Thiocoumarin Caged Nucleotides: Synthetic Access and Their Photophysical Properties
Abstract
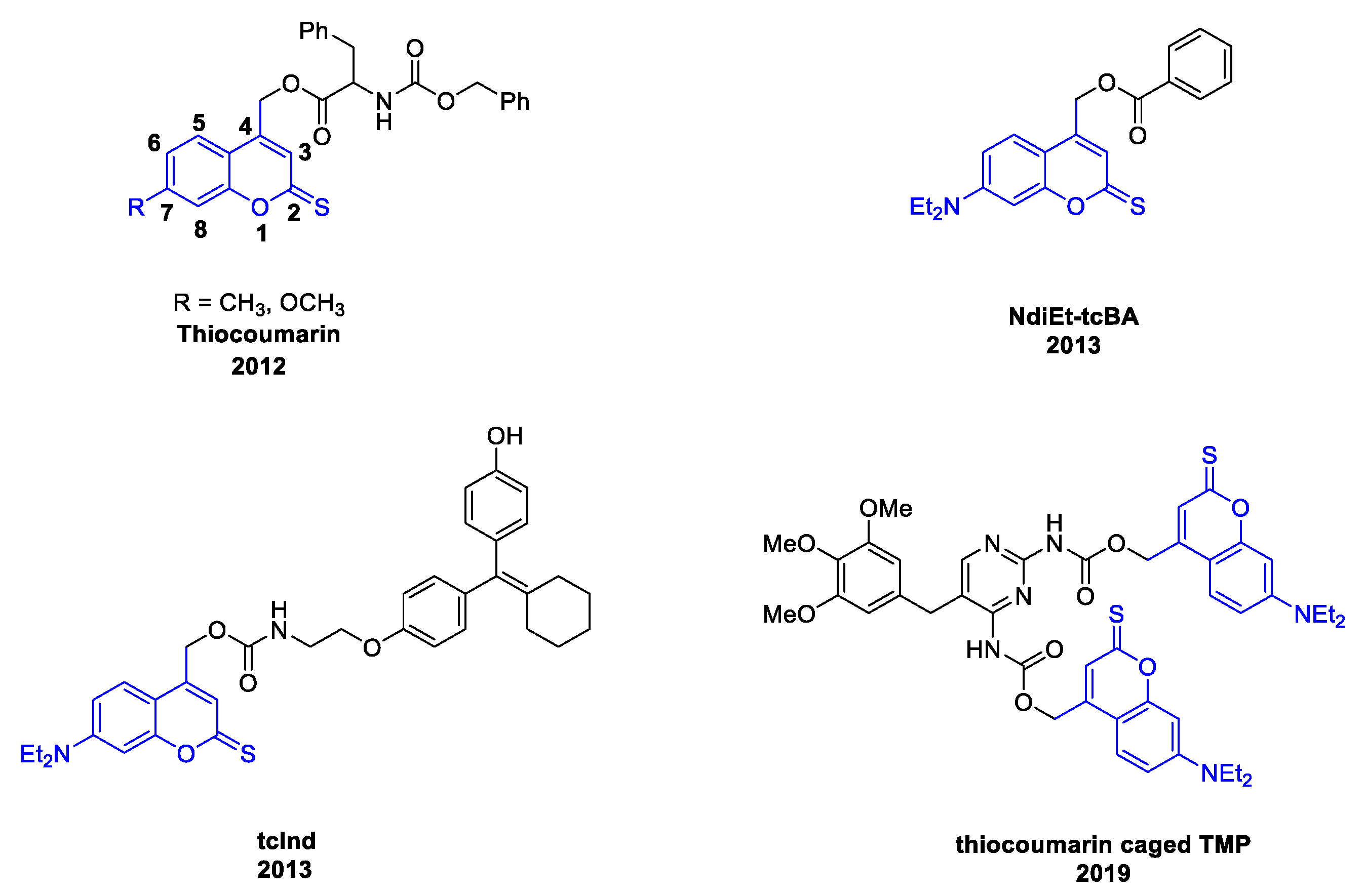
1. Introduction
2. Results
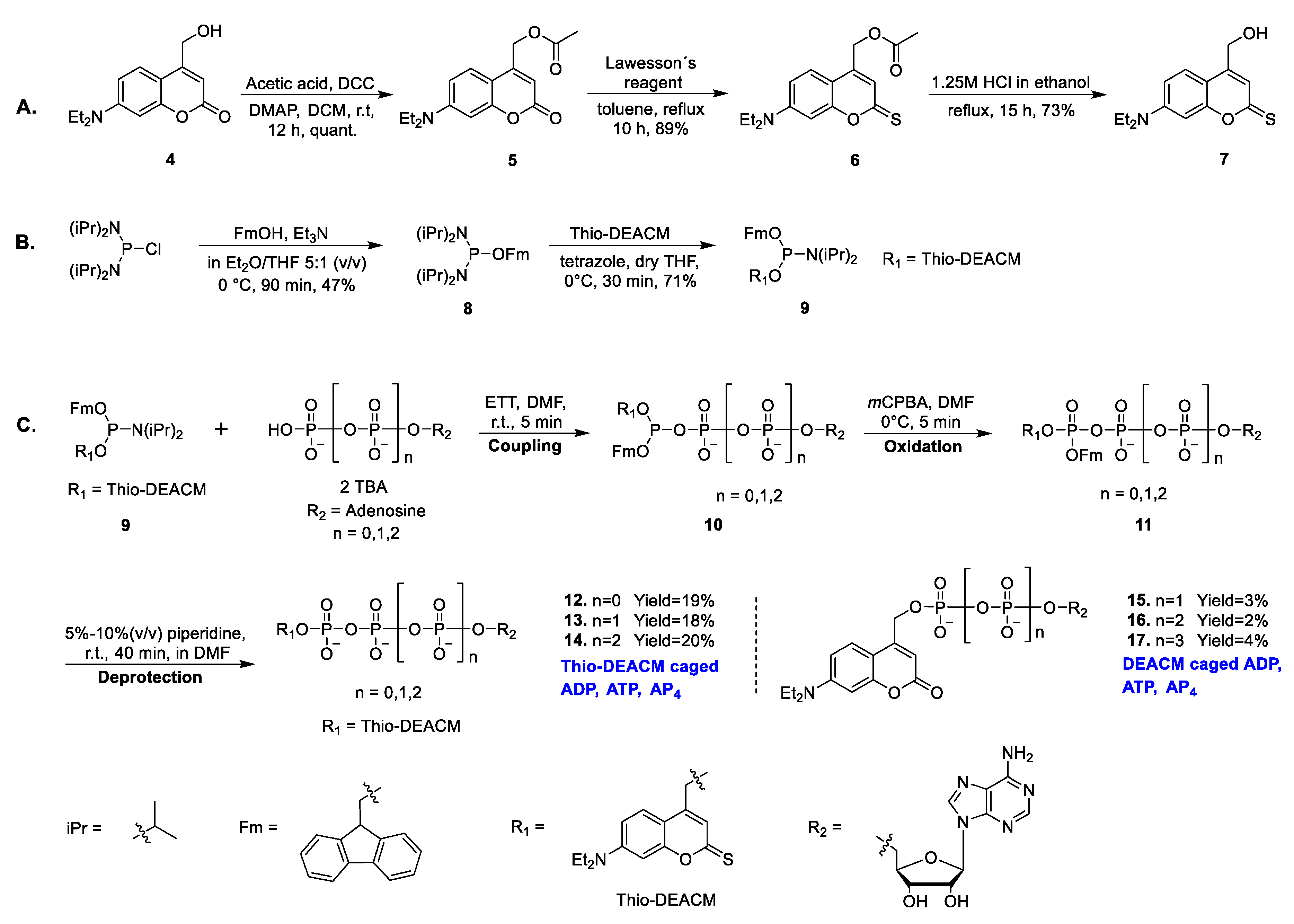
2.1. Chemistry
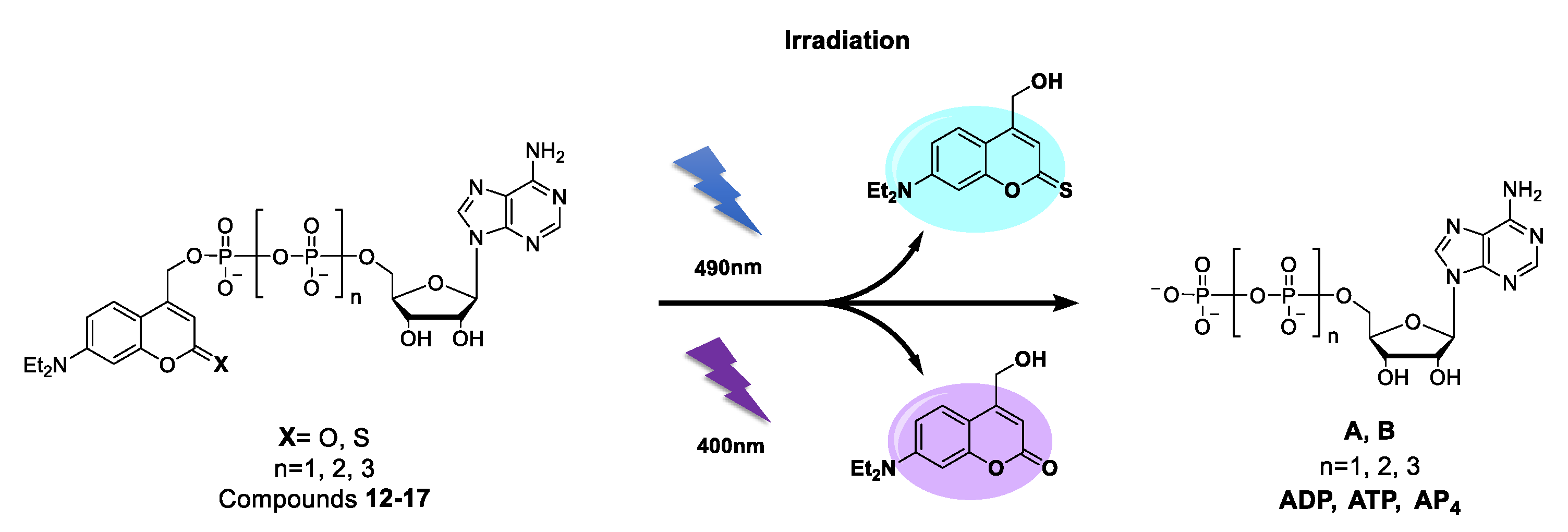
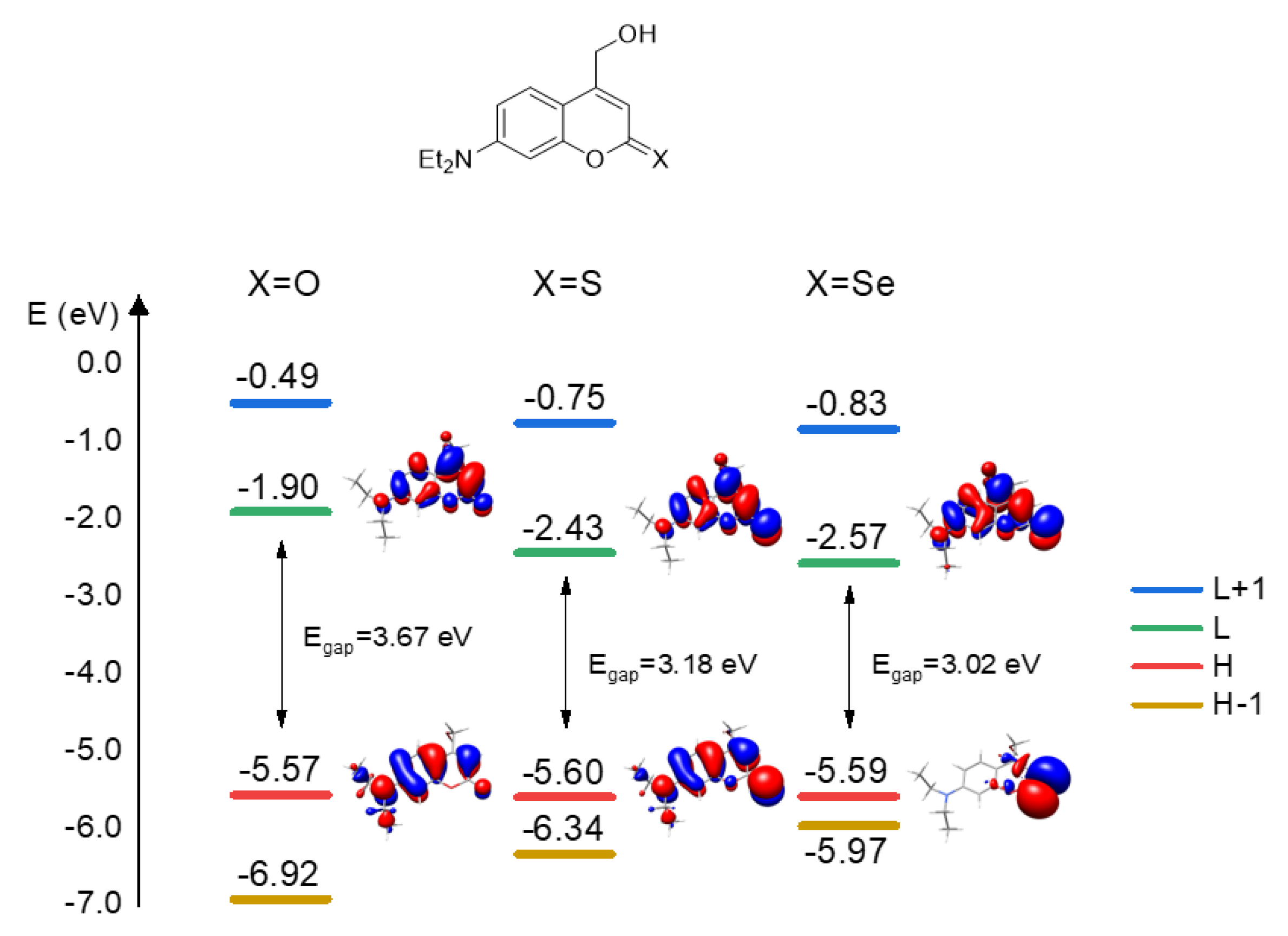
2.2. Photophysical Properties
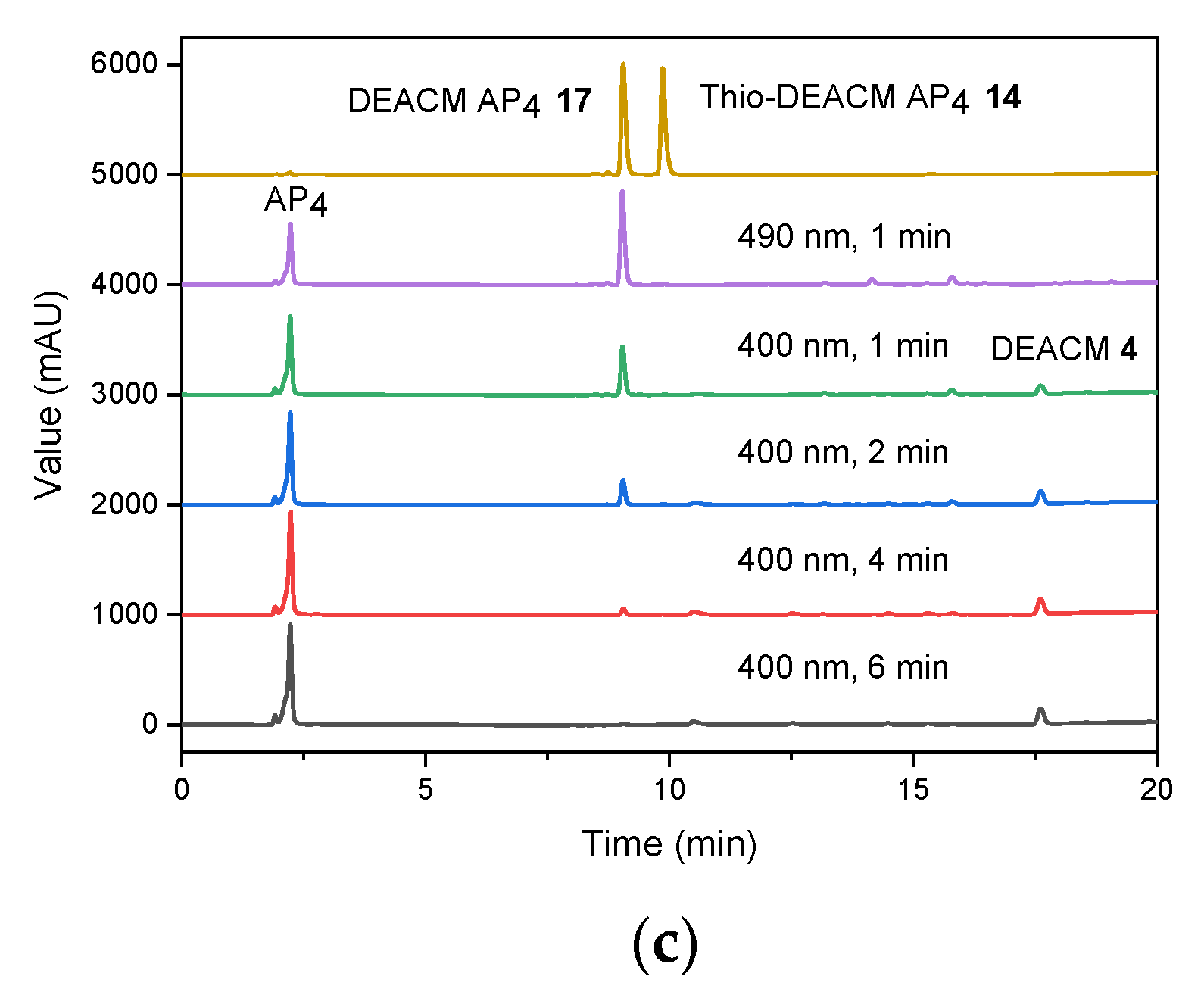
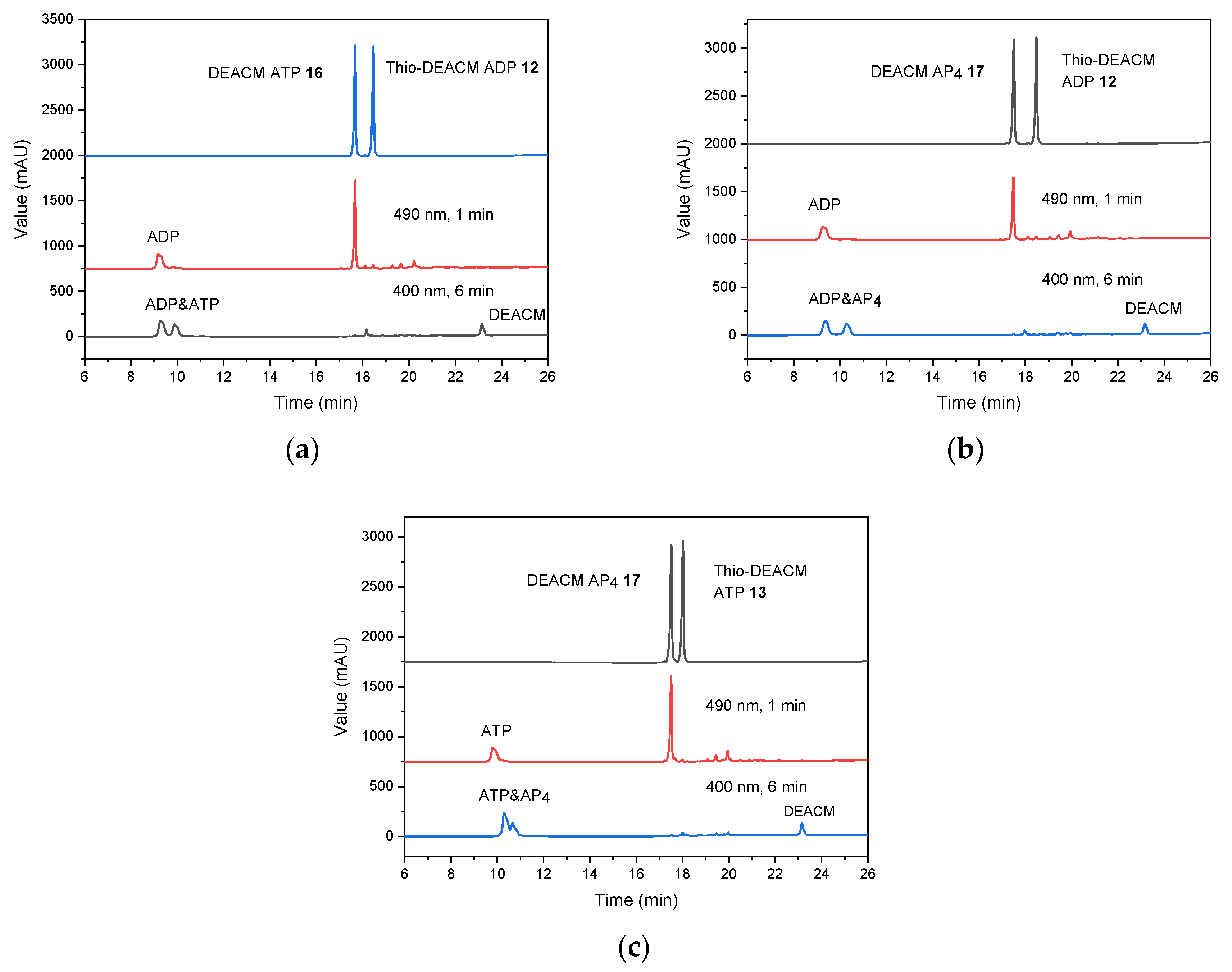
2.3. Photolysis Studies
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mayer, G.; Heckel, A. Biologically active molecules with a “light switch”. Angew. Chem. Int. Ed. 2006, 45, 4900–4921. [Google Scholar] [CrossRef]
- Kaplan, J.H.; Forbush, B.; Hoffman, J.F. Rapid Photolytic Release of Adenosine 5ʹ-Triphosphate from a Protected Analogue: Utilization by the Na:K Pump of Human Red Blood Cell Ghosts. Biochemistry 1978, 17, 1929–1935. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.J.; Velema, W.A.; Lerch, M.M.; Szymanski, W.; Feringa, B.L. Wavelength-selective cleavage of photoprotecting groups: Strategies and applications in dynamic systems. Chem. Soc. Rev. 2015, 44, 3358–3377. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.P.; Banghart, M.R.; Sabatini, B.L.; Ellis-Davies, G.C.R. Spectral evolution of a photochemical protecting group for orthogonal two-color uncaging with visible light. J. Am. Chem. Soc. 2013, 135, 15948–15954. [Google Scholar] [CrossRef]
- Bojtár, M.; Kormos, A.; Kis-Petik, K.; Kellermayer, M.; Kele, P. Green-Light Activatable, Water-Soluble Red-Shifted Coumarin Photocages. Org. Lett. 2019, 21, 9410–9414. [Google Scholar] [CrossRef] [PubMed]
