Nano Carrier Drug Delivery Systems for the Treatment of Neuropsychiatric Disorders: Advantages and Limitations
Abstract
1. Introduction
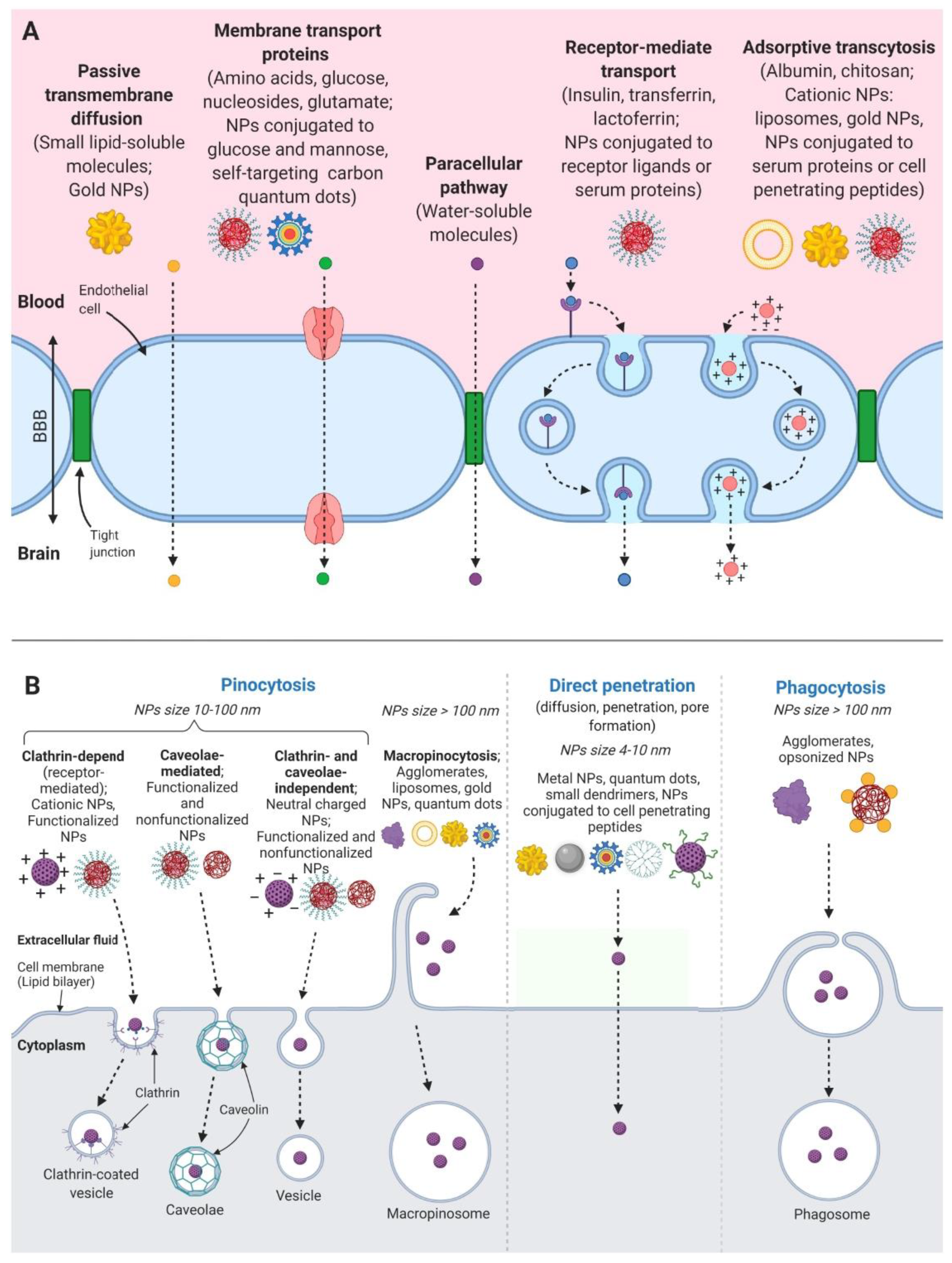
2. Drug Transport through the Blood Brain Barrier
- Transient opening of BBB is induced by a stimulus derived from bioactive components on the surface of nanoparticles, or by a stimulus derived from the “nanoeffects” or “nanotoxicity” of nanoparticles. This opening promotes the diffusion of drug conjugates into brain tissue;
- The adsorption of nanocarrier conjugates on the surface of capillary endothelium cells facilitates the release of the drug from carriers on the cell surface. This process increases the concentration gradient of the drug and promotes the diffusion of substances into the brain;
- Transcytosis, endocytosis and exocytosis of nanocarriers by capillary endothelial cells of the brain provides direct penetration of the substances into brain tissue.
3. Categories of Nanocarriers
3.1. Polymeric Nanoparticles
3.1.1. Dendrimers
3.1.2. Micelles
3.2. Lipid-Based NPs
3.2.1. Liposomes
3.2.2. Solid Lipid Nanoparticles
3.2.3. Nanoemulsion
3.3. Inorganic NPs
4. Drug Delivery for Depression
- Tricyclic antidepressants (TCAs) block the reuptake of monoamine neurotransmitters (primarily serotonin and norepinephrine) presynaptically.
- Monoamine oxidase inhibitors (MAOIs) amplify concentration of monoamines in the synaptic cleft by the blockage of monoamine oxidase enzymes.
- Selective serotonin reuptake inhibitors (SSRIs) selectively block the reuptake of serotonin. This group of drugs demonstrates decreased side-effects in comparison to other classes of antidepressants.
- Serotonin and norepinephrine reuptake inhibitors (SNRIs) produce dual inhibition of serotonin and norepinephrine reuptake.
- Norepinephrine-dopamine reuptake inhibitors block the action of norepinephrine and dopamine transporters thereby inducing reuptake suppression.
- Norepinephrine reuptake inhibitors block norepinephrine and epinephrine reuptake via norepinephrine transporter inhibition.
- Serotonin antagonists and reuptake inhibitors (SARIs) act by antagonizing serotonin receptors such as 5-HT2A and inhibiting the reuptake of serotonin, norepinephrine and/or dopamine. Most of them also act as α1-adrenergic receptor antagonists. The majority of the currently marketed SARIs belong to the phenylpiperazine class of substances.
- NMDA receptor antagonists deactivate NMDA glutamate receptors. This class of antidepressants characterizes as rapid-action drugs and includes such substances as ketamine and esketamine.
4.1. TCAs
4.2. MAOIs
4.3. SSRIs
4.4. SNRIs
4.5. SARIs
4.6. Other Drugs
5. Drug Delivery for Anxiety Disorders
6. Drug Delivery for Schizophrenia and Bipolar Disorders
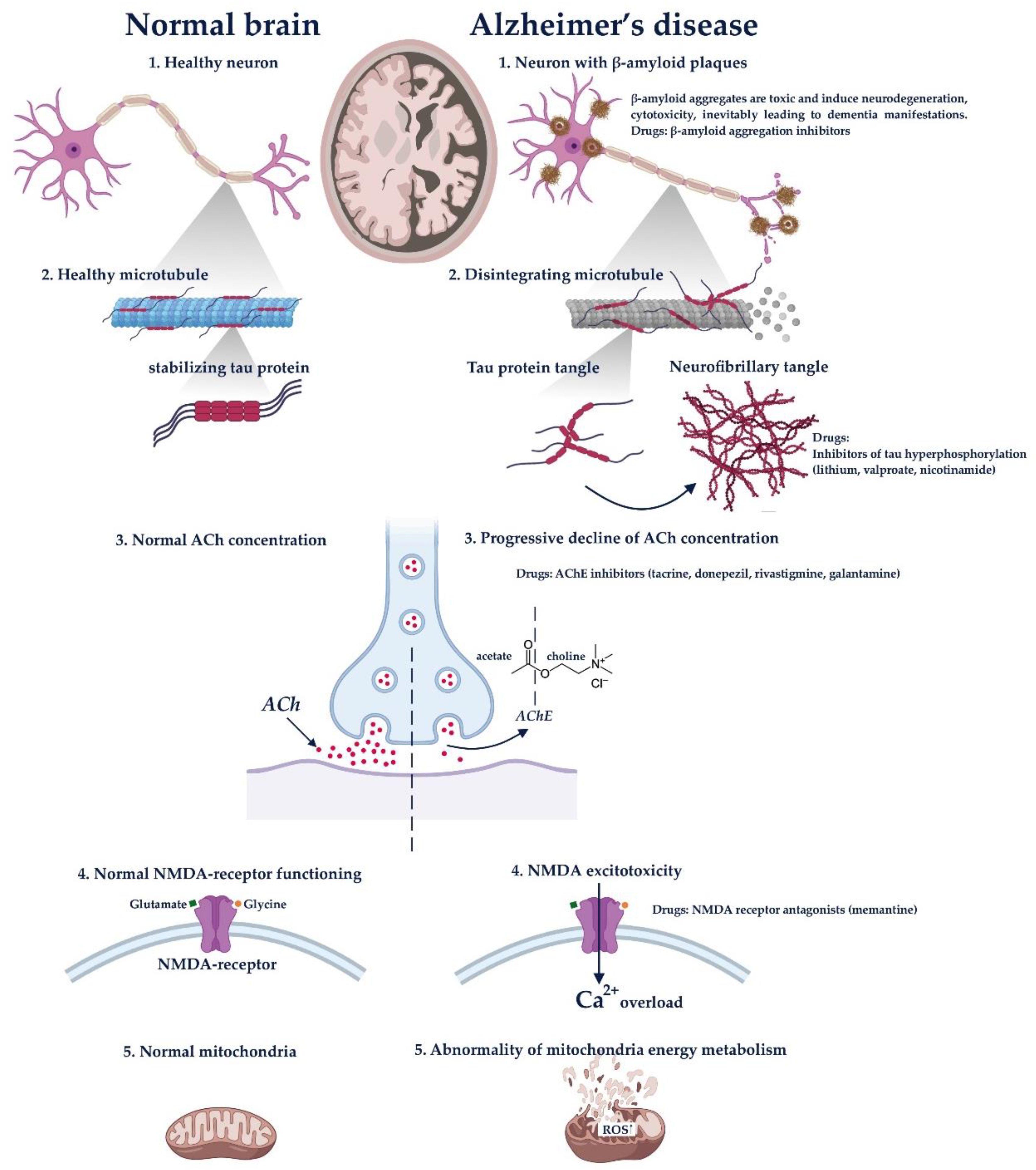
7. Drug Delivery for Alzheimer’s Disease
- Accumulation of β-amyloid (Aβ(1 → 40) and Aβ(1 → 42)), which is resulted from pathologic proteolysis of amyloid precursor protein (APP). The aggregates of this protein are toxic and induce neurodegeneration, cytotoxicity and inevitably leading to dementia manifestations.
- Tau accumulation. It is known that a normal mature neuron produces three microtubule-associated protein (MAP) taus; MAP1A, MAP1B and MAP2. These proteins are responsible for the promotion of the assembly and stability of microtubules. The biological activity of tau is regulated by its phosphorylation level. For example, in the brain of AD patients, tau hyperphosphorylates abnormally, which impairs its binding to microtubules; leading to the accumulation of neurofibrillary tangles and dementia development. Several anti-tau therapies including lithium, valproate and nicotinamide have been already investigated for the prevention of Tau protein hyperphosphorylation.
- Progressive decline of acetylcholine concentration. This is the earliest hypothesis of AD progression. It was demonstrated that AD is accompanied by substantial presynaptic cholinergic deficit, which expresses as a reduced choline uptake and acetylcholine release in the brain. That leads to memory and cognition impairment. Drugs that eliminate these symptoms include acetylcholinesterase inhibitors (AChEs) such as tacrine, donepezil, rivastigmine and galantamine.
- NMDA excitotoxicity. Some experimental and neuropathological evidence suggests that glutamatergic system is also involved in the neurodegenerative progression. NMDA receptor is essential for the control of synaptic plasticity and memory function. It is activated by glutamate and glycine, allowing Ca2+ and Na+ influx. It was demonstrated that the hyperexcitability of NMDA receptors induces Ca2+ overload, eventually leading to apoptosis and producing cognitive impairments. The drug that affects this pathogenesis block is memantine (NMDA inhibitor).
- Abnormality of mitochondria energy metabolism. It is suggested that genetic mutations alter the regulation of the electron transport chain complex enzymes, which are capable of generating ROS. That leads to cell apoptosis and neurodegeneration.
