Effect of Heat Exposure on Activity Degradation of Enzymes in Mango Varieties Sindri, SB Chaunsa, and Tommy Atkins during Drying
Abstract
1. Introduction
2. Results and Discussion
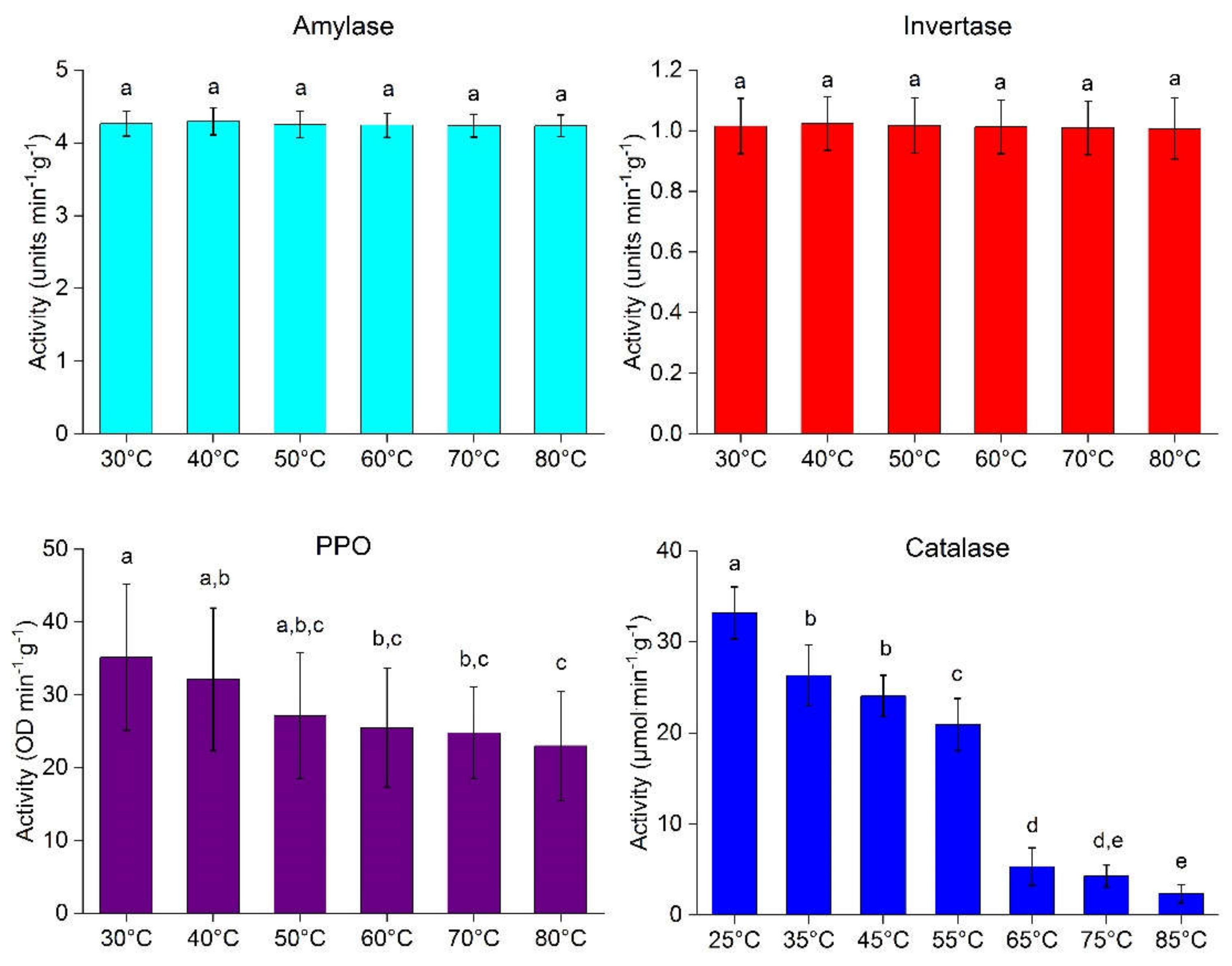
2.1. Effect of Incubation Temperature on Enzyme Activity in Fresh Mango
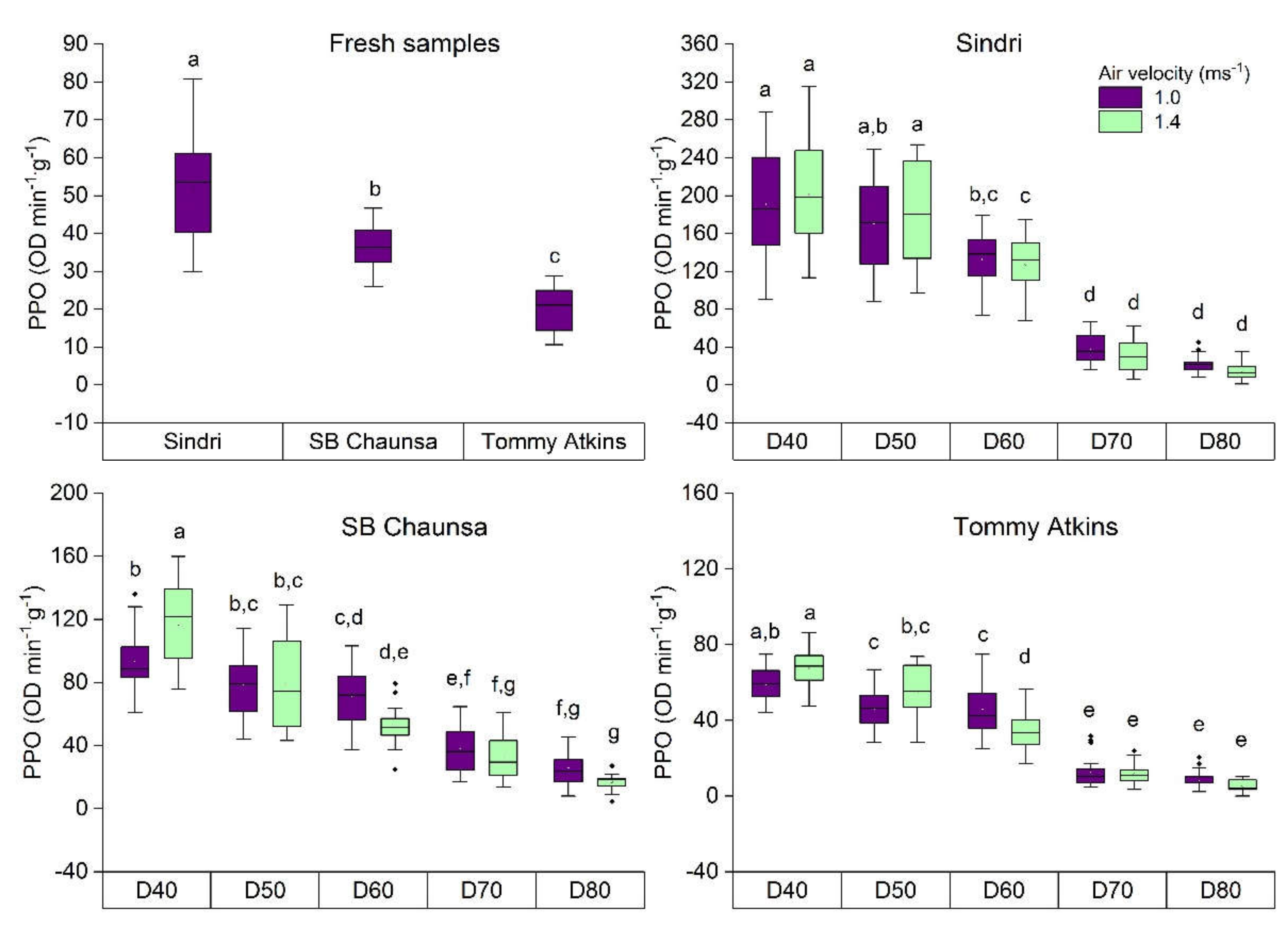
2.2. Polyphenoloxidase (PPO) Activity of Fresh and Dried Mango
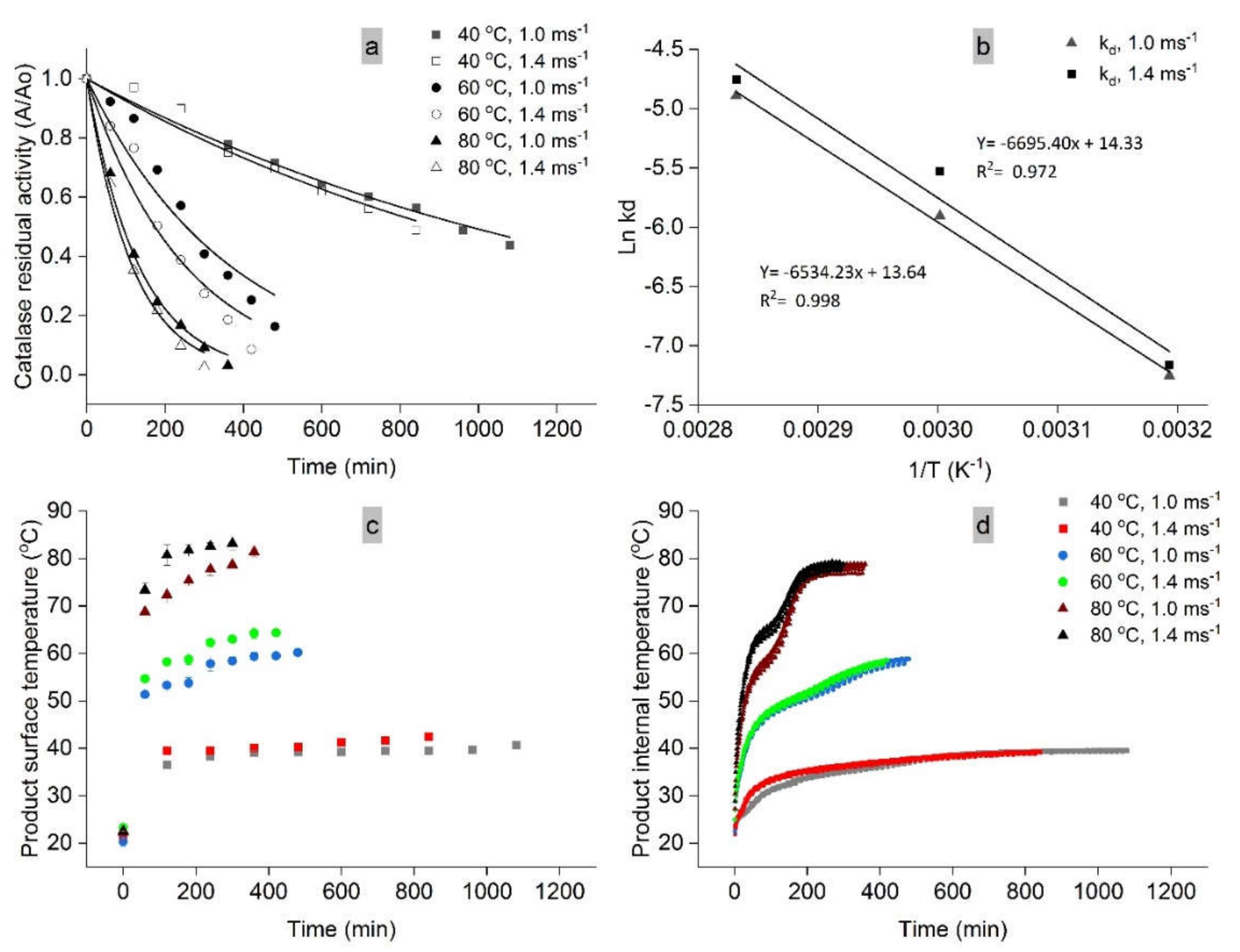
2.3. Catalase Activity of Fresh and Dried Mango
2.4. Drying Kinetics of Catalase Activity
3. Materials and Methods
3.1. Mango Varieties
3.2. Enzyme Activity Assays
3.2.1. Catalase
3.2.2. Polyphenol Oxidase (PPO)
3.2.3. Amylase
3.2.4. Invertase
3.3. Incubation Trials to Determine the Heat-Sensitivity of Enzymes in Fresh Mango
3.4. Hot Air Convective Thin-Layer Drying of Mango Slabs
3.5. Measurement of Enzyme Activity in Fresh and Dried Mango
3.6. Modeling of Enzyme Activity Kinetics during Drying
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Temperature | Mean Difference | Std. Error | Sig. | 95% Confidence Interval | ||
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| 30 °C | 40 °C | 3.0372 | 2.83829 | 0.892 | −5.2069 | 11.2814 |
| 50 °C | 8.0367 | 2.83829 | 0.060 | −0.2075 | 16.2808 | |
| 60 °C | 9.6667 * | 2.83829 | 0.012 | 1.4225 | 17.9108 | |
| 70 °C | 10.3822 * | 2.83829 | 0.005 | 2.1381 | 18.6264 | |
| 80 °C | 12.1972 * | 2.83829 | 0.001 | 3.9531 | 20.4414 | |
| 40 °C | 30 °C | −3.0372 | 2.83829 | 0.892 | −11.2814 | 5.2069 |
