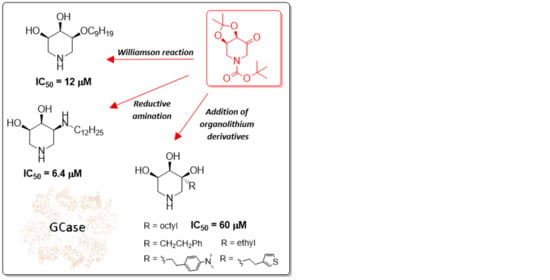
Synthesis of “All-Cis” Trihydroxypiperidines from a Carbohydrate-Derived Ketone: Hints for the Design of New β-Gal and GCase Inhibitors
Abstract
1. Introduction
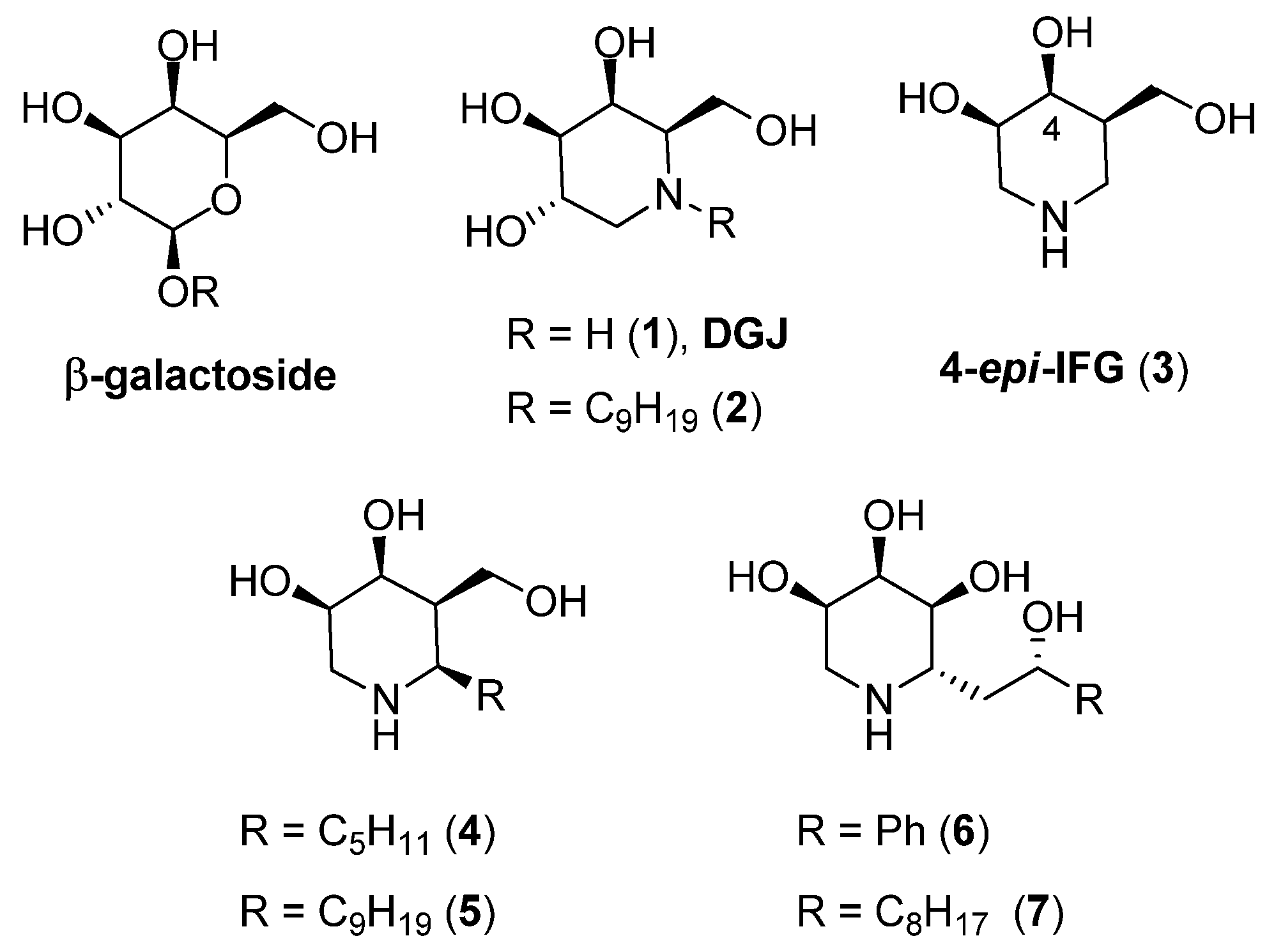
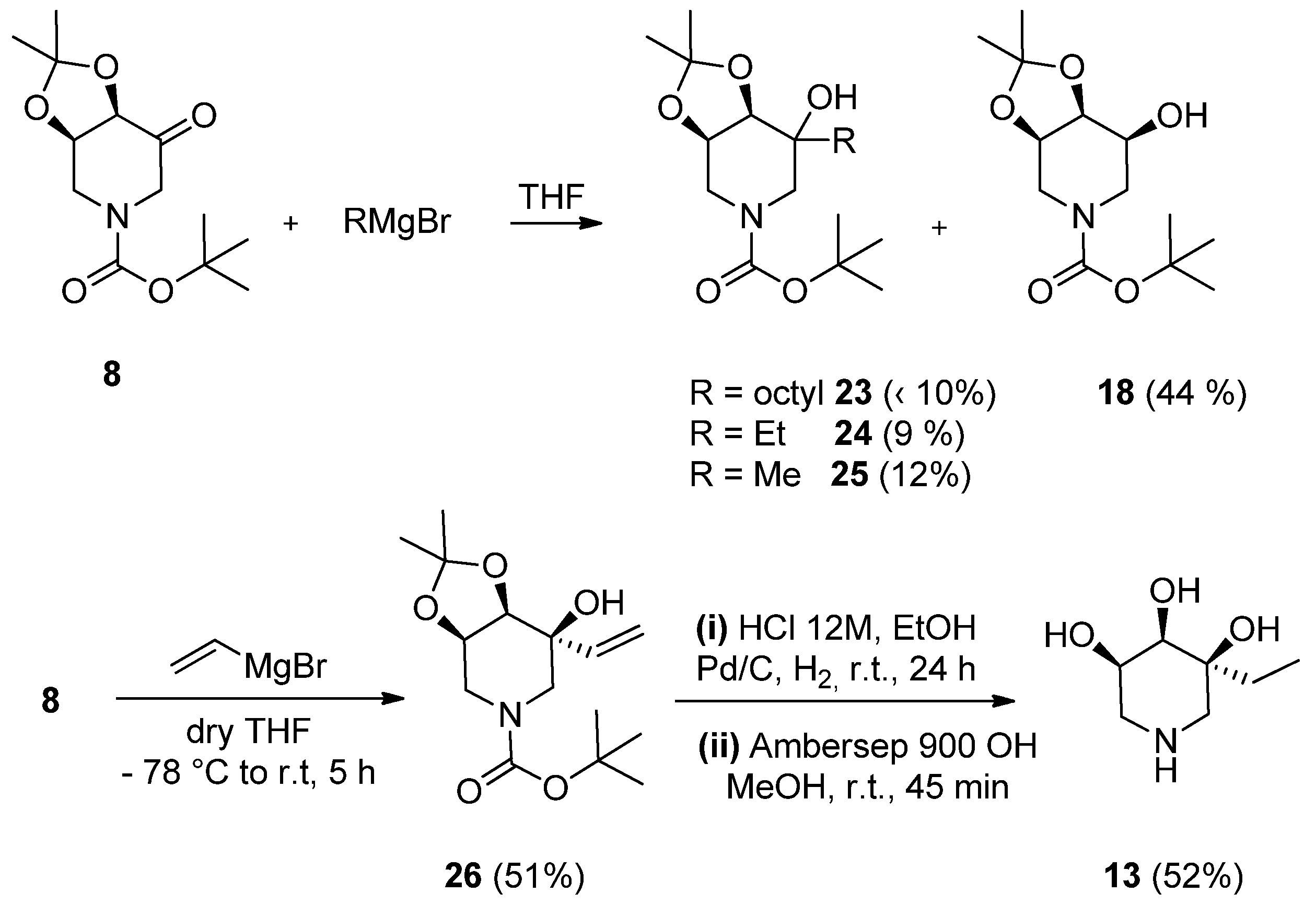
2. Results and Discussion
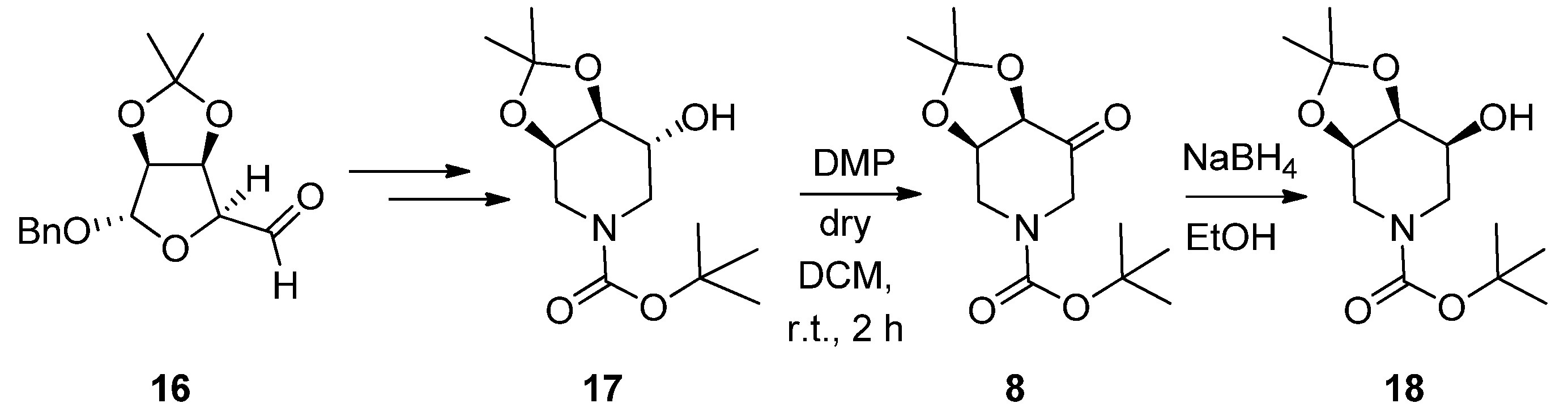
2.1. Synthesis
2.2. Configuration Assignment
2.3. Biological Screening
3. Materials and Methods
3.1. General Experimental Procedures for the Syntheses
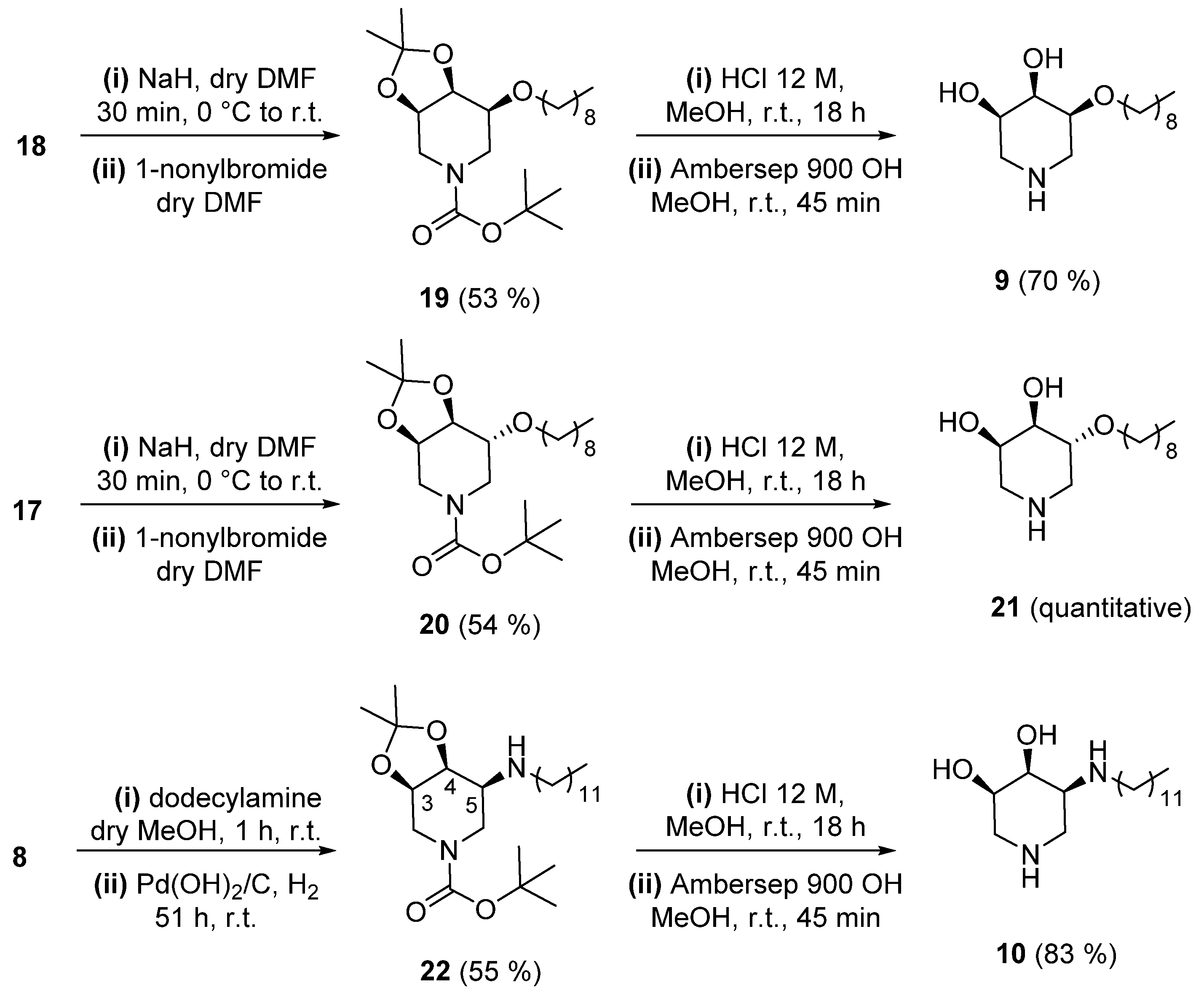
3.1.1. Synthesis of (3R, 4S, 5S)-3, 4-O-(1-Methylethylidene)-5-Nonyloxy-N-Boc-Piperidine (19)
3.1.2. Synthesis of (3S, 4R, 5R)-4, 5-Dihydroxy-3-(Nonyloxy) Piperidine (9)
3.1.3. Synthesis of (3R, 4S, 5R)-3, 4-O-(1-Methylethylidene)-5-Nonyloxy-N-Boc-Piperidine (20)
3.1.4. Synthesis of (3R, 4R, 5R)-4, 5-Dihydroxy-3-(Nonyloxy) Piperidine (21)
3.1.5. Synthesis of (3R, 4S, 5S)-5-Dodecylamino-3, 4-O-(1-Methylethylidene)-N-Boc-Piperidine (22)
3.1.6. Synthesis of (3R, 4S, 5S)-3, 4-Dihydroxy-5-(Dodecylamino) Piperidine (10)
3.1.7. Synthesis of (3S, 4R, 5R)-3-Hydroxy-4, 5-O-(1-Methylethylidene)-3-Vinyl-N-Boc-Piperidine (26)
3.1.8. Synthesis of (3S, 4R, 5R)-3-Ethyl-3, 4, 5-Trihydroxypiperidine (13)
3.1.9. General Procedure for the Addition of Lithium Acetylides to Ketone 8
3.1.10. Synthesis of (3S, 4R, 5R)-3-Hydroxy-4, 5-O-(1-Methylethylidene)-3-(Phenylethynyl)-N-Boc-Piperidine (27)