7.1. Nanoparticles with High Affinity to Aβ1–42 Peptide
7.2. Drugs Directed on the Inhibition of Tau Aggregation
7.3. Acetylcholinesterase Inhibitors (AChE)
7.4. Memantine, the NMDA Receptors Antagonist
7.5. Other Drugs for AD Treatment
7.6. Others Methods of AD Treatment
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AChE | acetylcholinesterase inhibitors |
| AD | Alzheimer’s disease |
| BACE1 | beta-Secretase 1 |
| BBB | blood–brain barrier |
| BDNF | brain-derived neurotrophic factor |
| DOPE | dioleylphosphatidylethanolamine |
| FDA | U.S. Food and Drug Administration |
| Lf | lactoferrin |
| MAOIs | monoamine oxidase inhibitors |
| MEM | memantine hydrochloride |
| MRI | magnetic resonance imaging |
| NMDA | N-methyl-d-aspartate |
| NPs | nanoparticles |
| PEG | polyethylene glycol |
| PLA | polylactide acid |
| PLGA | poly lactic-co-glycolic acid |
| RES | reticuloendothelial system |
| SARIs | serotonin antagonists and reuptake inhibitors |
| SLNs | solid lipid nanoparticles |
| SNRIs | serotonin and norepinephrine reuptake inhibitors |
| SPIONs | superparamagnetic iron oxide nanoparticles |
| SSRIs | selective serotonin reuptake inhibitors |
| TCAs | tricyclic antidepressants |
References
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Agrahari, V. The exciting potential of nanotherapy in brain-tumor targeted drug delivery approaches. Neural Regen. Res. 2017, 12, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Rakotoarisoa, M.; Angelov, B.; Garamus, V.M.; Angelova, A. Curcumin- and fish oil-loaded spongosome and cubosome nanoparticles with neuroprotective potential against H2O2-induced oxidative stress in differentiated human SH-SY5Y cells. ACS Omega 2019, 4, 3061–3073. [Google Scholar] [CrossRef]
- Deeken, J.F.; Löscher, W. The blood-brain barrier and cancer: Transporters, treatment, and Trojan horses. Clin. Cancer Res. 2007, 13, 1663–1674. [Google Scholar] [CrossRef] [PubMed]
- Van Tellingen, O.; Yetkin-Arik, B.; De Gooijer, M.; Wesseling, P.; Wurdinger, T.; De Vries, H.E. Overcoming the blood–brain tumor barrier for effective glioblastoma treatment. Drug Resist. Updates 2015, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Villabona-Rueda, A.; Erice, C.; Pardo, C.A.; Stins, M.F. The evolving concept of the blood brain barrier (BBB): From a single static barrier to a heterogeneous and dynamic relay center. Front. Cell. Neurosci. 2019, 13. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.K.; Dolman, D.E.M.; Yusof, S.R.; Begley, D.J. Structure and function of the blood–brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Weidle, U.H.; Niewöhner, J.; Tiefenthaler, G. The blood-brain barrier challenge for the treatment of brain cancer, secondary brain metastases, and neurological diseases. Cancer Genom. Proteom. 2015, 12, 167–177. [Google Scholar]
- Pardridge, W.M. Drug and gene targeting to the brain with molecular Trojan horses. Nat. Rev. Drug Discov. 2002, 1, 131–139. [Google Scholar] [CrossRef]
- Agarwal, S.; Sane, R.; Oberoi, R.; Ohlfest, J.R.; Elmquist, W.F. Delivery of molecularly targeted therapy to malignant glioma, a disease of the whole brain. Expert Rev. Mol. Med. 2011, 13, e17. [Google Scholar] [CrossRef]
- Lesniak, W.G.; Chu, C.; Jablonska, A.; Du, Y.; Pomper, M.G.; Walczak, P.; Janowski, M. A Distinct advantage to intraarterial delivery of 89Zr-bevacizumab in PET imaging of mice with and without osmotic opening of the blood–brain barrier. J. Nucl. Med. 2018, 60, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Côté, J.; Bovenzi, V.; Savard, M.; Dubuc, C.; Fortier, A.; Neugebauer, W.; Tremblay, L.; Müller-Esterl, W.; Tsanaclis, A.-M.; Lepage, M.; et al. Induction of selective blood-tumor barrier permeability and macromolecular transport by a biostable kinin B1 receptor agonist in a glioma rat model. PLoS ONE 2012, 7, e37485. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, B.K.; Cohen-Gadol, A.A.; Miller, J.C. Novel delivery methods bypassing the blood-brain and blood-tumor barriers. Neurosurg. Focus 2015, 38, E10. [Google Scholar] [CrossRef] [PubMed]
- Hersh, D.S.; Wadajkar, A.S.; Roberts, N.B.; Perez, J.G.; Connolly, N.P.; Frenkel, V.; Winkles, J.A.; Woodworth, G.F.; Kim, A.J. Evolving drug delivery strategies to overcome the blood brain barrier. Curr. Pharm. Des. 2016, 22, 1177–1193. [Google Scholar] [CrossRef]
- Alkhani, A.M.; Al-Shaar, H.A. Intrathecal baclofen therapy for spasticity: A compliance-based study to indicate effectiveness. Surg. Neurol. Int. 2016, 7, S539–S541. [Google Scholar] [CrossRef][Green Version]
- Lochhead, J.J.; Thorne, R.G. Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev. 2012, 64, 614–628. [Google Scholar] [CrossRef]
- Li, X.; Tsibouklis, J.; Weng, T.; Zhang, B.; Yin, G.; Feng, G.; Cui, Y.; Savina, I.N.; Mikhalovska, L.; Sandeman, S.R.; et al. Nano carriers for drug transport across the blood–brain barrier. J. Drug Target. 2017, 25, 17–28. [Google Scholar] [CrossRef]
- Adjei, I.M.; Sharma, B.; Labhasetwar, V. Nanoparticles: Cellular uptake and cytotoxicity. Atherosclerosis 2014, 811, 73–91. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, Y.; Zhao, S.-G.; Jiang, L.-Q.; Meng, Y.; Liu, P.; Kim, M.O.; Li, S. Selective neuronal targeting, protection and signaling network analysis via dopamine-mediated mesoporous silica nanoparticles. MedChemComm 2015, 6, 1117–1129. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef]
- Hasadsri, L.; Kreuter, J.; Hattori, H.; Iwasaki, T.; George, J.M. Functional protein delivery into neurons using polymeric nanoparticles. J. Biol. Chem. 2009, 284, 6972–6981. [Google Scholar] [CrossRef] [PubMed]
- Scuderi, C.; Stecca, C.; Iacomino, A.; Steardo, L. Role of astrocytes in major neurological disorders: The evidence and implications. IUBMB Life 2013, 65, 957–961. [Google Scholar] [CrossRef] [PubMed]
- Rittchen, S.; Boyd, A.; Burns, A.; Park, J.; Fahmy, T.M.; Metcalfe, S.; Williams, A. Myelin repair in vivo is increased by targeting oligodendrocyte precursor cells with nanoparticles encapsulating leukaemia inhibitory factor (LIF). Biomaterials 2015, 56, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Hutter, E.; Boridy, S.; Labrecque, S.; Lalancette-Hébert, M.; Kriz, J.; Winnik, F.M.; Maysinger, D. Microglial response to gold nanoparticles. ACS Nano 2010, 4, 2595–2606. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.K.; Janjic, J.M. Macrophage targeted theranostics as personalized nanomedicine strategies for inflammatory diseases. Theranostics 2015, 5, 150–172. [Google Scholar] [CrossRef]
- Papa, S.; Rossi, F.; Ferrari, R.; Mariani, A.; De Paola, M.; Caron, I.; Fiordaliso, F.; Bisighini, C.; Sammali, E.; Colombo, C.; et al. Selective nanovector mediated treatment of activated proinflammatory microglia/macrophages in spinal cord injury. ACS Nano 2013, 7, 9881–9895. [Google Scholar] [CrossRef]
- Kim, Y.-T.; Caldwell, J.-M.; Bellamkonda, R.V. Nanoparticle-mediated local delivery of methylprednisolone after spinal cord injury. Biomaterials 2009, 30, 2582–2590. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, M.; Zhang, J.; Maincent, P.; Xia, X.; Wu, W. Updated progress of nanocarrier-based intranasal drug delivery systems for treatment of brain diseases. Crit. Rev. Ther. Drug Carr. Syst. 2018, 35, 433–467. [Google Scholar] [CrossRef]
- Fonseca-Santos, B.; Chorilli, M.; Gremião, M.P.D. Nanotechnology-based drug delivery systems for the treatment of Alzheimer’s disease. Int. J. Nanomed. 2015, 10, 4981–5003. [Google Scholar] [CrossRef]
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as drug delivery systems. Pharmacol. Rep. 2012, 64, 1020–1037. [Google Scholar] [CrossRef]
- Zhou, Y.; Peng, Z.; Seven, E.S.; Leblanc, R.M. Crossing the blood-brain barrier with nanoparticles. J. Control. Release 2018, 270, 290–303. [Google Scholar] [CrossRef]
- Wen, M.M.; El-Salamouni, N.S.; El-Refaie, W.M.; Hazzah, H.A.; Ali, M.M.; Tosi, G.; Farid, R.M.; Blanco-Prieto, M.J.; Billa, N.; Hanafy, A.S. Nanotechnology-based drug delivery systems for Alzheimer’s disease management: Technical, industrial, and clinical challenges. J. Control. Release 2017, 245, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Saraf, S.; Saraf, S.K.; Antimisiaris, S.G.; Hamano, N.; Li, S.-D.; Chougule, M.; Shoyele, S.A.; Gupta, U.; Ajazuddin; et al. Recent advancements in the field of nanotechnology for the delivery of anti-Alzheimer drug in the brain region. Expert Opin. Drug Deliv. 2018, 15, 589–617. [Google Scholar] [CrossRef] [PubMed]
- Majumder, J.; Taratula, O.; Minko, T. Nanocarrier-based systems for targeted and site specific therapeutic delivery. Adv. Drug Deliv. Rev. 2019, 144, 57–77. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Chavda, K.; Vyas, B.; Patel, S. Formulation development of linagliptin solid lipid nanoparticles for oral bioavailability enhancement: Role of P-gp inhibition. Drug Deliv. Transl. Res. 2020, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.; Fukuda, T.; Nagaoka, Y.; Hasumura, T.; Morimoto, H.; Yoshida, Y.; Maekawa, T.; Venugopal, K.; Kumar, D.S. Curcumin loaded-PLGA nanoparticles conjugated with Tet-1 peptide for potential use in Alzheimer’s disease. PLoS ONE 2012, 7, e32616. [Google Scholar] [CrossRef] [PubMed]
- Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Volceanov, A.; Teleanu, R.I. Blood-brain delivery methods using nanotechnology. Pharmaceutics 2018, 10, 269. [Google Scholar] [CrossRef]
- Igartúa, D.E.; Martinez, C.S.; Temprana, C.F.; Alonso, S.D.V.; Prieto, M.J. PAMAM dendrimers as a carbamazepine delivery system for neurodegenerative diseases: A biophysical and nanotoxicological characterization. Int. J. Pharm. 2018, 544, 191–202. [Google Scholar] [CrossRef]
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Yu, B.; Lee, R.J.; Lee, L.J. Microfluidic methods for production of liposomes. Methods Enzymol. 2009, 465, 129–141. [Google Scholar] [CrossRef]
- Carugo, D.; Bottaro, E.; Owen, J.; Stride, E.; Nastruzzi, C. Liposome production by microfluidics: Potential and limiting factors. Sci. Rep. 2016, 6, 25876. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, P.P.; Biswas, S.; Torchilin, V. Current trends in the use of liposomes for tumor targeting. Nanomedicine 2013, 8, 1509–1528. [Google Scholar] [CrossRef] [PubMed]
- Stenehjem, D.D.; Hartz, A.M.; Bauer, B.; Anderson, G.W. Novel and emerging strategies in drug delivery for overcoming the blood–brain barrier. Futur. Med. Chem. 2009, 1, 1623–1641. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Suzuki, S.; Kawakami, S.; Yamashita, F.; Hashida, M. The role of dioleoylphosphatidylethanolamine (DOPE) in targeted gene delivery with mannosylated cationic liposomes via intravenous route. J. Control. Release 2005, 108, 484–495. [Google Scholar] [CrossRef]
- Luo, L.; Bian, Y.; Liu, Y.; Zhang, X.; Wang, M.; Xing, S.; Li, L.; Gao, D. Gold nanoshells: Combined near infrared photothermal therapy and chemotherapy using gold nanoshells coated liposomes to enhance antitumor effect (Small 30/2016). Small 2016, 12, 4102. [Google Scholar] [CrossRef]
- Lindqvist, A.; Rip, J.; Van Kregten, J.; Gaillard, P.J.; Hammarlund-Udenaes, M. In vivo functional evaluation of increased brain delivery of the opioid peptide DAMGO by glutathione-PEGylated liposomes. Pharm. Res. 2016, 33, 177–185. [Google Scholar] [CrossRef]
- Tapeinos, C.; Battaglini, M.; Ciofani, G. Advances in the design of solid lipid nanoparticles and nanostructured lipid carriers for targeting brain diseases. J. Control. Release 2017, 264, 306–332. [Google Scholar] [CrossRef]
- Karakoti, A.S.; Das, S.; Thevuthasan, S.; Seal, S. PEGylated inorganic nanoparticles. Angew. Chem. Int. Ed. 2011, 50, 1980–1994. [Google Scholar] [CrossRef]
- Joralemon, M.J.; McRae, S.; Emrick, T. PEGylated polymers for medicine: From conjugation to self-assembled systems. Chem. Commun. 2010, 46, 1377–1393. [Google Scholar] [CrossRef]
- Qiao, R.; Jia, Q.; Hüwel, S.; Xia, R.; Liu, T.; Gao, F.; Galla, H.-J.; Gao, M. Receptor-mediated delivery of magnetic nanoparticles across the blood–brain barrier. ACS Nano 2012, 6, 3304–3310. [Google Scholar] [CrossRef]
- Luo, S.; Ma, C.; Zhu, M.-Q.; Ju, W.-N.; Yang, Y.; Wang, X. Application of iron oxide nanoparticles in the diagnosis and treatment of neurodegenerative diseases with emphasis on Alzheimer’s disease. Front. Cell. Neurosci. 2020, 14, 21. [Google Scholar] [CrossRef] [PubMed]
- Frigell, J.; García, I.; Gómez-Vallejo, V.; Llop, J.; Penadés, S. 68Ga-Labeled gold glyconanoparticles for exploring blood–brain barrier permeability: Preparation, biodistribution studies, and improved brain uptake via neuropeptide conjugation. J. Am. Chem. Soc. 2013, 136, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Timberlake, M.A., II; Prall, K.; Dwivedi, Y. The recent progress in animal models of depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 77, 99–109. [Google Scholar] [CrossRef]
- McQuaid, R.J.; McInnis, O.A.; Abizaid, A.; Anisman, H. Making room for oxytocin in understanding depression. Neurosci. Biobehav. Rev. 2014, 45, 305–322. [Google Scholar] [CrossRef]
- O’Leary, O.F.; Dinan, T.G.; Cryan, J.F. Faster, better, stronger: Towards new antidepressant therapeutic strategies. Eur. J. Pharmacol. 2015, 753, 32–50. [Google Scholar] [CrossRef] [PubMed]
- Cirri, M.; Maestrini, L.; Maestrelli, F.; Mennini, N.; Mura, P.; Ghelardini, C.; Mannelli, L.D.C. Design, characterization and in vivo evaluation of nanostructured lipid carriers (NLC) as a new drug delivery system for hydrochlorothiazide oral administration in pediatric therapy. Drug Deliv. 2018, 25, 1910–1921. [Google Scholar] [CrossRef]
- Vitorino, C.; Silva, S.; Bicker, J.; Falcão, A.; Fortuna, A. Antidepressants and nose-to-brain delivery: Drivers, restraints, opportunities and challenges. Drug Discov. Today 2019, 24, 1911–1923. [Google Scholar] [CrossRef]
- Jani, P.; Vanza, J.; Pandya, N.; Tandel, H. Formulation of polymeric nanoparticles of antidepressant drug for intranasal delivery. Ther. Deliv. 2019, 10, 683–696. [Google Scholar] [CrossRef]
- Shinde, M.; Bali, N.R.; Rathod, S.; Karemore, M.N.; Salve, P. Effect of binary combinations of solvent systems on permeability profiling of pure agomelatine across rat skin: A comparative study with statistically optimized polymeric nanoparticles. Drug Dev. Ind. Pharm. 2020, 46, 826–845. [Google Scholar] [CrossRef]
- Chen, B.; Luo, M.; Liang, J.; Zhang, C.; Gao, C.; Wang, J.; Wang, J.; Li, Y.; Xu, D.; Liu, L.; et al. Surface modification of PGP for a neutrophil–nanoparticle co-vehicle to enhance the anti-depressant effect of baicalein. Acta Pharm. Sin. B 2018, 8, 64–73. [Google Scholar] [CrossRef]
- Harris, N.M.; Ritzel, R.; Mancini, N.S.; Jiang, Y.; Yi, X.; Manickam, D.S.; Banks, W.A.; Kabanov, A.V.; McCullough, L.D.; Verma, R. Nano-particle delivery of brain derived neurotrophic factor after focal cerebral ischemia reduces tissue injury and enhances behavioral recovery. Pharmacol. Biochem. Behav. 2016, 48–56. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhu, Y.; Wang, M.; Jing, G.; Zhu, R.; Wang, S. Antidepressant effects of curcumin and HU-211 coencapsulated solid lipid nanoparticles against corticosterone-induced cellular and animal models of major depression. Int. J. Nanomed. 2016, 11, 4975–4990. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Yang, L.; Wang, M.; Zhuang, X.; Huang, R.; Zhu, R.; Wang, S. Targeting the endocannabinoid/CB1 receptor system for treating major depression through antidepressant activities of curcumin and dexanabinol-loaded solid lipid nanoparticles. Cell Physiol. Biochem. 2017, 42, 2281–2294. [Google Scholar] [CrossRef] [PubMed]