- Schade, B.; Hagen, V.; Schmidt, R.; Herbrich, R.; Krause, E.; Eckardt, T.; Bendig, J. Deactivation behavior and excited-state properties of (coumarin-4- yl)methyl derivatives. 1. Photocleavage of (7-methoxycoumarin-4-yl)methyl- caged acids with fluorescence enhancement. J. Org. Chem. 1999, 64, 9109–9117. [Google Scholar] [CrossRef]
- Schmidt, R.; Geissler, D.; Hagen, V.; Bendig, J. Kinetics study of the photocleavage of (coumarin-4-yl)methyl esters. J. Phys. Chem. A 2005, 109, 5000–5004. [Google Scholar] [CrossRef]
- Schmidt, R.; Geissler, D.; Hagen, V.; Bendig, J. Mechanism of photocleavage of (coumarin-4-yl)methyl esters. J. Phys. Chem. A 2007, 111, 5768–5774. [Google Scholar] [CrossRef]
- Pavlovic, I.; Thakor, D.T.; Vargas, J.R.; McKinlay, C.J.; Hauke, S.; Anstaett, P.; Camunã, R.C.; Bigler, L.; Gasser, G.; Schultz, C.; et al. Cellular delivery and photochemical release of a caged inositol-pyrophosphate induces PH-domain translocation in cellulo. Nat. Commun. 2016, 7, 1–8. [Google Scholar] [CrossRef]
- Nadler, A.; Yushchenko, D.A.; Müller, R.; Stein, F.; Feng, S.; Mulle, C.; Carta, M.; Schultz, C. Exclusive photorelease of signalling lipids at the plasma membrane. Nat. Commun. 2015, 6, 1–10. [Google Scholar] [CrossRef]
- Wagner, N.; Stephan, M.; Höglinger, D.; Nadler, A. A Click Cage: Organelle-Specific Uncaging of Lipid Messengers. Angew. Chem. Int. Ed. 2018, 57, 13339–13343. [Google Scholar] [CrossRef] [PubMed]
- Kantevari, S.; Matsuzaki, M.; Kanemoto, Y.; Kasai, H.; Ellis-Davies, G.C.R. Two-color, two-photon uncaging of glutamate and GABA. Nat. Methods 2010, 7, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Amatrudo, J.M.; Olson, J.P.; Lur, G.; Chiu, C.Q.; Higley, M.J.; Ellis-Davies, G.C.R. Wavelength-selective one- and two-photon uncaging of Gaba. ACS Chem. Neurosci. 