Physico-Chemical and Phytochemical Characterization of Moroccan Wild Jujube “Zizyphus lotus (L.)” Fruit Crude Extract and Fractions
Abstract
1. Introduction
2. Results and Discussion
2.1. Variation of Physicochemical Parameters
2.2. Phytochemical Screening
2.3. Antioxidant Activity
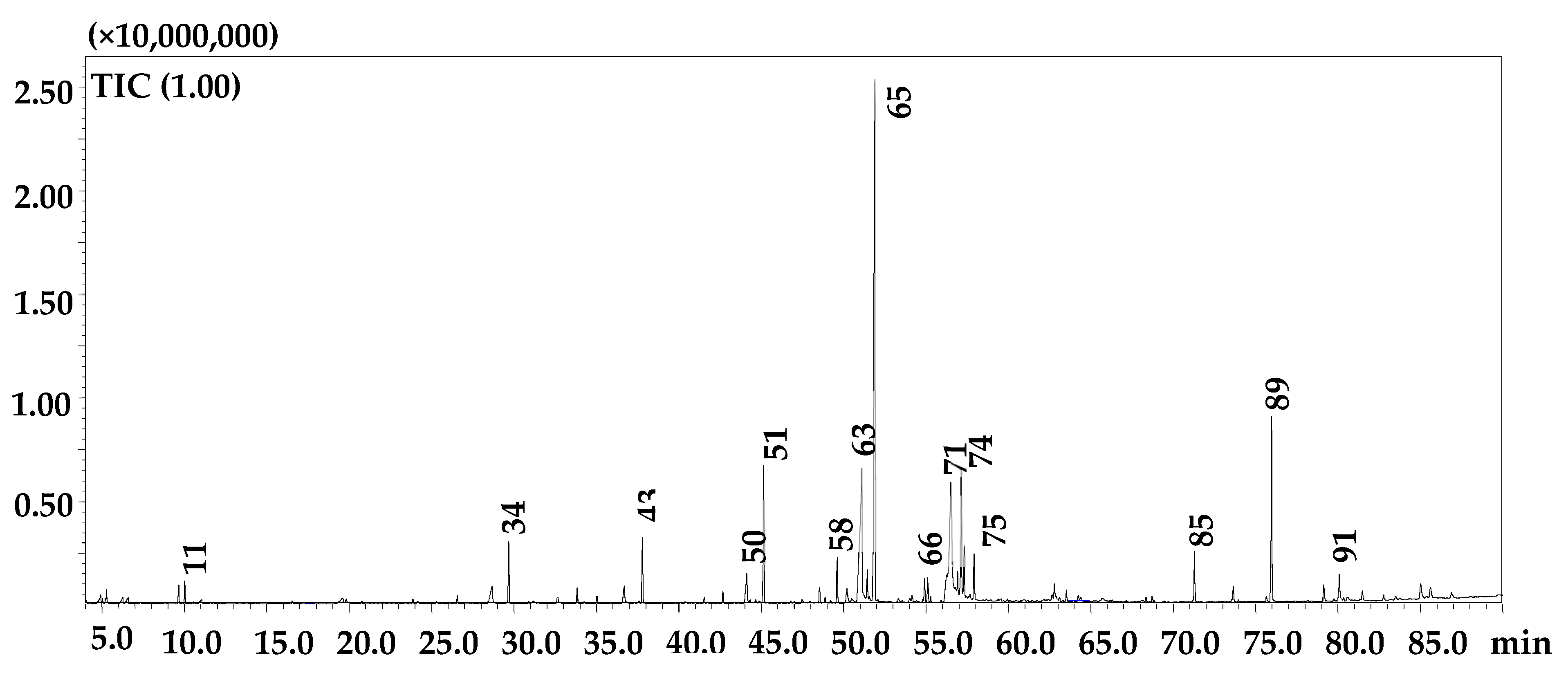
2.4. GC-MS Analyses
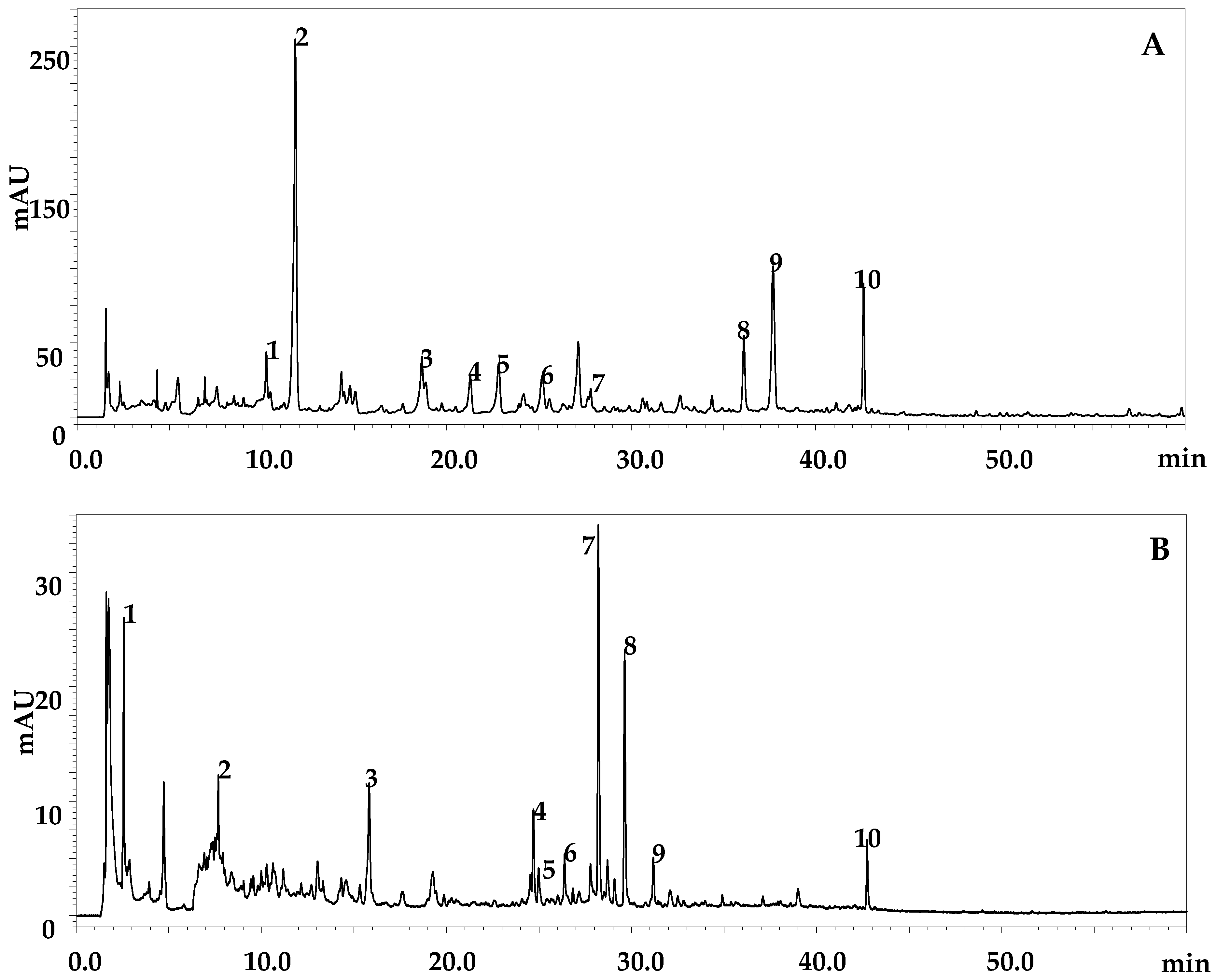
2.5. HPLC-DAD-ESI/MS Analyses
3. Materials and Methods
3.1. Samples
3.2. Chemical Reagents and Solvents
3.3. Extraction Method
3.4. Physico-Chemical Analyses
3.5. Phytochemical Screening
3.6. Determination of the Polyphenolic Content
3.6.1. Quantification of TPP, TFv, and TT in Z. lotus extracts
3.6.2. Quantification of Total Anthocyanin Content in Z. Lotus Extracts
3.7. Determination of Antioxidant Activity
3.8. GC-MS
3.9. HPLC-DAD/ESI-MS
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rsaissi, N.; Bouhache, M.; Bencharki, B. Importance and agro-economical impact of wild jujube (Ziziphus lotus) in Chaouia region. Revue. Maroc. Prot. Des. Plantes 2012, 3, 13–27. [Google Scholar]
- Chevalier, A. Les Jujubiers ou Ziziphus de l’Ancien monde et l’utilisation de leurs fruits. J. D’agric. Tradit. Bot. Appliquée 1947, 301–302, 470–483. [Google Scholar] [CrossRef]
- Hammiche, V.; Maiza, K. Traditional medicine in central Sahara: Pharmacopoeia of Tassili N’ajjer. J. Ethnopharmacol. 2006, 105, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Niamat, R.; Khan, M.A.; Khan, K.Y.; Mushtaq, A.; Barkat, A.; Paras, M.; Mazhar, M.; Hussain, A. Element content of some ethnomedicinal Ziziphus Linn. species using atomic absorption spectroscopy technique. J. Appl. Pharm. Sci. 2012, 2, 96–100. [Google Scholar]
- Glombitza, K.W.; Mahran, G.H.; Mirhom, Y.W.; Michel, K.G.; Motawi, T.K. Hypoglycemic and antihyperglycemic effects of Zizyphus spina-christi in rats. Planta Med. 1994, 60, 244–247. [Google Scholar] [CrossRef]
- Mathon, C.-C. Baumann Hellmut.—Le bouquet d’Athéna: Les plantes dans la mythologie et l’art grecs. Trad. de l’allemand par Roger Barbier, éd. allem. originale, 1982; éd. fr., La Maison rustique-Flammarion, 1984. J. D’agric. Tradit. Bot. Appliquée 1984, 31, 129. [Google Scholar]
- Borgi, W.; Ghedira, K.; Chouchane, N. Anti-inflammatory and analgesic activities of Zizyphus lotus L. root barks. Fitoterapia 2007, 78, 16–19. [Google Scholar] [CrossRef]
- Borgi, W.; Recio, M.C.; Rios, J.L.; Chouchane, N. Anti-inflammatory and analgesic activities of flavonoid and saponin fractions from Zizyphus lotus (L.) Lam. S. Afr. J. Bot. 2008, 74, 320–324. [Google Scholar] [CrossRef]
- Abdel-Zaher, A.O.; Salim, S.Y.; Assaf, M.H.; Abdel-Hady, R.H. Antidiabetic activity and toxicity of Zizyphus spina-christi leaves. J. Ethnopharmacol. 2005, 101, 129–138. [Google Scholar] [CrossRef]
- Hirsinger, F. New Annual Oil Crops, in Oils Crops of the World; McGraw-Hill: New York, NY, USA, 1989; pp. 518–532. [Google Scholar]
- Abdoul-Azize, S.; Bendahmane, M.; Hichami, A.; Dramane, G.; Simonin, A.M.; Benammar, C.; Sadou, H.; Akpona, S.; El Boustani, E.S.; Khan, N.A. Effects of Zizyphus lotus L. (Desf.) polyphenols on Jurkat cell signaling and proliferation. Int. Immunopharmacol. 2013, 15, 364–371. [Google Scholar] [CrossRef]
- Le Crouéour, G.; Thépenier, P.; Richard, B.; Petermann, C.; Ghédira, K.; Zèches-Hanrot, M. Lotusine G: A new cyclopeptide alkaloid from Zizyphus lotus. Fitoterapia 2002, 73, 63–68. [Google Scholar] [CrossRef]