- Fidelis, E.M.; Savall, A.S.P.; Abreu, E.D.L.; Carvalho, F.; Teixeira, F.E.G.; Haas, S.E.; Sampaio, T.B.; Pinton, S. Curcumin-loaded nanocapsules reverses the depressant-like behavior and oxidative stress induced by β-amyloid in mice. Neuroscience 2019, 423, 122–130. [Google Scholar] [CrossRef]
- Alam, M.I.; Baboota, S.; Ahuja, A.; Ali, M.; Ali, J.; Sahni, J.K.; Bhatnagar, A. Pharmacoscintigraphic evaluation of potential of lipid nanocarriers for nose-to-brain delivery of antidepressant drug. Int. J. Pharm. 2014, 470, 99–106. [Google Scholar] [CrossRef]
- Ravouru, N.; Kondreddy, P.; Korakanchi, D. Formulation and evaluation of niosomal nasal drug delivery system of folic acid for brain targeting. Curr. Drug Discov. Technol. 2013, 10, 270–282. [Google Scholar] [CrossRef]
- Margret, A.A.; Dhayabaran, V.V.; Kumar, A.G. Nanoparticulated polymeric composites enfolding lithium carbonate as brain drug in persuading depression: An in vivo study. Prog. Biomater. 2017, 6, 165–173. [Google Scholar] [CrossRef]
- Nagpal, K.; Singh, S.K.; Mishra, D. Evaluation of safety and efficacy of brain targeted chitosan nanoparticles of minocycline. Int. J. Biol. Macromol. 2013, 59, 20–28. [Google Scholar] [CrossRef]
- Singh, D.P.; Rashid, M.; Hallan, S.S.; Mehra, N.K.; Prakash, A.; Mishra, N. Pharmacological evaluation of nasal delivery of selegiline hydrochloride-loaded thiolated chitosan nanoparticles for the treatment of depression. Artif. Cells Nanomed. Biotechnol. 2015, 44, 1–13. [Google Scholar] [CrossRef]
- Ashraf, A.; Mahmoud, P.A.; Reda, H.; Mansour, S.; Helal, M.H.; Michel, H.E.; Nasr, M. Silymarin and silymarin nanoparticles guard against chronic unpredictable mild stress induced depressive-like behavior in mice: Involvement of neurogenesis and NLRP3 inflammasome. J. Psychopharmacol. 2019, 33, 615–631. [Google Scholar] [CrossRef]
- Alam, M.; Najmi, A.K.; Ahmad, I.; Ahmad, F.J.; Akhtar, J.; Imam, S.S.; Akhtar, M. Formulation and evaluation of nano lipid formulation containing CNS acting drug: Molecular docking, in-vitro assessment and bioactivity detail in rats. Artif. Cells Nanomed. Biotechnol. 2018, 46, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Garg, T.; Vaidya, B.; Prakash, A.; Rath, G.; Goyal, A.K. Brain delivery of intranasalin situgel of nanoparticulated polymeric carriers containing antidepressant drug: Behavioral and biochemical assessment. J. Drug Target. 2014, 23, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Yang, X.; Zhang, R.-X.; Luo, Y.-X.; Li, J.-L.; Hou, J.; Zhang, C.; Li, Y.-J.; Shi, J.; Lu, L.; et al. Monocyte mediated brain targeting delivery of macromolecular drug for the therapy of depression. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.; Shadab; Fazil, M.; Kumar, M.; Sahni, J.K.; Ali, J.; Baboota, S. Venlafaxine loaded chitosan NPs for brain targeting: Pharmacokinetic and pharmacodynamic evaluation. Carbohydr. Polym. 2012, 89, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Cayero-Otero, M.; Gomes, M.J.; Martins, C.; Álvarez-Fuentes, J.; Fernández-Arévalo, M.; Sarmento, B.; Martín-Banderas, L. In vivo biodistribution of venlafaxine-PLGA nanoparticles for brain delivery: Plain vs. functionalized nanoparticles. Expert Opin. Drug Deliv. 2019, 16, 1413–1427. [Google Scholar] [CrossRef]
- Haque, S.; Shadab; Sahni, J.K.; Ali, J.; Baboota, S. Development and evaluation of brain targeted intranasal alginate nanoparticles for treatment of depression. J. Psychiatr. Res. 2014, 48, 1–12. [Google Scholar] [CrossRef]
- Grabrucker, A.M.; Garner, C.C.; Boeckers, T.M.; Bondioli, L.; Ruozi, B.; Forni, F.; Vandelli, M.A.; Tosi, G. Development of novel Zn2+ loaded nanoparticles designed for cell-type targeted drug release in CNS neurons: In vitro evidences. PLoS ONE 2011, 6, e17851. [Google Scholar] [CrossRef][Green Version]
- Vinzant, N.; Scholl, J.L.; Wu, C.-M.; Kindle, T.; Koodali, R.; Forster, G.L. Iron oxide nanoparticle delivery of peptides to the brain: Reversal of anxiety during drug withdrawal. Front. Neurosci. 2017, 11, 608. [Google Scholar] [CrossRef]
- Bari, N.K.; Fazil, M.; Hassan, Q.; Haider, R.; Gaba, B.; Narang, J.K.; Baboota, S.; Ali, J. Brain delivery of buspirone hydrochloride chitosan nanoparticles for the treatment of general anxiety disorder. Int. J. Biol. Macromol. 2015, 81, 49–59. [Google Scholar] [CrossRef]
- Abdelnabi, D.M.; Abdallah, M.H.; Elghamry, H.A. Buspirone hydrochloride loaded in situ nanovesicular gel as an anxiolytic nasal drug delivery system: In vitro and animal studies. AAPS PharmSciTech 2019, 20, 134. [Google Scholar] [CrossRef]
- Bohrey, S.; Chourasiya, V.; Pandey, A. Polymeric nanoparticles containing diazepam: Preparation, optimization, characterization, in-vitro drug release and release kinetic study. Nano Converg. 2016, 3, 1–7. [Google Scholar] [CrossRef]
- Nagpal, K.; Singh, S.K.; Mishra, D. Optimization of brain targeted gallic acid nanoparticles for improved antianxiety-like activity. Int. J. Biol. Macromol. 2013, 57, 83–91. [Google Scholar] [CrossRef]
- Sinha, S.V. Enhancement of in vivo efficacy and oral bioavailability of aripiprazole with solid lipid nanoparticles. AAPS PharmSciTech 2018, 19, 1264–1273. [Google Scholar] [CrossRef]
- Sawant, K.; Pandey, A.; Patel, S. Aripiprazole loaded poly(caprolactone) nanoparticles: Optimization and in vivo pharmacokinetics. Mater. Sci. Eng. C 2016, 66, 230–243. [Google Scholar] [CrossRef]
- Singh, S.K.; Hidau, M.K.; Gautam, S.; Gupta, K.; Singh, K.P.; Singh, S.K. Glycol chitosan functionalized asenapine nanostructured lipid carriers for targeted brain delivery: Pharmacokinetic and teratogenic assessment. Int. J. Biol. Macromol. 2018, 108, 1092–1100. [Google Scholar] [CrossRef]
- Shreya, A.B.; Managuli, R.S.; Menon, J.; Kondapalli, L.; Hegde, A.R.; Avadhani, K.; Shetty, P.K.; Amirthalingam, M.; Kalthur, G.; Mutalik, S. Nano-transfersomal formulations for transdermal delivery of asenapine maleate: In vitro and in vivo performance evaluations. J. Liposome Res. 2015, 26, 221–232. [Google Scholar] [CrossRef]
- Piazza, J.; Hoare, T.; Molinaro, L.; Terpstra, K.; Bhandari, J.; Selvaganapathy, P.R.; Gupta, B.; Mishra, R.K. Haloperidol-loaded intranasally administered lectin functionalized poly(ethylene glycol)–block-poly(d,l)-lactic-co-glycolic acid (PEG–PLGA) nanoparticles for the treatment of schizophrenia. Eur. J. Pharm. Biopharm. 2014, 87, 30–39. [Google Scholar] [CrossRef]
- Narayan, S. Lithium entrapped chitosan nanoparticles to reduce toxicity and increase cellular uptake of lithium. Environ. Toxicol. Pharmacol. 2018, 61, 79–86. [Google Scholar] [CrossRef]
- Patel, M.H.; Mundada, V.P.; Sawant, K.K. Fabrication of solid lipid nanoparticles of lurasidone HCl for oral delivery: Optimization, in vitro characterization, cell line studies and in vivo efficacy in schizophrenia. Drug Dev. Ind. Pharm. 2019, 45, 1242–1257. [Google Scholar] [CrossRef]
- Jazuli, I.; Nabi, B.; Moolakkadath, T.; Alam, T.; Baboota, S.; Ali, J.; Annu, I.J. Optimization of nanostructured lipid carriers of lurasidone hydrochloride using box-behnken design for brain targeting: In vitro and in vivo studies. J. Pharm. Sci. 2019, 108, 3082–3090. [Google Scholar] [CrossRef]
- Pokharkar, V.; Suryawanshi, S.; Dhapte-Pawar, V. Exploring micellar-based polymeric systems for effective nose-to-brain drug delivery as potential neurotherapeutics. Drug Deliv. Transl. Res. 2019, 10, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, J.; Baskaran, M.; Humtsoe, L.C.; Vadivelan, R.; Justin, A. Enhanced brain targeting efficacy of Olanzapine through solid lipid nanoparticles. Artif. Cells Nanomed. Biotechnol. 2016, 45, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gadhave, D.G.; Tagalpallewar, A.A.; Kokare, C.R. Agranulocytosis-protective olanzapine-loaded nanostructured lipid carriers engineered for CNS delivery: Optimization and hematological toxicity studies. AAPS PharmSciTech 2019, 20, 22. [Google Scholar] [CrossRef] [PubMed]
- Abdelbary, G.A.; Tadros, M.I. Brain targeting of olanzapine via intranasal delivery of core–shell difunctional block copolymer mixed nanomicellar carriers: In vitro characterization, ex vivo estimation of nasal toxicity and in vivo biodistribution studies. Int. J. Pharm. 2013, 452, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, F.N.; Betti, A.H.; Carvalho, F.C.U.; Gremiao, M.P.D.U.; Dimer, F.A.; Guterres, S.S.; Tebaldi, M.L.; Rates, S.M.K.; Pohlmann, A.R. Mucoadhesive amphiphilic methacrylic copolymer-functionalized poly(ε-caprolactone) nanocapsules for nose-to-brain delivery of olanzapine. J. Biomed. Nanotechnol. 2015, 11, 1472–1481. [Google Scholar] [CrossRef] [PubMed]
- Baltzley, S.; Mohammad, A.; Malkawi, A.H.; Al-Ghananeem, A.M. Intranasal drug delivery of olanzapine-loaded chitosan nanoparticles. AAPS PharmSciTech 2014, 15, 1598–1602. [Google Scholar] [CrossRef] [PubMed]
- Seju, U.; Kumar, A.; Sawant, K. Development and evaluation of olanzapine-loaded PLGA nanoparticles for nose-to-brain delivery: In vitro and in vivo studies. Acta Biomater. 2011, 7, 4169–4176. [Google Scholar] [CrossRef]
- Helal, H.M.; Mortada, S.M.; Sallam, M.A. Paliperidone-loaded nanolipomer system for sustained delivery and enhanced intestinal permeation: Superiority to polymeric and solid lipid nanoparticles. AAPS PharmSciTech 2016, 18, 1946–1959. [Google Scholar] [CrossRef]
- Sherje, A.P.; Londhe, V. Development and evaluation of pH-responsive cyclodextrin-based in situ gel of paliperidone for intranasal delivery. AAPS PharmSciTech 2017, 19, 384–394. [Google Scholar] [CrossRef]
- Muthu, M.S.; Sahu, A.K.; Sonali; Abdulla, A.; Kaklotar, D.; Rajesh, C.V.; Singh, S.; Pandey, B.L. Solubilized delivery of paliperidone palmitate by d-alpha-tocopheryl polyethylene glycol 1000 succinate micelles for improved short-term psychotic management. Drug Deliv. 2014, 23, 230–237. [Google Scholar] [CrossRef]
- Patel, M.R.; Patel, R.B.; Bhatt, K.K.; Patel, B.G.; Gaikwad, R.V. Paliperidone microemulsion for nose-to-brain targeted drug delivery system: Pharmacodynamic and pharmacokinetic evaluation. Drug Deliv. 2014, 23, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Lugasi, L.; Grinberg, I.; Sabag, R.; Madar, R.; Einat, H.; Margel, S. Proteinoid nanocapsules as drug delivery system for improving antipsychotic activity of risperidone. Molecules 2020, 25, 4013. [Google Scholar] [CrossRef] [PubMed]
- Rukmangathen, R.; Yallamalli, I.M.; Yalavarthi, P.R. Formulation and biopharmaceutical evaluation of risperidone-loaded chitosan nanoparticles for intranasal delivery. Drug Dev. Ind. Pharm. 2019, 45, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Chavhan, S.; Soni, H.; Babbar, A.; Mathur, R.; Mishra, A.; Sawant, K. Brain targeting of risperidone-loaded solid lipid nanoparticles by intranasal route. J. Drug Target. 2010, 19, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Misra, A.; Mishra, A.K.; Mishra, P.; Pathak, K. Mucoadhesive nanoemulsion-based intranasal drug delivery system of olanzapine for brain targeting. J. Drug Target. 2008, 16, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Narayan, R.; Singh, M.; Ranjan, O.; Nayak, Y.; Garg, S.; Shavi, G.V.; Nayak, U.Y. Development of risperidone liposomes for brain targeting through intranasal route. Life Sci. 2016, 163, 38–45. [Google Scholar] [CrossRef]
- Qureshi, M.; Aqil, M.; Imam, S.S.; Ahad, A.; Sultana, Y. Formulation and evaluation of neuroactive drug loaded chitosan nanoparticle for nose to brain delivery: In-vitro characterization and in-vivo behavior study. Curr. Drug Deliv. 2018, 16, 123–135. [Google Scholar] [CrossRef]
- Li, J.; Zhang, W.-J.; Zhu, J.-X.; Zhu, N.; Zhang, H.-M.; Wang, X.; Zhang, J.; Wang, Q.-Q. Preparation and brain delivery of nasal solid lipid nanoparticles of quetiapine fumarate in situ gel in rat model of schizophrenia. Int. J. Clin. Exp. Med. 2015, 8, 17590–17600. [Google Scholar]
- Boche, M.; Pokharkar, V.B. Quetiapine nanoemulsion for intranasal drug delivery: Evaluation of brain-targeting efficiency. AAPS PharmSciTech 2016, 18, 686–696. [Google Scholar] [CrossRef]
- Upadhyay, P.; Trivedi, J.; Pundarikakshudu, K.; Sheth, N. Direct and enhanced delivery of nanoliposomes of anti schizophrenic agent to the brain through nasal route. Saudi Pharm. J. 2017, 25, 346–358. [Google Scholar] [CrossRef]
- Shah, B.; Khunt, D.; Misra, M.; Padh, H. Non-invasive intranasal delivery of quetiapine fumarate loaded microemulsion for brain targeting: Formulation, physicochemical and pharmacokinetic consideration. Eur. J. Pharm. Sci. 2016, 91, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.; Khunt, D.; Misra, M.; Padh, H. Application of box-behnken design for optimization and development of quetiapine fumarate loaded chitosan nanoparticles for brain delivery via intranasal route. Int. J. Biol. Macromol. 2016, 89, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Naik, A.; Nair, H. Formulation and evaluation of thermosensitive biogels for nose to brain delivery of doxepin. BioMed Res. Int. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pandey, Y.R.; Kumar, S.; Gupta, B.K.; Ali, J.; Baboota, S. Intranasal delivery of paroxetine nanoemulsion via the olfactory region for the management of depression: Formulation, behavioural and biochemical estimation. Nanotechnology 2016, 27, 25102. [Google Scholar] [CrossRef] [PubMed]
- Pathan, I.B.; Mene, H.; Bairagi, S. Quality by design (QbD) approach to formulate in situ gelling system for nose to brain delivery of Fluoxetine hydrochloride: Ex-vivo and In-vivo study. ARS Pharm. 2017, 58, 107–114. [Google Scholar]
- Kamel, R.; Abbas, H.; El-Naa, M. Composite carbohydrate interpenetrating polyelectrolyte nano-complexes (IPNC) as a controlled oral delivery system of citalopram HCl for pediatric use: In-vitro/in-vivo evaluation and histopathological examination. Drug Deliv. Transl. Res. 2018, 8, 657–669. [Google Scholar] [CrossRef]
- Rahman, M.A.; Harwansh, R.K.; Iqbal, Z. Systematic development of sertraline loaded solid lipid nanoparticle (SLN) by emulsification-ultrasonication method and pharmacokinetic study in sprague-dawley rats. Pharm. Nanotechnol. 2019, 7, 162–176. [Google Scholar] [CrossRef]
- Bhandwalkar, M.J.; Avachat, A.M. Thermoreversible nasal in situ gel of venlafaxine hydrochloride: Formulation, characterization, and pharmacodynamic evaluation. AAPS PharmSciTech 2012, 14, 101–110. [Google Scholar] [CrossRef]
- Aranaz, I.; Paños, I.; Peniche, C.; Heras, A.; Acosta, N. Chitosan spray-dried microparticles for controlled delivery of venlafaxine hydrochloride. Molecules 2017, 22, 1980. [Google Scholar] [CrossRef]
- Tong, G.-F.; Qin, N.; Sun, L.-W. Development and evaluation of Desvenlafaxine loaded PLGA-chitosan nanoparticles for brain delivery. Saudi Pharm. J. 2016, 25, 844–851. [Google Scholar] [CrossRef]
- Casolaro, M.; Casolaro, I. Controlled release of antidepressant drugs by multiple stimuli-sensitive hydrogels based on α-aminoacid residues. J. Drug Deliv. Sci. Technol. 2015, 30, 82–89. [Google Scholar] [CrossRef]
