Nickel Nanopillar Arrays Electrodeposited on Silicon Substrates Using Porous Alumina Templates
Abstract
1. Introduction
2. Results
3. Materials and Methods
3.1. Fabrication of Porous Alumina Membranes onto Si
3.2. Synthesis of Ni Nanopillars
3.3. Characterizations
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Izyumskaya, N.; Tahira, A.; Ibupoto, Z.H.; Lewinski, N.; Avrutin, V.; Özgür, Ü.; Topsakal, E.; Willander, M.; Morkoç, H. Review-Electrochemical biosensors based on ZnO. ECS J. Solid State Sci. Technol. 2017, 6, 84–100. [Google Scholar]
- Schopphoven, C.; Tschöpe, A. Magnetic anisotropy of nickel nanorods and the mechanical torque in an elastic environment. J. Phys. D Appl. Phys. 2018, 51, 115005. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, F.; Kannan, P.; Ji, S.; Wang, H. Nickel phosphate nanowires directly grown on Ni foam as binder-free electrode for pseudocapacitors. Mater. Lett. 2019, 257, 126742. [Google Scholar]
- Zhang, J.; Xiang, W.; Liu, Y.; Hu, M.; Zhao, K. Synthesis of high-aspect-ratio nickel nanowires by dropping method. Nanoscale Res. Lett. 2016, 11, 118. [Google Scholar] [PubMed]
- Hamidi, S.M.; Sobhani, A.; Aftabi, A.; Najafi, M. Optical and magneto-optical properties of aligned Ni nanowires embedded in polydimethylsiloxane. J. Magn. Magn. Mater. 2015, 374, 139–143. [Google Scholar]
- Nasirpouri, F.; Peighambari-Sattari, S.M.; Bran, C.; Palmero, E.M.; Eguiarte, E.B.; Marquez, M.; Patsopoulos, A.; Kechrakos, D. Geometrically designed domain wall trap in tri-segmented nickel magnetic nanowires for spintronics devices. Sci Rep. 2019, 9, 9010. [Google Scholar]
- Cheng, H.E.; Lin, S.Y.; Tian, D.C. Fabrication of nickel nanopillar-array electrodes for water electrolysis. J. Electrochem. Soc. 2014, 161, E202–E206. [Google Scholar]
- Lee, J.K.; Shin, J.H.; Lee, H.; Yoon, W.Y. Characterization of nano silicon on nanopillar-patterned nickel substrate for lithium ion batteries. J. Electrochem. Soc. 2014, 161, A1480–A1485. [Google Scholar]