| 50 °C | 4.9994 | 2.83829 | 0.495 | −3.2447 | 13.2436 | |
| 60 °C | 6.6294 | 2.83829 | 0.190 | −1.6147 | 14.8736 | |
| 70 °C | 7.3450 | 2.83829 | 0.110 | −0.8991 | 15.5891 | |
| 80 °C | 9.1600 * | 2.83829 | 0.020 | 0.9159 | 17.4041 | |
| 50 °C | 30 °C | −8.0367 | 2.83829 | 0.060 | −16.2808 | 0.2075 |
| 40 °C | −4.9994 | 2.83829 | 0.495 | −13.2436 | 3.2447 | |
| 60 °C | 1.6300 | 2.83829 | 0.992 | −6.6141 | 9.8741 | |
| 70 °C | 2.3456 | 2.83829 | 0.962 | −5.8986 | 10.5897 | |
| 80 °C | 4.1606 | 2.83829 | 0.687 | −4.0836 | 12.4047 | |
| 60 °C | 30 °C | −9.6667 * | 2.83829 | 0.012 | −17.9108 | −1.4225 |
| 40 °C | −6.6294 | 2.83829 | 0.190 | −14.8736 | 1.6147 | |
| 50 °C | −1.6300 | 2.83829 | 0.992 | −9.8741 | 6.6141 | |
| 70 °C | 0.7156 | 2.83829 | 1.000 | −7.5286 | 8.9597 | |
| 80 °C | 2.5306 | 2.83829 | 0.948 | −5.7136 | 10.7747 | |
| 70 °C | 30 °C | −10.3822 * | 2.83829 | 0.005 | −18.6264 | −2.1381 |
| 40 °C | −7.3450 | 2.83829 | 0.110 | −15.5891 | 0.8991 | |
| 50 °C | −2.3456 | 2.83829 | 0.962 | −10.5897 | 5.8986 | |
| 60 °C | −0.7156 | 2.83829 | 1.000 | −8.9597 | 7.5286 | |
| 80 °C | 1.8150 | 2.83829 | 0.988 | −6.4291 | 10.0591 | |
| 80 °C | 30 °C | −12.1972 * | 2.83829 | 0.001 | −20.4414 | −3.9531 |
| 40 °C | −9.1600 * | 2.83829 | 0.020 | −17.4041 | −0.9159 | |
| 50 °C | −4.1606 | 2.83829 | 0.687 | −12.4047 | 4.0836 | |
| 60 °C | −2.5306 | 2.83829 | 0.948 | −10.7747 | 5.7136 | |
| 70 °C | −1.8150 | 2.83829 | 0.988 | −10.0591 | 6.4291 | |
| Temperature | Mean Difference | Std. Error | Sig. | 95% Confidence Interval | ||