- Mitroshina, E.V.; Mishchenko, T.A.; Usenko, A.V.; Epifanova, E.A.; Yarkov, R.S.; Gavrish, M.S.; Babaev, A.A.; Vedunova, M.V. AAV-Syn-BDNF-EGFP virus construct exerts neuroprotective action on the hippocampal neural network during hypoxia in vitro. Int. J. Mol. Sci. 2018, 19, 2295. [Google Scholar] [CrossRef]
- Ma, X.-C.; Liu, P.; Zhang, X.-L.; Jiang, W.-H.; Jia, M.; Wang, C.-X.; Dong, Y.-Y.; Dang, Y.-H.; Gao, C.-G. intranasal delivery of recombinant AAV containing BDNF fused with HA2TAT: A potential promising therapy strategy for major depressive disorder. Sci. Rep. 2016, 6, 22404. [Google Scholar] [CrossRef]
- Mill, J.; Petronis, A. Molecular studies of major depressive disorder: The epigenetic perspective. Mol. Psychiatry 2007, 12, 799–814. [Google Scholar] [CrossRef]
- Bandelow, B.; Michaelis, S.; Wedekind, D. Treatment of anxiety disorders. Dialogues Clin. Neurosci. 2017, 19, 93–107. [Google Scholar]
- Pollack, M.; Otto, M.W.; Roy-Byrne, P.P.; Coplan, J.D.; Rothbaum, B.O.; Simon, N.M.; Gorman, J.M. Novel treatment approaches for refractory anxiety disorders. Depression Anxiety 2008, 25, 467–476. [Google Scholar] [CrossRef]
- Loane, C.; Politis, M. Buspirone: What is it all about? Brain Res. 2012, 1461, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Mendrek, A.; Mancini-Marïe, A. Sex/gender differences in the brain and cognition in schizophrenia. Neurosci. Biobehav. Rev. 2016, 67, 57–78. [Google Scholar] [CrossRef] [PubMed]
- Clark, C.T.; Wisner, K.L. Treatment of peripartum bipolar disorder. Obstet. Gynecol. Clin. N. Am. 2018, 45, 403–417. [Google Scholar] [CrossRef]
- Rabin, C.R.; Siegel, S.J. Antipsychotic dosing and drug delivery. In Behavioral Neurobiology of Schizophrenia and Its Treatment; Swerdlow, N., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 4, pp. 141–177. [Google Scholar]
- Cheng, Y.-H.; Illum, L.; Davis, S.S. Schizophrenia and drug delivery systems. J. Drug Target. 2000, 8, 107–117. [Google Scholar] [CrossRef]
- Kane, J.M.; García-Ribera, C. Clinical guideline recommendations for antipsychotic long-acting injections. Br. J. Psychiatry 2009, 195, s63–s67. [Google Scholar] [CrossRef] [PubMed]
- Joseph, E.; Reddi, S.; Rinwa, V.; Balwani, G.; Saha, R. Design and in vivo evaluation of solid lipid nanoparticulate systems of Olanzapine for acute phase schizophrenia treatment: Investigations on antipsychotic potential and adverse effects. Eur. J. Pharm. Sci. 2017, 104, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Jawahar, N.; Hingarh, P.K.; Arun, R.; Selvaraj, J.; Anbarasan, A.; Sathianarayanan, S.; Nagaraju, G. Enhanced oral bioavailability of an antipsychotic drug through nanostructured lipid carriers. Int. J. Biol. Macromol. 2018, 110, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Pervaiz, F.; Ahmad, M.; Hussain, T.; Idrees, A.; Yaqoob, A.; Abbas, K. Development of olanzapine loaded PNA microgels for depot drug delivery in treatment of schizophrenia: In vitro an in vivo release profile. Acta Pol. Pharm. Drug Res. 2016, 73, 175–181. [Google Scholar]
- De Freitas, M.R.; Rolim, L.A.; Soares, M.F.D.L.R.; Rolim-Neto, P.J.; De Albuquerque, M.M.; Soares-Sobrinho, J.L. Inclusion complex of methyl-β-cyclodextrin and olanzapine as potential drug delivery system for schizophrenia. Carbohydr. Polym. 2012, 89, 1095–1100. [Google Scholar] [CrossRef]
- Venkateswarlu, V.; Manjunath, K. Preparation, characterization and in vitro release kinetics of clozapine solid lipid nanoparticles. J. Control. Release 2004, 95, 627–638. [Google Scholar] [CrossRef]
- Shafaat, K.; Kumar, B.; Das, S.K.; Hasan, R.U.; Prajapati, S.K. Novel nanoemulsion as vehicles for transdermal delivery of clozapine, in vitro in vivo studies. Int. J. Pharm. Pharm. Sci. 2013, 5, 126–134. [Google Scholar]
- Iqbal, N.; Vitorino, C.; Taylor, K.M.G. How can lipid nanocarriers improve transdermal delivery of olanzapine? Pharm. Dev. Technol. 2017, 22, 587–596. [Google Scholar] [CrossRef]
- Dimer, F.A.; Ortiz, M.; Pase, C.S.; Roversi, K.; Friedrich, R.B.; Pohlmann, A.R.; Burger, M.E.; Guterres, S.S. Nanoencapsulation of olanzapine increases its efficacy in antipsychotic treatment and reduces adverse effects. J. Biomed. Nanotechnol. 2014, 10, 1137–1145. [Google Scholar] [CrossRef]
- Joseph, E.; Reddi, S.; Rinwa, V.; Balwani, G.; Saha, R. DoE based Olanzapine loaded poly-caprolactone nanoparticles decreases extrapyramidal effects in rodent model. Int. J. Pharm. 2018, 541, 198–205. [Google Scholar] [CrossRef]
- Al-Dhubiab, B.E. Aripiprazole nanocrystal impregnated buccoadhesive films for schizophrenia. J. Nanosci. Nanotechnol. 2017, 17, 2345–2352. [Google Scholar] [CrossRef] [PubMed]
- Çelik, B. Risperidone mucoadhesive buccal tablets: Formulation design, optimization and evaluation. Drug Des. Dev. Ther. 2017, 11, 3355–3365. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.; Kumar, A.; Wild, W.; Ferreira, D.; Santos, D.; Forbes, B. Long-term stability, biocompatibility and oral delivery potential of risperidone-loaded solid lipid nanoparticles. Int. J. Pharm. 2012, 436, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Alzubaidi, A.F.A.; El-Helw, A.-R.M.; Ahmed, T.A.; Ahmed, O.A.A. The use of experimental design in the optimization of risperidone biodegradable nanoparticles: In vitro and in vivo study. Artif. Cells Nanomed. Biotechnol. 2016, 45, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mandpe, L.; Pokharkar, V. Targeted brain delivery of iloperidone nanostructured lipid carriers following intranasal administration: In vivo pharmacokinetics and brain distribution studies. J. Nanopharm. Drug Deliv. 2013, 1, 212–225. [Google Scholar] [CrossRef]
- Đorđević, S.M.; Santrač, A.; Cekić, N.D.; Markovic, B.D.; Divović, B.; Ilić, T.M.; Savić, M.M.; Savic, S. Parenteral nanoemulsions of risperidone for enhanced brain delivery in acute psychosis: Physicochemical and in vivo performances. Int. J. Pharm. 2017, 533, 421–430. [Google Scholar] [CrossRef]
- Shan, G.W.; Makmor-Bakry, M.; Omar, M.S. Long term use of lithium and factors associated with treatment response among patients with bipolar disorder. Psychiatr. Danub. 2016, 28, 146–153. [Google Scholar]
- Won, E.; Kim, Y.-K. An oldie but goodie: Lithium in the treatment of bipolar disorder through neuroprotective and neurotrophic mechanisms. Int. J. Mol. Sci. 2017, 18, 2679. [Google Scholar] [CrossRef]
- Karthivashan, G.; Ganesan, P.; Park, S.-Y.; Kim, J.-S.; Choi, D.-K. Therapeutic strategies and nano-drug delivery applications in management of ageing Alzheimer’s disease. Drug Deliv. 2018, 25, 307–320. [Google Scholar] [CrossRef]
- Swerdlow, R.H.; Burns, J.M.; Khan, S.M. The Alzheimer’s disease mitochondrial cascade hypothesis: Progress and perspectives. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2014, 1842, 1219–1231. [Google Scholar] [CrossRef]
- Jojo, G.M.; Kuppusamy, G.; De, A.; Karri, V.V.S.N.R. Formulation and optimization of intranasal nanolipid carriers of pioglitazone for the repurposing in Alzheimer’s disease using Box-Behnken design. Drug Dev. Ind. Pharm. 2019, 45, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Rassu, G.; Soddu, E.; Posadino, A.M.; Pintus, G.; Sarmento, B.; Giunchedi, P.; Gavini, E. Nose-to-brain delivery of BACE1 siRNA loaded in solid lipid nanoparticles for Alzheimer’s therapy. Colloids Surf. B Biointerfaces 2017, 152, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zheng, X.; Guo, Q.; Yang, P.; Pang, X.; Qian, K.; Lu, W.; Zhang, Q.; Jiang, X. Systemic delivery of BACE1 siRNA through neuron-targeted nanocomplexes for treatment of Alzheimer’s disease. J. Control. Release 2018, 279, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; An, S.; Li, J.; Kuang, Y.; He, X.; Guo, Y.; Ma, H.; Zhang, Y.; Ji, B.; Jiang, C. Brain-targeted co-delivery of therapeutic gene and peptide by multifunctional nanoparticles in Alzheimer’s disease mice. Biomaterials 2016, 80, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Gu, Z.; Shen, L.; Liu, X.; Lin, H. A dual targeting drug delivery system for penetrating blood-brain barrier and selectively delivering siRNA to neurons for Alzheimer’s disease treatment. Curr. Pharm. Biotechnol. 2018, 18, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Agyare, E.; Jaruszewski, K.M.; Curran, G.L.; Rosenberg, J.T.; Grant, S.C.; Lowe, V.J.; Ramakrishnan, S.; Paravastu, A.K.; Poduslo, J.F.; Kandimalla, K.K. Engineering theranostic nanovehicles capable of targeting cerebrovascular amyloid deposits. J. Control. Release 2014, 185, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, J.A.; Gomes, B.; Fricker, G.; Coelho, M.A.; Rocha, S.; Pereira, M.C. Cellular uptake of PLGA nanoparticles targeted with anti-amyloid and anti-transferrin receptor antibodies for Alzheimer’s disease treatment. Colloids Surf. B Biointerfaces 2016, 145, 8–13. [Google Scholar] [CrossRef]
- Zhang, C.; Zheng, X.; Wan, X.; Shao, X.; Liu, Q.; Zhang, Q.; Zhang, Q. The potential use of H102 peptide-loaded dual-functional nanoparticles in the treatment of Alzheimer’s disease. J. Control. Release 2014, 192, 317–324. [Google Scholar] [CrossRef]
- Gobbi, M.; Re, F.; Canovi, M.; Beeg, M.; Gregori, M.F.; Sesana, S.; Sonnino, S.; Brogioli, D.; Musicanti, C.; Gasco, P.; et al. Lipid-based nanoparticles with high binding affinity for amyloid-β1–42 peptide. Biomaterials 2010, 31, 6519–6529. [Google Scholar] [CrossRef]
- Balducci, C.; Mancini, S.; Minniti, S.; La Vitola, P.; Zotti, M.; Sancini, G.; Mauri, M.; Cagnotto, A.; Colombo, L.; Fiordaliso, F.; et al. Multifunctional liposomes reduce brain—amyloid burden and ameliorate memory impairment in Alzheimer’s disease mouse models. J. Neurosci. 2014, 34, 14022–14031. [Google Scholar] [CrossRef]
- Tanifum, E.A.; Dasgupta, I.; Srivastava, M.; Bhavane, R.C.; Sun, L.; Berridge, J.; Pourgarzham, H.; Kamath, R.; Espinosa, G.; Cook, S.C.; et al. Intravenous delivery of targeted liposomes to amyloid-β pathology in APP/PSEN1 transgenic mice. PLoS ONE 2012, 7, e48515. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Huang, M.; Yao, L.; Wang, X.; Gu, X.; Chen, J.; Huang, J.; Hu, Q.; Kang, T.; Rong, Z.; et al. Lipoprotein-based nanoparticles rescue the memory loss of mice with Alzheimer’s disease by accelerating the clearance of amyloid-beta. ACS Nano 2014, 8, 2345–2359. [Google Scholar] [CrossRef]
- Poduslo, J.F.; Hultman, K.L.; Curran, G.L.; Preboske, G.M.; Chamberlain, R.; Marjańska, M.; Garwood, M.; Jack, C.R., Jr.; Wengenack, T.M. Targeting vascular amyloid in arterioles of Alzheimer disease transgenic mice with amyloid β protein antibody-coated nanoparticles. J. Neuropathol. Exp. Neurol. 2011, 70, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Prades, R.; Guerrero, S.; Araya, E.; Molina, C.; Salas, E.; Zurita, E.; Selva, J.; Egea, G.; López-Iglesias, C.; Teixidó, M.; et al. Delivery of gold nanoparticles to the brain by conjugation with a peptide that recognizes the transferrin receptor. Biomaterials 2012, 33, 7194–7205. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Sun, H.; Dong, K.; Ren, J.; Qu, X. Gold-nanoparticle-based multifunctional amyloid-β inhibitor against Alzheimer’s disease. Chem. A Eur. J. 2015, 21, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.; Chang, C.-W.; Chou, H.-H. Gold nanoparticles as amyloid-like fibrillogenesis inhibitors. Colloids Surf. B Biointerfaces 2013, 112, 525–529. [Google Scholar] [CrossRef]
- Cimini, A.; D’Angelo, B.; Das, S.; Gentile, R.; Benedetti, E.; Singh, V.; Monaco, A.; Santucci, S.; Seal, S. Antibody-conjugated PEGylated cerium oxide nanoparticles for specific targeting of Aβ aggregates modulate neuronal survival pathways. Acta Biomater. 2012, 8, 2056–2067. [Google Scholar] [CrossRef]
- Vakilinezhad, M.A.; Amini, A.; Javar, H.A.; Zarandi, B.F.B.B.; Montaseri, H.; Dinarvand, R. Nicotinamide loaded functionalized solid lipid nanoparticles improves cognition in Alzheimer’s disease animal model by reducing Tau hyperphosphorylation. DARU J. Pharm. Sci. 2018, 26, 165–177. [Google Scholar] [CrossRef]
- Jinwal, U.K.; Groshev, A.; Zhang, J.; Grover, A.; Sutariya, V.B. Preparation and characterization of methylene blue nanoparticles for Alzheimer’s disease and other tauopathies. Curr. Drug Deliv. 2014, 11, 541–550. [Google Scholar] [CrossRef]
- Sonawane, S.K.; Ahmad, A.; Chinnathambi, S. Protein-capped metal nanoparticles inhibit Tau aggregation in Alzheimer’s disease. ACS Omega 2019, 4, 12833–12840. [Google Scholar] [CrossRef]
- Misra, S.; Chopra, K.; Sinha, V.R.; Medhi, B. Galantamine-loaded solid–lipid nanoparticles for enhanced brain delivery: Preparation, characterization, in vitro and in vivo evaluations. Drug Deliv. 2015, 23, 1434–1443. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhou, Y.-Q.; Zhao, N.; Hao, B.; Wang, X.; Kong, P. Pharmacokinetic behavior and efficiency of acetylcholinesterase inhibition in rat brain after intranasal administration of galanthamine hydrobromide loaded flexible liposomes. Environ. Toxicol. Pharmacol. 2012, 34, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Fornaguera, C.; Feiner-Gracia, N.; Calderó, G.; García-Celma, M.J.; Solans, C. Galantamine-loaded PLGA nanoparticles, from nano-emulsion templating, as novel advanced drug delivery systems to treat neurodegenerative diseases. Nanoscale 2015, 7, 12076–12084. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, A.S.; Farid, R.M.; Helmy, M.W.; Elgamal, S.S. Pharmacological, toxicological and neuronal localization assessment of galantamine/chitosan complex nanoparticles in rats: Future potential contribution in Alzheimer’s disease management. Drug Deliv. 2016, 23, 3111–3122. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, A.S.; Farid, R.M.; Elgamal, S.S. Complexation as an approach to entrap cationic drugs into cationic nanoparticles administered intranasally for Alzheimer’s disease management: Preparation and detection in rat brain. Drug Dev. Ind. Pharm. 2015, 41, 2055–2068. [Google Scholar] [CrossRef]
- Ullah, Z.; Al Asmari, A.K.; Tariq, M.; Fatani, A. Preparation, characterization, and in vivo evaluation of intranasally administered liposomal formulation of donepezil. Drug Des. Dev. Ther. 2016, 205–215. [Google Scholar] [CrossRef]
- Bhavna, M.D.S.; Ali, M.; Ali, R.; Bhatnagar, A.; Baboota, S.; Ali, J. Donepezil nanosuspension intended for nose to brain targeting: In vitro and in vivo safety evaluation. Int. J. Biol. Macromol. 2014, 67, 418–425. [Google Scholar] [CrossRef]
- Jakki, S.L.; Ramesh, Y.V.; Gowthamarajan, K.; Senthil, V.; Jain, K.; Sood, S.; Pathak, D. Novel anionic polymer as a carrier for CNS delivery of anti-Alzheimer drug. Drug Deliv. 2016, 23, 3471–3479. [Google Scholar] [CrossRef]
- Baysal, I.; Ucar, G.; Gultekinoglu, M.; Ulubayram, K.; Yabanoglu-Ciftci, S. Donepezil loaded PLGA-b-PEG nanoparticles: Their ability to induce destabilization of amyloid fibrils and to cross blood brain barrier in vitro. J. Neural Transm. 2017, 124, 33–45. [Google Scholar] [CrossRef]
- Yang, C.-R.; Zhao, X.-L.; Hu, H.-Y.; Li, K.-X.; Sun, X.; Li, L.; Chen, D.-W. Preparation, optimization and characteristic of huperzine a loaded nanostructured lipid carriers. Chem. Pharm. Bull. 2010, 58, 656–661. [Google Scholar] [CrossRef]
