Effective Removal of Cyanide and Heavy Metals from an Industrial Electroplating Stream Using Calcium Alginate Hydrogels
Abstract
1. Introduction
2. Results and Discussion
2.1. Composition of the Raw Wastewater
2.2. Optima Bioadsorption of Pollutants
2.3. Effect of Two Successive Bioadsorption Cycles
2.4. Kinetic Studies
2.4.1. Influence of Contact Time on Bioadsorption
2.4.2. Kinetic Models
3. Materials and Methods
3.1. Industrial Wastewater
3.2. Preparation of Bioadsorbents
3.3. Bioadsorption of Pollutants
3.4. Analysis of Pollutants
3.5. Kinetic Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jaszczak, E.; Polkowska, Ż.; Narkowicz, S.; Namieśnik, J. Cyanides in the environment—analysis—problems and challenges. Environ. Sci. Pollut. Res. 2017, 24, 15929–15948. [Google Scholar] [CrossRef]
- USEPA (United States Environmental Protection Agency). Toxicological Review of Hydrogen Cyanide and Cyanide Salts (Final Report); EPA/635/R-08/016F; U.S. Environmental Protection Agency: Washington, DC, USA, 2010.
- Patil, Y.B.; Paknikar, K.M. Development of a process for biodetoxification of metal cyanides from wastewater. Process Biochem. 2000, 35, 1139–1151. [Google Scholar] [CrossRef]
- Botz, M.M. Overview of Cyanide Treatment Methods. Min. Environ. Manag. 2001, 1, 28–30. [Google Scholar]
- Wild, S.R.; Rudd, T.; Neller, A. Fate and effects of cyanide during wastewater treatment processes. Sci. Total Environ. 1994, 156, 93–107. [Google Scholar] [CrossRef]
- USEPA (United States Environmental Protection Agency). Electroplating and Metal Finishing Point Source Categories, Effluent Limitations Guidelines, Pretreatment Standards and New Source Performance Standards; 40 CFR, Part 413; Office of the Federal Register: College Park, MD, USA, 1983; pp. 32462–32488.
- Decree 141/2012 about Public Service of Sanitation and Wastewater Treatment of Galicia, Official Journal of Galicia, 2012, N° 129, 26924–26958. Available online: https://www.xunta.gal/diario-oficial-galicia/mostrarContenido.do?paginaCompleta=false&idEstado=5&rutaRelativa=true&ruta=/2012/20120706/Secciones1_es.html (accessed on 5 November 2020).