- Ghedira, K.; Chemli, R.; Richard, B.; Nuzillard, J.-M.; Zeches, M.; Le Men-Olivier, L. Two cyclopeptide alkaloids from Zizyphus lotus. Phytochemistry 1993, 32, 1591–1594. [Google Scholar] [CrossRef]
- Ghedira, K.; Chemli, R.; Caron, C.; Nuzilard, J.-M.; Zeches, M.; Le Men-Olivier, L. Four cyclopeptide alkaloids from Zizyphus lotus. Phytochemistry 1995, 38, 767–772. [Google Scholar] [CrossRef]
- El Maaiden, E.; El Kharrassi, Y.; Lamaoui, M.; Allai, L.; Essamadi, A.K.; Nasser, B.; Moustaid, K. Variation in minerals, polyphenolics and antioxidant activity of pulp, seed and almond of different Ziziphus species grown in Morocco. Braz. J. Food Technol. 2020. [Google Scholar] [CrossRef]
- El Maaiden, E.; El Kharrassi, Y.; Moustaid, K.; Essamadi, A.K.; Nasser, B. Comparative study of phytochemical profile between Ziziphus spina christi and Ziziphus lotus from Morocco. J. Food Meas. Charact. 2019, 13, 121–130. [Google Scholar] [CrossRef]
- Renault, J.-H.; Ghedira, K.; Thepenier, P.; Lavaud, C.; Zeches-Hanrot, M.; Le Men-Olivier, L. Dammarane saponins from Zizyphus lotus. Phytochemistry 1997, 44, 1321–1327. [Google Scholar] [CrossRef]
- El Hachimi, F.; El Antari, A.; Boujnah, M.; Bendrisse, A. Comparison of oils seed and fatty acid content of various Moroccan populations of jujube, grenadier and prickly pear. J. Mat. Env. Sci. 2015, 6, 1488–1502. [Google Scholar]
- Abdeddaim, M.; Lombarkia, O.; Bacha, A.; Fahloul, D.; Abdeddaim, D.; Farhat, R.; Saadoudi, M.; Noui, Y.; Lekbir, A. Biochemical Characterization and nutritional properties Zizyphus lotus L. fruits in Aures region, Northerastern of Algeria. Ann. Food Sci. Technol. 2014, 15, 75–81. [Google Scholar]
- Benammar, C.; Hichami, A.; Yessoufou, A.; Simonin, A.-M.; Belarbi, M.; Allali, H.; Khan, N.A. Zizyphus lotus L. (Desf.) modulates antioxidant activity and human T-cell proliferation. BMC Complement. Altern. Med. 2010, 20, 54. [Google Scholar] [CrossRef]
- Chen, K.; Fan, D.; Fu, B.; Zhou, J.; Li, H. Comparison of physical and chemical composition of three chinese jujube (Ziziphus jujuba Mill.) cultivars cultivated in four districts of Xinjiang region in China. Food Sci. Technol. 2019, 39, 912–921. [Google Scholar] [CrossRef]
- Maraghni, M.; Gorai, M.; Neffati, M. The Influence of Water-Deficit Stress on Growth, Water Relations and Solute Accumulation in WildJujube (Ziziphus lotus). J. Ornam. Hortic. 2011, 1, 63–72. [Google Scholar]
- Karumi, Y.; Onyeyili, P.A.; Ogugduaja, V.O. Identification des principles actifs de l’extrait de feuilles de M. balsamia (Baume de la pomme). J. Med. Sci. 2004, 4, 179–182. [Google Scholar]
- Sheng, J.P.; Shen, L. Chinese jujube (Ziziphus jujuba Mill.) and Indian jujube (Ziziphus mauritiana Lam.). In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Elsevier: Amsterdam, The Netherlands, 2011; pp. 299–326. [Google Scholar]
- Zia-Ul-Haq, M.; Riaz, M.; De Feo, V.; Jaafar, H.; Moga, M.; Rubus Fruticosus, L. Constituents, Biological Activities and Health Related Uses. Molecules 2014, 19, 10998–11029. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.-H.; Wu, P.-T.; Liu, J.-R.; Wu, C.-S.; Parry, J.W.; Wang, M. Physico-chemical properties and antioxidant capacity of different jujube (Ziziphus jujuba Mill.) cultivars grown in loess plateau of China. Sci. Hortic. 2011, 130, 67–72. [Google Scholar] [CrossRef]
- Li, J.-W.; Fan, L.-P.; Ding, S.-D.; Ding, X.-L. Nutritional composition of five cultivars of chinese jujube. Food Chem. 2007, 103, 454–460. [Google Scholar] [CrossRef]