3.1.11. Synthesis of (3S, 4R, 5R)-3-Hydroxy-4, 5-O-(1-Methylethylidene)-3-(oct-1-yn-1-yl)-N-Boc-Piperidine (28)
3.1.12. Synthesis of (3S, 4R, 5R)-3-Hydroxy-4, 5-O-(1-Methylethylidene)-3-(3, 3-Diethoxyprop-1-yn-1-yl)-N-Boc-Piperidine (29)
3.1.13. Synthesis of (3S, 4R, 5R)-3-Hydroxy-4, 5-O-(1-Methylethylidene)-3-(3-Thienylethynyl)-N-Boc-Piperidine (30)
3.1.14. Synthesis of (3S, 4R, 5R)-3-Hydroxy-4, 5-O-(1-Methylethylidene)-3-((4-(Dimethylamino) Phenyl) Ethynyl)-N-Boc-Piperidine (31)
3.1.15. General Procedure for the Synthesis of Trihydroxypiperidines 32, 33, and 34
3.1.16. Synthesis of (3S, 4R, 5R)-3, 4, 5-Trihydroxy-3-(Phenylethynyl)-Piperidine (32)
3.1.17. Synthesis of (3S, 4R, 5R)-3, 4, 5-Trihydroxy-3-(oct-1-yn-1-yl)-Piperidine (33)
3.1.18. Synthesis of (3S, 4R, 5R)-3, 4, 5-Trihydroxy-3-(3-Thienylethynyl))-Piperidine (34)
3.1.19. General Procedure for the Reduction of Triple Bond to Piperidines 11, 12, 14
3.1.20. Synthesis of (3S, 4R, 5R)-3, 4, 5-Trihydroxy-3-(2-Phenylethyl)-Piperidine (11)
3.1.21. Synthesis of (3S, 4R, 5R)-3, 4, 5-Trihydroxy-3-Octyl-Piperidine (12)
3.1.22. Synthesis of (3S, 4R, 5R)-3, 4, 5-Trihydroxy-3-((2-(3-Thienyl) Ethyl))-Piperidine (14)
3.1.23. Synthesis of (3S, 4R, 5R)-3-hydroxy-4, 5-O-(1-methylethylidene)-3-(2-((4-(dimethylamino) phenyl) ethyl)-N-Boc-piperidine (35)
3.1.24. Synthesis of (3S, 4R, 5R)-3, 4, 5-Trihydroxy-3-(2-((4-(Dimethylamino) Phenyl) Ethyl)-Piperidine (15)