2014, 5, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.P.; Kwon, H.B.; Takasaki, K.T.; Chiu, C.Q.; Higley, M.J.; Sabatini, B.L.; Ellis-Davies, G.C.R. Optically selective two-photon uncaging of glutamate at 900 nm. J. Am. Chem. Soc. 2013, 135, 5954–5957. [Google Scholar] [CrossRef] [PubMed]
- Richers, M.T.; Amatrudo, J.M.; Olson, J.P.; Ellis-Davies, G.C.R. Cloaked Caged Compounds: Chemical Probes for Two-Photon Optoneurobiology. Angew. Chem. Int. Ed. 2017, 56, 193–197. [Google Scholar] [CrossRef]
- Rovira, A.; Gandioso, A.; Goñalons, M.; Galindo, A.; Massaguer, A.; Bosch, M.; Marchán, V. Solid-Phase Approaches for Labeling Targeting Peptides with Far-Red Emitting Coumarin Fluorophores. J. Org. Chem. 2019, 84, 1808–1817. [Google Scholar] [CrossRef] [PubMed]
- Novohradsky, V.; Rovira, A.; Hally, C.; Galindo, A.; Vigueras, G.; Gandioso, A.; Svitelova, M.; Bresolí-Obach, R.; Kostrhunova, H.; Markova, L.; et al. Towards Novel Photodynamic Anticancer Agents Generating Superoxide Anion Radicals: A Cyclometalated IrIII Complex Conjugated to a Far-Red Emitting Coumarin. Angew. Chem. Int. Ed. 2019, 58, 6311–6315. [Google Scholar] [CrossRef] [PubMed]
- Gandioso, A.; Palau, M.; Nin-Hill, A.; Melnyk, I.; Rovira, C.; Nonell, S.; Velasco, D.; García-Amorós, J.; Marchán, V. Sequential Uncaging with Green Light can be Achieved by Fine-Tuning the Structure of a Dicyanocoumarin Chromophore. ChemistryOpen 2017, 6, 375–384. [Google Scholar] [CrossRef]
- Gandioso, A.; Contreras, S.; Melnyk, I.; Oliva, J.; Nonell, S.; Velasco, D.; García-Amorós, J.; Marchán, V. Development of Green/Red-Absorbing Chromophores Based on a Coumarin Scaffold That Are Useful as Caging Groups. J. Org. Chem. 2017, 82, 5398–5408. [Google Scholar] [CrossRef]