- Patel, P.A.; Patil, S.C.; Kalaria, D.R.; Kalia, Y.N.; Patravale, V. Comparative in vitro and in vivo evaluation of lipid based nanocarriers of Huperzine A. Int. J. Pharm. 2013, 446, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Liu, C.; Zhai, W.; Zhuang, N.; Han, T.; Ding, Z. The optimization design of lactoferrin loaded hupa nanoemulsion for targeted drug transport via intranasal route. Int. J. Nanomed. 2019, 14, 9217–9234. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Wang, A.; Hua, H.; Jiang, Y.; Wang, Y.; Mu, H.; Wu, Z.; Sun, K. Intranasal delivery of Huperzine A to the brain using lactoferrin-conjugated N-trimethylated chitosan surface-modified PLGA nanoparticles for treatment of Alzheimer’s disease. Int. J. Nanomed. 2018, 13, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Wong, Y.C.; Zuo, Z. Development, characterization and application of in situ gel systems for intranasal delivery of tacrine. Int. J. Pharm. 2014, 468, 272–282. [Google Scholar] [CrossRef]
- Luppi, B.; Bigucci, F.; Corace, G.; Delucca, A.; Cerchiara, T.; Sorrenti, M.; Catenacci, L.; Di Pietra, A.M.; Zecchi, V. Albumin nanoparticles carrying cyclodextrins for nasal delivery of the anti-Alzheimer drug tacrine. Eur. J. Pharm. Sci. 2011, 44, 559–565. [Google Scholar] [CrossRef]
- Wilson, B.; Samanta, M.K.; Santhi, K.; Kumar, K.S.; Ramasamy, M.; Suresh, B. Chitosan nanoparticles as a new delivery system for the anti-Alzheimer drug tacrine. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 144–152. [Google Scholar] [CrossRef]
- Wavikar, P.; Pai, R.; Vavia, P. Nose to brain delivery of rivastigmine by in situ gelling cationic nanostructured lipid carriers: Enhanced brain distribution and pharmacodynamics. J. Pharm. Sci. 2017, 106, 3613–3622. [Google Scholar] [CrossRef]
- Ismail, M.F.; ElMeshad, A.N.; Salem, N.A.-H. Potential therapeutic effect of nanobased formulation of rivastigmine on rat model of Alzheimer’s disease. Int. J. Nanomed. 2013, 8, 393–406. [Google Scholar] [CrossRef]
- Arumugam, K.; Subramanian, G.S.; Mallayasamy, S.R.; Averineni, R.K.; Reddy, M.S.; Udupa, N. A study of rivastigmine liposomes for delivery into the brain through intranasal route. Acta Pharm. 2008, 58, 287–297. [Google Scholar] [CrossRef]
- Yang, Z.-Z.; Zhang, Y.-Q.; Wang, Z.-Z.; Wu, K.; Lou, J.-N.; Qi, X.-R. Enhanced brain distribution and pharmacodynamics of rivastigmine by liposomes following intranasal administration. Int. J. Pharm. 2013, 452, 344–354. [Google Scholar] [CrossRef]
- Mutlu, N.B.; Değim, Z.; Yilmaz, Ş.; Eşsiz, D.; Nacar, A. New perspective for the treatment of Alzheimer diseases: Liposomal rivastigmine formulations. Drug Dev. Ind. Pharm. 2011, 37, 775–789. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Chavhan, S.S.; Sawant, K.K. Rivastigmine-loaded PLGA and PBCA nanoparticles: Preparation, optimization, characterization, in vitro and pharmacodynamic studies. Eur. J. Pharm. Biopharm. 2010, 76, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Khemariya, R.P.; Khemariya, P.S. New-fangled approach in the management of Alzheimer by formulation of polysorbate 80 coated chitosan nanoparticles of rivastigmine for brain delivery and their in vivo evaluation. Int. J. Cur. Res. Med. Sci. 2016, 2, 18–29. [Google Scholar]
- Fazil, M.; Md, S.; Haque, S.; Kumar, M.; Baboota, S.; Sahni, J.K.; Ali, J. Development and evaluation of rivastigmine loaded chitosan nanoparticles for brain targeting. Eur. J. Pharm. Sci. 2012, 47, 6–15. [Google Scholar] [CrossRef]
- Casadomé-Perales, Á.; De Matteis, L.; Alleva, M.; Infantes-Rodríguez, C.; Palomares-Pérez, I.; Saito, T.; Saido, T.C.; Esteban, J.A.; Nebreda, A.R.; De La Fuente, J.M.; et al. Inhibition of p38 MAPK in the brain through nasal administration of p38 inhibitor loaded in chitosan nanocapsules. Nanomedicine 2019, 14, 2409–2422. [Google Scholar] [CrossRef]
- Dara, T.; Vatanara, A.; Sharifzadeh, M.; Khani, S.; Vakilinezhad, M.A.; Vakhshiteh, F.; Meybodi, M.N.; Malvajerd, S.S.; Hassani, S.; Mosaddegh, M.H. Improvement of memory deficits in the rat model of Alzheimer’s disease by erythropoietin-loaded solid lipid nanoparticles. Neurobiol. Learn. Mem. 2019, 166, 107082. [Google Scholar] [CrossRef]
- Elnaggar, Y.S.R.; Etman, S.M.; Abdelmonsif, D.A.; Abdallah, O.Y. Novel piperine-loaded Tween-integrated monoolein cubosomes as brain-targeted oral nanomedicine in Alzheimer’s disease: Pharmaceutical, biological, and toxicological studies. Int. J. Nanomed. 2015, 10, 5459–5473. [Google Scholar] [CrossRef]
- Huang, M.; Hu, M.; Song, Q.; Song, H.; Huang, J.; Gu, X.; Wang, X.; Chen, J.; Kang, T.; Feng, X.; et al. GM1-modified lipoprotein-like nanoparticle: Multifunctional nanoplatform for the combination therapy of Alzheimer’s disease. ACS Nano 2015, 9, 10801–10816. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, M.; Kang, T.; Miao, D.; Gu, G.; Song, Q.; Yao, L.; Hu, Q.; Tu, Y.; Pang, Z.; et al. Lactoferrin-modified PEG-co-PCL nanoparticles for enhanced brain delivery of NAP peptide following intranasal administration. Biomaterials 2013, 34, 3870–3881. [Google Scholar] [CrossRef]
- Yang, L.; Yin, T.; Liu, Y.; Sun, J.; Zhou, Y.; Liu, J. Gold nanoparticle-capped mesoporous silica-based H2O2-responsive controlled release system for Alzheimer’s disease treatment. Acta Biomater. 2016, 46, 177–190. [Google Scholar] [CrossRef]
- Yin, T.; Yang, L.; Liu, Y.; Zhou, X.; Sun, J.; Liu, J. Sialic acid (SA)-modified selenium nanoparticles coated with a high blood–brain barrier permeability peptide-B6 peptide for potential use in Alzheimer’s disease. Acta Biomater. 2015, 25, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, J.A.; Andrade, S.; Duarte, A.; Neves, A.; Queiroz, J.F.; Nunes, C.; Sevin, E.; Fenart, L.; Gosselet, F.; Coelho, M.A.; et al. Resveratrol and grape extract-loaded solid lipid nanoparticles for the treatment of Alzheimer’s disease. Molecules 2017, 22, 277. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wei, C.; Liu, Y.; Xie, W.; Xu, M.; Zhou, H.; Liu, J. Progressive release of mesoporous nano-selenium delivery system for the multi-channel synergistic treatment of Alzheimer’s disease. Biomaterials 2019, 197, 417–431. [Google Scholar] [CrossRef]
- Gothwal, A.; Kumar, H.; Nakhate, K.T.; Ajazuddin; Dutta, A.; Borah, A.; Gupta, U. Lactoferrin coupled lower generation PAMAM dendrimers for brain targeted delivery of memantine in aluminum-chloride-induced Alzheimer’s disease in mice. Bioconjugate Chem. 2019, 30, 2573–2583. [Google Scholar] [CrossRef] [PubMed]
- Laserra, S.; Basit, A.; Sozio, P.; Marinelli, L.; Fornasari, E.; Cacciatore, I.; Ciulla, M.; Turkez, H.; Geyikoğglu, F.; Di Stefano, A. Solid lipid nanoparticles loaded with lipoyl–memantine codrug: Preparation and characterization. Int. J. Pharm. 2015, 485, 183–191. [Google Scholar] [CrossRef]
- Alawdi, S.H.; Eidi, H.; Safar, M.M.; Abdel-Wahhab, M.A. Loading amlodipine on diamond nanoparticles: A novel drug delivery system. Nanotechnol. Sci. Appl. 2019, 12, 47–53. [Google Scholar] [CrossRef]
- Meng, F.; Asghar, S.; Gao, S.; Su, Z.; Song, J.; Huo, M.; Meng, W.; Ping, Q.; Xiao, Y. A novel LDL-mimic nanocarrier for the targeted delivery of curcumin into the brain to treat Alzheimer’s disease. Colloids Surf. B Biointerfaces 2015, 134, 88–97. [Google Scholar] [CrossRef]
- Gao, C.; Wang, Y.; Sun, J.; Han, Y.; Gong, W.; Li, Y.; Feng, Y.; Wang, H.; Yang, M.; Li, Z.; et al. Neuronal mitochondria-targeted delivery of curcumin by biomimetic engineered nanosystems in Alzheimer’s disease mice. Acta Biomater. 2020, 108, 285–299. [Google Scholar] [CrossRef]
- Kuo, Y.-C.; Lin, C.-Y.; Li, J.-S.; Lou, Y.-I. Wheat germ agglutinin-conjugated liposomes incorporated with cardiolipin to improve neuronal survival in Alzheimer’s disease treatment. Int. J. Nanomed. 2017, 12, 1757–1774. [Google Scholar] [CrossRef]
- Huo, X.; Zhang, Y.; Jin, X.; Li, Y.; Zhang, L. A novel synthesis of selenium nanoparticles encapsulated PLGA nanospheres with curcumin molecules for the inhibition of amyloid β aggregation in Alzheimer’s disease. J. Photochem. Photobiol. B Biol. 2019, 190, 98–102. [Google Scholar] [CrossRef]
- Jaruszewski, K.M.; Curran, G.L.; Swaminathan, S.K.; Rosenberg, J.T.; Grant, S.C.; Ramakrishnan, S.; Lowe, V.J.; Poduslo, J.F.; Kandimalla, K.K. Multimodal nanoprobes to target cerebrovascular amyloid in Alzheimer’s disease brain. Biomaterials 2013, 35, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Bilia, A.R.; Nardiello, P.; Piazzini, V.; Leri, M.; Bergonzi, M.C.; Bucciantini, M.; Casamenti, F. Successful brain delivery of andrographolide loaded in human albumin nanoparticles to TgCRND8 mice, an Alzheimer’s disease mouse model. Front. Pharmacol. 2019, 10, 910. [Google Scholar] [CrossRef] [PubMed]
- Al-Azzawi, S.; Masheta, D.; Guildford, A.; Phillips, G.; Santin, M. Dendrimeric poly(epsilon-lysine) delivery systems for the enhanced permeability of flurbiprofen across the blood-brain barrier in Alzheimer’s disease. Int. J. Mol. Sci. 2018, 19, 3224. [Google Scholar] [CrossRef] [PubMed]
- Muntimadugu, E.; Dhommati, R.; Jain, A.; Challa, V.G.S.; Shaheen, M.; Khan, W. Intranasal delivery of nanoparticle encapsulated tarenflurbil: A potential brain targeting strategy for Alzheimer’s disease. Eur. J. Pharm. Sci. 2016, 92, 224–234. [Google Scholar] [CrossRef]
- Saffari, P.M.; Alijanpour, S.; Takzaree, N.; Sahebgharani, M.; Etemad-Moghadam, S.; Noorbakhsh, F.; Partoazar, A. Metformin loaded phosphatidylserine nanoliposomes improve memory deficit and reduce neuroinflammation in streptozotocin-induced Alzheimer’s disease model. Life Sci. 2020, 255, 117861. [Google Scholar] [CrossRef]
- Fernandes, J.; Ghate, V.M.; Mallik, S.B.; Lewis, S. Amino acid conjugated chitosan nanoparticles for the brain targeting of a model dipeptidyl peptidase-4 inhibitor. Int. J. Pharm. 2018, 547, 563–571. [Google Scholar] [CrossRef]
- Kuo, Y.-C.; Wang, C.-T. Protection of SK-N-MC cells against β-amyloid peptide-induced degeneration using neuron growth factor-loaded liposomes with surface lactoferrin. Biomaterials 2014, 35, 5954–5964. [Google Scholar] [CrossRef]
- Kuo, Y.-C.; Lin, C.-Y. Targeting delivery of liposomes with conjugated p-aminophenyl-α-d-manno-pyranoside and apolipoprotein E for inhibiting neuronal degeneration insulted with β-amyloid peptide. J. Drug Target. 2014, 23, 147–158. [Google Scholar] [CrossRef]
- Kuo, Y.-C.; Chou, P.-R. Neuroprotection against degeneration of SK-N-MC cells using neuron growth factor-encapsulated liposomes with surface cereport and transferrin. J. Pharm. Sci. 2014, 103, 2484–2497. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, J.; Feng, C.; Shao, X.; Liu, Q.; Zhang, Q.; Pang, Z.; Jiang, X. Intranasal nanoparticles of basic fibroblast growth factor for brain delivery to treat Alzheimer’s disease. Int. J. Pharm. 2014, 461, 192–202. [Google Scholar] [CrossRef]
- Angelova, A.; Drechsler, M.; Garamus, V.M.; Angelov, B. Pep-lipid cubosomes and vesicles compartmentalized by micelles from self-assembly of multiple neuroprotective building blocks including a large peptide hormone PACAP-DHA. ChemNanoMat 2019, 5, 1381–1389. [Google Scholar] [CrossRef]
- Leblanc, R.M.; Liyanage, P.Y.; Devadoss, D.; Guevara, L.R.R.; Cheng, L.; Graham, R.M.; Chand, H.S.; Al-Youbi, A.O.; Bashammakh, A.S.; El-Shahawi, M.S.; et al. Nontoxic amphiphilic carbon dots as promising drug nanocarriers across the blood–brain barrier and inhibitors of β-amyloid. Nanoscale 2019, 11, 22387–22397. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, H.; Xu, C.; Hu, W.; Yu, B. Efficient cholera toxin B subunit-based nanoparticles with MRI capability for drug delivery to the brain following intranasal administration. Macromol. Biosci. 2018, 19, e1800340. [Google Scholar] [CrossRef]
- Cui, N.; Lu, H.; Li, M. Magnetic nanoparticles associated PEG/PLGA block copolymer targeted with anti-transferrin receptor antibodies for Alzheimer’s disease. J. Biomed. Nanotechnol. 2018, 14, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Do, T.D.; Amin, F.U.; Noh, Y.; Kim, M.O.; Yoon, J.; Duc, D.T.; Ul, A.F.; YeongIl, N.; Ok, K.M.; Jungwon, Y. Guidance of magnetic nanocontainers for treating Alzheimer’s disease using an electromagnetic, targeted drug-delivery actuator. J. Biomed. Nanotechnol. 2016, 12, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Sanati, M.; Khodagholi, F.; Aminyavari, S.; Ghasemi, F.; Gholami, M.; Kebriaeezadeh, A.; Sabzevari, O.; Hajipour, M.J.; Imani, M.; Mahmoudi, M.; et al. Impact of gold nanoparticles on amyloid β-induced Alzheimer’s disease in a rat animal model: Involvement of STIM proteins. ACS Chem. Neurosci. 2019, 10, 2299–2309. [Google Scholar] [CrossRef]
- Pardridge, W.M. Blood–brain barrier drug delivery of IgG fusion proteins with a transferrin receptor monoclonal antibody. Expert Opin. Drug Deliv. 2014, 12, 207–222. [Google Scholar] [CrossRef]
- Liu, W.; Sun, F.; Wan, M.; Jiang, F.; Bo, X.; Lin, L.; Tang, H.; Xu, S. β-Sheet breaker peptide-HPYD for the treatment of Alzheimer’s disease: Primary studies on behavioral test and transcriptional profiling. Front. Pharmacol. 2018, 8, 969. [Google Scholar] [CrossRef]
- Zheng, X.; Shao, X.; Zhang, C.; Tan, Y.; Liu, Q.; Wan, X.; Zhang, Q.; Xu, S.; Jiang, X. Intranasal H102 peptide-loaded liposomes for brain delivery to treat Alzheimer’s disease. Pharm. Res. 2015, 32, 3837–3849. [Google Scholar] [CrossRef]
- Hu, B.; Dai, F.; Fan, Z.; Ma, G.; Tang, Q.; Zhang, X. Nanotheranostics: Congo Red/Rutin-MNPs with enhanced magnetic resonance imaging and H2O2-responsive therapy of alzheimer’s disease in APPswe/PS1dE9 transgenic mice. Adv. Mater. 2015, 27, 5499–5505. [Google Scholar] [CrossRef]
- Gao, C.; Chu, X.; Gong, W.; Zheng, J.; Xie, X.; Wang, Y.; Yang, M.; Li, Z.; Gao, C.; Yang, Y. Neuron tau-targeting biomimetic nanoparticles for curcumin delivery to delay progression of Alzheimer’s disease. J. Nanobiotechnol. 2020, 18, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.A.; Mandal, A.K.A.; Khan, Z.A. Potential neuroprotective properties of epigallocatechin-3-gallate (EGCG). Nutr. J. 2015, 15, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.; Saraf, S.; Saraf, S.; Antimisiaris, S.G.; Chougule, M.B.; Shoyele, S.A.; Alexander, A. Nose-to-brain drug delivery: An update on clinical challenges and progress towards approval of anti-Alzheimer drugs. J. Control. Release 2018, 281, 139–177. [Google Scholar] [CrossRef] [PubMed]
- Md, S.; Haque, S.; Fazil, M.; Kumar, M.; Baboota, S.; Narang, J.K.; Ali, J. Optimised nanoformulation of bromocriptine for direct nose-to-brain delivery: Biodistribution, pharmacokinetic and dopamine estimation by ultra-HPLC/mass spectrometry method. Expert Opin. Drug Deliv. 2014, 11, 827–842. [Google Scholar] [CrossRef]
- Lauzon, M.-A.; Daviau, A.; Marcos, B.; Faucheux, N. Nanoparticle-mediated growth factor delivery systems: A new way to treat Alzheimer’s disease. J. Control. Release 2015, 206, 187–205. [Google Scholar] [CrossRef]
| Property | NPs | Intranasal Adm. | Transdermal Adm. | Oral Adm. | Intravenous Adm. |
|---|---|---|---|---|---|
| Controlled release | Available | Not available | Available | Not available | Not available |
| BBB penetration |
|
| Depends on the properties of drug | Depends on the properties of drug | Depends on the properties of drug |
| Ability to targeted delivery | High | Not available | Not available | Not available | Not available |
| Bioavailability | High | High | Poor | Poor | Poor |
