)
4.11. Cytotoxicity
4.12. Parameters of Oxidative Stress
4.12.1. Lipid Peroxidation Measurement
4.12.2. Carbonyl Group Measurement
4.12.3. Thiol Group Determination
4.13. Data analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Gale, A.J. Continuing education course #2: Current understanding of hemostasis. Toxicol. Pathol. 2011, 39, 273–280. [Google Scholar] [PubMed]
- Kluft, C.; Burggraaf, J. Introduction to hemostasis from a pharmacodynamic perspective. Br. J. Clin. Pharm. 2011, 72, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Arthur, J.F.; Gardiner, E.E.; Andrews, R.K.; Zeng, L.; Xu, K. Regulation of platelet activation and thrombus formation by reactive oxygen species. Redox Biol. 2018, 14, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Bartosz, G. Druga Twarz Tlenu. Wyd. 2; Państwowe Wydawnictwo Naukowe (PWN): Warszawa, Poland, 2013; pp. 99–120. [Google Scholar]
- Ivanov, I.G. Polyphenols content and antioxidant activities of Taraxacum officinale F.H. Wigg (dandelion) leaves. Int. J. Pharm. Phytochem. Res. 2014, 6, 889–893. [Google Scholar]
- DRUGBANK. Available online: https://www.drugbank.ca/categories/DBCAT000368 (accessed on 28 October 2019).
- Lis, B.; Olas, B. Pro-health activity of dandelion (Taraxacum officinale L.) and its food products–history and present. J. Funct. Foods 2019, 59, 40–48. [Google Scholar] [CrossRef]
- Martinez, M.; Poirrier, P.; Chamy, R.; Prüfer, D.; Schulze-Gronover, C.; Jorquera, L.; Ruiz, G. Taraxacum officinale and related species—An ethnopharmacological review and its potential as a commercial medicinal plant. J. Ethnopharmacol. 2015, 169, 244–262. [Google Scholar] [CrossRef]
- Majewski, M.; Lis, B.; Juśkiewicz, J.; Ognik, K.; Borkowska-Sztachańska, M.; Jedrejek, D.; Stochmal, A.; Olas, B. Phenolic fractions from dandelion leaves and petals as modulators the lipid profile and antioxidant status in an in vivo study. Antioxidants 2020, 9, 131. [Google Scholar] [CrossRef]
- Jedrejek, D.; Lis, B.; Rolnik, A.; Stochmal, A.; Olas, B. Comparative phytochemical, cytotoxicity, antioxidant and hemostatic studies of Taraxacum officinale root preparations. Food Chem. Toxicol. 2019, 126, 233–247. [Google Scholar] [CrossRef]
- Xue, Y.; Zhang, S.; Du, M.; Zhu, M.J. Dandelion extract suppresses reactive oxidative species and inflammasome in intestinal epithelial cells. J. Funct. Foods 2017, 29, 10–18. [Google Scholar] [CrossRef]
- Williams, C.A.; Goldstone, F.; Greenham, J. Flavonoids, cinnamic acids and coumarins from the different tissues and medicinal preparations of Taraxacum officinale. Phytochemistry 1996, 42, 121–127. [Google Scholar] [CrossRef]
- Sareedenchai, V.; Zidorn, C. Flavonoids as chemosystematic markers in the tribe Cichorieae of the Asteraceae. Biochem. Syst. Ecol. 2010, 38, 935–957. [Google Scholar] [CrossRef]
- Colle, D.; Arantes, L.P.; Rauber, R.; Campos De Mattos, S.E.; Teixeira Rocha, J.B.; Nogueira, C.W.; Soares, F.A. Antioxidant properties of Taraxacum officinale fruit extract are involved in the protective effect against cellular death induced by sodium nitroprusside in brain of rats. Pharm. Biol. 2012, 50, 883–891. [Google Scholar] [CrossRef] [PubMed]
- SIRIUS 4. Available online: https://bio.informatik.uni-jena.de/sirius/ (accessed on 14 October 2019).