- Gandioso, A.; Bresolí-Obach, R.; Nin-Hill, A.; Bosch, M.; Palau, M.; Galindo, A.; Contreras, S.; Rovira, A.; Rovira, C.; Nonell, S.; et al. Redesigning the Coumarin Scaffold into Small Bright Fluorophores with Far-Red to Near-Infrared Emission and Large Stokes Shifts Useful for Cell Imaging. J. Org. Chem. 2018, 83, 1185–1195. [Google Scholar] [CrossRef]
- Gandioso, A.; Palau, M.; Bresolí-Obach, R.; Galindo, A.; Rovira, A.; Bosch, M.; Nonell, S.; Marchán, V. High Photostability in Nonconventional Coumarins with Far-Red/NIR Emission through Azetidinyl Substitution. J. Org. Chem. 2018, 83, 11519–11531. [Google Scholar] [CrossRef] [PubMed]
- Bassolino, G.; Nançoz, C.; Thiel, Z.; Bois, E.; Vauthey, E.; Rivera-Fuentes, P. Photolabile coumarins with improved efficiency through azetidinyl substitution. Chem. Sci. 2018, 9, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Fournier, L.; Gauron, C.; Xu, L.; Aujard, I.; Le Saux, T.; Gagey-Eilstein, N.; Maurin, S.; Dubruille, S.; Baudin, J.B.; Bensimon, D.; et al. A blue-absorbing photolabile protecting group for in vivo chromatically orthogonal photoactivation. ACS Chem. Biol. 2013, 8, 1528–1536. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Inscho, E.W. Role of adenosine 5′-triphosphate in regulating renal microvascular function and in hypertension. Hypertension 2011, 58, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Thirlwell, H.; Corrie, J.E.; Reid, G.P.; Trentham, D.R.; Ferenczi, M.A. Kinetics of relaxation from rigor of permeabilized fast-twitch skeletal fibers from the rabbit using a novel caged ATP and apyrase. Biophys. J. 1994, 67, 2436–2447. [Google Scholar] [CrossRef]