- Sakamura, F.; Suga, T. Changes in chemical components of ripening oleaster fruits. Phytochemistry 1987, 26, 2481–2484. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Julkunen-Tiitto, R. Phenolic constituents in the leaves of northern willows: Methods for the analysis of certain phenolics. J. Agric. Food Chem. 1985, 33, 213–217. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and Measurement of Anthocyanins by UV-Visible Spectroscopy. Curr. Protoc. Food Anal. Chem. 2001. [Google Scholar] [CrossRef]
- Braca, A.; Sortino, C.; Politi, M.; Morelli, I.; Mendez, J. Antioxidant activity of flavonoids from Licania licaniaeflora. J. Ethnopharmacol. 2002, 79, 379–381. [Google Scholar] [CrossRef]
- Irakli, M.; Chatzopoulou, P.; Ekateriniadou, L. Optimization of ultrasound-assisted extraction of phenolic compounds: Oleuropein, phenolic acids, phenolic alcohols and flavonoids from olive leaves and evaluation of its antioxidant activities. Ind. Crop. Prod. 2018, 124, 382–388. [Google Scholar] [CrossRef]
- Chouaibi, M.; Mahfoudhi, N.; Rezig, L.; Donsì, F.; Ferrari, G.; Hamdi, S. Nutritional composition of Zizyphus lotus L. seeds. J. Sci. Food Agric. 2012, 92, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Rached, W.; Barros, L.; Ziani, B.E.C.; Bennaceur, M.; Calhelha, R.C.; Heleno, S.A.; Alves, M.J.; Abderrazak, M.; Ferreira, I.C.F.R. HPLC-DAD-ESI-MS/MS screening of phytochemical compounds and the bioactive properties of different plant parts of Zizyphus lotus (L.) Desf. Food Funct. 2019, 10, 5898–5909. [Google Scholar] [CrossRef] [PubMed]
- Diallo, D.; Sanogo, R.; Yasambou, H.; Traoré, A.; Coulibaly, K.; Maïga, A. Étude des constituants des feuilles de Ziziphus mauritiana Lam. (Rhamnaceae), utilisées traditionnellement dans le traitement du diabète au Mali. Comptes Rendus Chim. 2004, 7, 1073–1080. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Patras, A.; Brunton, N.; Cullen, P.J.; O’Donnell, C.P. Effect of ultrasound processing on anthocyanins and color of red grape juice. Ultrason. Sonochem. 2010, 17, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Haminiuk, C.W.I.; Maciel, G.M.; Plata-Oviedo, M.S.V.; Peralta, R.M. Phenolic compounds in fruits—An overview: Phenolic compounds in fruits. Int. J. Food Sci. Technol. 2012, 47, 2023–2044. [Google Scholar] [CrossRef]
- Chu, W.; Cheung, S.C.M.; Lau, R.A.W.; Benzie, I.F.F. Bilberry (Vaccinium myrtillus L.) in Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Nair, S.K.P.; Ganesan, K.; Sinaga, M.; Letha, N.; Gani, B. Preliminary phytochemical screening of different solvent extracts of leaves of Echeveria elegans rose, an endangered mexican succulent herb. J. Glob. Biosci. 2016, 5, 3429–3432. [Google Scholar]
- Mishra, B.; Kar, D.M.; Maharana, L.; Mishra, G.P. Physicochemical and phytochemical investigation of different fractions from hydroalcoholic extract of Tectona grandis (Linn) barks. Der Pharm. Lett. 2016, 8, 80–85. [Google Scholar]
- Ribéreau-Gayon, J.; Peynaud, E. Les Composés Phénoliques des Végétaux, Traité D’oenologie; Dunod: Paris, France, 1968. [Google Scholar]
- Macheix, J.J.; Fleuriet, A. Phenolics in fruit products: Progress and prospects. In Polyphenolic Phenomena; Scalbert, A., Ed.; INRA: Paris, France, 1993. [Google Scholar]
- Dib, M.E.A.; Allali, H.; Bendiabdellah, A.; Meliani, N.; Tabti, B. Antimicrobial activity and phytochemical screening of Arbutus unedo L. J. Saudi Chem. Soc. 2013, 17, 381–385. [Google Scholar] [CrossRef]
- Hadi, S.M.; Asad, S.F.; Singh, S.; Ahmad, A. Putative. Mechanism for Anticancer and Apoptosis-Inducing Properties of Plant-Derived Polyphenolic Compounds. IUBMB Life 2000, 50, 167–171. [Google Scholar]