- Wedl, D.J.; Fulk, R.J. Cyanide destruction in plating sludges by hot alkaline chlorination. Met. Finish. 1991, 89, 33–37. [Google Scholar]
- Lee, T.Y.; Kwon, Y.S.; Kim, D.S. Oxidative treatment of cyanide in wastewater using hydrogen peroxide and homogeneous catalyst. J. Environ. Sci. Health Part A 2004, 39, 787–801. [Google Scholar] [CrossRef]
- Ghosh, R.S.; Dzombak, D.A.; Luthy, R.G. Equilibrium precipitation and dissolution of iron cyanide solids in water. Environ. Eng. Sci. 1999, 16, 293–313. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Liang, Y.; Zhou, J.; Xu, Y.; Ruan, X.; Lu, Y.; Xu, Z.; Qian, G. Enhanced precipitation of cyanide from electroplating wastewater via self-assembly of bimetal cyanide complex. Sep. Purif. Technol. 2015, 150, 179–185. [Google Scholar] [CrossRef]
- Dwivedi, N.; Balomajumder, C.; Mondal, P. Comparative investigation on the removal of cyanide from aqueous solution using two different bioadsorbents. Water Resour. Ind. 2016, 15, 28–40. [Google Scholar] [CrossRef]
- Dash, R.R.; Gaur, A.; Balomajumder, C. Cyanide in industrial wastewaters and its removal: A review on biotreatment. J. Hazard. Mater. 2009, 163, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Murthy, Z.V.P.; Gupta, S.K. Sodium cyanide separation and parameter estimation for reverse osmosis thin film composite polyamide membrane. J. Membr. Sci. 1999, 154, 9–103. [Google Scholar] [CrossRef]
- Scarazzato, T.; Buzzi, D.C.; Bernardes, A.M.; Espinosa, D.C.R. Treatment of wastewaters from cyanide-free plating process by electrodialysis. J. Clean. Prod. 2015, 91, 241–250. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, W.; Lv, Y.; Li, B.; Ying, W. Enhanced carbon adsorption treatment for removing cyanide from coking plant effluent. J. Hazard. Mater. 2010, 184, 135–140. [Google Scholar] [CrossRef]