- Sokolov, V.S.; Apell, H.J.; Corrie, J.E.T.; Trentham, D.R. Fast transient currents in Na,K-ATPase induced by ATP concentration jumps from the P3-[1-(3’,5’-dimethoxyphenyl)-2-phenyl-2-oxo]ethyl ester of ATP. Biophys. J. 1998, 74, 2285–2298. [Google Scholar] [CrossRef]
- Park, C.H.; Givens, R.S. New photoactivated protecting groups. 6. p-Hydroxyphenacyl: A phototrigger for chemical and biochemical probes. J. Am. Chem. Soc. 1997, 119, 2453–2463. [Google Scholar] [CrossRef]
- Geißler, D.; Kresse, W.; Wiesner, B.; Bendig, J.; Kettenmann, H.; Hagen, V. DMACM-caged adenosine nucleotides: Ultrafast phototriggers for ATP, ADP, and AMP activated by long-wavelength irradiation. ChemBioChem 2003, 4, 162–170. [Google Scholar] [CrossRef]
- Pinheiro, A.; Baptistap, P.; Lima, J.C. Light activation of transcription: Photocaging of nucleotides for control over RNA polymerization. Nucleic Acids Res. 2008, 36. [Google Scholar] [CrossRef]
- Fonseca, A.S.C.; Soares, A.M.S.; Gonçalves, M.S.T.; Costa, S.P.G. Thionated coumarins and quinolones in the light triggered release of a model amino acid: Synthesis and photolysis studies. Tetrahedron 2012, 68, 7892–7900. [Google Scholar] [CrossRef]
- Fournier, L.; Aujard, I.; Le Saux, T.; Maurin, S.; Beaupierre, S.; Baudin, J.B.; Jullien, L. Coumarinylmethyl caging groups with redshifted absorption. Chem. A Eur. J. 2013, 19, 17494–17507. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Sun, W.; Butt, H.J.; Wu, S. Upconverting-nanoparticle-assisted photochemistry induced by low-intensity near-infrared light: How low can we go? Chem. A Eur. J. 2015, 21, 9165–9170. [Google Scholar] [CrossRef] [PubMed]