- Bate-Smith, E.C. The phenolic constituents of plants and their taxonomic significance. I. Dicotyledons. J. Linn. Soc. Lond. Bot. 1962, 58, 95–173. [Google Scholar] [CrossRef]
- Di Carlo, G.; Mascolo, N.; Izzo, A.A.; Capasso, F. Flavonoids: Old and new aspects of a class of natural therapeutic drugs. Life Sci. 1999, 65, 337–353. [Google Scholar] [CrossRef]
- Alhakmani, F.; Khan, S.A.; Ahmad, A. Determination of total phenol, in-vitro antioxidant and anti-inflammatory activity of seeds and fruits of Zizyphus spina-christi grown in Oman. Asian Pac. J. Trop. Biomed. 2014, 4, S656–S660. [Google Scholar] [CrossRef]
- Khadhri, A.; Neffati, M.; Aschi-Smiti, S.; Falé, P.; Lino, A.R.L.; Serralheiro, M.L. Machado Araùjo ME. Antioxidant, antiacetylcholinesterase and antimicrobial activities of Cymbopogon schoenanthus L. Spreng (lemon grass) from Tunisia. Lwt-Food Sci. Technol. 2010, 43, 331–336. [Google Scholar] [CrossRef]
- Hsouna, A.B.; Saoudi, M.; Trigui, M.; Jamoussi, K.; Boudawara, T.; Jaoua, S.; ElFeki, A. Characterization of bioactive compounds and ameliorative effects of Ceratonia siliqua leaf extract against CCl4 induced hepatic oxidative damage and renal failure in rats. Food Chem. Toxicol. 2011, 49, 3183–3191. [Google Scholar] [CrossRef]
- Choi, C.W.; Kim, S.C.; Hwang, S.S.; Choi, B.K.; Ahn, H.J.; Lee, M.Y.; Park, S.H.; Kim, S.K. Antioxidant activity and free radical scavenging capacity between Korean medicinal plants and flavonoids by assay-guided comparison. Plant. Sci. 2002, 163, 1161–1168. [Google Scholar] [CrossRef]
- Nićiforović, N.; Mihailović, V.; Mašković, P.; Solujić, S.; Stojković, A.; Muratspahić, D.P. Antioxidant activity of selected plant species; potential new sources of natural antioxidants. Food Chem. Toxicol. 2010, 48, 3125–3130. [Google Scholar] [CrossRef]
- Alothman, M.; Bhat, R.; Karim, A.A. Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia, extracted with different solvents. Food Chem. 2009, 115, 785–788. [Google Scholar] [CrossRef]
- Vinatoru, M.; Toma, M.; Radu, O.; Filip, P.I.; Lazurca, D.; Mason, T.J. The use of ultrasound for the extraction of bioactive principles from plant materials. Ultrason. Sonochem. 1997, 4, 135–139. [Google Scholar] [CrossRef]
- Goltz, C.; Ávila, S.; Barbieri, J.B.; Igarashi-Mafra, L.; Mafra, M.R. Ultrasound-assisted extraction of phenolic compounds from Macela (Achyrolcine satureioides) extracts. Ind. Crop. Prod. 2018, 115, 227–234. [Google Scholar] [CrossRef]
- Vega Arroy, J.D.; Ruiz-Espinosa, H.; Luna-Guevara, J.J.; Luna-Guevara, M.L.; Hernández-Carranza, P.; Ávila-Sosa, R.; Ochoa-Velasco, C.E. Effect of Solvents and Extraction Methods on Total Anthocyanins, Phenolic Compounds and Antioxidant Capacity of Renealmia alpinia (Rottb.) Maas Peel. Czech J. Food Sci. 2017, 35, 456–465. [Google Scholar] [CrossRef]
- Ju, Z.Y.; Howard, L.R. Effects of solvent and temperature on pressurized liquid extraction of anthocyanins and total phenolics from dried red grape skin. J. Agric. Food Chem. 2003, 51, 5207–5213. [Google Scholar] [CrossRef] [PubMed]
- Khonkarn, R.; Okonogi, S.; Ampasavate, C.; Anuchapreeda, S. Investigation of fruit peel extracts as sources for compounds with antioxidant and antiproliferative activities against human cell lines. Food Chem. Toxicol. 2010, 48, 2122–2129. [Google Scholar] [CrossRef] [PubMed]