- Wang, S.; Chen, K.; Wang, M.; Li, H.; Chen, G.; Liu, J.; Xu, L.; Jian, Y.; Meng, C.; Zheng, X.; et al. Controllable synthesis of nickel nanowires and its application in high sensitivity, stretchable strain sensor for body motion sensing. J. Mater. Chem. C. 2018, 6, 4737–4745. [Google Scholar]
- Guiliani, J.; Cadena, J.; Monton, C. Template-assisted electrodeposition of Ni and Ni/Au nanowires on planar and curved substrates. Nanotechnology 2018, 29, 075301. [Google Scholar] [CrossRef]
- Lee, J.D.; Kim, H.S.; Jeong, S.Y.; Kim, K.H.; Lee, J.J.; Kim, J.E. Magnetic characteristics of thin Ni films electrodeposited on n-Si(1 1 1). Curr. Appl. Phys. 2010, 10, 249–254. [Google Scholar]
- Sofiah, A.G.N.; Kananathan, J.; Samykano, M.; Ulakanathan, S.; Lah, N.A.C.; Harun, W.S.W.; Sudhakar, K.; Kadirgama, K.; Ngui, W.K.; Siregar, J.P. Electrochemical deposited nickel nanowires: Influence of deposition bath temperature on the morphology and physical properties. IOP Conf. Ser.-Mater. Sci. Eng. 2017, 257, 012032. [Google Scholar] [CrossRef]
- Proenca, M.P.; Sousa, C.T.; Ventura, J.; Vazquez, M.; Araujo, J.P. Ni growth inside ordered arrays of alumina nanopores: Enhancing the deposition rate. Electrochim. Acta 2012, 72, 215–221. [Google Scholar] [CrossRef]
- Banerjee, A.; Halder, N. Electrochemical growth of ordered nickel nano-rods within a composite structure of anodic-alumina-membrane/metal/silicon substrate. J. Nanosci. Nanotechnol. 2010, 10, 4252–4258. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.W.; Banerjee, A.N. FESEM studies of densely packed aligned nickel nanopillars on silicon substrate by electrochemical deposition through porous alumina membrane. Mater. Sci. Eng. B. 2010, 175, 36–40. [Google Scholar] [CrossRef]
- Cassoux, P.; Valade, L.; Fabre, P.L. Electrochemical methods, electrocrystallization. In Comprehensive Coordination Chemistry II; McCleverty, J.A., Meyer, T.J., Eds.; Elsevier Ltd.: Amsterdam, The Netherland, 2003; pp. 761–773. [Google Scholar]