3.2. Preliminary Biological Screening towards Commercial Glycosidases
3.3. Biological Screening towards Human Lysosomal β-Galactosidase (β-Gal) and β-Glucosidase (GCase)
3.3.1. Human Lysosomal β-Galactosidase (β-Gal) Activity
3.3.2. Human Lysosomal β-Glucosidase (GCase) Activity
3.4. Pharmacological Chaperoning Activity of Compound 10
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marques, A.R.A.; Saftig, P. Lysosomal storage disorders—challenges, concepts and avenues for therapy: Beyond rare diseases. J. Cell Sci. 2019, 132, jcs221739. [Google Scholar] [CrossRef] [PubMed]
- Platt, F.M.; D’Azzo, A.; Davidson, B.L.; Neufeld, E.F.; Tifft, C.J. Lysosomal storage diseases. Nat. Rev. Dis. Prim. 2018, 4, 27. [Google Scholar] [CrossRef] [PubMed]
- Parenti, G.; Andria, G.; Ballabio, A. Lysosomal Storage Diseases: From Pathophysiology to Therapy. Annu. Rev. Med. 2015, 66, 471–486. [Google Scholar] [CrossRef] [PubMed]
- Ortolano, S.; Viéitez, I.; Navarro, C.; Spuch, C. Treatment of lysosomal storage diseases: Recent patents and future strategies. Recent Patents Endocr. Metab. Immune Drug Discov. 2014, 8, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Caciotti, A.; Garman, S.C.; Rivera-Colón, Y.; Procopio, E.; Catarzi, S.; Ferri, L.; Guido, C.; Martelli, P.; Parini, R.; Antuzzi, D.; et al. GM1 gangliosidosis and Morquio B disease: An update on genetic alterations and clinical findings. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2011, 1812, 782–790. [Google Scholar] [CrossRef] [PubMed]