- Benabdeljalil, C.; Cheynier, V.; Fulcrand, H.; Hafiki, A.; Mosaddak, M.; Moutounet, M. Mise en évidence de nouveaux pigments formés par réaction des anthocyanes avec des métabolites de levure. Sci. Aliment. 2000, 20, 203–220. [Google Scholar] [CrossRef]
- Mensor, L.L.; Mnezes, F.S.; Leitão, G.G.; Reis, A.S.; dos Santos, T.C.; Coube, C.S.; Leitão, S.G. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef]
- Mokhtarpour, A.; Naserian, A.A.; Valizadeh, R.; Danesh Mesgaran, M.; Pourmollae, F. Extraction of Phenolic Compounds and Tannins from Pistachio By-products. Annu. Res. Rev. Biol. 2014, 4, 1330–1338. [Google Scholar] [CrossRef]
- Ghasemi, S.; Naserian, A.A.; Valizadeh, R.; Vakil, A.R.; Behgar, M.; Tahmasebi, A.M.; Ghovvati, S. Partial and total substitution of alfalfa hay by pistachio byproduct modulated the counts of selected cellulolytic ruminal bacteria attached to alfalfa hay in sheep. Livest. Sci. 2012, 150, 342–348. [Google Scholar] [CrossRef]
- Naima, R.; Oumam, M.; Hannache, H.; Sesbou, A.; Charrier, B.; Pizzi, A.; Charrier–El Bouhtoury, F. Comparison of the impact of different extraction methods on polyphenols yields and tannins extracted from Moroccan Acacia mollissima barks. Ind. Crop. Prod. 2015, 70, 245–252. [Google Scholar] [CrossRef]
- Najjaa, H.; Ben Arfa, A.; Elfalleh, W.; Zouari, N.; Neffati, M. Jujube (Zizyphus lotus L.): Benefits and its effects on functional and sensory properties of sponge cake. PLoS ONE 2020, 15, e0227996. [Google Scholar] [CrossRef]
- Ghazghazi, H.; Aouadhi, C.; Riahi, L.; Maaroufi, A.; Hasnaoui, B. Fatty acids composition of Tunisian Ziziphus lotus L. (Desf.) fruits and variation in biological activities between leaf and fruit extracts. Nat. Prod. Res. 2014, 28, 1106–1110. [Google Scholar] [CrossRef]
- Barros, L.; Carvalho, A.M.; Morais, J.S.; Ferreira, I.C.F.R. Strawberry-tree, blackthorn and rose fruits: Detailed characterisation in nutrients and phytochemicals with antioxidant properties. Food Chem. 2010, 120, 247–254. [Google Scholar] [CrossRef]
- Kintzios, S.; Papageorgiou, K.; Yiakoumettis, I.; Baričevič, D.; Kušar, A. Evaluation of the antioxidants activities of four Slovene medicinal plant species by traditional and novel biosensory assays. J. Pharm. Biomed. Anal. 2010, 53, 773–776. [Google Scholar] [CrossRef]
- Hammi, K.M.; Jdey, A.; Abdelly, C.; Majdoub, H.; Ksouri, R. Optimization of ultrasound-assisted extraction of antioxidant compounds from Tunisian Zizyphus lotus fruits using response surface methodology. Food Chem. 2015, 184, 80–89. [Google Scholar] [CrossRef]
- El Cadi, H.; El Cadi, A.; Kounnoun, A.; Oulad El Majdoub, Y.; Lovillo, M.P.; Brigui, J.; Dugo, P.; Mondello, L.; Cacciola, F. Wild strawberry (Arbutus unedo): Phytochemical screening and antioxidant properties of fruits collected in northern Morocco. Arab. J. Chem. 2020, 13, 6299–6311. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Phenolics in cereals, fruits and vegetables: Occurrence, extraction and analysis. J. Pharm. Biomed. Anal. 2006, 41, 1523–1542. [Google Scholar] [CrossRef]
- Trease, E.; Evans, W.C. Pharmacognosie; Billiaire Tindall: London, UK, 1987. [Google Scholar]
- Singleton, V.; Rossi, J. Colorimetry of Total Phenolic Compounds with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |
| Parameter | Crude Extract | Solvent Fractions | |
|---|---|---|---|
| EtOAc | MeOH-H2O | ||
| pH | 4.9 ± 0.23 | - | - |
| Acidity | 1.5 ± 0.06 | - | - |
| RI | 1.3 ± 0.02 | 2.8 ± 0.00 | 2.7 ± 0.02 |
| TSS | 6.5 ± 0.92 | 60.8 ± 0.20 | 16.7 ± 0.48 |
| S/A | 4.2 ± 0.40 | - | - |
| DM (%) | 87.1 ± 0.25 | - | - |
| Ash (%) | 3.2 ± 0.54 | - | - |