- Shi, J.B.; Chen, Y.C.; Lee, C.W.; Lin, Y.T.; Wu, C.; Chen, C.J. Optical and magnetic properties of 30 and 60 nm Ni nanowires. Mater. Lett. 2008, 62, 15–18. [Google Scholar] [CrossRef]
- Rahman, I.Z.; Razeeb, K.M.; Rahman, M.A.; Kamruzzaman, M. Fabrication and characterization of nickel nanowires depositedon metal substrate. J. Magn. Magn. Mater. 2003, 262, 166–169. [Google Scholar]
- Fuentes, G.P.; Holanda, J.; Guerra, Y.; Silva, D.B.O.; Farias, B.V.M.; Padrón-Hernández, E. Micromagnetic simulation and the angular dependence of coercivity and remanence for array of polycrystalline nickel nanowires. J. Magn. Magn. Mater. 2017, 423, 262–266. [Google Scholar] [CrossRef]
- Evans, P.; Hendren, W.R.; Atkinson, R.; Wurtz, G.A.; Dickson, W.; Zayats, A.V.; Pollard, R.J. Growth and properties of gold and nickel nanorods in thin film alumina. Nanotechnology 2006, 17, 5749–5753. [Google Scholar] [CrossRef]
- Oh, S.L.; Kim, Y.R.; Malkinski, L.; Vovk, A.; Whittenburg, S.L.; Kim, E.M.; Jung, J.S. Magnetic properties of nickel nanostructures grown in AAO membrane. J. Magn. Magn. Mater. 2007, 310, e827–e829. [Google Scholar] [CrossRef]
- Kartopu, G.; Yalçın, O.; Choy, K.L.; Topkaya, R.; Kazan, S.; Aktaş, B. Size effects and origin of easy-axis in nickel nanowire arrays. J. Appl. Phys. 2011, 109, 033909. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, S.; Zhang, L.; He, X. 3D Si@Cu-Ni nano-pillars array composite as carbon/binder free anode for lithium ion battery. J. Matter Res. Technol. 2020, 9, 1549–1558. [Google Scholar] [CrossRef]
- Kang, L.; Zhang, S.; Lin, R. Preparation of tin nano-spheres film anode based on copper-nickel nano-pillars for lithium ion batteries. Adv. Mater. Res. 2012, 399, 1467–1472. [Google Scholar] [CrossRef]
- Batail, P. Electrochemical crystal growth. In Encyclopedia of Materials: Science and Technology, 2nd ed.; Jürgen-Buschow, K.H., Cahn, R.W., Flemings, M.C., Ilschner, B., Kramer, E.J., Mahajan, S., Veyssiere, P., Eds.; Elsevier Ltd.: Nantes, France, 2001; pp. 2518–2520. [Google Scholar]