- Lis, B.; Jedrejek, D.; Moldoch, J.; Stochmal, A.; Olas, B. The anti-oxidative and hemostasis-related multifunctionality of L-chicoric acid, the main component of dandelion: An in vitro study of its cellular safety, antioxidant and anti-platelet properties, and effect on coagulation. J. Funct. Foods 2019, 62, 103524. [Google Scholar] [CrossRef]
- Lis, B.; Rolnik, A.; Jedrejek, D.; Soluch, A.; Stochmal, A.; Olas, B. Dandelion (Taraxacum officinale L.) root components exhibit anti-oxidative and antiplatelet action in an in vitro study. J. Funct. Foods 2019, 59, 16–24. [Google Scholar] [CrossRef]
- Jedrejek, D.; Kontek, B.; Lis, B.; Stochmal, A.; Olas, B. Evaluation of antioxidant activity of phenolic fractions from the leaves and petals of dandelion in human plasma treated with H2O2 and H2O2/Fe. Chem. Biol. Interact. 2017, 262, 29–37. [Google Scholar] [CrossRef]
- Schütz, K.; Carle, R.; Schieber, A. Taraxacum: A review on its phytochemical and pharmacological profile. J. Ethnopharmacol. 2006, 107, 313–323. [Google Scholar] [CrossRef]
- Schütz, K.; Kammerer, D.R.; Carle, R.; Schieber, A. Characterization of phenolic acids and flavonoids in dandelion (Taraxacum officinale WEB. ex WIGG.) root and herb by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 179–186. [Google Scholar] [CrossRef]
- Mercader, A.G.; Pomilio, A.B. Biflavonoids: Occurrence, Structural Features and Bioactivity; Nova Science Publishers, Inc.: New York, NY, USA, 2012. [Google Scholar]
- Geiger, H.; Bokel, M. Die Biflavonoidausstattung von Chimarrhis turbinate (Hedw.). Z. Nat. 1989, 44c, 559–562. [Google Scholar]
- Seeger, T.; Geiger, H.; Zinsmeister, H.D. Bartramiaflavone, a macrocyclic biflavonoid from the moss Bartramia pomiformis. Phytochemistry 1991, 30, 1653–1656. [Google Scholar] [CrossRef]
- Zhang, B.B.; Dai, Y.; Liao, Z.X. Chemical Constituents of Saussurea eopygmaea. Chin. J. Nat. Med. 2011, 9, 33–37. [Google Scholar] [CrossRef]
- Lone, S.H.; Khuroo, M.A. Biflavanoids: Chemical and Pharmacological Aspects; Elsevier Ltd.: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Choi, J.; Yoon, K.D.; Kim, J. Chemical constituents from Taraxacum officinale and their α-glucosidase inhibitory activities. Bioorganic Med. Chem. Lett. 2018, 28, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Karolczak, K.; Olas, B.; Kołodziejczyk, J. The role of thiols in blood platelet activation. Post. Biol. Komorki 2009, 36, 101–120. [Google Scholar]
- Łapaciuk, S. Zakrzepy i Zatory. Wyd. 1; PZWL: Warszawa, Poland, 1996; pp. 95–112. [Google Scholar]
- Windyga, J. Skazy Krwotoczne. Wyd. 1; PZWL: Warszawa, Poland, 2006; pp. 33–67. [Google Scholar]
- Kim, K.; Park, K. A review of antiplatelet activity of traditional medicinal herbs on integrative medicine studies. Evid. Based Complement. Altern. Med. 2019, 2019, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Kaikita, K.; Hosokawa, K.; Dahlen, J.R.; Tsujita, K. Total thrombus-formation analysis system (T-TAS): Clinical application of quantitative analysis of thrombus formation in cardiovascular disease. Thromb. Hemost. 2019, 119, 1554–1562. [Google Scholar] [CrossRef]
- Versteeg, H. New fundamentals in hemostasis. Physiol. Rev. 2013, 93, 327–358. [Google Scholar] [CrossRef]