|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | |||||
| 25 °C | 35 °C | 6.8794 * | 0.78998 | 0.000 | 4.5100 | 9.2489 |
| 45 °C | 9.1500 * | 0.78998 | 0.000 | 6.7805 | 11.5195 | |
| 55 °C | 12.2600 * | 0.78998 | 0.000 | 9.8905 | 14.6295 | |
| 65 °C | 27.8828 * | 0.78998 | 0.000 | 25.5133 | 30.2523 | |
| 75 °C | 28.9306 * | 0.78998 | 0.000 | 26.5611 | 31.3000 | |
| 85 °C | 30.8728 * | 0.78998 | 0.000 | 28.5033 | 33.2423 | |
| 35 °C | 25 °C | −6.8794 * | 0.78998 | 0.000 | −9.2489 | −4.5100 |
| 45 °C | 2.2706 | 0.78998 | 0.070 | −0.0989 | 4.6400 | |
| 55 °C | 5.3806 * | 0.78998 | 0.000 | 3.0111 | 7.7500 | |
| 65 °C | 21.0033 * | 0.78998 | 0.000 | 18.6339 | 23.3728 | |
| 75 °C | 22.0511 * | 0.78998 | 0.000 | 19.6816 | 24.4206 | |
| 85 °C | 23.9933 * | 0.78998 | 0.000 | 21.6239 | 26.3628 | |
| 45 °C | 25 °C | −9.1500 * | 0.78998 | 0.000 | −11.5195 | −6.7805 |
| 35 °C | −2.2706 | 0.78998 | 0.070 | −4.6400 | 0.0989 | |
| 55 °C | 3.1100 * | 0.78998 | 0.003 | 0.7405 | 5.4795 | |
| 65 °C | 18.7328 * | 0.78998 | 0.000 | 16.3633 | 21.1023 | |
| 75 °C | 19.7806 * | 0.78998 | 0.000 | 17.4111 | 22.1500 | |
| 85 °C | 21.7228 * | 0.78998 | 0.000 | 19.3533 | 24.0923 | |
| 55 °C | 25 °C | −12.2600 * | 0.78998 | 0.000 | −14.6295 | −9.8905 |
| 35 °C | −5.3806 * | 0.78998 | 0.000 | −7.7500 | −3.0111 | |
| 45 °C | −3.1100 * | 0.78998 | 0.003 | −5.4795 | −0.7405 | |
| 65 °C | 15.6228 * | 0.78998 | 0.000 | 13.2533 | 17.9923 | |
| 75 °C | 16.6706 * | 0.78998 | 0.000 | 14.3011 | 19.0400 | |