- Lambers, E.S.; Dykstal, C.N.; Seo, J.M.; Rowe, J.E.; Holloway, P.H. Room-temerature oxidation of Ni(110) at low and atmospheric oxygen pressures. Oxid. Met. 1996, 45, 301–321. [Google Scholar] [CrossRef]
- Mateos, D.; Valdez, B.; Castillo, J.R.; Nedev, N.; Cueriel, M.; Perez, O.; Arias, A.; Tiznado, H. Synthesis of high purity nickel oxide by a modified sol-gel method. Ceram. Int. 2019, 45, 11403–11407. [Google Scholar] [CrossRef]
- Liu, C.; Li, C.; Ahmed, K.; Mutlu, Z.; Ozkan, C.S.; Ozkan, M. Template free and binderless NiO nanowire foam for Li-ion battery anodes with long cycle life and ultrahigh rate capability. Sci. Rep. 2016, 6, 29183. [Google Scholar] [CrossRef]
- Richardson, J.T.; Scates, R.; Twigg, M.V. X-ray diffraction study of nickel oxide reduction by hydrogen. Appl. Catal. A. 2003, 246, 137–150. [Google Scholar] [CrossRef]
- Santos, A.; Vojkuvka, L.; Pallarés, J.; Ferré-Borrull, J.; Marsal, L.F. Cobalt and nickel nanopillars on aluminium substrates by direct current electrodeposition process. Nanoscale Res. Lett. 2009, 4, 1021–1028. [Google Scholar] [CrossRef]
- Qin, J.; Nogués, J.; Mikhaylova, M.; Roig, A.; Muñoz, J.S.; Muhammed, M. Differences in the magnetic properties of Co, Fe, and Ni 250-300 nm wide nanowires electrodeposited in amorphous anodized alumina templates. Chem. Mater. 2005, 17, 1829–1834. [Google Scholar] [CrossRef]
- Chandra, S.; Kumar, A.; Kumar-Tomar, P. Synthesis of Ni nanoparticles and their characterizations. J. Saudi Chem. Soc. 2014, 18, 437–442. [Google Scholar] [CrossRef]
- Guerra, Y.; Peña-Garcia, R.; Padrón-Hernández, E. Magnetic reversion in real nickel and cobalt nanowires and the angulardependence of coercivity. J. Magn. Magn. Mater. 2018, 452, 17–22. [Google Scholar] [CrossRef]