| TS (%) | 80.2 ± 3.81 | 6.2 ± 0.75 | 76.5 ± 1.21 |
| RS (%) | 9.6 ± 0.39 | - | - |
| Lipids (mg/g) | 2.3 ± 0.09 | - | - |
| Proteins (mg/g) | 0.9 ± 0.02 | 0.9 ± 0.00 | 0.00 |
| Vitamin C (mg/g) | 34.5 ± 0.30 | 12.7 ± 0.51 | 33.6 ± 0.45 |
| Compounds Group/Solvent of Extraction | Crude Extract | EtOAc | MeOH-H2O | |
|---|---|---|---|---|
| Alkaloids | - | ± | ± | |
| Polyphenols | Flavonoids | C++ | B | A+ |
| Tannins | + | - | ++ | |
| Anthocyanins | + | - | ± | |
| Catechic tannins | + | - | + | |
| Gallic tannins | + | - | + | |
| Coumarins | + | - | - | |
| Steroids | Soponosides | + | - | + |
| Unsaturated Sterols/Terpenes | - | + | - | |
| Sterols and Steroids | ++ | - | ++ | |
| Sugars | Deoxysugars | + | - | - |
| Glycosides | - | + | ± | |
| Mucilages | + | - | + | |
| Extract | Vit. C | TPP | TFv | TA | TT | IC50 |
|---|---|---|---|---|---|---|
| EtOAc | 12.7 ± 1.01 | 3.0 ± 0.10 | 2.0 ± 0.10 | 0.1 ± 0.00 | 5.2 ± 0.10 | 1.5 ± 0.00 |
| MeOH-H20 | 33.6 ± 2.50 | 4.8 ± 1.05 | 5.7 ± 0.05 | 0.1 ± 0.00 | 11.1 ± 0.50 | 1.3 ± 0.00 |
| Peak | Compound | LRI (lib) | LRI (exp) | Similarity (%) | Library |
|---|---|---|---|---|---|
| 1 | Isobutyric acid | 752 | 740 | 83 | FFNSC 4.0 |
| 2 | 3-Hexanone | 782 | 781 | 93 | FFNSC 4.0 |
| 3 | Butyl methyl ketone | 786 | 787 | 98 | FFNSC 4.0 |
| 4 | 3-Hexanol | 795 | 798 | 91 | FFNSC 4.0 |
| 5 | 2-Hexanol | 802 | 801 | 92 | FFNSC 4.0 |
| 6 | Isovaleric acid | 842 | 838 | 97 | FFNSC 4.0 |
| 7 | 2-methylbutanoic acid | 881 | 849 | 94 | FFNSC 4.0 |
| 8 | n-Hexanol | 867 | 867 | 88 | FFNSC 4.0 |
| 9 | n-Pentanoic acid | 911 | 876 | 96 | FFNSC 4.0 |
| 10 | n-Heptanal | 906 | 903 | 90 | FFNSC 4.0 |
| 11 | (E)-Hept-2-enal | 956 | 957 | 93 | FFNSC 4.0 |
| 12 | n-Hexanoic acid | 997 | 980 | 96 | FFNSC 4.0 |
| 13 | 2-pentyl Furan | 991 | 992 | 86 | FFNSC 4.0 |
| 14 | n-Octanal | 1006 | 1004 | 91 | FFNSC 4.0 |
| 15 | Limonene | 1030 | 1030 | 93 | FFNSC 4.0 |
| 16 | Oct-3-en-2-one | 1036 | 1039 | 90 | FFNSC 4.0 |
| 17 | (E)-Oct-2-enal | 1058 | 1059 | 93 | FFNSC 4.0 |
| 18 | n-Nonanal | 1107 | 1105 | 96 | FFNSC 4.0 |
| 19 | methyl-Octanoate | 1125 | 1124 | 93 | FFNSC 4.0 |
| 20 | Benzenecarboxylic acid | 1213 | 1171 | 97 | FFNSC 4.0 |
| 21 | n-Octanoic acid | 1192 | 1176 | 96 | FFNSC 4.0 |
| 22 | ethyl-Octanoate | 1202 | 1196 | 95 | FFNSC 4.0 |
| 23 | n-Decanal | 1208 | 1207 | 91 | FFNSC 4.0 |
| 24 | methyl-Nonanoate | 1224 | 1224 | 88 | FFNSC 4.0 |
| 25 | (Z)-Dec-2-enal | 1250 | 1250 | 89 | FFNSC 4.0 |
| 26 | (E)-Dec-2-enal | 1265 | 1264 | 97 | FFNSC 4.0 |
| 27 | n-Nonanoic acid | 1289 | 1270 | 94 | FFNSC 4.0 |
| 28 | ethyl-Nonanoate | 1297 | 1295 | 93 | FFNSC 4.0 |
| 29 | Carvacrol | 1300 | 1302 | 92 | FFNSC 4.0 |
| 30 | n-Undecanal | 1309 | 1309 | 91 | FFNSC 4.0 |
| 31 | (E,E)-2,4-Decadienal | 1322 | 1321 | 89 | FFNSC 4.0 |
| 32 | methyl-Decanoate | 1327 | 1324 | 96 | FFNSC 4.0 |
| 33 | n-Decanoic acid | 1398 | 1372 | 97 | FFNSC 4.0 |
| 34 | ethyl-Decanoate | 1399 | 1395 | 97 | FFNSC 4.0 |
| 35 | methyl-Undecanoate | 1423 | 1424 | 95 | FFNSC 4.0 |
| 36 | n-Undecanoic acid | 1473 | 1466 | 95 | FFNSC 4.0 |
| 37 | ethyl-Undecanoate | 1498 | 1494 | 96 | FFNSC 4.0 |
| 38 | ethyl 9-oxononanoate | - | 1505 | - | W11N17 |
| 39 | methyl-Dodecanoate | 1527 | 1524 | 96 | FFNSC 4.0 |
| 40 | isobutyl-Decanoate | 1545 | 1545 | 92 | FFNSC 4.0 |
| 41 | n-Dodecanoic acid | 1581 | 1566 | 96 | FFNSC 4.0 |
| 42 | butyl-Decanoate | 1585 | 1586 | 88 | FFNSC 4.0 |
| 43 | ethyl-Dodecanoate | 1598 | 1594 | 97 | FFNSC 4.0 |
| 44 | n-Tetradecanal | 1614 | 1614 | 91 | FFNSC 4.0 |