| 85 °C | 18.6128 * | 0.78998 | 0.000 | 16.2433 | 20.9823 | |
| 65 °C | 25 °C | −27.8828 * | 0.78998 | 0.000 | −30.2523 | −25.5133 |
| 35 °C | −21.0033 * | 0.78998 | 0.000 | −23.3728 | −18.6339 | |
| 45 °C | −18.7328 * | 0.78998 | 0.000 | −21.1023 | −16.3633 | |
| 55 °C | −15.6228 * | 0.78998 | 0.000 | −17.9923 | −13.2533 | |
| 75 °C | 1.0478 | 0.78998 | 0.838 | −1.3217 | 3.4173 | |
| 85 °C | 2.9900 * | 0.78998 | 0.004 | 0.6205 | 5.3595 | |
| 75 °C | 25 °C | −28.9306 * | 0.78998 | 0.000 | −31.3000 | −26.5611 |
| 35 °C | −22.0511 * | 0.78998 | 0.000 | −24.4206 | −19.6816 | |
| 45 °C | −19.7806 * | 0.78998 | 0.000 | −22.1500 | −17.4111 | |
| 55 °C | −16.6706 * | 0.78998 | 0.000 | −19.0400 | −14.3011 | |
| 65 °C | −1.0478 | 0.78998 | 0.838 | −3.4173 | 1.3217 | |
| 85 °C | 1.9422 | 0.78998 | 0.184 | −0.4273 | 4.3117 | |
| 85 °C | 25 °C | −30.8728 * | 0.78998 | 0.000 | −33.2423 | −28.5033 |
| 35 °C | −23.9933 * | 0.78998 | 0.000 | −26.3628 | −21.6239 | |
| 45 °C | −21.7228 * | 0.78998 | 0.000 | −24.0923 | −19.3533 | |
| 55 °C | −18.6128 * | 0.78998 | 0.000 | −20.9823 | −16.2433 | |
| 65 °C | −2.9900 * | 0.78998 | 0.004 | −5.3595 | −0.6205 | |
| 75 °C | −1.9422 | 0.78998 | 0.184 | −4.3117 | 0.4273 | |
References
- Tharanathan, R.N.; Yashoda, H.M.; Prabha, T.N. Mango (Mangifera indica L.), “The king of fruits”—An overview. Food Rev. Int. 2006, 22, 95–123. [Google Scholar] [CrossRef]
- FAO. Food and Agriculture Commodities Production. 2018. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 4 June 2020).