- Benavente-Garcia, O.; Castillo, J. Update on uses and properties of citrus flavonoids: New findings in anticancer, cardiovascular, and anti-inflammatory activity. J. Agric. Food Chem. 2008, 56, 6185–6205. [Google Scholar] [CrossRef]
- Guerrero, J.A.; Navarro-Nunez, L.; Lozano, M.L.; Martinez, C.; Vicente, V.; Gibbins, J.M.; Rivera, J. Flavonoids inhibit the platelet TxA(2) signaling pathway and antagonize TxA(2) receptors (TP) in platelets and smooth muscle cells. Br. J. Clin. Pharm. 2007, 64, 133–144. [Google Scholar] [CrossRef]
- Dell’Agli, M.; Maschi, O.; Galli, G.V.; Fagnani, R.; Da Cero, E.; Caruso, D.; Bosino, E. Inhibition of platelet aggregation by olive oil phenols via cAMP-phosphodiesterase. Br. J. Nutr. 2008, 99, 945–951. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230–242. [Google Scholar] [CrossRef]
- Makowska-Wąs, J.; Janeczko, Z. Bioavailability of plant polyphenols. Post. Fitoter. 2004, 3, 126–137. [Google Scholar]
- Baeza, G.; Bachmair, E.M.; Wood, S.; Mateos, R.; Bravo, L.; De Roos, B. The colonic metabolites dihydrocaffeic acid and dihydroferulic acid are more effective inhibitors of in vitro platelet activation than their phenolic precursors. Food Funct. 2017, 8, 1333–1342. [Google Scholar] [CrossRef] [PubMed]
- Doolittle, R.F.; Schubert, D.; Schwartz, S.A. Amino acid sequence studies on artiodactyl fibrinopeptides I Dromedary camel, mule deer, and cape buffalo. Arch. Biochem. Biophys. 1967, 118, 456–467. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Walkowiak, B.; Michalak, E.; Koziołkiewicz, W.; Cierniewski, C.S. Rapid photometric method for estimation of platelet count in blood plasma or platelet suspension. Thromb. Res. 1989, 56, 763–766. [Google Scholar] [CrossRef]
- Whitaker, J.R.; Granum, P.E. An absolute method for protein determination based on difference in absorbance at 235 and 280 nm. Anal. Biochem. 1980, 109, 156–159. [Google Scholar] [CrossRef]
- Bellavite, P.; Andrioli, G.; Guzzo, P.; Arigliano, P.; Chirumbolo, S.; Manzato, F.; Santonastaso, C. A colorimetric method for the measurement of platelet adhesion in microtiter plates. Anal. Biochem. 1994, 216, 444–450. [Google Scholar] [CrossRef]
- Rywaniak, J.; Luzak, B.; Watała, C. The advantage of flow cytometry technique for evaluation of viability of blood platelets stained with calcein. J. Lab. Diagn. 2012, 48, 279–285. [Google Scholar]
- Malinowska, J.; Kołodziejczyk-Czepas, J.; Moniuszko-Szajwaj, B.; Kowalska, I.; Oleszek, W.; Stochmal, A.; Olas, B. Phenolic fractions from Trifolium pallidum and Trifolium scabrum aerial parts in human plasma protect against changes induced by hyperhomocysteinemia. Food Chem. Toxicol. 2012, 50, 4023–4027. [Google Scholar] [CrossRef]
- Hosokawa, K.; Ohnishi, T.; Kondo, T.; Fukasawa, M.; Koide, T.; Maruyama, I.; Tanaka, K. A novel automated microchip flow-chamber system to quantitatively evaluate thrombus formation and antithrombotic agents under blood flow conditions. J. Thromb. Hemost. 2011, 9, 2029–2037. [Google Scholar] [CrossRef] [PubMed]
- Wroblewski, F.; Ladue, J.S. Lactic dehydrogenase activity in blood. Proc. Soc. Exp. Biol. Med. 1955, 90, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Wachowicz, B. Adenine nucleotides in thrombocytes of birds. Cell Biochem. Funct. 1984, 2, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.-G.; Ahn, B.-W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar] [PubMed]