| 45 | n-Tridecanoic acid | 1668 | 1663 | 93 | FFNSC 4.0 |
| 46 | Apiole | 1683 | 1679 | 92 | FFNSC 4.0 |
| 47 | Ethyl tridecanoate | - | 1694 | - | W11N17 |
| 48 | Tridecyl methyl ketone | 1697 | 1698 | 92 | FFNSC 4.0 |
| 49 | methyl-Tetradecanoate | 1727 | 1725 | 97 | FFNSC 4.0 |
| 50 | n-Tetradecanoic acid | 1773 | 1765 | 90 | FFNSC 4.0 |
| 51 | ethyl-Tetradecanoate | 1794 | 1794 | 98 | FFNSC 4.0 |
| 52 | Hexadecanal | - | 1818 | - | W11N17 |
| 53 | methyl pentadecanoate | - | 1825 | - | W11N17 |
| 54 | Neophytadiene | 1836 | 1837 | 93 | FFNSC 4.0 |
| 55 | Phytone | 1841 | 1842 | 94 | FFNSC 4.0 |
| 56 | Pentadecylic acid | 1869 | 1862 | 96 | FFNSC 4.0 |
| 57 | ethyl-Pentadecanoate | 1893 | 1893 | 94 | FFNSC 4.0 |
| 58 | methyl (Z)-9-hexadecenoate | - | 1904 | - | W11N17 |
| 59 | methyl (Z)-11-hexadecenoate | - | 1913 | - | W11N17 |
| 60 | methyl-Hexadecanoate | 1925 | 1926 | 96 | FFNSC 4.0 |
| 61 | 9-Hexadecenoic acid | - | 1944 | - | W11N17 |
| 62 | (Z)-11-Hexadecenoic acid | - | 1953 | - | W11N17 |
| 63 | n-Hexadecanoic acid | 1977 | 1971 | 95 | FFNSC 4.0 |
| 64 | Ethyl 9-hexadecenoate | - | 1982 | - | W11N17 |
| 65 | ethyl-Palmitate | 1993 | 1996 | 97 | FFNSC 4.0 |
| 66 | propyl hexadecanoate | - | 2090 | - | W11N17 |
| 67 | ethyl heptadecanoate | - | 2094 | - | W11N17 |
| 68 | methyl-Oleate | 2098 | 2100 | 93 | FFNSC 4.0 |
| 69 | methyl-Octadecanoate | 2127 | 2127 | 93 | FFNSC 4.0 |
| 70 | Linoleic acid | 2144 | 2139 | 95 | FFNSC 4.0 |
| 71 | Oleic acid | 2147 | 2142 | 90 | FFNSC 4.0 |
| 72 | (Z)-Vaccenic acid | - | 2150 | - | W11N17 |
| 73 | ethyl-Linoleate | 2164 | 2161 | 93 | FFNSC 4.0 |
| 74 | ethyl-Oleate | 2166 | 2168 | 87 | FFNSC 4.0 |
| 75 | ethyl-Stearate | 2198 | 2194 | 96 | FFNSC 4.0 |
| 76 | (Z)-9-Octadecenamide | - | 2363 | - | W11N17 |
| 77 | hexyl hexadecanoate | - | 2380 | - | W11N17 |
| 78 | ethyl-Eicosanoate | 2394 | 2395 | 90 | FFNSC 4.0 |
| 79 | n-Tetracosane | 2400 | 2400 | 87 | FFNSC 4.0 |
| 80 | n-Pentacosane | 2500 | 2500 | 90 | FFNSC 4.0 |
| 81 | benzyl hexadecanoate | - | 2581 | - | W11N17 |
| 82 | ethyl-Docosanoate | 2595 | 2595 | 87 | FFNSC 4.0 |
| 83 | n-Hexacosane | 2600 | 2600 | 90 | FFNSC 4.0 |
| 84 | ethyl docosanoate | - | 2581 | - | W11N17 |
| 85 | n-Heptacosane | 2700 | 2700 | 95 | FFNSC 4.0 |
| 86 | ethyl-Tetracosanoate | 2796 | 2796 | 88 | FFNSC 4.0 |
| 87 | n-Octacosane | 2800 | 2800 | 94 | FFNSC 4.0 |
| 88 | Squalene | 2810 | 2814 | 87 | FFNSC 4.0 |
| 89 | n-Nonacosane | 2900 | 2902 | 92 | FFNSC 4.0 |
| 90 | n-Triacontane | 3000 | 3000 | 85 | FFNSC 4.0 |
| 91 | Octacosanal | - | 3045 | - | W11N17 |
| 92 | 10-Nonacosanone | - | 3088 | - | W11N17 |
| 93 | n-Hentriacontane | 3100 | 3100 | 92 | FFNSC 4.0 |
| 94 | Octacosanol | - | 3111 | - | W11N17 |
| 95 | Vitamin E | - | 3131 | - | W11N17 |
| 96 | Triacontanal | - | 3250 | - | W11N17 |
| 97 | γ-Sitosterol | - | 3323 | - | W11N17 |
| Peak | Tentative Identification | tR (min) | Identification Type | λMAX (nm) | m/z | Fragments |
|---|---|---|---|---|---|---|
| Phenolic acid and derivatives | ||||||
| 1 | synapic acid | 10.23 | DAD/MS | 309 | 223 | 193, 161 |
| 2 | p-Hydroxybenzoic acid | 11.80 | DAD/MS | 254 | 137 | - |
| 4 | p-coumaric acid | 21.27 | DAD/MS | 308 | 163 | - |
| 5 | p-Coumaroyl glucose | 22.81 | DAD/MS | 293 | 325 | 163 |
| 6 | benzoic acid | 25.17 | DAD/MS | 273 | 121 | - |
| 9 | cinnamic acid derivative | 37.68 | DAD/MS | 277 | 650 | 616, 147 |
| Flavonol | ||||||
| 7 | Rutin | 27.80 | DAD/MS | 255–353 | 609 | - |