- Ramírez-Muñiz, K.; Song, S.; Berber-Mendoza, S.; Tong, S. Adsorption of the complex ion Au(CN)2− onto sulfur-impregnated activated carbon in aqueous solutions. J. Colloid Interface Sci. 2010, 349, 602–606. [Google Scholar] [CrossRef]
- Tyagi, M.; Rana, A.; Kumari, S.; Jagadevan, S. Adsorptive removal of cyanide from coke oven wastewater onto zero-valent iron: Optimization through response surface methodology, isotherm and kinetic studies. J. Clean. Prod. 2018, 178, 398–407. [Google Scholar] [CrossRef]
- Ravuru, S.S.; Jana, A.; De, S. Synthesis of NiAl- layered double hydroxide with nitrate intercalation: Application in cyanide removal from steel industry effluent. J. Hazard. Mater. 2019, 373, 791–800. [Google Scholar] [CrossRef]
- Yazici, E.Y.; Deveci, H.; Alp, I. Treatment of cyanide effluents by oxidation and adsorption in batch and column studies. J. Hazard. Mater. 2009, 166, 1362–1366. [Google Scholar] [CrossRef]
- Moussavi, G.; Khosravi, R. Removal of cyanide from wastewater by adsorption onto pistachio hull wastes: Parametric experiments, kinetics and equilibrium analysis. J. Hazard Mater. 2010, 183, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Asgari, G.; Dayari, A. Experimental dataset on acid treated eggshell for removing cyanide ions from synthetic and industrial wastewaters. Data Brief 2018, 16, 442–452. [Google Scholar] [CrossRef]
- Villaescusa, I.; Fiol, N.; Martínez, M.; Miralles, N.; Poch, J.; Serarols, J. Removal of copper and nickel ions from aqueous solutions by grape stalks wastes. Water Res. 2004, 38, 992–1002. [Google Scholar] [CrossRef]
- Vilardi, G.; Di Palma, L.; Verdone, N. Heavy metals adsorption by banana peels micro-powder: Equilibrium modeling by non-linear models. Chin. J. Chem. Eng. 2018, 26, 455–464. [Google Scholar] [CrossRef]
- Agarwal, R.M.; Singh, K. Heavy metal removal from wastewater using various adsorbents: A review. J. Water Reuse Desal. 2017, 7, 387–410. [Google Scholar]