- Manna, D.; Maji, B.; Gangopadhyay, S.A.; Cox, K.J.; Zhou, Q.; Law, B.K.; Mazitschek, R.; Choudhary, A. A Singular System with Precise Dosing and Spatiotemporal Control of CRISPR-Cas9. Angew. Chem. Int. Ed. 2019, 58, 6285–6289. [Google Scholar] [CrossRef] [PubMed]
- Weinrich, T.; Gränz, M.; Grünewald, C.; Prisner, T.F.; Göbel, M.W. Synthesis of a Cytidine Phosphoramidite with Protected Nitroxide Spin Label for EPR Experiments with RNA. Eur. J. Org. Chem. 2017, 2017, 491–496. [Google Scholar] [CrossRef]
- Caruthers, M.H. Chemical Synthesis of DNA and DNA Analogues. Acc. Chem. Res. 1991, 24, 278–284. [Google Scholar] [CrossRef]
- Hofer, A.; Cremosnik, G.S.; Müller, A.C.; Giambruno, R.; Trefzer, C.; Superti-Furga, G.; Bennett, K.L.; Jessen, H.J. A Modular Synthesis of Modified Phosphoanhydrides. Chem. A Eur. J. 2015, 21, 10116–10122. [Google Scholar] [CrossRef]
- Singh, J.; Ripp, A.; Haas, T.M.; Qiu, D.; Keller, M.; Wender, P.A.; Siegel, J.S.; Baldridge, K.K.; Jessen, H.J. Synthesis of Modified Nucleoside Oligophosphates Simplified: Fast, Pure, and Protecting Group Free. J. Am. Chem. Soc. 2019, 141, 15013–15017. [Google Scholar] [CrossRef]
- Haas, T.M.; Ebensperger, P.; Eisenbeis, V.B.; Nopper, C.; Dürr, T.; Jork, N.; Steck, N.; Jessen-Trefzer, C.; Jessen, H.J. Magic spot nucleotides: Tunable target-specific chemoenzymatic synthesis. Chem. Commun. 2019, 55, 5339–5342. [Google Scholar] [CrossRef]