- Samardak, A.S.; Sukovatitsina, E.V.; Ognev, A.V.; Chebotkevich, L.A.; Mahmoodi, R.; Hosseini, M.G.; Peighambari, S.M.; Nasirpouri, F. Geometry dependent magnetic properties of Ni nanowires embedded in self-assembled arrays. Physcs. Proc. 2011, 22, 549–556. [Google Scholar] [CrossRef]
- Luo, Y.; Zhang, J.C.; Shen, Y.; Jiang, S.T.; Liu, G.Y.; Wang, L.J. Preparation and magnetic properties of nickel nanorods by thermal decomposition reducing methods. Trans. Nonferrous Met. Soc. China. 2006, 16, s96–s100. [Google Scholar] [CrossRef]
- Meneses, F.; Urret, S.E.; Escrig, J.; Bercoff, P.G. Temperature dependence of the effective anisotropy in Ni nanowire arrays. Curr. Appl. Phys. 2018, 18, 1240–1247. [Google Scholar] [CrossRef]
- Moreno, R.; Poyser, S.; Meilak, D.; Meo, A.; Jenkins, S.; Lazarov, V.K.; Vallejo-Fernandez, G.; Majetich, S.; Evans, R.L. The role of faceting and elongation on the magnetic anisotropy of magnetite Fe3O4 nanocrystals. Sci. Rep. 2020, 10, 2722. [Google Scholar] [CrossRef] [PubMed]
- Rashidi, A.M. A galvanostatic modeling for preparation of electrodeposited nanocrystalline coatings by control of current density. J. Mater. Sci. Technol. 2012, 28, 1071–1076. [Google Scholar] [CrossRef]
- Quiroga-Arganaraz, M.P.; Ribotta, S.B.; Folquer, M.E.; Zelaya, E.; Llorente, C.; Ramallo-López, J.M.; Benítez, G.; Rubert, A.; Gassa, L.M.; Vela, M.E.; et al. The chemistry and structure of nickel-tungsten coatings obtained by pulse galvanostatic electrodeposition. Electrochim. Acta 2012, 72, 87–93. [Google Scholar] [CrossRef]
- García-Merino, J.A.; Jímenez-Marín, E.; Mercado-Zúñiga, C.; Trejo-Valdez, M.; Vargas-García, J.; Torres-Torres, C. Quantum and bistable magneto-conductive signatures in multiwall carbon nanotubes decorated with bimetallic Ni and Pt nanoparticles driven by phonons. OSA Continuum. 2019, 2, 1285–1295. [Google Scholar] [CrossRef]
- Celik, E.; Guven, I.; Madenci, E. Mechanical characterization of nickel nanowires by using a customized atomic force microscope. Nanotechnology 2011, 22, 155702. [Google Scholar] [CrossRef]