- Papageorgiou, S.K.; Katsaros, F.K.; Kouvelos, E.P.; Nolan, J.W.; Deit, H.L.; Kanellopoulos, N.K. Heavy metal sorption by calcium alginate beads from Laminaria digitate. J. Hazard. Mater. B 2006, 137, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, G.; Ceyhan, N.; Manav, E. Utilization in alginate beads for Cu(II) and Ni(II) adsorption of an exopolysaccharide produced by Chryseomonas luteola TEM05. World J. Microbiol. Biotechnol. 2005, 21, 163–176. [Google Scholar] [CrossRef]
- Bustos, G.; Calvar, S.; Vecino, X.; Cruz, J.M.; Moldes, A.B. Industrial Symbiosis Between the Winery and Environmental Industry Through the Utilization of Grape Marc for Water, Desalination Containing Copper(II). Water Air Soil Pollut. 2018, 229, 36. [Google Scholar] [CrossRef]
- Vecino, X.; Devesa-Rey, R.; Cruz, J.M.; Moldes, A.B. Entrapped peat in alginate beads as green adsorbent for the elimination of dye compounds from vinasses. Water Air Soil Pollut. 2013, 224, 1448. [Google Scholar] [CrossRef]
- Vecino, X.; Devesa-Rey, R.; Moldes, A.B.; Cruz, J.M. Formulation of an alginate-vineyard pruning waste composite as a new eco-friendly adsorbent to remove micronutrients from agroindustrial effluents. Chemosphere 2014, 111, 24–31. [Google Scholar] [CrossRef]
- Pal, P.; Edathil, A.A.; Banat, F. Calcium alginate gel and hard beads for the removal of total organic acid anions and heavy metal ions from industrial lean methyldiethanolamine solvent. Polym. Bull. 2019, 76, 103–118. [Google Scholar] [CrossRef]
- Dotto, G.L.; Cunha, J.M.; Calgaro, C.O.; Tanabe, E.H.; Bertuol, D.A. Surface modification of chitin using ultrasound-assisted and supercritical CO2 technologies for cobalt adsorption. J. Hazard. Mater. 2015, 295, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Dotto, G.L.; Rodrigues, F.K.; Tanabe, E.H.; Fröhlich, R.; Bertuol, D.A.; Foletto, E.L. Development of chitosan/bentonite hybrid composite to remove hazardous anionic and cationic dyes from colored effluents. J. Environ. Chem. Eng. 2016, 4, 3230–3239. [Google Scholar] [CrossRef]
- Dotto, G.L.; Esquerdo, V.M.; Vieira, M.L.G.; Pinto, L.A.A. Optimization and kinetic analysis of food dyes biosorption by Spirulina platensis. Colloids Surf. B Biointerfaces 2012, 91, 234–241. [Google Scholar] [CrossRef]