- Mitra, S.K. Mango production in the world—Present situation and future prospect. Acta Hortic. 2016, 1111, 287–296. [Google Scholar] [CrossRef]
- Niranjana, P.; Gopalakrishna, K.; Sudhakar, D.; Madhusudhan, B. Effect of pre-cooling and heat treatment on antioxidant enzymes profile of mango and banana. Afr. J. Food Agric. Nutr. Dev. 2009, 9, 1210–1225. [Google Scholar] [CrossRef]
- Hossain, M.A.; Rana, M.M.; Kimura, Y.; Roslan, H.A. Changes in biochemical characteristics and activities of ripening associated enzymes in mango fruit during the storage at different temperatures. Biomed. Res. Int. 2014, 14, 1–11. [Google Scholar] [CrossRef]
- Matés, J.M.; Pérez-Gómez, C.; de Castro, I.N. Antioxidant enzymes and human diseases. Clin. Biochem. 1999, 32, 595–603. [Google Scholar]
- Janiak, M.C. Digestive enzymes of human and nonhuman primates. Evol. Anthropol. 2016, 25, 253–266. [Google Scholar] [CrossRef]
- Putra, R.N.; Ajiwiguna, T.A. Influence of air temperature and velocity for drying process. Procedia Eng. 2017, 170, 516–519. [Google Scholar] [CrossRef]
- Elamin, O.M.A. Effect of drying temperature on some quality attributes of mango slices. Int. J. Innov. Sci. Res. 2014, 4, 91–99. [Google Scholar]
- Kowalski, S.J.; Łechtańska, J.M.; Szadzińska, J. Quality aspects of fruit and vegetables dried convectively with osmotic pretreatment. Chem. Process Eng. 2013, 34, 51–62. [Google Scholar] [CrossRef]
- Mercer, D. A comparison of the kinetics of mango drying in open-air, solar, and forced-air dryers. Afr. J. Food Agric. Nutr. 2013, 12, 6835–6852. [Google Scholar] [CrossRef]
- Kanyinda, J.N.M. Comparison of two drying methods of mango (oven and solar drying). MOJ Food Process. Technol. 2017, 5, 1–4. [Google Scholar] [CrossRef]
- López, J.; Uribe, E.; Vega-Gálvez, A.; Miranda, M.; Vergara, J.; Gonzalez, E.; Di Scala, K. Effect of air temperature on drying kinetics, vitamin c, antioxidant activity, total phenolic content, non-enzymatic browning and firmness of blueberries variety óneil. Food Bioprocess Technol. 2010, 3, 772–777. [Google Scholar] [CrossRef]
- Leite, J.B.; Mancini, M.C.; Borges, S.V. Effect of drying temperature on the quality of dried bananas cv. prata and d’água. LWT Food Sci. Technol. 2007, 40, 319–323. [Google Scholar] [CrossRef]
- Maskan, M. Drying, shrinkage and rehydration characteristics of kiwifruits during hot air and microwave drying. J. Food Eng. 2001, 48, 177–182. [Google Scholar] [CrossRef]
- Udomkun, P.; Argyropoulos, D.; Nagle, M.; Mahayothee, B.; Janjai, S.; Müller, J. Single layer drying kinetics of papaya amidst vertical and horizontal airflow. LWT Food Sci. Technol. 2015, 64, 67–73. [Google Scholar] [CrossRef]
- Vega-Gálvez, A.; Ah-Hen, K.; Chacana, M.; Vergara, J.; Martínez-Monzó, J.; García-Segovia, P.; Lemus-Mondaca, R.; di Scala, K. Effect of temperature and air velocity on drying kinetics, antioxidant capacity, total phenolic content, colour, texture and microstructure of apple (var. Granny Smith) slices. Food Chem. 2012, 132, 51–59. [Google Scholar] [CrossRef]
- Guarte, R.C.; Pott, I.; Mühlbauer, W. Influence of drying parameters on β-carotene retention in mango leather. Fruits 2005, 60, 255–265. [Google Scholar] [CrossRef][Green Version]
- Russo, P.; Adiletta, G.; Matteo, M.D.; Farina, V.; Corona, O.; Cinquanta, L. Drying kinetics and physico-chemical quality of mango slices. Chem. Eng. Trans. 2019, 75, 109–114. [Google Scholar]
- Adepoju, L.A.; Osunde, Z.D. Effect of pretreatments and drying methods on some qualities of dried mango (Mangifera indica) fruit. Agric. Eng. Int. CIGR J. 2017, 19, 187–194. [Google Scholar]
- Omolola, A.O.; Jideani, A.I.O.; Kapila, P.F. Quality properties of fruits as affected by drying operation. Crit. Rev. Food Sci. Nutr. 2017, 57, 95–108. [Google Scholar] [CrossRef]
- Izli, N.; Izli, G.; Taskin, O. Influence of different drying techniques on drying parameters of mango. Food Sci. Technol. 2017, 37, 604–612. [Google Scholar] [CrossRef]
- Sehrawat, R.; Nema, P.K.P.K.; Kaur, B.P.B.P. Quality evaluation and drying characteristics of mango cubes dried using low-pressure superheated steam, vacuum and hot air drying methods. LWT 2018, 92, 548–555. [Google Scholar] [CrossRef]