- Il’ina, M.V.; Il’in, O.I.; Smirnov, V.A.; Blinov, Y.F.; Konoplev, B.G.; Ageev, O.A. Scanning probe techniques for characterization of vertically aligned carbon nanotubes. In Atomic-force Microscopy and Its Applications; Tański, T., Staszuk, M., Ziębowicz, B., Eds.; IntechOpen: London, UK, 2018; pp. 49–68. [Google Scholar]
- Bentley, A.K.; Ellis, A.B.; Lisensky, G.C.; Crone, W.C. Suspensions of nickel nanowires as magneto-optical switches. Nanotechnology 2005, 16, 10. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, W.; Xu, S.; Fei, G.; Xiao, Y.; Hu, J. Optical properties of Ni and Cu nanowire arrays and Ni/Cu superlattice nanowire arrays. Nanoscale Res. Lett. 2012, 7, 569. [Google Scholar] [CrossRef] [PubMed]
- Payne, B.P.; Grosvenor, A.P.; Biesinger, M.C.; Kobe, B.A.; Stewart-McIntyre, N. Structure and growth of oxides on polycrystalline nickel surfaces. Surf. Interface Anal. 2007, 39, 582–592. [Google Scholar] [CrossRef]
- Khalil, A.; Lalia, B.S.; Hashaikeh, R. Nickel oxide nanocrystals as a lithium-ion battery anode: Structure-performance relationship. J. Mater. Sci. 2016, 51, 6624–6638. [Google Scholar] [CrossRef]
- Kim, J.; Kang, H.; Hwang, K.; Yoon, S. Thermal decomposition study on Li2O2 for Li2NiO2 synthesis as a sacrificing positive additive of lithium-ion batteries. Molecules 2019, 24, 4624. [Google Scholar] [CrossRef]
- Cheng, M.; Ye, Y.; Chiu, T.; Pan, C.; Hwang, B. Size effect of nickel oxide for lithium ion battery anode. J. Power Sources 2014, 253, 27–34. [Google Scholar] [CrossRef]
- Hevia, S.A.; Homm, P.; Guzmán, F.; Ruiz, H.M.; Muñoz, G.; Caballero, L.S.; Favre, M.; Flores, M. Pulsed laser deposition of carbon nanodot arrays using porous alumina membranes as a mask. Surf. Coat. Technol. 2014, 253, 161–165. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
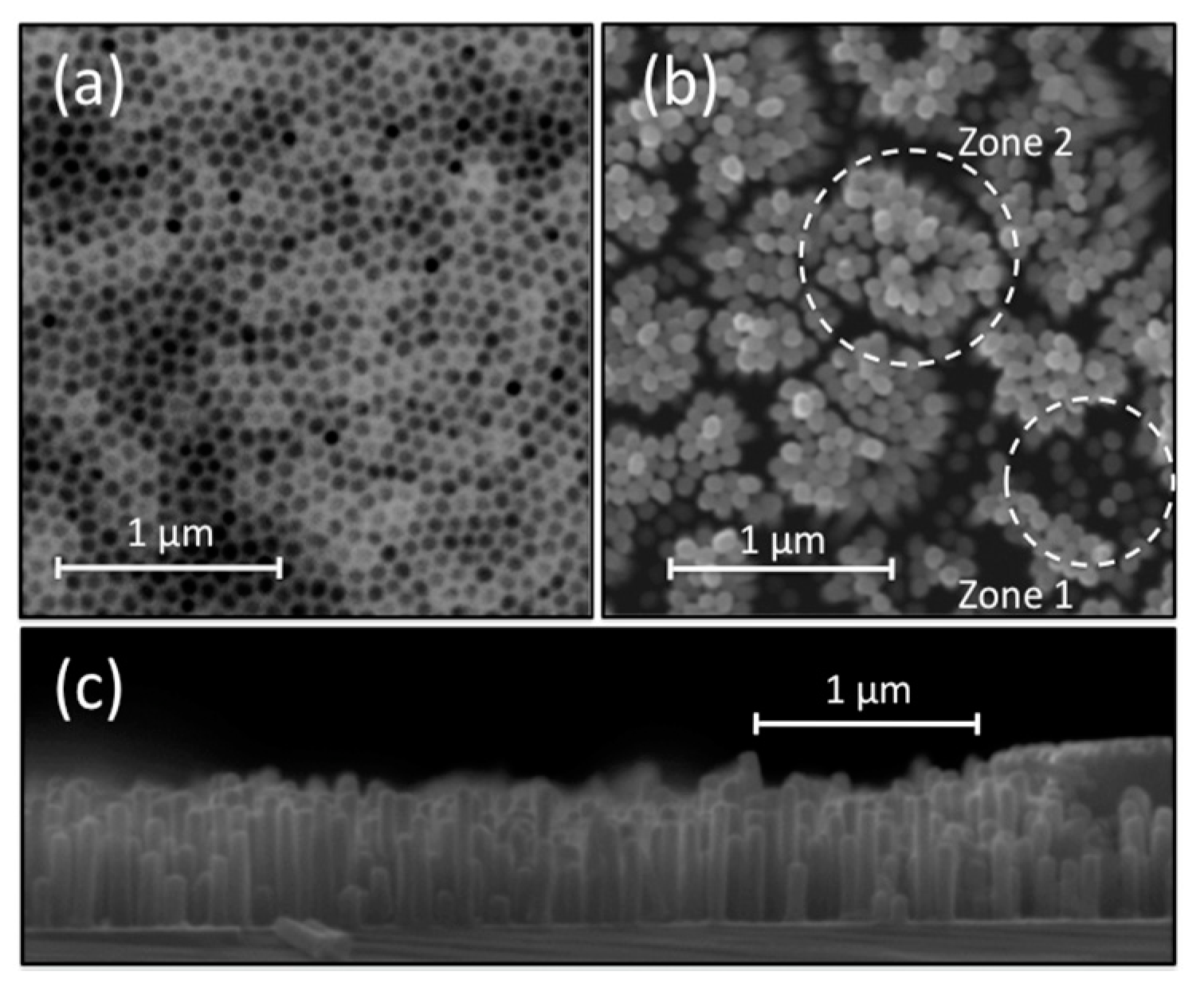
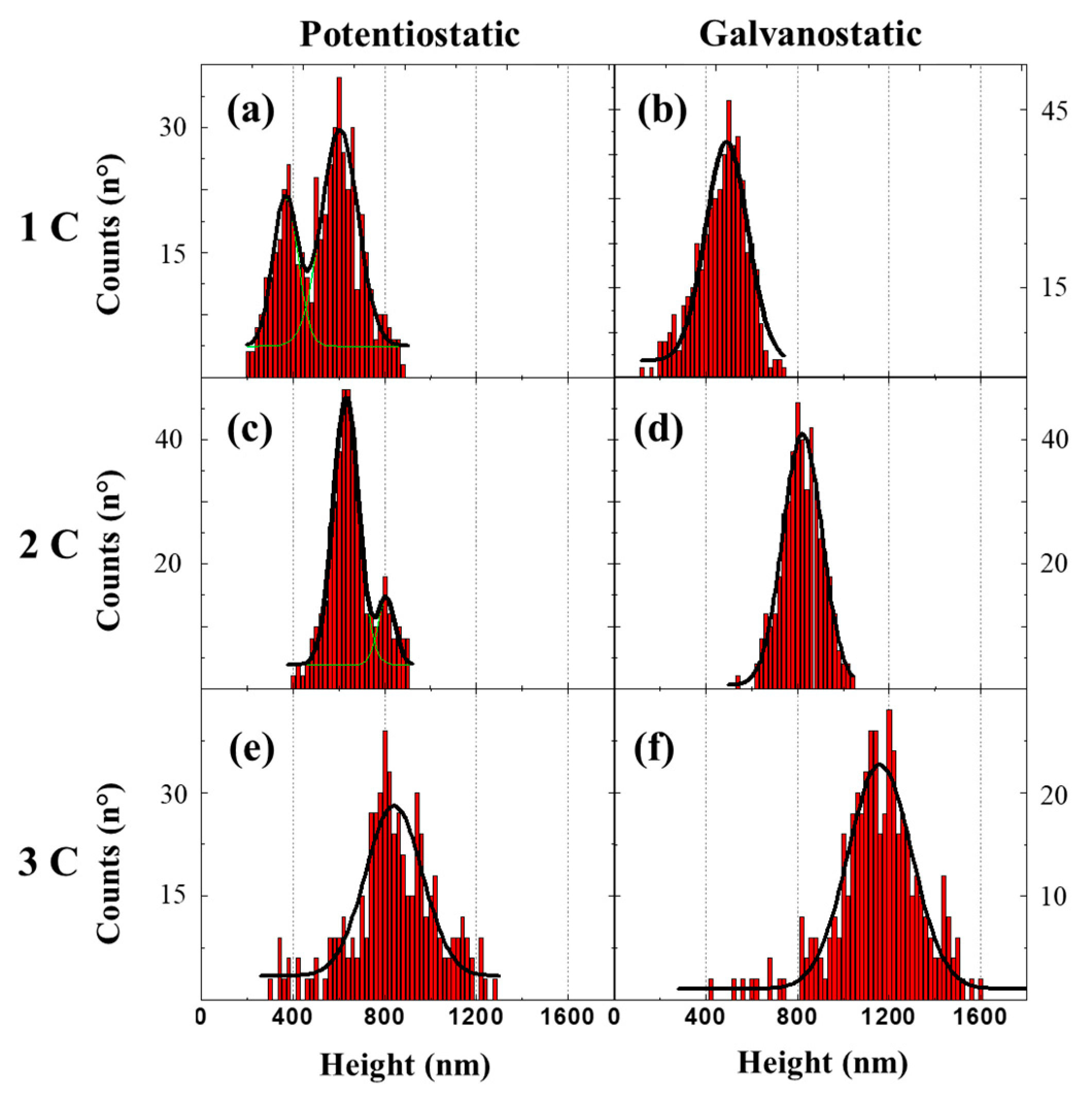
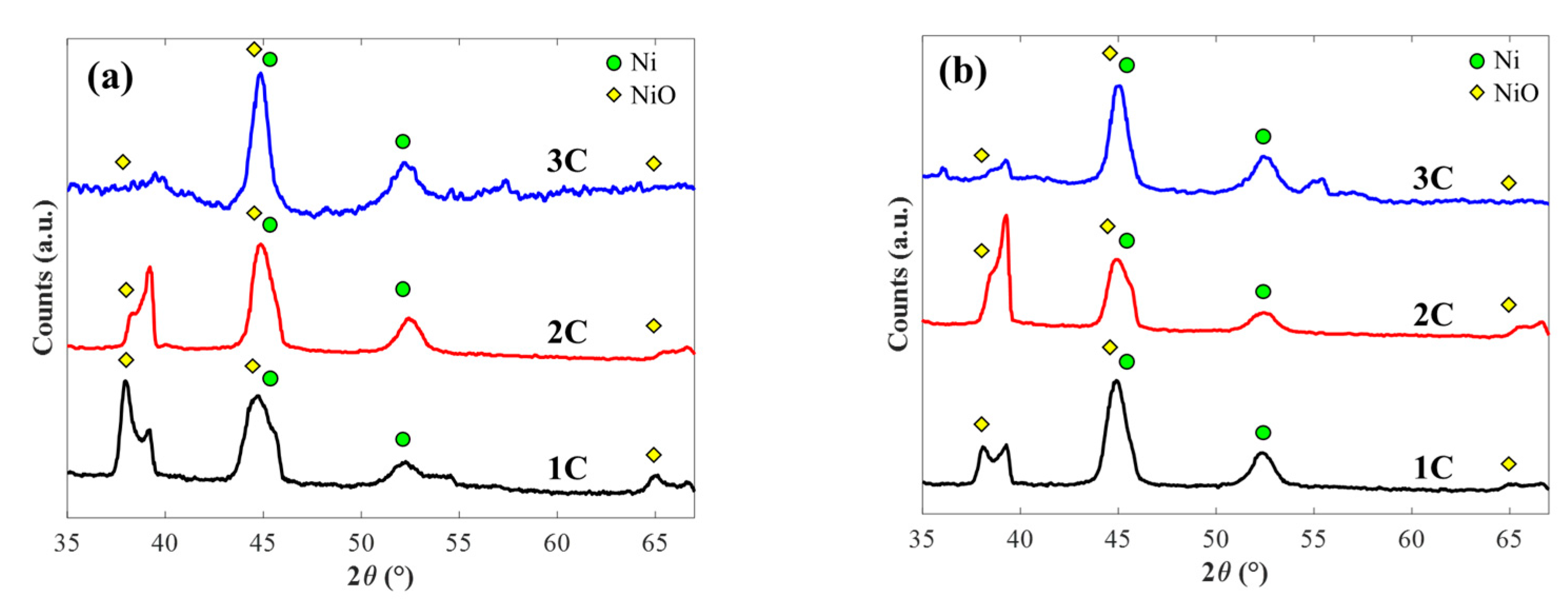

| Method | Potentiostat | Galvanostat | Correlation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 C | 2 C | 3 C | 1 C | 2 C | 3 C | L | ||Hc | ⊥Hc | ||Mr | ⊥Mr | ||Hs | |