- Brunetti-Pierri, N.; Scaglia, F.F. GM1 gangliosidosis: Review of clinical, molecular, and therapeutic aspects. Mol. Genet. Metab. 2008, 94, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.M.; Valentão, P.; Andrade, P.B. Tuning protein folding in lysosomal storage diseases: The chemistry behind pharmacological chaperones. Chem. Sci. 2018, 9, 1740–1752. [Google Scholar] [CrossRef]
- Convertino, M.; Das, J.; Dokholyan, N.V. Pharmacological Chaperones: Design and Development of New Therapeutic Strategies for the Treatment of Conformational Diseases. ACS Chem. Boil. 2016, 11, 1471–1489. [Google Scholar] [CrossRef]
- Parenti, G.; Andria, G.; Valenzano, K.J. Pharmacological Chaperone Therapy: Preclinical Development, Clinical Translation, and Prospects for the Treatment of Lysosomal Storage Disorders. Mol. Ther. 2015, 23, 1138–1148. [Google Scholar] [CrossRef]
- Boyd, R.E.; Lee, G.; Rybczynski, P.; Benjamin, E.R.; Khanna, R.; Wustman, B.A.; Valenzano, K.J. Pharmacological Chaperones as Therapeutics for Lysosomal Storage Diseases. J. Med. Chem. 2013, 56, 2705–2725. [Google Scholar] [CrossRef]
- Aguilar-Moncayo, M.; Takai, T.; Higaki, K.; Mena-Barragán, T.; Hirano, Y.; Yura, K.; Li, L.; Yu, Y.; Ninomiya, H.; Garcıa-Moreno, M.; et al. Tuning glycosidase inhibition through aglycone interactions: Pharmacological chaperones for Fabry disease and GM1 gangliosidosis. Chem. Commun. 2012, 48, 6514–6516. [Google Scholar] [CrossRef] [PubMed]