- Ando, Y.; Steiner, M. Sulphydryl and disulphide groups of platelet membranes: Determination of sulphydryl groups. Biochim. Biophys. Acta 1973, 311, 38–44. [Google Scholar] [CrossRef]
- Ando, Y.; Steiner, M. Sulphydryl and disulphide groups of platelet membranes: Determination of disulphide groups. Biochim. Biophys. Acta 1973, 311, 26–37. [Google Scholar] [CrossRef]
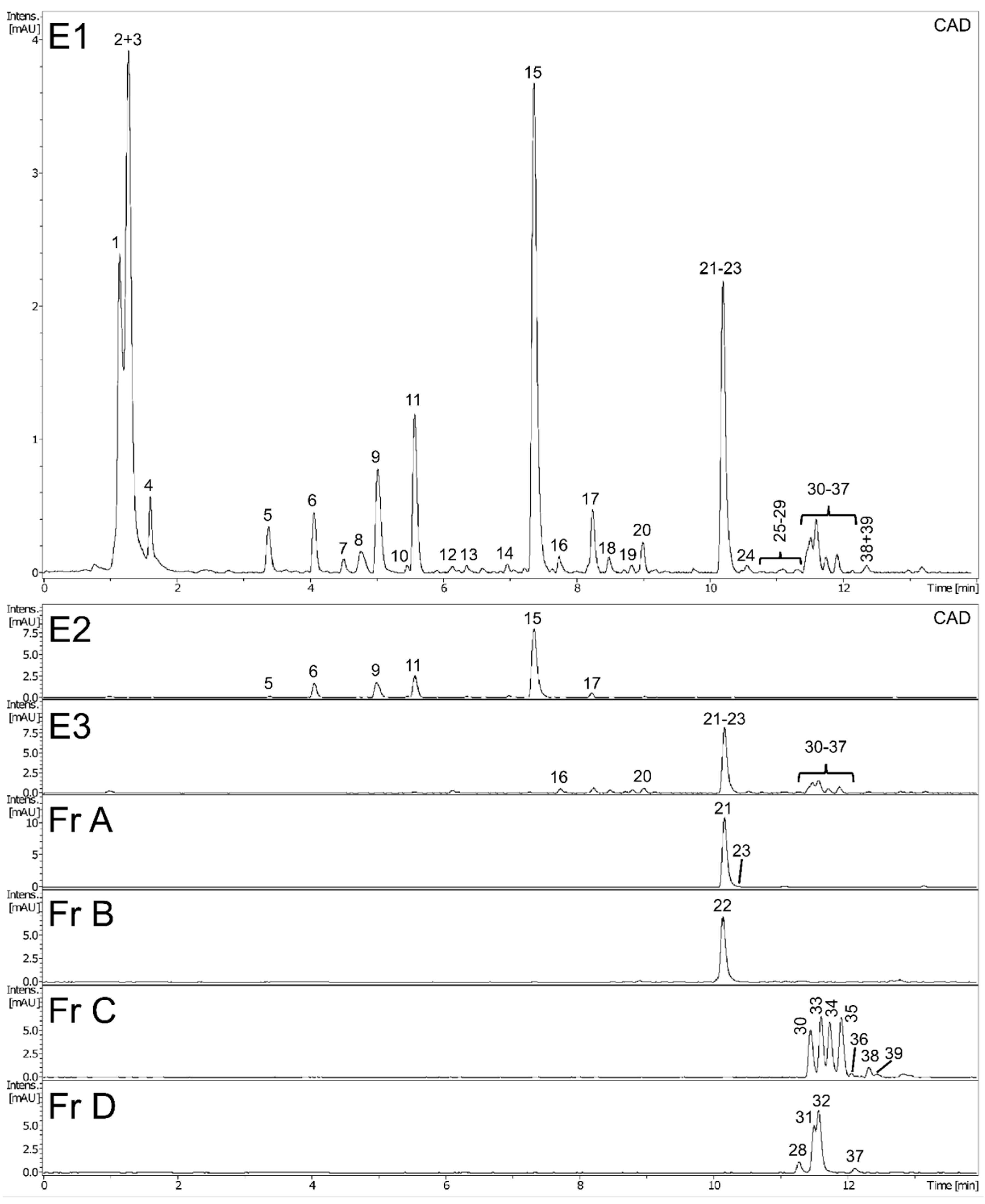










| No | Identity | RT (min) | Formula | UVmax (nm) | Error (ppm) | mσ | Observed [M − H]− | Major Fragments (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | unidentified | 1.10 | C13H9N4O6 | 260 | −5.2 | 28.6 | 317.0544 | 225.0072 (23), 164.9851 (3) |
| 2 | di-hexose (sucrose) | 1.27 | C13H23O13 | 220 | 1.6 | 2.3 | 387.1138 [M + HCO2H − H]−, 341.1085 | 179.0563 (17), 161.0449 (5) |
| 3 | tri-hexose (raffinose) | 1.27 | C19H33O18 | 220 | 1.1 | 7.1 | 549.1666 [M + HCO2H − H]−, 503.1610 | 179.0563 (8), 341.1085 (5) |
| 4 | unidentified | 1.63 | C9H11N2O6 | 275 | 0.6 | 5.7 | 243.0621 | - |
| 5 | unidentified | 3.39 | C10H11N4O6 | 262 | 1.2 | 9.9 | 283.0681 | 151.0256 (100) |
| 6 | caffeoyl-di-O-hexoside | 4.08 | C22H29O16 | 290br | 1.8 | 6.7 | 549.1455 [M + HCO2H − H]− | 341.0875 (100), 179.0349 (10) |
| 7 | di-hydroxy-benzoic acid hexoside | 4.52 | C13H15O9 | 305 | 0.5 | 15.2 | 315.0720 | 153.0186 (100) |
| 8 | caftaric acid | 4.78 | C13H11O9 | 328, 295sh | −0.3 | 15.7 | 311.0410 | 179.0348 (100), 135.0438 (51), 149.0079 (15) |
| 9 | unidentified | 4.97 | C15H20NO6 | 328, 295sh | 2.6 | 16.1 | 310.1288 [M + HCO2H − H]− | 250.1084 (100), 161.0237 (4), 179.0356 (2) |
| 10 | caffeoyl-O-hexoside | 5.48 | C15H17O9 | 320, 290sh | −0.3 | 15.8 | 341.0879 | 179.0351 (100), 135.0444 (16) |
| 11 | 5-O-caffeoylquinic acid * | 5.59 | C16H17O9 | 325, 295sh | 0.3 | 5.1 | 353.0877 | 191.0562 (100) |
| 12 | caffeic acid | 6.11 | C9H7O4 | 323, 295sh | −0.8 | 7.6 | 179.0351 | 135.0440 (100) |
| 13 | unidentified | 6.37 | C18H27O9 | 308br | −0.3 | 28.9 | 387.1664 | 207.1029 (15) |
| 14 | luteolin-O-di-hexoside | 6.95 | C27H29O16 | 255, 345 | 2.7 | 6.8 | 609.1445 | 285.0394 (12) |
| 15 | L-chicoric acid | 7.38 | C22H17O12 | 329, 295sh | 1.8 | 5.8 | 473.0727 | 179.0354 (100), 293.0306 (49), 311.0412 (44), 149.0088 (33), 219.0301 (26) |
| 16 | luteolin-7-O-glucoside | 7.76 | C21H19O11 | 255, 267, 345 | −0.9 | 19.7 | 447.0937 | 285.0408 (96) |
| 17 | 3,5-di-caffeoylquinic acid | 8.26 | C25H23O12 | 328, 295sh | 1.2 | 4.5 | 515.1189 | 353.0871 (100), 191.0557 (26), 179.0347 (12) |
| 18 | luteolin-4’-O-glucoside | 8.50 | C21H19O11 | 269, 337 | −1.6 | 18.1 | 447.0937 | 285.0408 (100) |
| 19 | luteolin-3’-O-glucoside # | 8.85 | C21H19O11 | 267, 337 | −2.0 | 27.4 | 447.0942 | 285.0407 (100) |
| 20 | taraxinic acid-1’-O-glucoside | 9.01 | C22H29O11 | 220 | −0.5 | 4.0 | 469.1718 [M + HCO2H − H]− | 261.1135 (100), 217.1235 (59) |
| 21 | luteolin | 10.23 | C15H9O6 | 255, 267, 345 | 0.3 | 3.1 | 285.0404 | 199.0399 (4), 217.0506 (3), 241.0505 (3) |
| 22 | philonotisflavone # | 10.23 | C30H17O12 | 255, 342 | −1.5 | 5.7 | 569.0734 | 391.0469 (8), 433.0574 (4), 459.0371 (3) |
| 23 | methyltricetin | 10.27 | C16H11O7 | 267, 347 | 0.4 | 15.0 | 315.0509 | 300.0274 (100), 272.0322 (7) |