| L (nm) | 486 | 655 | 818 | 481 | 812 | 1130 | - | - | - | - | - | - |
| ||Hc (Oe) | 633 | 814 | 741 | 703 | 690 | 1036 | 0.81 | - | - | - | - | - |
| ⊥Hc (Oe) | 133 | 263 | 427 | 187 | 212 | 288 | 0.5 | 0.39 | - | - | - | - |
| ||Mr (M/Ms) | 0.13 | 0.28 | 0.25 | 0.21 | 0.18 | 0.43 | 0.81 | 0.99 | 0.48 | - | - | - |
| ⊥ Mr (M/Ms) | 0.11 | 0.13 | 0.14 | 0.12 | 0.13 | 0.15 | 0.92 | 0.79 | 0.75 | 0.83 | - | - |
| ||Hs (Oe) | 3641 | 3000 | 2738 | 3391 | 3296 | 2440 | −0.87 | −0.87 | −0.79 | −0.91 | −0.94 | - |
| ⊥Hs (Oe) | 2380 | 2293 | 2738 | 2811 | 2640 | 2851 | 0.49 | 0.37 | 0.34 | 0.41 | 0.49 | −0.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bejide, M.; Contreras, P.; Homm, P.; Duran, B.; García-Merino, J.A.; Rosenkranz, A.; Denardin, J.C.; del Río, R.; Hevia, S.A. Nickel Nanopillar Arrays Electrodeposited on Silicon Substrates Using Porous Alumina Templates. Molecules 2020, 25, 5377. https://doi.org/10.3390/molecules25225377
Bejide M, Contreras P, Homm P, Duran B, García-Merino JA, Rosenkranz A, Denardin JC, del Río R, Hevia SA. Nickel Nanopillar Arrays Electrodeposited on Silicon Substrates Using Porous Alumina Templates. Molecules. 2020; 25(22):5377. https://doi.org/10.3390/molecules25225377
Chicago/Turabian StyleBejide, Matías, Patricio Contreras, Pia Homm, Boris Duran, José Antonio García-Merino, Andreas Rosenkranz, Juliano C. Denardin, Rodrigo del Río, and Samuel A. Hevia. 2020. "Nickel Nanopillar Arrays Electrodeposited on Silicon Substrates Using Porous Alumina Templates" Molecules 25, no. 22: 5377. https://doi.org/10.3390/molecules25225377
APA StyleBejide, M., Contreras, P., Homm, P., Duran, B., García-Merino, J. A., Rosenkranz, A., Denardin, J. C., del Río, R., & Hevia, S. A. (2020). Nickel Nanopillar Arrays Electrodeposited on Silicon Substrates Using Porous Alumina Templates. Molecules, 25(22), 5377. https://doi.org/10.3390/molecules25225377