- Lee, D.R.; Lee, K.H.; Shao, W.; Kim, C.L.; Kim, J.; Lee, J.Y. Heavy Atom Effect of Selenium for Metal-Free Phosphorescent Light-Emitting Diodes. Chem. Mater. 2020, 32, 2583–2592. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Mordhorst, S.; Singh, J.; Mohr, M.K.F.; Hinkelmann, R.; Keppler, M.; Jessen, H.J.; Andexer, J.N. Several Polyphosphate Kinase 2 Enzymes Catalyse the Production of Adenosine 5′-Polyphosphates. ChemBioChem 2019, 20, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Bittner, T.; Wittwer, C.; Hauke, S.; Wohlwend, D.; Mundinger, S.; Dutta, A.K.; Bezold, D.; Dürr, T.; Friedrich, T.; Schultz, C.; et al. Photolysis of Caged Inositol Pyrophosphate InsP8Directly Modulates Intracellular Ca2+Oscillations and Controls C2AB Domain Localization. J. Am. Chem. Soc. 2020, 142, 10606–10611. [Google Scholar] [CrossRef]
- Peterson, J.A.; Wijesooriya, C.; Gehrmann, E.J.; Mahoney, K.M.; Goswami, P.P.; Albright, T.R.; Syed, A.; Dutton, A.S.; Smith, E.A.; Winter, A.H. Family of BODIPY Photocages Cleaved by Single Photons of Visible/Near-Infrared Light. J. Am. Chem. Soc. 2018, 140, 7343–7346. [Google Scholar] [CrossRef]
- Kand, D.; Liu, P.; Navarro, M.X.; Fischer, L.J.; Rousso-Noori, L.; Friedmann-Morvinski, D.; Winter, A.H.; Miller, E.W.; Weinstain, R. Water-Soluble BODIPY Photocages with Tunable Cellular Localization. J. Am. Chem. Soc. 2020, 142, 4970–4974. [Google Scholar] [CrossRef]
- Shrestha, P.; Dissanayake, K.C.; Gehrmann, E.J.; Wijesooriya, C.S.; Mukhopadhyay, A.; Smith, E.A.; Winter, A.H. Efficient Far-Red/Near-IR Absorbing BODIPY Photocages by Blocking Unproductive Conical Intersections. J. Am. Chem. Soc. 2020, 142, 15505–15512. [Google Scholar] [CrossRef]
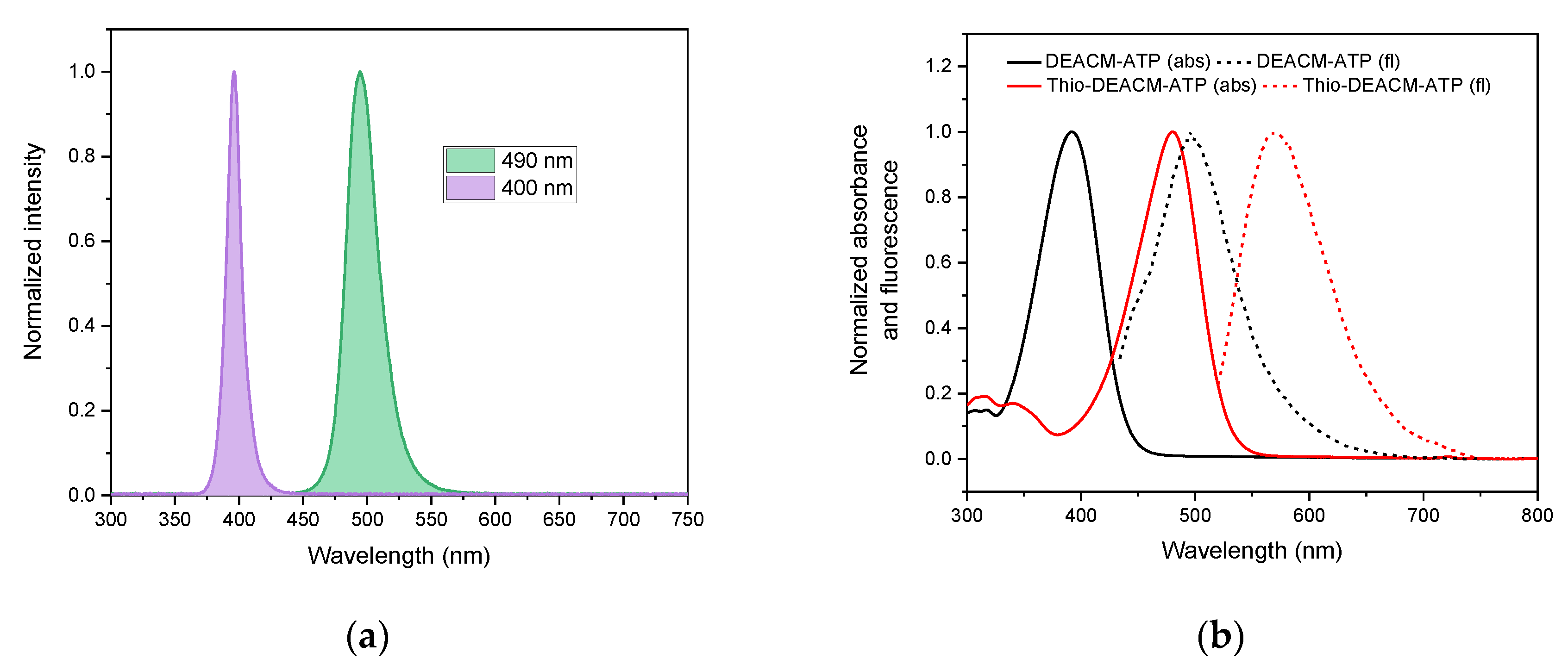

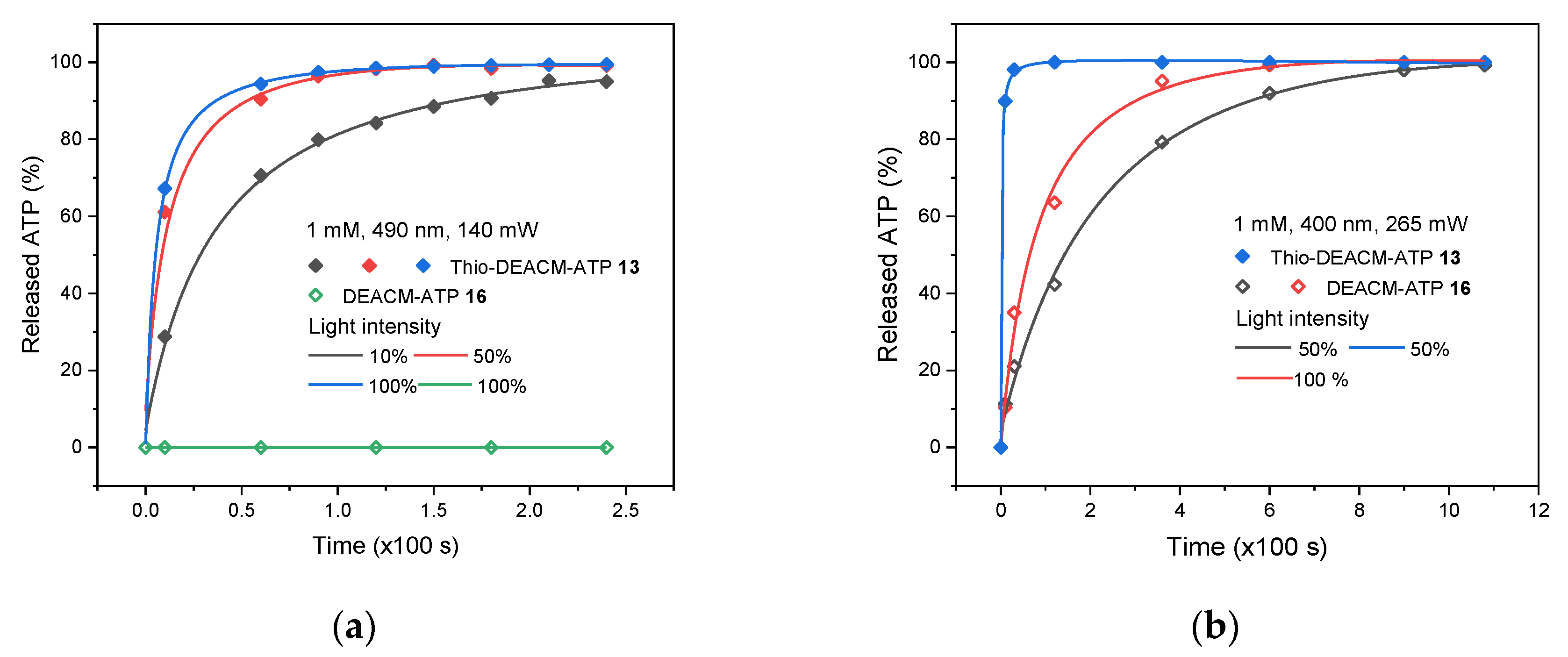
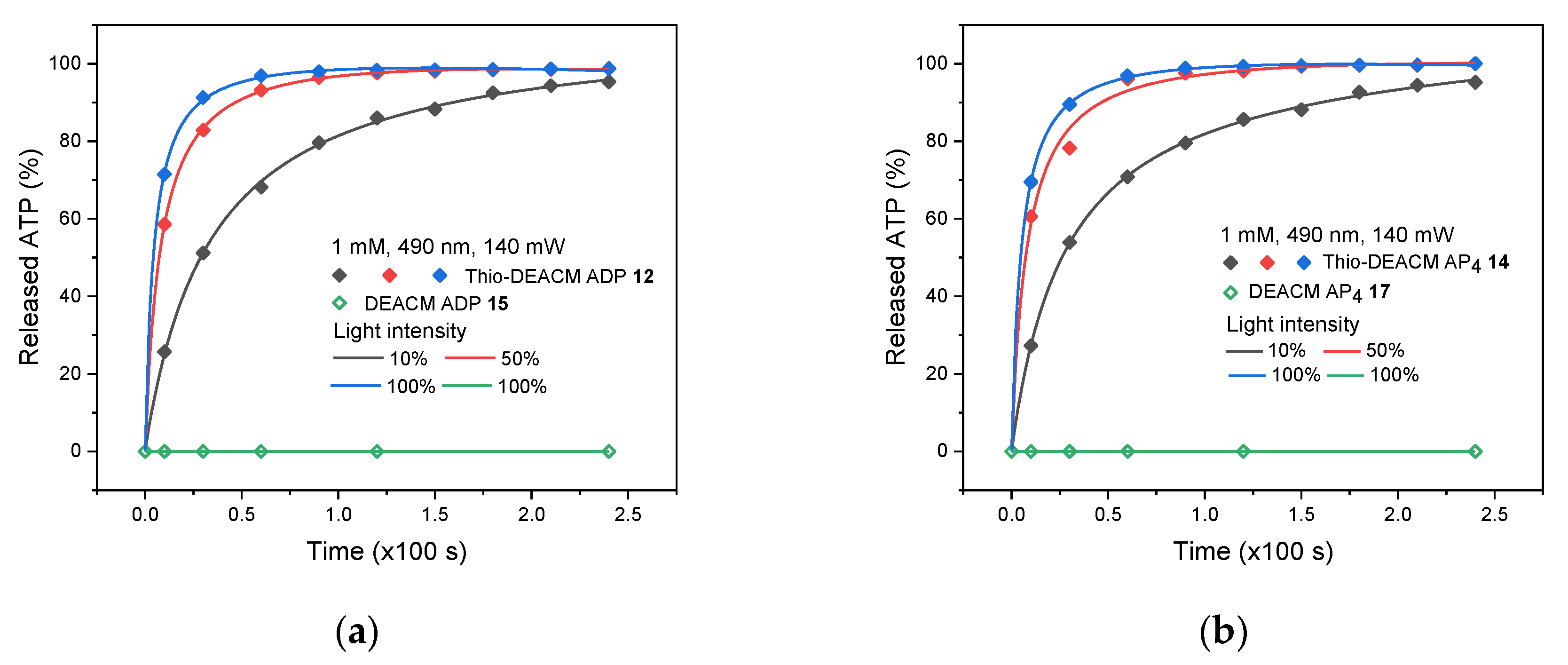
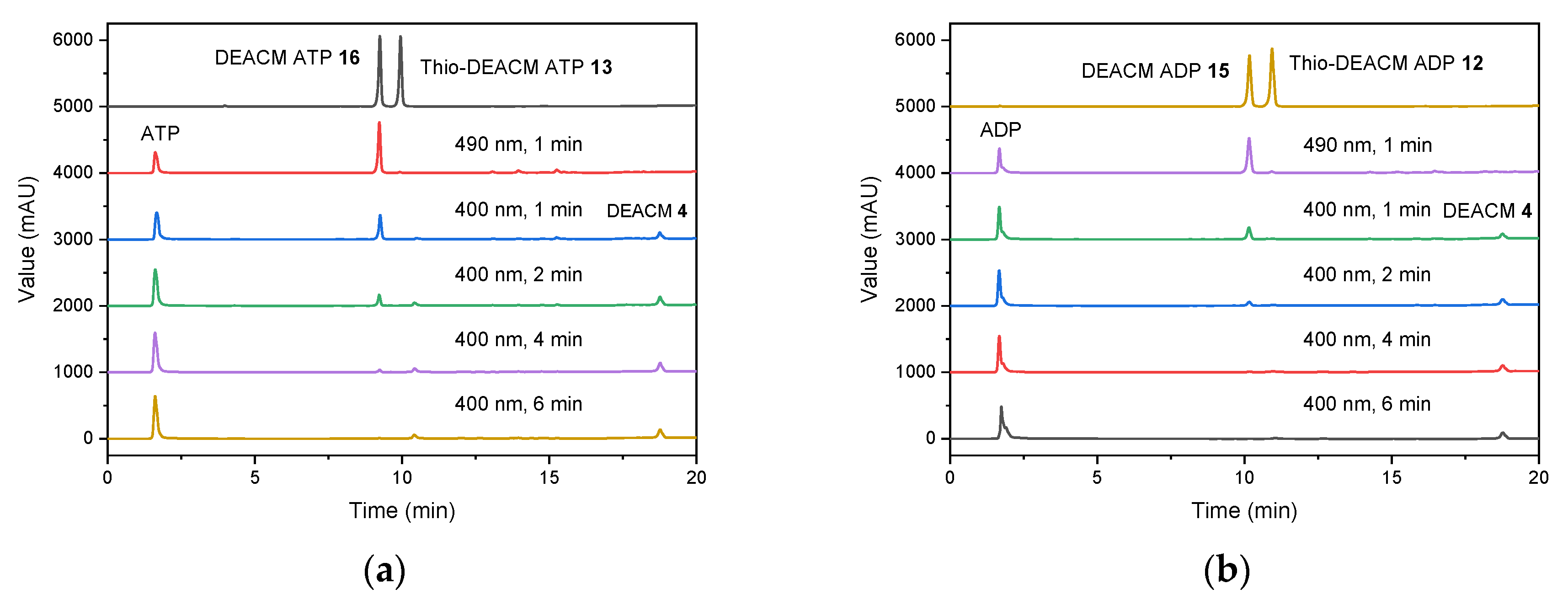
| Compound | [nm] | [nm] | ε () [mM−1 cm−1] | Φfl (%) |