- Lee, C.G.; Song, M.K.; Ryu, J.C.; Park, C.; Choi, J.W.; Lee, S.H. Application of carbon foam for heavy metal removal from industrial plating wastewater and toxicity evaluation of the adsorbent. Chemosphere 2016, 153, 1–9. [Google Scholar] [CrossRef]
- Perez-Ameneiro, M.; Vecino, X.; Cruz, J.M.; Moldes, A.B. Physicochemical study of a bio-based adsorbent made from grape marc. Ecol. Eng. 2015, 84, 190–193. [Google Scholar] [CrossRef]
- Vecino, X.; Devesa-Rey, R.; Cruz, J.M.; Moldes, A.B. Study of the physical properties of calcium alginate hydrogel beads containing vineyard pruning waste for dye removal. Carbohydr. Polym. 2015, 115, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Nastaj, J.; Przewłocka, A.; Rajkowska-yśliwiec, M. Biosorption of Ni(II), Pb(II) and Zn(II) on calcium alginate beads: Equilibrium, kinetic and mechanism studies. Pol. J. Chem. Tech. 2016, 18, 81–87. [Google Scholar] [CrossRef]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. K. Sven. Vetensk. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Chien, S.H.; Clayton, W.R. Application of Elovich equation to the kinetics of phosphate release and sorption in soils. Soil Sci. Soc. Am. J. 1980, 44, 265–268. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Advances in water pollution research: Removal of biologically resistant pollutants from waste waters by adsorption. Proc. Int. Conf. Water Pollut. Symp. 1962, 2, 231–266. [Google Scholar]
- Moldes, A.B.; Vázquez, M.; Domínguez, J.M.; Díaz-Fierros, F.; Barral, M.T. Evaluation of mesophilic biodegraded grape marc as soil fertilizer. Appl. Biochem. Biotechnol. 2007, 141, 27–36. [Google Scholar] [CrossRef]
- APHA-AWWA-WEF. Method 3500-Cr B. In Standard Methods for the Examination of Water and Wastewater, 23th ed.; Rice, E.W., Baird, R.B., Eaton, A.D., Eds.; APHA-AWWA-WEF: Washington, DC, USA, 2017; pp. 3–71. [Google Scholar]