| Not identified | ||||||
| 3 | Unknown | 18.65 | - | 266 | 281 | 265+ |
| 10 | Unknown | 42.58 | - | 294–381 | 698 | - |
| 8 | Unknown | 36.10 | - | 264 | 263 | - |
| Peak | Tentative Identification | tR (min) | Identification Type | λMAX (nm) | m/z | Fragments |
|---|---|---|---|---|---|---|
| Organic acid | ||||||
| 1 | Malic acid derivative | 2.51 | DAD/MS | - | 503 | 191,133 |
| Phenolic acid and derivatives | ||||||
| 3 | Galloyl shikimic acid | 15.3 | DAD/MS | 252–286 | 325 | |
| Flavan-3-ols | ||||||
| 2 | (-)-Catechin 3-O-gallate | 7.35 | DAD/MS | 258 | 441 | - |
| Flavonols | ||||||
| 4 | Quercetin rhamnosyl-rhamnosyl-glucoside | 24.98 | DAD/MS | 253–357 | 755 | 303+ |
| 5 | Quercetin di-glucoside | 25.25 | DAD/MS | 254–357 | 625 | 303+ |
| 7 | Quercetin rhamnoside-glucoside | 28.53 | DAD/MS | 286 | 609 | 303+ |
| 8 | Eriodictyol derivative | 29.80 | DAD/MS | 285 | 597 | 287 |
| Non-identified | ||||||
| 6 | Unknown | 26.51 | DAD/MS | 351 | 613 | - |
| 9 | Unknown | 31.31 | DAD/MS | 255–352 | 141 | - |
| 10 | Unknown | 43.02 | DAD/MS | 277–373 | 698 | - |
| Compound | EtOAc | MeOH-Water | Standard Used |
|---|---|---|---|
| Phenolic acid and derivatives | |||
| p-Hydroxybenzoic acid | 185.7 ± 0.50 | - | Gallic acid |
| benzoic acid | 13.7 ± 0.50 | - | Gallic acid |
| galloyl shikimic acid | - | 2.4 ± 0.02 | Gallic acid |
| Total of Hydroxybenzoic acids | 199.4 ± 0.80 | 2.4 ± 0.02 | |
| sinapic acid | 60.0 ± 0.10 | - | Cinnamic acid |
| p-coumaric acid | 3.7 ± 0.04 | - | Cinnamic acid |
| p-coumaroyl glucose | 6.5 ± 0.01 | - | Cinnamic acid |
| cinnamic acid derivative | 14.5 ± 0.50 | - | Cinnamic acid |
| Total of hydroxycinnamic acid | 84.7 ± 0.50 | - | |
| Flavonols | |||
| Rutin | 14.4 ± 0.01 | - | Rutin |
| Quercetin rhamnosyl-rhamnosyl-glucoside | - | 4.1 ± 0.02 | Rutin |
| Quercetin di-glucoside | - | 2.5 ± 0.05 | Rutin |
| Quercetin rhamnoside-glucoside | - | 25.4 ± 0.03 | Rutin |
| Total flavonols | 14.4 ± 0.01 | 32.0 ± 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cadi, H.E.; Bouzidi, H.E.; Selama, G.; Cadi, A.E.; Ramdan, B.; Oulad El Majdoub, Y.; Alibrando, F.; Dugo, P.; Mondello, L.; Fakih Lanjri, A.; et al. Physico-Chemical and Phytochemical Characterization of Moroccan Wild Jujube “Zizyphus lotus (L.)” Fruit Crude Extract and Fractions. Molecules 2020, 25, 5237. https://doi.org/10.3390/molecules25225237
Cadi HE, Bouzidi HE, Selama G, Cadi AE, Ramdan B, Oulad El Majdoub Y, Alibrando F, Dugo P, Mondello L, Fakih Lanjri A, et al. Physico-Chemical and Phytochemical Characterization of Moroccan Wild Jujube “Zizyphus lotus (L.)” Fruit Crude Extract and Fractions. Molecules. 2020; 25(22):5237. https://doi.org/10.3390/molecules25225237
Chicago/Turabian StyleCadi, Hafssa El, Hajar EL Bouzidi, Ginane Selama, Asmae El Cadi, Btissam Ramdan, Yassine Oulad El Majdoub, Filippo Alibrando, Paola Dugo, Luigi Mondello, Asmae Fakih Lanjri, and et al. 2020. "Physico-Chemical and Phytochemical Characterization of Moroccan Wild Jujube “Zizyphus lotus (L.)” Fruit Crude Extract and Fractions" Molecules 25, no. 22: 5237. https://doi.org/10.3390/molecules25225237
APA StyleCadi, H. E., Bouzidi, H. E., Selama, G., Cadi, A. E., Ramdan, B., Oulad El Majdoub, Y., Alibrando, F., Dugo, P., Mondello, L., Fakih Lanjri, A., Brigui, J., & Cacciola, F. (2020). Physico-Chemical and Phytochemical Characterization of Moroccan Wild Jujube “Zizyphus lotus (L.)” Fruit Crude Extract and Fractions. Molecules, 25(22), 5237. https://doi.org/10.3390/molecules25225237