- Compain, P.; Martin, O.R. (Eds.) Iminosugars: From Synthesis to Therapeutic Applications; John Wiley & Sons Ltd.: Chichester, UK, 2007; ISBN 978-0-470-03391-3. [Google Scholar] [CrossRef]
- Sánchez-Fernández, E.M.; Fernández, J.M.G.; Mellet, C.O. Glycomimetic-based pharmacological chaperones for lysosomal storage disorders: Lessons from Gaucher, GM1-gangliosidosis and Fabry diseases. Chem. Commun. 2016, 52, 5497–5515. [Google Scholar] [CrossRef] [PubMed]
- McCafferty, E.H.; Scott, L.J. Migalastat: A Review in Fabry Disease. Drugs 2019, 79, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, J.; Suzuki, O.; Oshima, A.; Yamamoto, Y.; Noguchi, A.; Takimoto, K.; Itoh, M.; Matsuzaki, Y.; Yasuda, Y.; Ogawa, S.; et al. Chemical chaperone therapy for brain pathology in GM1-gangliosidosis. Proc. Natl. Acad. Sci. USA 2003, 100, 15912–15917. [Google Scholar] [CrossRef] [PubMed]
- Rigat, B.A.; Tropak, M.B.; Buttner, J.; Crushell, E.; Benedict, D.; Callahan, J.W.; Martin, D.R.; Mahuran, D.J. Evaluation of N-nonyl-deoxygalactonojirimycin as a pharmacological chaperone for human GM1 gangliosidosis leads to identification of a feline model suitable for testing enzyme enhancement therapy. Mol. Genet. Metab. 2012, 107, 203–212. [Google Scholar] [CrossRef][Green Version]