- APHA-AWWA-WEF. Method 4500-CN− D. In Standard Methods for the Examination of Water and Wastewater, 23th ed.; Rice, E.W., Baird, R.B., Eaton, A.D., Eds.; APHA-AWWA-WEF: Washington, DC, USA, 2017; pp. 3–45. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |
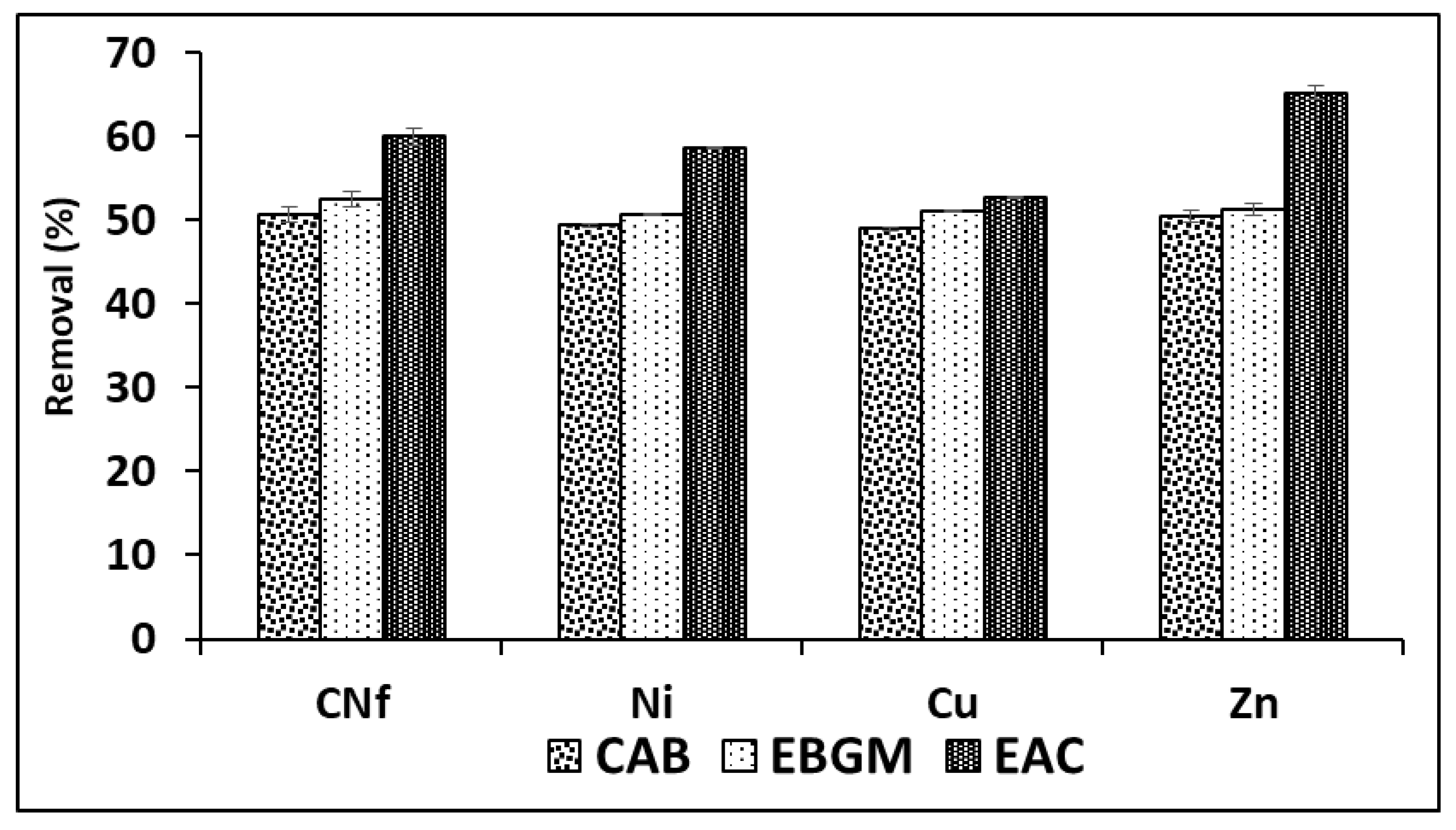
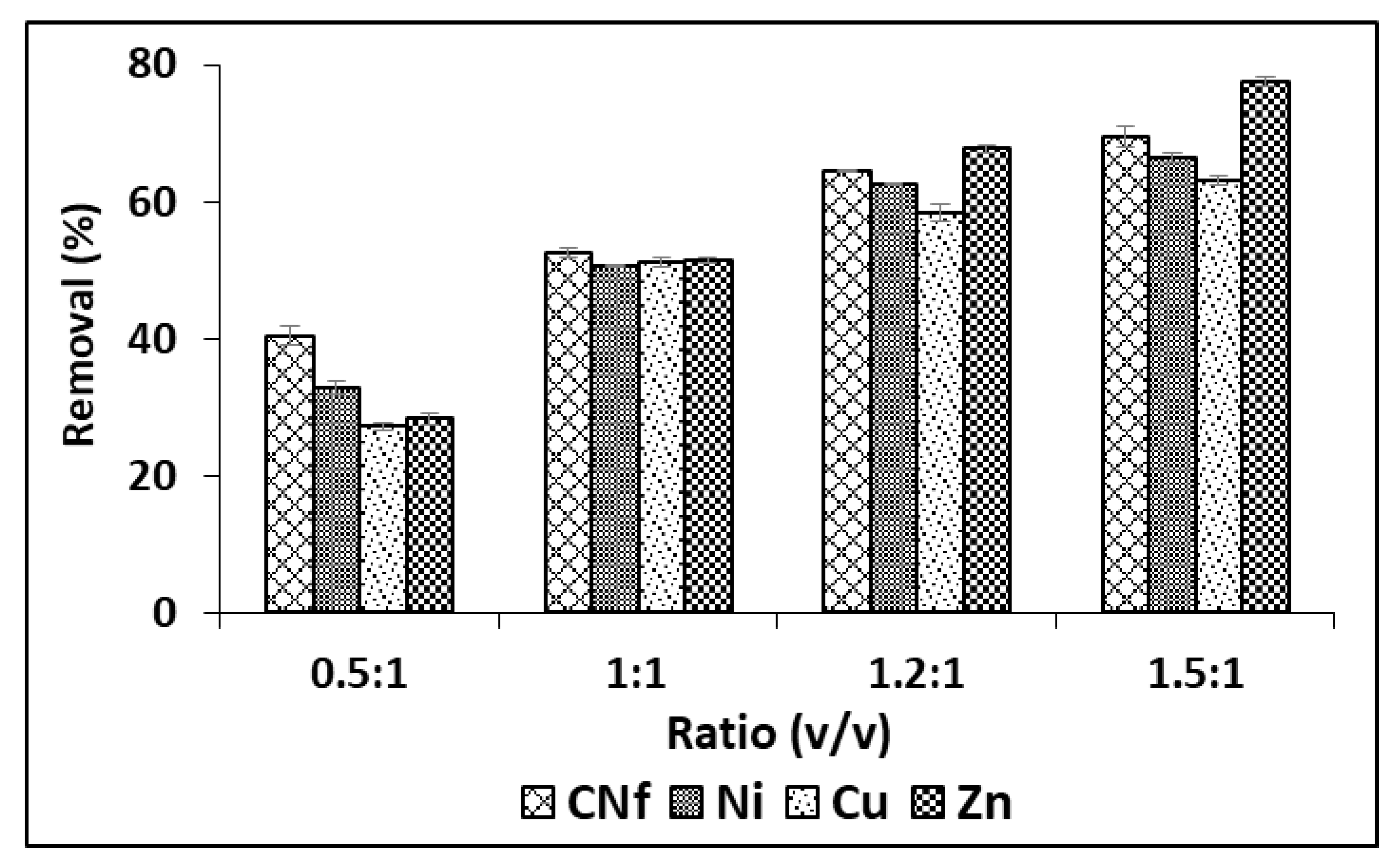
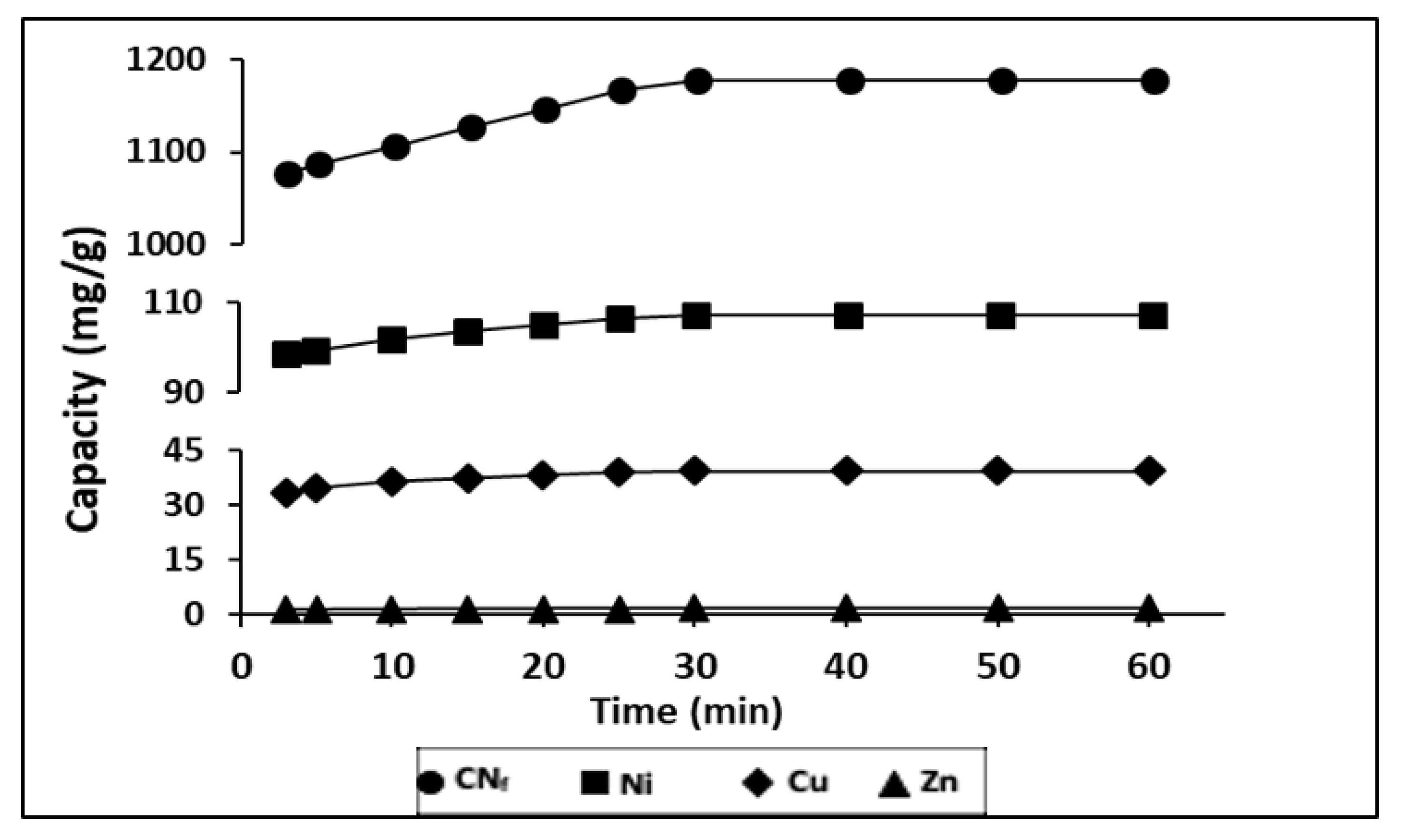

| Pollutant | Raw Wastewater (mg/L) | Maximum Values in Discharges (mg/L) b | Maximum Values in Discharges (mg/L) c |
|---|---|---|---|
| CNf | 51,190 ± 415 | 0.50 | - |
| CNTotal | - | 1.0 | 1.0 |
| Ni | 4899 ± 13 | 2.00 | 2.6 |
| Cu | 1904 ± 6 | 3.00 | 2.7 |
| Zn | 58.8 ± 0.5 | 2.00 | 2.6 |
| Pb | N.D. a | 1.00 | 0.4 |
| Cr (VI) | N.D. a | 0.5 | - |
| Cr (Total) | N.D. a | 2.00 | 4.00 |
| pH | 12.45 ± 0.1 | 5.5–9 | 7.5–10 |
| ANOVA | t-Test | ||
|---|---|---|---|
| F Value a | p Value | t Value b | |
| CNf | 86.135 | <0.001 | 2.585 |
| Ni | 4412.065 | <0.001 | 10.11 |
| Cu | 416.796 | <0.001 | 15.05 |
| Zn | 416.796 | <0.001 | 1.532 |
| First Treatment Cycle (Ratio 1.5:1) | |||
| Raw Water (mg/L) | Water after 1st Cycle (mg/L) | Removal (%) | |
| CNf | 51,190 ± 415 | 15,659 ± 105 | 69.41 ± 0.21 |
| Ni | 4899 ± 13 | 1641 ± 5 | 66.50 ± 0.14 |
| Cu | 1904 ± 6 | 702 ± 2 | 63.13 ± 0.15 |
| Zn | 58.8 ± 0.5 | 13.2 ± 0.1 | 77.55 ± 0.24 |
| Second Treatment Cycle Ratio 0.5:1 (v/v) | |||
| Removal (%) | |||
| Water after 2nd Cycle (mg/L) | Regarding Pre-Treated Water a | Regarding Raw Water | |
| CNf | 10,913 ± 79 | 30.31 ± 0.51 b | 78.68 ± 0.15 c |
| Ni | 105.3 ± 0.9 | 93.59 ± 0.12 b | 97.85 ± 0.04 c |
| Cu | 52.4 ± 0.5 | 92.53 ± 0.21 b | 97.25 ± 0.08 c |
| Zn | 6.01 ± 0.07 | 54.44 ± 0.64 b | 89.81 ± 0.14 c |
| Second Treatment Cycle Ratio 1:1 (v/v) | |||
| Removal (%) | |||
| Water after 2nd Cycle (mg/L) | Regarding Pre-Treated Water a | Regarding Raw Water | |
| CNf | 7667 ± 79 | 51.04± 0.50 b | 85.02 ± 0.15 c |
| Ni | 87.5 ± 0.5 | 94.67 ± 0.04 b | 98.21 ± 0.01 c |
| Cu | 38.7 ± 0.4 | 94.49 ± 0.05 b | 97.97 ± 0.02 c |
| Zn | 3.90 ± 0.02 | 70.46 ± 0.16 b | 93.40 ± 0.04 c |
| Pseudo-First-Order | ||||