| 24 | bi-flavone (luteolin-luteolin) # | 10.52 | C30H17O12 | 255, 345 | −0.2 | 20.7 | 569.0727 | 417.0610 (10), 285.0411 (2) |
| 25 | bi-flavone (luteolin-apigenin) # | 10.93 | C30H17O11 | 257, 345 | −2.3 | 25.2 | 553.0789 | 391.0478 (13), 433.0572 (3), 459.0370 (3) |
| 26 | bi-flavone (luteolin-apigenin) # | 11.09 | C30H17O11 | 257, 345 | −1.4 | 6.4 | 553.0791 | 401.0682 (19) |
| 27 | bi-flavone (luteolin-chrysoeriol) # | 11.28 | C31H19O12 | 257, 343 | −0.9 | 9.3 | 583.0891 | 431.0781 (11) |
| 28 | apigenin | 11.32 | C15H9O5 | 267, 337 | −1.6 | 9.7 | 269.0460 | 225.0562 (10) |
| 29 | bi-flavone (luteolin-chrysoeriol) # | 11.38 | C31H19O12 | 257, 343 | −1.0 | 7.6 | 583.0892 | 431.0779 (11) |
| 30 | flavonolignan (tricin-lignan (m/z 170) conjugate) # | 11.50 | C26H23O10 | 269, 338 | 2.7 | 1.7 | 495.1283, 541.1340 [M + HCO2H − H]− | 329.0658 (100), 447.1076 (8) |
| 31 | tricin | 11.54 | C17H13O7 | 255, 267, 351 | −1.7 | 0.5 | 329.0672 | 299.0204 (100), 314.0438 (71), 271.0256 (10) |
| 32 | chrysoeriol | 11.62 | C16H11O6 | 251, 267, 345 | −2.2 | 7.0 | 299.0568 | 284.0333 (100), 256.0384 (13) |
| 33 | flavonolignan (salcolin A/B) | 11.62 | C27H25O11 | 271, 337 | 2.7 | 3.4 | 525.1388, 571.1445 [M + HCO2H − H]− | 329.0659 (100), 314.0426 (14), 195.0659 (10), 165.0551 (7) |
| 34 | flavonolignan (tricin-lignan (m/z 170) conjugate) # | 11.77 | C26H23O10 | 271, 337 | 2.4 | 4.3 | 495.1285, 541.1342 [M + HCO2H − H]− | 329.0659 (100), 314.0425 (24), 135.0441 (4) |
| 35 | flavonolignan (salcolin A/B) | 11.94 | C27H25O11 | 271, 337 | 2.1 | 1.4 | 525.1391, 571.1448 [M + HCO2H − H]− | 329.0660 (100), 314.0425 (14), 195.0659 (9), 165.0551 (6) |
| 36 | flavonolignan (tricin derivative) # | 12.07 | C37H35O14 | 271, 340 | 2.7 | 18.1 | 703.2013, 749.2069 [M + HCO2H − H]− | 329.0657 (100), 373.1282 (41), 673.1911 (30), 343.1178 (18), 685.1909 (16) |
| 37 | apometzgerin | 12.12 | C17H13O7 | 269, 335 | −2.5 | 2.2 | 329.0675 | 314.0441 (100), 299.0206 (95), 271.0257 (11) |
| 38 | flavonolignan (tricin-lignan (m/z 194) conjugate) # | 12.33 | C27H23O11 | 271, 327 | 1.9 | 11.2 | 523.1236, 569.1291 [M + HCO2H − H]− | 329.0659 (100), 314.0422 (6) |
| 39 | flavonolignan (tricin derivative) # | 12.43 | C37H35O14 | 271, 340 | 2.8 | 14.1 | 703.2013, 749.2069 [M + HCO2H − H]− | 329.0658 (100), 373.1286 (53), 673.1913 (20), 343.1178 (17), 685.1911 (5) |
| No | Compound | Content [mg Standard eq/g DW] (Mean ± SD) | ||||||
|---|---|---|---|---|---|---|---|---|
| E1 (Total Extract) | E2 (Phenolic Acid Extract) | E3 (Flavonoid Extract) | Fr A (Luteolin) | Fr B (Philonotisflavone) | Fr C (Flavonolignans) | Fr D (Flavone Aglycones) | ||
| 1 | unidentified | + a | + | ND b | ND | ND | ND | ND |
| 2 | di-hexose (sucrose) | + | + | + | ND | ND | ND | ND |
| 3 | tri-hexose (raffinose) | + | + | + | ND | ND | ND | ND |
| 4 | unidentified | + | + | + | ND | ND | ND | ND |
| 5 | unidentified | + | + | ND | ND | ND | ND | ND |
| 6 | caffeoyl-di-O-hexoside | 6.02 ± 0.14 | 27.63 ± 0.20 | ND | ND | ND | ND | ND |
| 7 | di-hydroxy-benzoic acid hexoside | + | + | ND | ND | ND | ND | ND |
| 8 | caftaric acid | 4.97 ± 0.33 | ND | ND | ND | ND | ND | ND |
| 9 | unidentified | + | + | + | ND | ND | ND | ND |
| 10 | caffeoyl-O-hexoside | 1.02 ± 0.03 | 4.68 ± 0.26 | ND | ND | ND | ND | ND |