| Hepatic first-pass metabolism | Prevent | Prevent | Prevent | Not prevent | Not prevent |
| Toxicity | Less pronounced side effects because of lower concentrations of therapeutic drugs and more stable release characteristics. | Less pronounced side effects because of lower concentrations of therapeutic drugs and direct transport through BBB. | Less pronounced side effects because of lower concentrations of therapeutic drugs and more stable release characteristic | High chance and dose-depended side effects | High chance and dose-depended side effects |
| Nanocarrier | Description | Materials | BBB Penetration | Advantages | Disadvantages | References |
|---|---|---|---|---|---|---|
| Polymer-Based Nanocarrier Systems | ||||||
| Polymeric NPs | The colloidal carriers are obtained from biodegradable and biocompatible natural or synthetic polymers into which drugs are loaded in either solid-state or solution. | Alginate, chitosan, gelatin, cellulose, polyacrylate, polyanhydride, PLGA, PACA, PCL, PEI, PLA, PEG. |
|
|
| [28,29,30,31,32,33] |
| Polymeric micelles | The core–shell structure from amphiphilic blocks copolymers that aggregate in aqueous solutions to form spheroidal NPs with a hydrophobic core and hydrophilic surface. | PEG, PLGA, cholesterol conjugated polyoxyethylene sorbitol oleate. | The surface functionalization with targeting ligands. |
| The efficiency delivering across the intact BBB still needs further investigation. | [17] |
| Dendrimers | The monodisperse symmetric molecules that comprise a series of branching units around an inner core, which have a spheroidal shape and radially crowded layers. | Poly(amido amide) (PAMAM) | The internalization by brain capillary endothelial cells through a clathrin- and caveolane-mediated energy-depending endocytosis, also partly through macropinocytosis. |
| Potential toxicity. | [17,30,31,32] |
| Lipid-Based Nanocarrier System | ||||||
| Less or no toxicity, biodegradability, and ability to successfully deliver biomolecules, DNA, RNA, genes, antibodies, etc. | ||||||
| Liposomes | The spherical vesicles with different sizes (20 nm to 100 μm), with an aqueous inner core enclosed by unilamellar or multilamellar phospholipid bilayers. Liposomes may have different surface charges and uni-, bi- or multi-lamellar structures. | Sphingomyelin, phosphatidyl choline, glycerophospholipids, cholesterol, phosphatidylcholine, cardiolipin. |
|
|
| [28,29,30,32,33] |
| Solid lipid NPs | Nano-sized dispersions of biocompatible lipids | The lipid core consists of triglycerides, diglycerides, monoglycerides, fatty acids, steroids, stearic acid or waxes stabilized by various surfactants |
|
|
| [29,30,33] |
| Nanoemulsions | Colloidal droplet system of oil-in-water (O/W) or water-in-oil (W/O) formulations stabilized with surface-active agents Oil droplet size of nanoemulsions ranges from 10 to 100 nm, making them interesting systems to improve drug delivery | Edible oils, such as flaxseed oil, pine-nut oil, hemp oil, fish oil as well as safflower oil and wheat-germ oil, biocompatible surfactants such as egg phosphatidylcholine which is one of the components of cell membrane lipids, deoxycholic acid, stearylamine, dioleoyltrimethyl ammonium propane. |
|
|
| [17,32,33] |
| Inorganic Nanoparticles | ||||||
| Mesoporous Silica NPs | Silica xerogels and mesoporous silica nanoparticles | N-Cetyltrimethylammonium bromide, Tetraethoxysilane | Transcytosis of vascular endothelial cells (PEGylated NPs) |
|
| [30,34] |
| Gold NPs |
|
| Drug delivery efficiency needs further investigation. | [34] | ||
| Carbon nanotubes | Graphitic sheets rolled into single-walled or multiple walled tubes with an enormous surface area | Graphitic sheets |
|
|
| [30] |
| Iron oxide NPs | Iron oxide nanoparticles, which are also known as super paramagnetic nanoparticles | Iron oxide | Ability to cross the blood-brain barrier. Mechanisms of internalization and overall biodistribution are closely associated with their surface chemistry and hydrodynamic sizes. |
| Tend to aggregate into larger clusters. | [17,30,31,34] |
| Drug | Nanocarrier | Components | PS, nm | ZP, mV | EE, % | LC, % | PDI | In Vivo Route of Administration | Outcomes | References |
|---|---|---|---|---|---|---|---|---|---|---|
| Depression | ||||||||||
| Agomelatine | PLGA NPs | PLGA, PM407 | 116,06 ± 3523 | −22.7 | 96 | - | <0.3 | i.n. | Prominent antidepressant activity | [58] |
| Agomelatine | Polymeric NPs | Propylene glycol, Plu F68, Ethanol, Transcutol HP. | 107.64 | −15 | 81.91 | - | 0.209 | t.d. | Penetration enhancement of drug | [59] |
| Baicalein | Solid lipid NPs. | N-Acetyl Pro-Gly-Pro (to binding to neutrophil CXCR2 receptor), GMS, PM188, 1,2-dipalmitoyl-sn-glycero-3-phospho choline. | <100 | −13.5, −12.6 | 98.7, 99.1 | - | - | i.v. | The enhanced concentration of drug in the basolateral amygdala. Antidepressant effect in vitro and in vivo. | [60] |
| BDNF | Polyion complex formulation of BDNF (nano-BDNF) | Human recombinant BDNF, PEG-poly(l-glutamate) diblock copolymer | 95 | - | - | - | 0.165 | i.v. | Significant reduction in cerebral tissue loss. Improved memory/cognition, reduced post-stroke depressive phenotypes, and maintained myelin basic protein and brain BDNF levels. | [61] |
| HU-211 and curcumin | Solid lipid dual-drug NPs | Polyoxyethylene (40) stearate, stearic acid, and lecithin. | 58.77 ± 1.7 | −21.7 ± 0.4 | - | 0.74 ± 0.02, 18.34 ± 1.06 | - | i.p. | Protection of PC12 cells from corticosterone-induced apoptosis. Antidepressant-like effect and enhanced fall latency in rotarod test, improved level of dopamine in the mice blood. NPs can deliver more curcumin to the brain and thus produce a significant increase in neurotransmitters level in brain tissue, especially in the hippocampus and striatum. | [62] |
| Curcumin and dexanabinol | Solid lipid NPs | Stearic acid, lecithin, polyoxyethylene stearate. | - | −22.6 ± 0.9 | 19.12 ± 1.43, 0.81 ± 0.04 | - | - | - | NPs exerted antidepressant activity by targeting the endocannabinoid/CB1 receptor system. | [63] |
| Curcumin | Nanocapsules | Organic phase PCL, capric caprylic triglycerides, 106 sorbitan monoestearate, acetone | 291–312 | From −22 to −36 | Close to 100% | - | - | via gavage | Antidepressant-like and antioxidant effects in a mouse model of Alzheimer’s disease. NPs were more effective than the free curcumin. | [64] |
| Duloxetine | Nanostructured lipid carriers | GMS, capryol 136 PGMC, Plu F-68, sodium taurocholate | 80.17–127.73 | - | - | - | - | i.n., i.v. | Better brain targeting efficiency than DLX solution when administered intranasally. Decreased side effects. | [65] |
| Folic acid | Niosomes | Different nonionic surfactants (Span 20, Span 60, Span 80, Tween 20, Tween 80, CL) | 3050–5625 | - | 69.42 | - | - | i.n. | Niosomes prepared with span 60 and CL in the ratio of 1:1 (50 mg: 50 mg) showed higher EE and better in vitro drug release of 64.2% at the end of 12 hrs and therefore considered as optimized formulation. About 48.15% of the drug was found to be absorbed through the nasal cavity at the end of 6 hrs. | [66] |
| Lithium carbonate | CS nanocomposites | CS, sodium tripolyphosphate anions, Tween 80. | 90.68–220.81 | +37.9 | 87 ± 1.21 | 28.87 | - | p.o. | Reversed degenerative changes and gliosis in depression-induced animal models. Fortified targeted drug delivery and restrained adverse effects. | [67] |
| Minocycline hydrochloride (MH) | CS NPs | Tween 80, CS. Two types of NPs: MH loaded NPs, Tween 80 coated MH encapsulated NPs. | - | - | - | - | - | i.p. | NPs improved the therapeutic efficacy as well as safety of MH. | [68] |
| Selegiline hydrochloride | Thiolated CS NPs | CS, thioglycolic acid. | 215 ± 34.71 | +17.06 | 70 ± 2.71 | - | 0.214 ± 0.042 | i.n. | Attenuation of the oxidative stress and restoring of the activity of the mitochondrial complex. Antidepressant-like effect in vivo | [69] |
| Silymarin | Nanostructured lipid carriers | Precirol solid lipid, Labrafac Lipophile oil. | 519.00 ± 28.67 | −12.95 ± 1.58 | 90.00 ± 3.20 | - | 0.66 ± 0.05 | p.o. | Antidepressant-like effect, comparable with fluoxetine in mice. Significantly higher brain concentration by 12.46 fold superior to silymarin. | [70] |
| Thymoquinone (TQ) | Solid lipid NPs | Tween 80, GMS, PM188. | 188.66 ± 8.94 | −12.32 ± 1.04 | 68.60 ± 4.82 | - | 0.319 ± 0.04 | p.o. | Higher amount of TQ reached the target region after administration. Higher levels of monoamines 5 hydroxytryptamine, dopamine and norepinephrine as compared to TQ suspension were demonstrated. | [71] |
| Tramadol HCl | CS NPs with mucoadhesive thermo-reversible gel. | CS, Thermo reversible mucoadhesive Plu-HPMC | 152.0 ± 9.56 | +31 ± 2.21 | 85 ± 3.23 | - | 0.143 ± 0.003 | i.n. | Antidepressant-like effect in rat model of depression. | [72] |
| Trefoil factor 3 (TFF3) | cRGD-modified liposomes | Cyclic RGD (cRGD) peptide with high affinity for integrin receptors of leukocytes, soybean phosphatidylcholine, DSPE-PEG (PEG 2000), DSPG, CL | 133 | −21.8 | 27.6 | - | - | i.p. | Brain targeted delivery in a murine model. Antidepressant-like effect of direct intra-basolateral amygdala administration of TFF3 solution in rats subjected to chronic mild stress. | [73] |
| Venlafaxine | CS NPs | CS glutamate. | 167 ± 6.5 | +23.83 ± 1.76 | 79.3 ± 2.6 | 32.25 ± 1.63 | 0.367 ± 0.045 | i.n. | Enhanced uptake of venlafaxine to the brain. | [74] |
| Venlafaxine | PLGA NPs | PLGA, two ligands (Tf and TfRp) against transferrin receptor (TfR) to enhance access to brain across BBB. | 206.3 ± 3.7 | −25 | 48–50 | 10–12 | 0.041 ± 0.017 | i.n. | Plain NPs demonstrated the highest ability to reach the brain vs. functionalized NPs. | [75] |
| Venlafaxine | Alginate NPs | - | - | - | - | - | - | i.n., i.v., p.o. | Improved antidepressant-like activity in comparison with the venlafaxine (i.n. and oral form). The greater brain/blood ratios for VNPs (i.n.) | [76] |
| Zn(2+) | PLGA NPs conjugated with glycopeptides | Antibodies against NCAM1 and CD44, PLGA conjugated with tetramethylrhodamine and glycopeptides. | 190–210 | From −0.5 to −10 | - | - | - | - | NPs were able to cross the BBB and to deliver Zn(2+) ions at non-toxic concentrations. Easily modified for preferential targeting of specific cell populations. | [77] |
| Anxiety Disorders | ||||||||||
| Peptide antisauvagine-30 (ASV-30) | Iron oxide NPs | Fe2O3, 3-aminopropyltriethoxysilane | 5 ± 1 | - | - | - | - | i.p. | Systemically administered NPs were observed in the brain. Association with neurons and reduced amphetamine withdrawal-induced anxiety in rats without affecting locomotion were demonstrated. | [78] |
| BUH | Thiolated CS NPs | CS, thiolated CS. | 226.7 ± 2.52 | - | 81.13 ± 2.8 | 49.67 ± 5.5 | - | i.n. | The brain concentration achieved after intranasal administration was significantly higher than after administration of BUH (i.v. and i.n.). Excellent bioadhesion. | [79] |
| BUH | Nanovesicles | Two types of nanovesicular in situ gels (P407-based and carbopol 974P-based). Span 20, Span 40, Span 60, CL, stearylamine, dicetylphosphate. | - | - | 56.67–70.57 | - | - | i.n. | Higher level of permeability for carbopol formulation in comparison to PM formulations. A 3.26 times increase of BUH bioavailability when loaded into the carbopol nanovesicular in situ gel in comparison to control (i.n.). | [80] |
| Diazepam | PLGA NPs | PLGA, PVA. | 250 | −23.3 | 66 | - | - | - | In vitro drug release analysis showed sustained release of drug from nanoparticles. Correspondence to Korsmeyer–Peppas model. | [81] |
| Gallic acid (GA) | CS NPs | CS, Tween 80 (for coating (cGANP batch)). Two types of NPs: Ligand coated NPs (cGANP) and uncoated NPs (GANP). | 103.33 ± 2.28 | - | 93.62 | - | - | i.p. | Improved anxiolytic activity in mice. The plasma nitrite level decreased in GA, GANP and cGANP (most pronounced for cGANP) treated group as compared to saline treated control group while no change in plasma corticosterone levels was observed after any treatment. | [82] |
| Schizophrenia and Bipolar Disorders | ||||||||||
| Aripiprazole (ARP) | Solid lipid NPs | Tristearin, Tween 80, sodium taurocholate. | 85.05–2042 | From -4.25 to −17.5 | 57.65–81.70 | 9.28–31.54 | 0.241–0.682 | p.o. | Improved bioavailability of aripiprazole (1.6-fold compared to plain drug suspension). Enhancement of absorption and minimizing of the first-pass metabolism. | [83] |
| Aripiprazole | PCL NPs | PCL | 199.2 ± 5.65 | −21.4 ± 4.6 | 69.2 ± 2.34 | - | - | i.n., i.v. | The enhanced brain targeting efficiency | [84] |
| Asenapine maleate | CS coated nanostructured lipid carrier | GMS, oleic acid, Tween 80, glycol CS. | 184.2 ± 5.59 | +18.83 ± 1.19 | 83.52 ± 2.59 | - | - | i.n. | 2.3 and 4 fold higher systemic and brain bioavailability compared to pure asenapine solution (i.n.). | [85] |
| Asenapine maleate | Transfersomes | SPC and sodium deoxycholate (SDC). | 126.0 | −43.7 | 54.96 | - | 0.232 | t.d. | Significant increase of bioavailability after transdermal application compared with oral route. | [86] |
| Haloperidol | Lectin-functionalized, PEG–PLGA NPs | Mixture of Maleimide-PEG–PLGA and Me-PEG–PLGA, Solanum tuberosum lectin (STL). | 132 ± 20 | 14.4 ± 0.1 | 73.2 ± 0.8 | 0.85 ± 0.01 | 0.07–0.22 | i.n. | Increase of the brain tissue haloperidol concentrations by 1.5-3-fold compared to non-STL-NPs and other routes of administration. | [87] |
| Lithium carbonate | CS NPs | CS | 162–419 | + 30 | - | - | - | - | CS increased cellular uptake of lithium. | [88] |
| Lurasidone hydrochloride (LH) | Solid lipid NPs | GMS, PM188, sodium deoxycholate. | 139.8 ± 5.5 | −30.8 ± 3.5 | 79.10 ± 2.50 | - | - | p.o. | Improved bioavailability of LH via lymphatic uptake along with improved therapeutic effect in MK-801 induced schizophrenia model in rats. | [89] |
| Lurasidone hydrochloride | Nanolipid carrier | Capryol 90, Tween 80, and Transcutol P. | 207.4 ± 1.5 | - | 92.12 ± 1.0 | - | 0.392 ± 0.15 | i.n. | 2-fold increase of the drug concentration in the brain when compared with pure drug solution (i.n.) | [90] |
| Lurasidone | Mixed polymeric micelles | Plu F127, Gelucire 44/14. | 175 | - | 97.8 | - | - | i.n., i.v. | Improved brain distribution as well as kinetics of lurasidone via the intranasal route. The sustained release of lurasidone hydrochloride from micelles with better permeability and bioavailability. | [91] |
| Olanzapine | Solid lipid NPs | Two types of NPs with GMS and tripalmitin. Stearylamine, Tween 80. | 165, 110 | +66.50, +35.3 | 96.3, 67.2 | - | 0.34, 0.726 | i.v. | 23-fold increased bioavailability of olanzapine in brain and decreased clearance. | [92] |
| Olanzapine | Nanostructured lipid carriers | Mucoadhesive NPs was prepared by using carbopol 974P (MNLC (C)) and the combination of PM 407 and of HPMC K4M (MNLC (P + H)). | 88.95 ± 1.7 | −22.62 ± 1.9 | 88.94 ± 3.9 | - | 0,31 ± 0,01 | i.n. | Nose-to-brain delivery of olanzapine MNLC (P + H) was considered as an effective and safe for CNS disorders. | [93] |
| Olanzapine | Micellar nanocarriers | Plu® mixture of L121 and P123 in different ratios. | 58.55 ± 2.47 | - | 75.03 ± 2.35 | 1.84 ± 0.06 | 0.27 ± 0.03 | i.n., i.v. | The micelles (i.n.) demonstrated a conciliation between kinetic and thermodynamic stability, controlled drug-release characteristics and evoked minor histopathological changes in sheep nasal mucosa. | [94] |