- Prichard, K.; Campkin, D.; O’Brien, N.; Kato, A.; Fleet, G.W.J.; Simone, M.I. Biological activities of 3,4,5-trihydroxypiperidines and their N- and O- derivatives. Chem. Boil. Drug Des. 2018, 92, 1171–1197. [Google Scholar] [CrossRef] [PubMed]
- Biela-Banaś, A.; Oulaïdi, F.; Front, S.; Gallienne, E.; Ikeda-Obatake, K.; Asano, N.; Wenger, D.A.; Martin, O.R. Iminosugar-Based Galactoside Mimics as Inhibitors of Galactocerebrosidase: SAR Studies and Comparison with Other Lysosomal Galactosidases. ChemMedChem 2014, 9, 2647–2652. [Google Scholar] [CrossRef]
- Front, S.; Biela-Banaś, A.; Burda, P.; Ballhausen, D.; Higaki, K.; Caciotti, A.; Morrone, A.; Charollais-Thoenig, J.; Gallienne, E.; Demotz, S.; et al. (5aR)-5a-C-Pentyl-4-epi-isofagomine: A powerful inhibitor of lysosomal β-galactosidase and a remarkable chaperone for mutations associated with GM1-gangliosidosis and Morquio disease type B. Eur. J. Med. Chem. 2017, 126, 160–170. [Google Scholar] [CrossRef]
- Front, S.; Almeida, S.; Zoete, V.; Charollais-Thoenig, J.; Gallienne, E.; Marmy, C.; Pilloud, V.; Marti, R.; Wood, T.; Martin, O.R.; et al. 4-epi-Isofagomine derivatives as pharmacological chaperones for the treatment of lysosomal diseases linked to β-galactosidase mutations: Improved synthesis and biological investigations. Bioorg. Med. Chem. 2018, 26, 5462–5469. [Google Scholar] [CrossRef]