| 11 | 5-O-caffeoylquinic acid | 21.97 ± 0.56 | 92.50 ± 1.23 | 1.88 ± 0.06 | ND | ND | ND | ND |
| 12 | caffeic acid | 1.41 ± 0.03 | 2.60 ± 0.08 | 9.19 ± 0.14 | ND | ND | ND | ND |
| 13 | unidentified | + | ND | + | ND | ND | ND | ND |
| 14 | luteolin-O-di-hexoside | ++ c | 2.42 ± 0.05 | ND | ND | ND | ND | ND |
| 15 | L-chicoric acid | 104.60 ± 3.33 | 384.40 ± 4.43 | 1.41 ± 0.10 | ND | ND | ND | ND |
| 16 | luteolin-7-O-glucoside | 1.42 ± 0.06 | 1.81 ± 0.05 | 6.13 ± 0.09 | ND | ND | ND | ND |
| 17 | 3,5-di-caffeoylquinic acid | 7.54 ± 0.26 | 18.75 ± 0.50 | 17.19 ± 0.21 | ND | ND | ND | ND |
| 18 | luteolin-4’-O-glucoside | 1.00 ± 0.03 | 0.76 ± 0.04 | 3.85 ± 0.08 | ND | ND | ND | ND |
| 19 | luteolin-3’-O-glucoside | 0.64 ± 0.03 | ND | 4.46 ± 0.09 | ND | ND | ND | ND |
| 20 | taraxinic acid-1’-O-glucoside | + | ND | + | ND | ND | ND | ND |
| 21 | luteolin | 36.03 ± 1.12 | ND | 203.89 ± 2.58 | 985.86 ± 13.50 | ND | ND | ND |
| 22 | philonotisflavone | ND | ++ | 472.21 ± 1.51 | ND | ND | ||
| 23 | methyltricetin | ND | 8.56 ± 0.20 | ND | ND | ND | ||
| 24 | bi-flavone (luteolin-luteolin) | 0.85 ± 0.02 | ND | 3.73 ± 0.02 | ND | ND | ND | ND |
| 25 | bi-flavone (luteolin-apigenin) | ++ | ND | ++ | ND | 3.80 ± 0.22 | ND | ND |
| 26 | bi-flavone (luteolin-apigenin) | ++ | ND | 0.35 ± 0.03 | ND | 2.60 ± 0.09 | ND | ND |
| 27 | bi-flavone (luteolin-chrysoeriol) | ++ | ND | 1.50 ± 0.05 | ND | ++ | ND | ND |
| 28 | apigenin | 0.37 ± 0.04 | ND | 2.88 ± 0.18 | ND | ND | ND | 69.60 ± 1.10 |
| 29 | bi-flavone (luteolin-chrysoeriol) | ++ | ND | 1.20 ± 0.08 | ND | 5.44 ± 0.08 | ND | ND |
| 30 | flavonolignan (tricin-lignan (m/z 170) conjugate) | ++ | ND | ++ | ND | ND | 87.03 ± 5.18 | ND |
| 31 | tricin | 6.53 ± 0.17 | ND | 37.13 ± 0.41 | ND | ND | ND | 799.09 ± 8.19 |
| 32 | chrysoeriol | ND | ND | ND | ND | |||
| 33 | flavonolignan (salcolin A/B) | 0.75 ± 0.01 | ND | 4.97 ± 0.12 | ND | ND | 107.28 ± 6.60 | ND |
| 34 | flavonolignan (tricin-lignan (m/z 170) conjugate) | 0.77 ± 0.01 | ND | 4.57 ± 0.14 | ND | ND | 96.93 ± 5.80 | ND |
| 35 | flavonolignan (salcolin A/B) | 0.89 ± 0.03 | ND | 6.02 ± 0.21 | ND | ND | 109.89 ± 6.39 | ND |
| 36 | flavonolignan (tricin derivative) | ++ | ND | ++ | ND | ND | 4.37 ± 0.42 | ND |
| 37 | apometzgerin | ++ | ND | ++ | ND | ND | ND | 18.01 ± 0.80 |
| 38 | flavonolignan (tricin-lignan (m/z 194) conjugate) | ++ | ND | 0.62 ± 0.01 | ND | ND | 11.74 ± 0.67 | ND |
| 39 | flavonolignan (tricin derivative) | ++ | ND | ++ | ND | ND | ++ | ND |
| Total caffeic acid derivatives | 147.53 ± 4.68 | 530.56 ± 6.70 | 29.67 ± 0.51 | ND | ND | ND | ND | |
| Total flavonoids | 48.48 ± 1.52 | 4.99 ± 0.14 | 281.30 ± 4.03 | 994.42 ± 13.70 | 484.05 ± 1.90 | 417.24 ± 25.06 | 886.71 ± 10.09 | |
| Total phenolic compounds | 196.01 ± 6.20 | 535.55 ± 6.84 | 310.97 ± 4.60 | 994.42 ± 13.70 | 484.05 ± 1.90 | 417.24 ± 25.06 | 886.71 ± 10.09 | |
| Total phenolic content (mg GAE/g DW) | 187.70 ± 0.22 | 447.58 ± 2.21 | 377.42 ± 1.77 | 879.55 ± 2.76 | 516.13 ± 3.31 | 384.27 ± 2.21 | 631.71 ± 2.95 | |
| Sample | Trolox Equivalents (TE) | IC50 (mg DW/mL) |
|---|---|---|
| E1 (total extract) | 0.26 ± 0.00b | 0.424 ± 0.01d |
| E2 (phenolic acid extract) | 0.48 ± 0.00c | 0.215 ± 0.01c |
| E3 (flavonoid extract) | 0.55 ± 0.03d | 0.202 ± 0.01c |
| Fr A (luteolin) | 2.01 ± 0.01g | 0.055 ± 0.00a |