| Olanzapine | Nanocapsules | Copolymer-functionalized PCL | 254.9 ± 12.1 | +22.2 ± 1.2 | 99.00 ± 0.05 | - | 0.03 ± 0.01 | i.n. | Controlled release, improved retention of the drug on the nasal mucosa under continuous wash was demonstrated. Increased brain uptake and improved prepulse inhibition deficit induced by apomorphine in rats. | [95] |
| Olanzapine | CS NPs | CS. NPs were prepared with 20 or 60% loading. | 208 ± 29 322 ± 18 | - | 86.7 ± 7.1, 87.6 ± 5.2 | 17.2 ± 1.4, 52.3 ± 3.1 | - | i.n., i.v. | Olanzapine administered via intranasal CS NPs demonstrated the potential to improve the efficacy of systemic absorption thereby offering an efficient method of administration in noncompliant patients. | [96] |
| Olanzapine | PLGA NPs | PLGA, PM407, acetone, acetonitrile, tetrahydrofuran. | 91.2 ± 5.2 | −23.7 ± 2.1 | 68.91 ± 2.31 | 8.613 ± 0.288 | 0.120 ± 0.018 | i.n., i.v. | 6.35 and 10.86 times higher uptake than pure olanzapine solution (i.v. and i.n.) in vivo | [97] |
| Paliperidone | Nanolipomers | PCL as a polymeric core, Lipoid S75, and Gelucire® 50/13 as a lipid shell and PVA as a stabilizing agent. | 168.2 ± 0.7 | −23.1 | 87.27 ± 0.098 | - | 0.22 | p.o. | Sustained release up to 24 h and better ex vivo intestinal permeation for paliperidone compared to the corresponding polymeric, solid lipid NPs and drug suspension. | [98] |
| Paliperidone | in situ Gels | Carbopol 934, HPMC K4M, HP-β-CD in the form of inclusion complex of PLPD as nasal absorption enhancer. | - | - | - | - | - | i.n. | In vitro and ex vivo drug permeation, exhibited mucoadhesion and sustained drug release. The formulation containing HP-β-CD complex of paliperidone demonstrated higher level of drug permeation through sheep nasal mucosa without injury of nasal mucosa architecture. | [99] |
| Paliperidone palmitate | Micelles | d-alpha-tocopheryl polyethylene glycol 1000 succinate | 26.5 ± 4.8 | 92.61 ± 2.5 | - | - | - | i.m. | Improved antipsychotic effect and decreased adverse effects after micellar formulation application. | [100] |
| Paliperidone | Microemulsion | Muco-adhesive polymer, oleic acid | 27.31 ± 1.86 | −38.65 ± 2.39 | - | - | 0.241 ± 0.05 | i.n., i.v. | Higher brain paliperidone concentrations compared to pure drug (i.v.) | [101] |
| Risperidone | Proteinoid NPs | Amino acids l-glutamic acid, l-phenylalanine, l-histidine, poly (l-lactic acid), PEG | 86 ± 3 | −16 ± 1 | - | 20 ± 0.1 | - | i.v. | Enhanced antipsychotic activity compared to pure risperidone in mice | [102] |
| Risperidone | CS NPs | CS, tween 80, PM 188. | 86 | +36.6 | 77.96 ± 1.50 | 13–37 | 0.287 | i.n. | Reduced stereotypical behavior score in experimental animals and reversed amphetamine effect. | [103] |
| Risperidone | Solid lipid NPs | Compritol 888 ATO, Plu F-127. | 0.148 ± 0.028 | −25.35 ± 0.45 | 59.65 ± 1.18 | 59.65 ± 1.18 | 0.148 ± 0.03 | i.n., i.v. | Effective brain targeting in mice in vivo | [104] |
| Risperidone | Nanoemulsion | CS, capmul MCM, transcutol, propylene glycol | 16.7 ± 1.21 | −9.15 ± 2.14 | 98.86 ± 1.21 | - | 0.191 ± 0.04 | i.n., i.v. | Effective delivery of significant amount of risperidone to the brain after intranasal administration. | [105] |
| Risperidone | Functionalized liposomes | Conventional liposomes consisting of SPC/CL, cationic liposomes containing SPC/CL/stearylamine, PEGylated liposomes consists of SPC/CL/distearylphosphatidylethanolamine-mPEG-2000 | 98.51 ± 6.82 | −28.6 ± 3.62 | 58.86 ± 1.38 | - | 0.103–0.324 | i.n., i.v. | Liposomal formulations provided enhanced bioavailability, less clearance rate, higher mean residential time and better response in vivo compared to conventional formulations. | [106] |
| Risperidone | CS lipid NPs | CS | 132.7 | - | - | 7.6 | - | i.n., i.v. | Increased nose to brain drug delivery compared to pure drug suspension of equivalent dose. | [107] |
| Quetiapine fumarate | Solid lipid NPs in situ gel | Heat-melting GMS, Span 80, butanol, PM407, PM188. | 307.1 ± 17.7 | + 57.2 ± 0.24 | 97.6 ± 0.58 | - | - | i.n., i.v., p.o. | Stable and effective brain delivery, amelioration of the damages induced by MK-801 in rat model of schizophrenia. | [108] |
| Quetiapine fumarate | Nanoemulsion system | HLBs of Emalex LWIS 10, PEG 400, Transcutol P, Capmul MCM, Tween 80. | 144 ± 0.5 | −8.131 ± 1.8 | - | - | 0.193 ± 0.04 | i.n. | 2-fold increase of the drug release compared to pure drug. Better direct nose-to-brain drug transport. | [109] |
| Quetiapine Fumarate | Liposome | Egg phosphatidylcholine, CL | 139.6 | −32.1 | 75.63 ± 3.77 | - | - | i.n. | Higher level of diffusion. Better potential to deliver drugs to the brain than by the pure solution. | [110] |
| Quetiapine fumarate | Microemulsion with and without CS | CS, methyl-β-cyclodextrin, Capmul MCM EP, labrasol, Tween 80, Transcutol-P. | 35.31 ± 1.71 | 20.29 ± 1.23 | - | - | 0.249 ± 0.03 | i.n., i.v. | Enhanced brain uptake of quetiapine and improved bioavailability. | [111] |
| Quetiapine fumarate | CS NPs | CS, sodium tripolyphosphate | 131.08 ± 7.45 | + 34.4 ± 1.87 | 89.93 ± 3.85 | - | 0.252 ± 0.064 | i.n., i.v. | Significantly higher brain/blood ratio and 2 folds higher nasal bioavailability in the brain in comparison to pure drug solution (i.n.). | [112] |
| Drug | Nanocarrier | Targeting Ligand | Components | PS, nm | ZP, mV | EE, % | LC, % | PDI | In Vivo Route of Administration | Outcomes | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Amyloid—Related Nanoparticles | |||||||||||
| Pioglitazone | Nano lipid carriers | - | Tripalmitin, MCM, stearyl amine | 211.4 ± 3.54 | +14.9 ± 1.09 | 70.18 ± 4.5 | - | 0.257 ± 0.108 | i.n. | The NC significantly improved the nasal permeability of pioglitazone ex-vivo. | [152] |
| BACE1 siRNA | Solid lipid NPs | Peptide derived from rabies virus glycoprotein (RVG-9R) | CS-coated and uncoated NPs | 419.47 ± 24.36, 469.71 ± 49.07 | −12.52 ± 0.99, +14.47 ± 0.19 | - | - | 0.26 ± 0.09, 0.30 ± 0.04 | i.n. | The siRNA permeated the monolayer (Caco-2) to a greater extent when released from any of the studied formulations than from bare siRNA, and primarily from CS-coated NPs. | [153] |
| siRNA against BACE1 | Nano complexes CT/siRNA, composed of CGN-PEG-PDMAEMA and Tet1-PEG-PDMAEMA (1:1) | CGN peptide, Tet1 peptide | PEGylated poly(2-(N,N-dimethylamino) ethyl methacrylate) (PEG-PDMAEMA) | 70–80 | +10 | - | - | - | i.v. | The NC entered the cytoplasm of the neuron cells, inducing effective gene silence (about 50% decrease in BACE1 mRNA levels) and reversing synaptic injury. The NC significantly decreased BACE1 mRNA and the amyloid plaques, suppressed phosphorylated tau protein levels, and promoted hippocampal neurogenesis. NC restored the cognitive performance of the AD transgenic mice to the level of wild-type control. | [154] |
| Plasmid DNA encoding BACE1-AS siRNA, d-peptide | PEGylated DGL NPs | Peptide from rabies virus glycoprotein (RVG29) | Dendrigraft poly-l-lysines 10 (DGL), α-Malemidyl-ω-N-hydroxysuccinimidylpolyethyleneglycol. | 110 | + 7.72 ± 2.80 | - | - | 0.3 | i.v. | Successful codelivery of drugs crossed the BBB. Simultaneous delivery of the therapeutic peptide into brain led to the reduction of neurofibrillary tangles. The memory loss rescue in AD mice was also observed. | [155] |
| BACE1 siRNA | Dendrimer NPs | Apolipoprotein A-I (ApoA-I), NL4 peptide. | Dendrigraft poly-l-lysines, α-Maleimide-ω-N-hydroxysuccinimidylPEG | 79.26 | +3.53 | 97.05 | - | 0.216 | - | The NC effectively targeted both BBB and PC12 cells and down-regulated BACE1 gene expression in PC12 cells. | [156] |
| Cyclophosphamide | Theranostic nanovehicles | Putrescine modified F(ab′)2 fragment of antiamyloid antibody, IgG4 | CS, MRI contrast agent, pentasodiumtripolyphosphate, | 239 ± 4.1 | 11.9 ± 0.5 | - | 21,7 ± 1,31 | - | i.v. | NPs successfully targeted cerebrovascular amyloid. | [157] |
| Peptide iA5 | PLGA NPs | Anti-transferrin receptor monoclonal antibody (OX26), anti-A (DE2B4) | PLGA | 166 ± 2 | −13 ± 1 | 63 ± 9 | - | 0.10 ± 0.04 | - | The uptake of NPs with a controlled delivery of the peptide iA5 was substantially increased compared to the NPs without monoclonal antibody functionalization. | [158] |
| Peptide H102 | PEG-PLA NPs | TGN and QSH peptides. | PEG-PLA | 125.5.10 ± 2.26 | −29.33 ± 0.15 | 58.49 ± 0.86 | 0.54 | 0.127. ± 0.010 | i.v. | This dual-functional drug delivery system effectively increased the H102 accumulation at brain Aβ42 concentrated in the hippocampal region and provided better neuroprotective effects in the AD model mice. | [159] |
| - | Liposomes and solid lipid NPs | Phosphatidicacid, cardiolipin | Monosialogangliosides, disialogangliosides, trisialoganglioside, sphingomyelin, CL, diphosphatidylglycerol, 1-palmitoyl-oleoyl-PC, phosphatidylethanolamine, Sephadex G75. | 145, 76 | −37.89, −43.30 | - | - | - | - | NPs with surfacephosphatidic acid and cardiolipin demonstrated pronounced affinity to Aβ1e42 fibrils | [160] |
| - | Liposomes | Peptide from the apolipoprotein-E receptor- binding domain, phosphatedic acid. | Dimyristoyl-PA, sphingomyelin, CL. | 121 ± 7 | −18.7 ± 4 | - | - | 0.15 | i.p. | Decrease of the total brain-insoluble amyloid peptide was demonstrated. Amelioration of mice impaired memory was observed. | [161] |
| - | Liposomes | MethoxyXO4 | DSPE-PEG, CL, DPPC. | 150 | - | - | - | - | i.v. | The NPs appeared to cross the BBB and to bind with Aβ plaque deposits, marketing the parenchymal amyloid deposits and vascular amyloid. | [162] |
| - | Apolipoprotein E3 NPs | High density lipoprotein | Lipid free ApoE3, DMPC. | 27.9 ± 8.9 | −4.07 ± 0.83 | - | - | 0.30 | i.v. | Four-week daily treatment with NPs decreased Aβ deposition, attenuated microgliosis, ameliorated neurologic changes, and rescued memory deficits in an AD animal model. | [163] |
| - | Monodisperse iron Oxide NPs | Monoclonal antibody against fibrillar human amyloid-β | - | 8 | - | - | - | - | i.v. | The targeting ability of NPs to cerebrovascular amyloid was demonstrated. | [164] |
| - | Gold NPs | peptide CLPFFD, peptide sequence THRPPMWSPVWP | Gold (III) chloride hydrate. | 13 ± 1.7 | −41 ± 2 | - | - | - | i.p. | Increase of the permeability of the conjugate into the brain was shown. | [165] |
| - | Gold NPs | - | Gold NPs, polyoxometalate with Wells–Dawson structure, Aβ1-40 peptide | 21.7 | −36.8 | - | - | - | i.v. | NPs inhibited Aβ aggregation, dissociated Aβ fibrils and decreased Aβ -mediated peroxidase activity and Aβ -induced cytotoxicity. | [166] |
| - | Gold NPs | - | Gold colloid solution | 10 ± 2 | - | - | - | - | - | NPs disrupted insulin amyloid fibrillation resulting in construction of fibrils that are shorter and more compact. | [167] |
| - | PEG-coated cerium oxide NPs | Aβ antibody | PEG | - | −37 | - | - | - | - | The rescue of neuronal survival was demonstrated. | [168] |
| Tau–Related Nanoparticles | |||||||||||
| Nicotin-amide | Solid lipid NPs | - | Stearic acid, phospholipon® 90G, sodium taurocholate. Three types of particles functionalized by phosphatidic acid, polysorbate 80, phosphatidylserine. | 124 ± 0.8 | −46.1 ± 0.65 | 41.3 ± 0.41 | - | - | i.p., i.v. | The phosphatidylserine-functionalized NPs improved the cognition, preserving the neuronal cells and reducing tau hyperphosphorylation in a rat model of AD. | [169] |
| Methylene blue | PLGA NPs | - | PLGA-b-PEG | 136.5 ± 4.4 | - | - | - | - | - | The reduction of both endogenous and over expressed tau protein levels in human neuroblastoma SHSY-5Y and HeLa cells was shown. | [170] |
| - | Protein-capped metal NPs | - | Two types of NPs: iron oxide (Fe3O4) NPsare capped with hydrolytic proteins from fungi, cadmium sulfide (CdS) NPs are capped with the mixture of four different proteins. | 10–20 | - | - | - | - | - | CdS NPs demonstrated dual properties of inhibition and disaggregation of Tau. | [171] |
| Acetylcholinesterase Inhibitors | |||||||||||
| Gal | Solid lipid NPs | - | Plu F127, Tween 80, glyceryl behenate. | 92.0 ± 3.51 | −17.22 ± 1.1 | 83.42 ± 0.63 | - | 0.380 ± 0.16 | p.o. | NPs restored memory in cognitive deficit rats. Increased bioavailability compared to the plain drug was demonstrated. | [172] |
| Gal | Flexible liposomes | - | Propylene glycol, soya PC, CL | 112 ± 8 | −49.2 ± 0.7 | 83.6 ± 1.8 | - | - | i.n., p.o. | The efficiency of acetylcholinesterase inhibition was greatly enhanced by i.n. administration compared with p.o. administration, especially after loading of the drug in flexible liposomes. | [173] |
| Gal | Nano-emulsions | - | PLGA | 21.5 ± 0.25 | −11.18 ± 0.89 | 98.47 ± 0.43 | 56.87 ± 3.48 | - | - | Non-cytotoxic drug-loaded NPs have been obtained with high encapsulation efficiencies and sustained drug release, maintaining drug pharmacological activity. | [174] |
| Gal | CS complex NPs | - | CS, Tween 80, sodium tripolyphosphate | - | - | - | - | - | i.n. | NPs exhibited a significant decrease of AChE protein level and activity in rat brains. | [175] |
| Gal | CS NPs | - | CS, sodium tripolyphosphate. | 190 ± 1.159 | + 31.6 ± 9.75 | 23.34 | 9.86 | 0.276 ± 0.006 | i.n. | NPs were detected in the olfactory bulb, hippocampus, orbitofrontal and parietal cortices 1 h after i.n. administration. | [176] |
| Donepezil | Liposomes | - | CL, PEG, DSPC. | 102 ± 3.3 | −28.31 ± 0.85 | 84.91 ± 3.31 | - | 0.28 ± 0.03 | i.n. | The bioavailability of drug in the plasma and in the brain increased significantly. The formulation was safe and free from toxicity | [177] |
| Donepezil | Nano suspension | - | CS, tripolyphosphate. | 150–200 | - | 92–96 | 40–48 | 0.341 | i.n. | No mortality, hematological changes, body weight variations and toxicity in animals were observed. | [178] |
| Donepezil | Mango gum polymeric NPs | - | CS, mango gum, Span 80. NPs were prepared by two methods. | 135. 55, 95.1 2 | + 8.2 5, + 25.9 2 | 72 ± 3.62, 85 ± 2.14 | 51 ± 5.42, 76 ± 2.26 | 0.53, 0.26 | i.v. | The brain targeting was achieved. | [179] |
| Donepezil | PLGA-b-PEG nanoparticles | - | PLGA, PEG, Plu F68. | 174-240 | From −11.32 to −20.49 | 52–61 | - | 0.20–0.36 | - | NPs crossed the BBB and showed a controlled release profile in this system. NPs administration caused a significant dose-dependent decrease in both gene and protein expression levels of IL-1b, IL-6, GM-CSF and TNF-a. | [180] |
| Hup | Nano structured lipid carriers | - | Cetyl Palmitate, Miglyol®812, soybean PC, Solutol HS15® | 120 | −22.93 ± 0.91 | 89.180.28 | 1.460.05 | - | - | A burst release at the initial stage and followed by a prolonged release of drug from NPs was up to 96 h. | [181] |
| Hup | Micro emulsion, solid lipid NPs, nano-structured lipid carriers. | - | Glyceryl monostearate, glyceryl mono-dicaprylate, TranscutolP, Tween 80, triethanolamine. | 119–148 | From −4 to −20 | 60.05 ± 5.84 | - | 0.3–0.422 | t.d. | In vitro permeation profiles in rat skin exhibited zero-order kinetics. The significant improvement in cognitive function in mice was observed. | [182] |
| Hup | Nanoemulsion | Lf | Isopropyl myristate, Capryol 90, Cremophor EL, Labrasol. | 15.24 ± 0.67 | −4.48 ± 0.97 | - | - | 0.128 ± 0.025 | i.n. | Intranasal Lf-nanoemulsion significantly enhanced drug delivery to the brain compared to pure nanoemulsion. | [183] |