| k1 (1/min) | qe Exp (mg/g) | qe Calc (mg/g) | r2 | |
| CNf | 0.0960 | 1177 | 160.1 | 0.9104 |
| Ni | 0.1018 | 107.3 | 13.83 | 0.9353 |
| Cu | 0.1326 | 39.5 | 10.44 | 0.9264 |
| Zn | 0.1108 | 1.52 | 0.415 | 0.9615 |
| Pseudo-Second-Order | ||||
| CNf | 0.00128 | 1177 | 1250 | 0.9999 |
| Ni | 0.01881 | 107.3 | 108.7 | 0.9999 |
| Cu | 0.03280 | 39.5 | 40.16 | 0.9999 |
| Zn | 0.65082 | 1.52 | 1.55 | 0.9998 |
| Chien-Clayton | ||||
| α (min mg/g) | β (g/mg) | r2 | α (min mg/g) | |
| CNf | 5.43 × 1011 | 0.023 | 0.9421 | 5.43 × 1011 |
| Ni | 1.58 × 1011 | 0.261 | 0.9632 | 1.58 × 1011 |
| Cu | 2.66 × 105 | 0.377 | 0.9969 | 2.66 × 105 |
| Zn | 1247.3 | 8.382 | 0.9961 | 1247.3 |
| Intraparticle Diffusion | ||||
| kp (mg/g min0.5) | C (mg/g) | r2 | kp (mg/g min0.5) | |
| CNf | 27.487 | 1024.9 | 0.9903 | 27.487 |
| Ni | 2.385 | 94.35 | 0.9958 | 2.385 |
| Cu | 1.609 | 31.11 | 0.9832 | 1.609 |
| Zn | 0.0727 | 1.130 | 0.9870 | 0.0727 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Cid, B.; Calvar, S.; Moldes, A.B.; Manuel Cruz, J. Effective Removal of Cyanide and Heavy Metals from an Industrial Electroplating Stream Using Calcium Alginate Hydrogels. Molecules 2020, 25, 5183. https://doi.org/10.3390/molecules25215183
Pérez-Cid B, Calvar S, Moldes AB, Manuel Cruz J. Effective Removal of Cyanide and Heavy Metals from an Industrial Electroplating Stream Using Calcium Alginate Hydrogels. Molecules. 2020; 25(21):5183. https://doi.org/10.3390/molecules25215183
Chicago/Turabian StylePérez-Cid, Benita, Sergio Calvar, Ana Belén Moldes, and Jose Manuel Cruz. 2020. "Effective Removal of Cyanide and Heavy Metals from an Industrial Electroplating Stream Using Calcium Alginate Hydrogels" Molecules 25, no. 21: 5183. https://doi.org/10.3390/molecules25215183
APA StylePérez-Cid, B., Calvar, S., Moldes, A. B., & Manuel Cruz, J. (2020). Effective Removal of Cyanide and Heavy Metals from an Industrial Electroplating Stream Using Calcium Alginate Hydrogels. Molecules, 25(21), 5183. https://doi.org/10.3390/molecules25215183