- Yao, L.; Fan, L.; Duan, Z. Effect of different pretreatments followed by hot-air and far-infrared drying on the bioactive compounds, physicochemical property and microstructure of mango slices. Food Chem. 2020, 305, 125477. [Google Scholar] [CrossRef] [PubMed]
- Mattoo, A.K.; Modi, V.V. Ethylene and ripening of mangoes. Plant Physiol. 1969, 44, 308–310. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cheema, S.; Sommerhalter, M. Characterization of polyphenol oxidase activity in Ataulfo mango. Food Chem. 2015, 171, 382–387. [Google Scholar] [CrossRef]
- De Lima, L.C.O.; Chitarra, A.B.; Chitarra, M.I.F. Changes in amylase activity starch and sugars contents in mango fruits pulp Cv. Tommy Atkins with spongy tissue. Braz. Arch. Biol. Technol. 2001, 44, 59–62. [Google Scholar] [CrossRef]
- Castrillo, M.; Kruger, N.J.; Whatley, F.R. Sucrose metabolism in mango fruit during ripening. Plant Sci. 1992, 84, 45–51. [Google Scholar] [CrossRef]
- Mehrnoush, A.; Mohd Yazid, A.M. Characterization of novel amylase enzyme from mango (Mangifera indica cv. Chokanan) peel. J. Food Agric. Environ. 2013, 11, 47–50. [Google Scholar]
- Alegre, A.; Polizeli, M.D.L.; Terenzi, H.; Jorge, J.; Guimarães, L. Production of thermostable invertases by Aspergillus caespitosus under submerged or solid state fermentation using agroindustrial residues as carbon source. Braz. J. Microbiol. 2009, 40, 612–622. [Google Scholar] [CrossRef]
- Holderbaum, D.F. Enzymatic browning, polyphenol oxidase activity, and polyphenols in four apple cultivars: Dynamics during fruit development. HortScience 2010, 45, 1150–1154. [Google Scholar] [CrossRef]
- Korbel, E.; Servent, A.; Billaud, C.; Brat, P. Heat inactivation of polyphenol oxidase and peroxidase as a function of water activity: A case study of mango drying. Dry. Technol. 2013, 31, 1675–1680. [Google Scholar] [CrossRef]
- Illera, A.E.; Sanz, M.T.; Benito-Román, O.; Varona, S.; Beltrán, S.; Melgosa, R.; Solaesa, A.G. Effect of thermosonication batch treatment on enzyme inactivation kinetics and other quality parameters of cloudy apple juice. Innov. Food Sci. Emerg. Technol. 2018, 47, 71–80. [Google Scholar] [CrossRef]
- Wang, C.Y. Effect of temperature preconditioning on catalase, peroxidase, and superoxide dismutase in chilled zucchini squash. Postharvest Biol. Technol. 1995, 5, 67–76. [Google Scholar] [CrossRef]
- Vishwasrao, C.; Ananthanarayan, L. Kinetics of inactivation of quality-deteriorating enzymes and degradation of selective phytoconstituents in pink guava pulp during thermal processing. J. Food Sci. Technol. 2018, 55, 3273–3280. [Google Scholar] [CrossRef] [PubMed]
- Ndiaye, C.; Xu, S.Y.; Wang, Z. Steam blanching effect on polyphenoloxidase, peroxidase and colour of mango (Mangifera indica L.) slices. Food Chem. 2009, 113, 92–95. [Google Scholar] [CrossRef]
- Andrade, N.; Araújo, J.R.; Correia-Branco, A.; Carletti, J.V.; Martel, F. Effect of dietary polyphenols on fructose uptake by human intestinal epithelial (Caco-2) cells. J. Funct. Foods 2017, 36, 429–439. [Google Scholar] [CrossRef]
- Lule, S.U.; Xia, W. Food phenolics, pros and cons: A review. Food Rev. Int. 2005, 21, 367–388. [Google Scholar] [CrossRef]
- Calhau, C.; Faria, A.; Keating, E.; Martel, F. Interaction of polyphenols with the Intestinal and Placental absorption of some nutrients and other compounds. Polyphen. Hum. Health Dis. 2013, 1, 523–536. [Google Scholar]
- Selvarajan, E.; Veena, R.; Manoj Kumar, N. Polyphenol oxidase, beyond enzyme browning. Microb. Bioprospect. Sustain. Dev. 2018, 203–222. [Google Scholar] [CrossRef]
- Esposito, E.; Durán, N. Potential applications of oxidative enzymes and phenoloxidase-like compounds in wastewater and soil treatment: A review. Appl. Catal. B Environ. 2000, 28, 83–99. [Google Scholar]
- Boonyaritthongchai, P.; Supapvanich, S.; Wongaree, C.; Uthairatanakij, A.; Jitareerat, P.; Pongprasert, N.; Kaewmanee, N. Application of natural extracts from pineapple juice on inhibiting browning symptom of fresh-cut “Nam Dok Mai” mango. Acta Hortic. 2018, 1210, 235–240. [Google Scholar] [CrossRef]