| Fr B (philonotisflavone) | 1.09 ± 0.01f | 0.099 ± 0.00b |
| Fr C (flavonolignans) | 0.06 ± 0.00a | 1.368 ± 0.04e |
| Fr D (flavone aglycones) | 0.05 ± 0.00a | 1.789 ± 0.04f |
| Trolox | 1.00e | 0.113 ± 0.00b |
| Sample Name | Tested Concentration (µg/mL) | (TT) | (PT) | (APTT) |
|---|---|---|---|---|
| Mean ± SD | ||||
| control | 0 | 15.5 ± 1.2 n.s. | 15.0 ± 0.6 n.s. | 43.1 ± 5.2 n.s. |
| E1 (total extract) | 10 | 15.5 ± 1.6 n.s. | 14.9 ± 0.5 n.s. | 43.5 ± 4.8 n.s. |
| 50 | 15.6 ± 1.6 n.s. | 14.8 ± 0.5 n.s. | 43.4 ± 5.2 n.s. | |
| E2 (phenolic acid extract) | 10 | 15.3 ± 1.5 n.s. | 14.8 ± 0.6 n.s. | 42.6 ± 4.4 n.s. |
| 50 | 15.4 ± 1.5 n.s. | 14.9 ± 0.6 n.s. | 42.9 ± 4.7 n.s. | |
| E3 (flavonoid extract) | 10 | 15.5 ± 1.4 n.s. | 14.7 ± 0.6 n.s. | 43.1 ± 4.5 n.s. |
| 50 | 15.4 ± 1.5 n.s. | 15.0 ± 0.4 n.s. | 43.3 ± 4.5 n.s. | |
| Fr A (luteolin) | 10 | 15.5 ± 1.3 n.s. | 14.9 ± 0.4 n.s. | 42.8 ± 4.3 n.s. |
| 50 | 15.3 ± 1.5 n.s. | 14.9 ± 0.5 n.s. | 42.5 ± 4.4 n.s. | |
| Fr B (philonotisflavone) | 10 | 15.5 ± 1.4 n.s. | 15.1 ± 0.7 n.s. | 42.5 ± 4.3 n.s. |
| 50 | 15.3 ± 1.3 n.s. | 14.9 ± 0.7 n.s. | 43.1 ± 4.4 n.s. | |
| Fr C (flavonolignans) | 10 | 15.4 ± 1.3 n.s. | 15.0 ± 0.6 n.s. | 42.3 ± 4.5 n.s. |
| 50 | 15.7 ± 1.3 n.s. | 15.1 ± 0.7 n.s. | 42.6 ± 4.4 n.s. | |
| Fr D (flavone aglycones) | 10 | 15.6 ± 1.2 n.s. | 15.1 ± 0.6 n.s. | 42.3 ± 5.0 n.s. |
| 50 | 15.7 ± 1.1 n.s. | 15.2 ± 0.6 n.s. | 42.7 ± 5.5 n.s. |
| Experiment | E1 (Total Extract) | E2 (Phenolic Acid Extract) | E3 (Flavonoid Extract) | Fr A (Luteolin) | Fr B (Philonotisflavone) | Fr C (Flavonolignans) | Fr D (Flavone Aglycones) |
|---|---|---|---|---|---|---|---|
| Plasma | |||||||
| Lipid peroxidation induced by H2O2/Fe | Positive action (antioxidative potential) | Positive action (antioxidative potential) | Positive action (antioxidative potential) | Positive action (antioxidative potential) | No effect | No effect | Positive action (antioxidative potential) |
| Oxidation of protein thiols induced by H2O2/Fe | No effect | No effect | No effect | No effect | Positive action (antioxidative potential) | No effect | No effect |
| Protein carbonylation induced by H2O2/Fe | Positive action (antioxidative potential) | Positive action (antioxidative potential) | Positive action (antioxidative potential) | Positive action (antioxidative potential) | Positive action (antioxidative potential) | Positive action (antioxidative potential) | Positive action (antioxidative potential) |
| Blood platelets | |||||||
| Lipid peroxidation in resting platelets | No effect | No effect | No effect | No effect | No effect | No effect | No effect |
| Lipid peroxidation in thrombin-activated platelets | No effect | No effect | Positive action (anti-platelet potential) | Positive action (anti-platelet potential) | Positive action (anti-platelet potential) | Positive action (anti-platelet potential) | Positive action (anti-platelet potential) |
| Lipid peroxidation in platelets treated with H2O2/Fe | Positive action (antioxidative potential) | Positive action (antioxidative potential) | Positive action (antioxidative potential) | Positive action (antioxidative potential) | Positive action (antioxidative potential) | Positive action (antioxidative potential) | Positive action (antioxidative potential) |