|---|---|---|---|---|
| DEACM (4) | 375 1 (374 6) | 472 1 | 16.8 1 | 25.6 1 |
| Thio-DEACM (7) | 457 1 (435 6) | 534 1 | 19.2 1 | <1 1 |
| DEACM ADP (15) 2 | 392 3 | 498 | 16.3 4 | 17.7 5 |
| Thio-DEACM ADP (12) 2 | 480 3 | 572 | 13.5 4 | 2.7 5 |
| DEACM ATP (16) 2 | 390 3 | 498 | 25.9 4 (24.7) 7 | 12.4 5 |
| Thio-DEACM ATP (13) 2 | 480 3 | 568 | 25.6 4 (4.6) 7 | 2.2 5 |
| DEACM AP4 (17) 2 | 392 3 | 498 | 16.6 4 | 13.3 5 |
| Thio-DEACM AP4 (14) 2 | 480 3 | 570 | 15.9 4 | 2.2 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Ripp, A.; Wassy, D.; Dürr, T.; Qiu, D.; Häner, M.; Haas, T.; Popp, C.; Bezold, D.; Richert, S.; et al. Thiocoumarin Caged Nucleotides: Synthetic Access and Their Photophysical Properties. Molecules 2020, 25, 5325. https://doi.org/10.3390/molecules25225325
Ma J, Ripp A, Wassy D, Dürr T, Qiu D, Häner M, Haas T, Popp C, Bezold D, Richert S, et al. Thiocoumarin Caged Nucleotides: Synthetic Access and Their Photophysical Properties. Molecules. 2020; 25(22):5325. https://doi.org/10.3390/molecules25225325
Chicago/Turabian StyleMa, Jiahui, Alexander Ripp, Daniel Wassy, Tobias Dürr, Danye Qiu, Markus Häner, Thomas Haas, Christoph Popp, Dominik Bezold, Sabine Richert, and et al. 2020. "Thiocoumarin Caged Nucleotides: Synthetic Access and Their Photophysical Properties" Molecules 25, no. 22: 5325. https://doi.org/10.3390/molecules25225325
APA StyleMa, J., Ripp, A., Wassy, D., Dürr, T., Qiu, D., Häner, M., Haas, T., Popp, C., Bezold, D., Richert, S., Esser, B., & Jessen, H. J. (2020). Thiocoumarin Caged Nucleotides: Synthetic Access and Their Photophysical Properties. Molecules, 25(22), 5325. https://doi.org/10.3390/molecules25225325