- Mondal, K.; Malhotra, S.P.; Jain, V.; Singh, R. Oxidative stress and antioxidant systems in Guava (Psidium guajava L.) fruits during ripening. Physiol. Mol. Biol. Plants 2009, 15, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, S.; Mallik, A.K. A study of the catalase reaction, with special reference to respiration in plants. New Phytol. 1931, 30, 355. [Google Scholar] [CrossRef]
- Peterson, M.E.; Daniel, R.M.; Danson, M.J.; Eisenthal, R. The dependence of enzyme activity on temperature: Determination and validation of parameters. Biochem. J. 2007, 402, 331–337. [Google Scholar] [CrossRef]
- Chong, C.H.; Law, C.L.; Cloke, M.; Hii, C.L.; Abdullah, L.C.; Daud, W.R.W. Drying kinetics and product quality of dried Chempedak. J. Food Eng. 2008, 88, 522–527. [Google Scholar] [CrossRef]
- Guiné, R.P.F.; Fernandes, R.M.C. Analysis of the drying kinetics of chestnuts. J. Food Eng. 2006, 76, 460–467. [Google Scholar] [CrossRef]
- Vinson, J.A.; Zubik, L.; Bose, P.; Samman, N.; Proch, J. Dried fruits: Excellent in vitro and in vivo antioxidants. J. Am. Coll. Nutr. 2005, 24, 44–50. [Google Scholar] [CrossRef]
- Djendoubi Mrad, N.; Boudhrioua, N.; Kechaou, N.; Courtois, F.; Bonazzi, C. Influence of air drying temperature on kinetics, physicochemical properties, total phenolic content and ascorbic acid of pears. Food Bioprod. Process. 2012, 90, 433–441. [Google Scholar] [CrossRef]
- Erenturk, S.; Gulaboglu, M.S.; Gultekin, S. The effects of cutting and drying medium on the vitamin C content of rosehip during drying. J. Food Eng. 2005, 68, 513–518. [Google Scholar] [CrossRef]
- Bennett, L.E.; Jegasothy, H.; Konczak, I.; Frank, D.; Sudharmarajan, S.; Clingeleffer, P.R. Total polyphenolics and anti-oxidant properties of selected dried fruits and relationships to drying conditions. J. Funct. Foods 2011, 3, 115–124. [Google Scholar] [CrossRef]
- Nandi, A.; Yan, L.J.; Jana, C.K.; Das, N. Role of catalase in oxidative stress and age associated degenerative diseases. Oxid. Med. Cell. Longev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Ratti, C. Hot air and freeze-drying of high-value foods: A review. J. Food Eng. 2001, 49, 311–319. [Google Scholar] [CrossRef]
- De Jesus, S.S.; Maciel Filho, R. Drying of α-amylase by spray drying and freeze-drying—A comparative study. Braz. J. Chem. Eng. 2014, 31, 625–631. [Google Scholar] [CrossRef]
- Bassetti, F.J.; Bergamasco, R.; Moraes, F.F.; Zanin, G.M. Thermal stability and deactivation energy of free and immobilized invertase. Braz. J. Chem. Eng. 2000, 17, 867–872. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, W.; Bi, Y.; Luo, Y. Postharvest BTH treatment induces resistance of peach (Prunus persica L. cv. Jiubao) fruit to infection by Penicillium expansum and enhances activity of fruit defense mechanisms. Postharvest Biol. Technol. 2005, 35, 263–269. [Google Scholar] [CrossRef]
- Vamos-Vigyázó, L. Polyphenol oxidase and peroxidase in fruits and vegetables. Crit. Rev. Food Sci. Nutr. 1981, 15, 49–127. [Google Scholar] [CrossRef]
- Zakir, H.M.; Fardush, J.; Hossain, M.S.; Islam, M.Z.; Shariar, S.M.S.; Rokshana, K.; Hossian, M. Effects of storage temperatures on different biochemical characteristics of 1-methylcyclopropene treated mango (Mangifera Indica L.) variety Khirshapat. Am. J. Food Sci. Technol. 2018, 6, 76–82. [Google Scholar] [CrossRef]
- Doǧaç, Y.I.; Çinar, M.; Teke, M. Improving of catalase stability properties by encapsulation in alginate/Fe3O4 magnetic composite beads for enzymatic removal of H2O2. Prep. Biochem. Biotechnol. 2015, 45, 144–157. [Google Scholar] [CrossRef]
- Zhang, X.; Shao, X. Characterisation of polyphenol oxidase and peroxidase and the role in browning of Loquat fruit. Czech J. Food Sci 2016, 33, 109–117. [Google Scholar] [CrossRef]
- Cordeiro, C.A.M.; Martins, M.L.L.; Luciano, A.B. Production and properties of alpha-amylase from thermophilic Bacillus sp. Braz. J. Microbiol. 2002, 33, 57–61. [Google Scholar] [CrossRef]
- Effect of pH and Temperature on the Rate of Reaction Catalysed by Yeast Invertase (Sucrase)|Fresh Nutrition. Available online: https://savannajackson.wordpress.com/2016/06/16/effect-of-ph-and-temperature-on-the-rate-of-reaction-catalysed-by-yeast-invertase-sucrase/ (accessed on 4 November 2020).