| Oxidation of protein thiols in platelets treated with H2O2/Fe | No effect | No effect | Positive action (antioxidative potential) | Positive action (antioxidative potential) | No effect | No effect | No effect |
| Protein carbonylation in platelets treated with H2O2/Fe | Positive action (antioxidative potential) | Positive action (antioxidative potential) | No effect | No effect | No effect | No effect | No effect |
| Adhesion of thrombin-activated platelets to fibrinogen | No effect | Positive action (anti-platelet potential) | Positive action (anti-platelet potential) | Positive action (anti-platelet potential) | Positive action (anti-platelet potential) | Positive action (anti-platelet potential) | No effect |
| Adhesion of ADP-activated platelets to fibrinogen | No effect | No effect | No effect | Positive action (anti-platelet potential) | Positive action (anti-platelet potential) | Positive action (anti-platelet potential) | No effect |
| GPIIb/IIIa expression–non-stimulated platelets | ND | No effect | No effect | No effect | ND | ND | ND |
| GPIIb/IIIa expression–platelets activated by 10 µM ADP | ND | No effect | No effect | No effect | ND | ND | ND |
| GPIIb/IIIa expression–platelets activated by 20 µM ADP | ND | Positive action (anti-platelet potential) | No effect | No effect | ND | ND | ND |
| GPIIb/IIIa expression–platelets activated by 10 µg/mL collagen | ND | Positive action (anti-platelet potential) | No effect | Positive action (anti-platelet potential) | ND | ND | ND |
| P-selectin expression–non-stimulated platelets | ND | No effect | No effect | No effect | ND | ND | ND |
| P-selectin expression–platelets activated by 10 µM ADP | ND | No effect | No effect | Positive action (anti-platelet potential) | ND | ND | ND |
| P-selectin expression–platelets activated by 20 µM ADP | ND | No effect | No effect | No effect | ND | ND | ND |
| P-selectin expression–platelets activated by 10 µg/mL collagen | ND | Positive action (anti-platelet potential) | No effect | Positive action (anti-platelet potential) | ND | ND | ND |
Sample Availability: Samples of dandelion fruit preparations E1–E3 and A–D are available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lis, B.; Jedrejek, D.; Rywaniak, J.; Soluch, A.; Stochmal, A.; Olas, B. Flavonoid Preparations from Taraxacum officinale L. Fruits—A Phytochemical, Antioxidant and Hemostasis Studies. Molecules 2020, 25, 5402. https://doi.org/10.3390/molecules25225402
Lis B, Jedrejek D, Rywaniak J, Soluch A, Stochmal A, Olas B. Flavonoid Preparations from Taraxacum officinale L. Fruits—A Phytochemical, Antioxidant and Hemostasis Studies. Molecules. 2020; 25(22):5402. https://doi.org/10.3390/molecules25225402
Chicago/Turabian StyleLis, Bernadetta, Dariusz Jedrejek, Joanna Rywaniak, Agata Soluch, Anna Stochmal, and Beata Olas. 2020. "Flavonoid Preparations from Taraxacum officinale L. Fruits—A Phytochemical, Antioxidant and Hemostasis Studies" Molecules 25, no. 22: 5402. https://doi.org/10.3390/molecules25225402
APA StyleLis, B., Jedrejek, D., Rywaniak, J., Soluch, A., Stochmal, A., & Olas, B. (2020). Flavonoid Preparations from Taraxacum officinale L. Fruits—A Phytochemical, Antioxidant and Hemostasis Studies. Molecules, 25(22), 5402. https://doi.org/10.3390/molecules25225402