| Hup | Mucoadhesive and targeted PLGA NPs | Lf | N-trimethylated CS, PLGA 5050 2A. | 153.2 ± 13.7 | +35.6 ± 5.2 | 73.8 ± 5.7 | - | 0.229 ± 0.078 | i.n. | The NPs facilitated the distribution of drug in the brain. | [184] |
| Tacrine | In situ gels | - | Plu F127, Plu F68, CS, PEG 8000 | - | - | - | - | - | i.n. | The enhanced nasal residence time, improved bioavailability, increased brain uptake of the drug and decreased exposure of metabolites were shown. | [185] |
| Tacrine | Albumin NPs carrying beta cyclodextrin and two beta cyclodextrin derivatives | - | Beta cyclodextrinderivatives, hydroxypropyl beta cyclodextrin, sulphobutylether beta cyclodextrin. | 177–266 | From −10 to −10.9 | 85–91 | 12.5–22.0 | 0.228–0.327 | i.n. | The presence of the different beta cyclodextrins affected drug loading and differently modulated NPs mucoadhesiveness and drug permeation. | [186] |
| Tacrine | CS NPs | - | CS, Polysorbate 80 | 41 ± 7 | +34.7 ± 1.5 | 77–83 | 10,86 ± 0,30 | - | i.v. | The NPs coated with 1% Polysorbate 80 altered the biodistribution pattern of NPs. | [187] |
| Riv | Nano structured lipid carriers | - | Glyceryl monosterate, Capmul MCM C8, lecithin, stearylamine, Tween 80 | 123.2 ± 2.3 | 32 ± 1.2 | 68.3 ± 3.4 | - | - | i.n., i.v. | The faster regain of memory loss in amnesic mice with 5-fold decrease in escape latency with NC compared to plain rivastigmine solution was shown. | [188] |
| Riv | Liposomes | - | 1,2-Diacyl-sn-glycero-3-phosphocholine, dihexadecyl phosphate, CL | 67,51–528.7 | From −6.6 to −25.1 | 75–97 | - | 0.612–0.755 | Subcutaneously | NPs resulted in faster memory regain and amelioration of metabolic disturbances in AD rats. | [189] |
| Riv | Liposomes | - | Soya lecithin, CL | 10.0 ± 2.8 µm | - | 80.0 ± 5.0 | - | - | i.n., p.o. | Intranasal liposomes demonstrated a longer half-life in the brain than intranasally or orally administered pure drug. | [190] |
| Riv | Liposomes | Cell penetrating peptide | DSPE-PEG, CL, egg PC | 178.9 ± 11.7 | −8.6 ± 2.4 | 30.5 ± 8.0 | - | 0.333 ± 0.032 | i.n., i.v. | i.n. administration demonstrated the capacity to improve rivastigmine distribution and adequate retention in CNS regions compared to i.v. administration. | [191] |
| Riv | Liposomes | - | CL, DPPC, methyl cellulose, dimethyl-β-CD, sodium taurocholate | 3.37 ± 0.00 µm | −4.30 ± 0.66 | 25,2 | - | - | p.o., i.p. | The highest acetylcholinesterase inhibition was observed for rivastigmine-sodium-taurocholate liposomes. | [192] |
| Riv | PLGA NPs, PBCA NPs | - | PLGA, PBCA | 135.6 ± 4.2, 146.8 ± 2.7 | −23.7 ± 1.18, −3.9 ± 0.58 m | 74.46 ± 0.76, 57.32 ± 0.91 | - | - | i.v. | The faster regain of memory loss in amnesic mice with both PLGA and PBCA NPs compared to rivastigmine solution was demonstrated. | [193] |
| Riv | CS NPs | - | CS, Polysorbate 80 | 45,16 ± 1,56 | 35.08 ± 1. 5 | 83.26 ± 3.1 | 43.48 ± 1.3 0 | - | i.v. | The Polysorbate 80 Coated CS NPs of rivastigmine was formulated and evaluated for brain delivery. | [194] |
| Riv | CS NPs | - | CS | 185.4 ± 8.4 | 38.4 ± 2.85 | 85.3 ± 3.5 | 43.37 ± 3.9 | 0.391 ± 0.065 | i.n., i.v. | NPs demonstrated better brain targeting efficiency and represented a promising approach for i.n. delivery of rivastigmine for the treatment and prevention of AD. | [195] |
| Others | |||||||||||
| p38 MAPK inhibitor PH797804 | Nanoemulsion-based CS nanocapsules | - | Span 85, oleic acid, Tween 20, CS. | 406.1 | −18.5 ± 2.9 | 21.5 ± 2.7 | 3.5 ± 0.5 | 0.287 | i.n. | The p38 MAPK inhibitor encapsulated in CS nanocapsules reduces p38 MAPK activity in brain cells in the cerebral cortex and hippocampus to a lower extent. | [196] |
| Erythropoie-tin | Solid lipid NPs | - | Glycerin monostearate, Span 80, Span 60, dichloromethane, Tween 80 | 219.9 ± 15.6 | −22.4 ± 0.8 | - | - | (0.187 ± 0.03 | i.p. | NPs reduced the oxidative stress, and beta-amyloid plaque deposition in the hippocampus more effectively than the pure drug. The memory was significantly restored in cognitive deficit rats treated with NPs. | [197] |
| Piperine | Monoolein cubosomes | - | Peceol®, Tween 80, poloxamer, Cremophor. | 167.00 ± 10.49 | −34.60 ± 0.47 | 86.67 ± 0.62 | - | 0.18 ± 0.01 | p.o. | The NPs significantly enhanced drug cognitive effect and even restored cognitive function to the normal level. | [198] |
| NAP | High density lipoprotein nanostructure | Mono sialotetrahexosyl ganglioside (GM1) | apoE3-reconstituted high density lipoprotein (rHDL), 1,2-dimyristoyl-sn-glycero3-phosphocholine (DMPC) liposome | 25.42 ± 1.18 | −15.70 ± 0.93 | 64.39 ± 12.84 | - | 0.218 | i.n. | Protection of neurons from cell toxicity, a reduction of Aβ deposition and a rescue of memory in AD model mice were demonstrated. | [199] |
| NAP | PEG-PCL NPs | Lf | PEG-PCL, coumarin-6. | 88.4 ± 7.8 | 23.56 ± 0.96 | 47.61 ± 2.36 | 0.62 ± 0.013 | 0.22 ± 0.033 | i.n. | The neuroprotective and memory improvement effect of Lf-NP was observed. These results were also confirmed by the evaluation of acetylcholinesterase, choline acetyltransferase activity and neuronal degeneration in the mice hippocampus. | [200] |
| Metal chelatorclio-quinol | Gold NP-capped mesoporous silica | - | Mesoporous silica NPs (N-Cetyl trimethylammonium bromide, tetraethoxysilane) | 52.13 | +26.7 | - | - | - | i.v. | The NPs reduced the cell membrane disruption, microtubular defects and apoptosis. | [201] |
| Selenium, sialic acid | Sialic acid modified selenium NPs | Peptide-B6 | Sodiumselenite, | 95 | −14.4 | - | - | - | - | The high permeability across the BBB was shown. The effectively inhibition Aβ aggregation was demonstrated. Therefore, NC did not disaggregate preformed Aβ fibrils into non-toxic amorphous oligomers. | [202] |
| Resveratrol, grape extract | Solid lipid NPs | Antitransferrin receptor mono clonal antibody (OX26 mAb) | Cetylpalmitate, polysorbate 80. | 254 ± 17 | −4.0 ± 0.1 | 92 ± 7, 95 ± 2 | - | 0.23 ± 0.05 | - | The cellular uptake of the OX26 NPs was substantially more efficient than that of normal NPs and NPs functionalized with an unspecific antibody. | [203] |
| Resveratrol (Res) | Delivery system MSe-Res/Fc-β-CD/Bor | - | Mesoporous nano-selenium (MSe), β-CD, borneolBor) | 160 | - | >70 | - | - | i.v. | NC inhibited aggregation of Aβ, mitigated oxidative stress, suppressed tau hyperphosphorylation and improving memory impairment in AD mice. | [204] |
| Memantine | Polyamido amine (PAMAM) dendrimers | Lf | PAMAM | 131.72 ± 4.73 | + 20.13 ± 0.94 | 71.1 ± 4.84 | - | 0.16 ± 0.025 | i.v. | A significant improvement in behavioral responses was demonstrated. | [205] |
| Lipoyl–memantine | Solid lipid NPs | - | Stearic acid | 170 | −33.8 | 88 | - | 0.072 | Through the gastrointestinal tract | The NPs demonstrated low toxicity. | [206] |
| Amlodipine | Diamond NPs | - | Nanodiamond powder | 31.1 ± 8.2 | - | 41 | - | - | - | The highest percentage of loaded amlodipine onto nanodiamond particles was achieved in alkaline medium using 2 mMNaOH at a corresponding pH of 8.5. | [207] |
| Curcumin | Low density lipoprotein mimic nanostructured lipid carrier | Lf | Carboxylated PEG (100) monostearate, CL, glycerol trioleate | 103.8 ± 0.6 | −5.80 ± 0.73 | 96.51 ± 1.87 | 2.60 ± 0.17 | 0.15 ± 0.022 | i.v. | The Lf NPs could effectively permeate BBB and preferentially accumulate in the brain. Superior efficacy of Lf NPs in controlling the damage associated with AD. | [208] |
| Curcumin | RBC membrane camouflaged HSANPs | T807, tri phenyl phosphine (TPP) | Amino-T807, carboxy-TPP, PEG, red blood cell (RBC) membrane, human serum albumin (HAS). | <120 | - | 88.43 ± 1.25 | 4.87 ± 1.04 | - | i.v. | The NPs relieved AD symptoms by mitigating mitochondrial oxidative stress and suppressing neuronal death. | [209] |
| Curcumin, NGF | Liposomes | Wheat germ agglutinin (WGA), cardiolipin (CL) | CL, soybean PC, 1,2-Dipalmitoylsn-glycero-3-phosphocholine. | 122–142 | −5.2 to −18.3 | 20.5–56.7 | - | - | i.v. | The NPs inhibited the expression of phosphorylated p38, prevented neurodegeneration of cells, enhanced the quantities of p-neurotrophic tyrosine kinase receptor type 1 and p-extracellular signal-regulated kinase 5. | [210] |
| Curcumin, selenium | PLGA nanospheres | - | PLGA, selenium NPs | 160 ± 5 | - | - | 11.5 | Low | i.v. | The NPs provided the enhanced therapeutic efficacy in AD lesions. | [211] |
| Curcumin or dexamethasone | Polymeric nanocore | Antiamyloid antibody IgG4.1 | CS, gadolinium-diethylene triamine pentaacetic acid, hydroxypropyl-beta-cyclodextran, Magnevist®, 125I | 145 ± 5,4, 157,6 ± 3,4 | 7,7 ± 0,4, 4,5 ± 0,5 | - | - | - | i.v. | The NPs targeted cerebrovascular amyloid deposits selectively. | [212] |
| Andrographolide | Human albumin NPs | - | Human albumin | 210.4 ± 3.2 | −20.3 ± 1.5 | 99.1 ± 0.2 | - | 0.10 ± 0.01 | i.v., i.p. | In the step-down inhibitory avoidance test, NPs improved mice performance. The presence of NPs both in the pE3-Aβ plaque surroundings and inside the pE3-Aβ plaque was observed. The anti-inflammatory activity was shown. | [213] |
| Flurbiprofen | Dendrimer NPs | - | Phenylalanine | - | - | - | - | - | - | Efficiency of NC influence on the γ-secretase enzyme in target cells was shown. Eventual drug release by hydrolysis of the carrier was demonstrated. | [214] |
| Tarenflurbil (TFB) | Solid lipid NPs, PLGA NPs | - | Monostearate, stearic acid, soya lecithin, Tween 20, PLGA, DMAB, PF-68, PVA | 169.87 ± 10.98, 133.13 ± 7.82 | −23.13 ± 2.32, −30.25 ± 2.11 | 57.81 ± 5.32, 64.11 ± 2.21 | −10 | 0.24 ± 0.04, 0.21 ± 0.02 | i.n. | The absolute bioavailability of the NPs was higher than TFB solution suspension. | [215] |
| Metformin | Nano liposomes | - | Phosphatidylserine. | 148.3 ± 5.6 | −34.8 ± 2.8 | 37 ± 2.3 | - | <0.3 | i.p. | The learning and memory parameters significantly improved in AD-rats treated with NPs. The decreased cytokine levels of IL1-β, TNF-α and TGF-β in hippocampal tissues were shown. A reduction in inflammatory and necrotic neural cells, and an increase neurogenesis was demonstrated. | [216] |
| Saxagliptin | CS-l-valine based NPs | l-valin | CS, BocL-valine | 385 ± 21 | + 0.554 ± 0.110 | 56.23 ± 13.44 | - | 0.574 ± 0.125 | i.p. | The NPs were highly stable in the plasma releasing only a minute after administration of the drug. Pronounced accumulation of drug from the NPs was demonstrated. The pure substance showed no detectable amount of the drug after 24 h. | [217] |
| NGF | Liposomes | Lf | CL, DSPE-PEG, DPPC, PEPEG | About 110 | From −3 to −11 | - | - | - | - | Lf/NGF-liposomes comprising CL and DPPC were physically stable with high biocompatibility to HBMECs and HAs cells. | [218] |
| NGF | Liposomes | P-aminophenyl-a D manno-pyrano-side, apolipo protein E | Soybean PC, phosphatidic acid, DPPC, cardiolipin, DSPE-PEG, CL | <170 | From −4 to −10 | 35.1 ± 3.2 | - | - | - | NPs were capable to enhance the NGF delivery across the BBB. NPs recognized a low-density lipoprotein receptor expressed by SK-N-MC cells and yielded neuroprotective effect on the neuronal degeneration induced by fibrillar Aβ1–42. | [219] |
| NGF | Liposomes | Cereport trans-ferrin | l-α-PC, DPPC, CL, DSPE-PEG-carboxy. | 152-189 | from −4.5 to −8.5 | 31.2 ± 2.8 | - | - | - | Covering of NPs with cereport and transferrin was effective in carrying NGF across the BBB and rescued the apoptosis of SK-N-MC cells after neurotoxic treatment with Aβ1–42. | [220] |
| Fibroblast growth factor | PEG-PLGA NPs | Solanum tubero-sum lectin | PEG-PLGA | 118.7 | −31,18 | 69.21 | 0.0462 | - | i.n. | Neuroprotective effect in AD rats was demonstrated. | [221] |
| Pituitary adenylate cyclaseactivating Polypeptide (PACAP38) coupled to a docosahexaenoic acid (DHA: an ω-3 polyunsaturated fatty acid) | Pep-lipid nanostructures and liquid crystalline nanocarriers | - | PACAP-DHA/MO (nonlamellar lipid monoolein)/DHA/vitamin E/VPGS-PEG1000 and MO/DHA/vitamin E/VPGS-PEG1000 | ~100 | - | - | - | - | - | Multicompartment nanocarriers with PACAP and DHA are promising therapy strategies for neurodegenerative disorders. PACAP is neuropeptide ligand of the PAC1 membrane receptor (a class B GPCR). PACAP demonstrate antiapoptotic effects in neuronal and non-neuronal cells, neuroprotive and neurotransmitter properties, can act as neurotrophic factor, attenuates Aβ amyloid toxicity. DHA plays a crucial role in the control of the phospholipid membrane organization, act as lipid trophic factor, demonstrated antiapoptotic, neuroprotective effect, take part in inflammatory cell signaling. Vitamin E reduces oxidative stress, demonstrates anti-inflammatory, cardio and neuroprotective effects, inhibit lipid peroxidation. | [222] |
| - | Amphiphilic yellow-emissive carbon dots (Y-CDs) | - | Citric acid, o-phenylenediamine. | 3.4 ± 1.0 | −15.3 | - | - | - | Injected into the heart. | Y-CDs entered cells to inhibit the overexpression of human amyloid precursor protein and β-amyloid. | [223] |
| - | Nanosystem CB-Gd-Cy5.5 | Holera toxin B subunit (CB) | Chelated gadolinium (Gd), Cyanine5.5 (Cy5.5). | 110 | −11.0 | - | - | - | i.n. | The nanosystem was accumulated in the hippocampus and demonstrated good magnetic resonance imaging capability satisfying the monitoring of AD at the different stages. | [224] |
| - | Fe3O4 NPs loaded with PEG-PLGA nano composite | Anti-transferrin mono clonal antibody (OX26) receptor | Fe3O4NPs, PEG, PLGA. | 95 | - | 76.2 | 18.1 | - | - | The significant in vitro drug release and cell viability were shown. | [225] |
| - | Carboxyl magnetic nano containers | - | Fluorescent Carboxyl Magnetic Particles, Yellow, 1% w/v | 700–900 | - | - | - | - | i.v. | The magnetic NPs crossed the normal BBB in mice after subjection to external electromagnetic fields of 28 mT (0.43 T/m) and 79.8 mT (1.39 T/m). | [226] |
| - | Gold NPs | - | Gold NPs suspension | 5 | −47.7 ± 10.9 | - | - | - | i.p. | The NPs improved the acquisition and retention of spatial learning and memory in Aβ treated rats. Expression of BDNF, cAMP, CREB and stromal interaction molecules, e.g., STIM1 and STIM2 was increased. | [227] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zorkina, Y.; Abramova, O.; Ushakova, V.; Morozova, A.; Zubkov, E.; Valikhov, M.; Melnikov, P.; Majouga, A.; Chekhonin, V. Nano Carrier Drug Delivery Systems for the Treatment of Neuropsychiatric Disorders: Advantages and Limitations. Molecules 2020, 25, 5294. https://doi.org/10.3390/molecules25225294
Zorkina Y, Abramova O, Ushakova V, Morozova A, Zubkov E, Valikhov M, Melnikov P, Majouga A, Chekhonin V. Nano Carrier Drug Delivery Systems for the Treatment of Neuropsychiatric Disorders: Advantages and Limitations. Molecules. 2020; 25(22):5294. https://doi.org/10.3390/molecules25225294
Chicago/Turabian StyleZorkina, Yana, Olga Abramova, Valeriya Ushakova, Anna Morozova, Eugene Zubkov, Marat Valikhov, Pavel Melnikov, Alexander Majouga, and Vladimir Chekhonin. 2020. "Nano Carrier Drug Delivery Systems for the Treatment of Neuropsychiatric Disorders: Advantages and Limitations" Molecules 25, no. 22: 5294. https://doi.org/10.3390/molecules25225294
APA StyleZorkina, Y., Abramova, O., Ushakova, V., Morozova, A., Zubkov, E., Valikhov, M., Melnikov, P., Majouga, A., & Chekhonin, V. (2020). Nano Carrier Drug Delivery Systems for the Treatment of Neuropsychiatric Disorders: Advantages and Limitations. Molecules, 25(22), 5294. https://doi.org/10.3390/molecules25225294