- Nurul Izzah, A. Characterization and Thermostability Study of Invertase by Aspergillus niger in Submerged Culture. Bachelor Thesis, University Malaysia Pahang, Pekan, Malaysia, 2013. [Google Scholar]
- Argyropoulos, D.; Heindl, A.; Müller, J. Assessment of convection, hot-air combined with microwave-vacuum and freeze-drying methods for mushrooms with regard to product quality. Int. J. Food Sci. Technol. 2011, 46, 333–342. [Google Scholar] [CrossRef]
- AOAC Int. Official Methods of Analysis, 17th ed.; AOAC Int.: Arlington, VA, USA, 2000. [Google Scholar]
- Segel, I.H. Enzyme Kinetics; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 216–220. [Google Scholar]
- Li, R.; Shang, H.; Wu, H.; Wang, M.; Duan, M.; Yang, J. Thermal inactivation kinetics and effects of drying methods on the phenolic profile and antioxidant activities of chicory (Cichorium intybus L.) leaves. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

| Mango Varieties Fresh Samples | Drying Duration (Min) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brix | MC | aw | 40 °C | 50 °C | 60 °C | 70 °C | 80 °C | ||||||
| (% wb) | 1.0 ms−1 | 1.4 ms−1 | 1.0 ms−1 | 1.4 ms−1 | 1.0 ms−1 | 1.4 ms−1 | 1.0 ms−1 | 1.4 ms−1 | 1.0 ms−1 | 1.4 ms−1 | |||
| Sindri | 17.33 b ± 1.71 | 86.96 b ± 2.05 | 0.922 b ± 0.012 | 915 ± 28.99 | 750 ± 26.87 | 660 ± 23.33 | 540 ± 20.51 | 465 ± 17.68 | 410 ± 16.97 | 350 ± 15.56 | 300 ± 12.73 | 280 ± 10.61 | 250 ± 9.90 |
| SB Chaunsa | 20.69 a ± 1.46 | 82.12 c ± 2.62 | 0.920 b ± 0.010 | 1155 ± 36.77 | 960 ± 31.11 | 750 ± 28.28 | 615 ± 25.46 | 540 ± 22.63 | 465 ± 21.21 | 390 ± 18.38 | 330 ± 16.26 | 310 ± 13.44 | 280 ± 11.31 |
| Tommy Atkins | 13.95 c ± 2.81 | 89.08 a ± 2.22 | 0.937 a ± 0.012 | 825 ± 26.16 | 705 ± 24.04 | 570 ± 21.92 | 465 ± 19.80 | 410 ± 14.14 | 360 ± 14.85 | 310 ± 12.73 | 270 ± 12.02 | 240 ± 7.78 | 210 ± 8.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukhtar, A.; Latif, S.; Mueller, J. Effect of Heat Exposure on Activity Degradation of Enzymes in Mango Varieties Sindri, SB Chaunsa, and Tommy Atkins during Drying. Molecules 2020, 25, 5396. https://doi.org/10.3390/molecules25225396
Mukhtar A, Latif S, Mueller J. Effect of Heat Exposure on Activity Degradation of Enzymes in Mango Varieties Sindri, SB Chaunsa, and Tommy Atkins during Drying. Molecules. 2020; 25(22):5396. https://doi.org/10.3390/molecules25225396
Chicago/Turabian StyleMukhtar, Adnan, Sajid Latif, and Joachim Mueller. 2020. "Effect of Heat Exposure on Activity Degradation of Enzymes in Mango Varieties Sindri, SB Chaunsa, and Tommy Atkins during Drying" Molecules 25, no. 22: 5396. https://doi.org/10.3390/molecules25225396
APA StyleMukhtar, A., Latif, S., & Mueller, J. (2020). Effect of Heat Exposure on Activity Degradation of Enzymes in Mango Varieties Sindri, SB Chaunsa, and Tommy Atkins during Drying. Molecules, 25(22), 5396. https://doi.org/10.3390/molecules25225396