- Siriwardena, A.; Sonawane, D.P.; Bande, O.P.; Markad, P.R.; Yonekawa, S.; Tropak, M.B.; Ghosh, S.; Chopade, B.A.; Mahuran, N.J.; Dhavale, D.D. Synthesis of 1,5-Dideoxy-1,5-iminoribitol C-Glycosides through a Nitrone–Olefin Cycloaddition Domino Strategy: Identification of Pharmacological Chaperones of Mutant Human Lysosomal β-Galactosidase. J. Org. Chem. 2014, 79, 4398–4404. [Google Scholar] [CrossRef]
- Parmeggiani, C.; Catarzi, S.; Matassini, C.; D’Adamio, G.; Morrone, A.; Goti, A.; Paoli, P.; Cardona, F. Human Acid β-Glucosidase Inhibition by Carbohydrate Derived Iminosugars: Towards New Pharmacological Chaperones for Gaucher Disease. ChemBioChem 2015, 16, 2054–2064. [Google Scholar] [CrossRef] [PubMed]
- Clemente, F.; Matassini, C.; Goti, A.; Morrone, A.; Paoli, P.; Cardona, F. Stereoselective Synthesis of C-2 Alkylated Trihydroxypiperidines: Novel Pharmacological Chaperones for Gaucher Disease. ACS Med. Chem. Lett. 2019, 10, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Clemente, F.; Matassini, C.; Faggi, C.; Giachetti, S.; Cresti, C.; Morrone, A.; Paoli, P.; Goti, A.; Martínez-Bailén, M.; Cardona, F. Glucocerebrosidase (GCase) activity modulation by 2-alkyl trihydroxypiperidines: Inhibition and pharmacological chaperoning. Bioorg. Chem. 2020, 98, 103740. [Google Scholar] [CrossRef] [PubMed]
- Matassini, C.; Mirabella, S.; Goti, A.; Cardona, F. Double Reductive Amination and Selective Strecker Reaction of a D-Lyxaric Aldehyde: Synthesis of Diversely Functionalized 3,4,5-Trihydroxypiperidines. Eur. J. Org. Chem. 2012, 3920–3924. [Google Scholar] [CrossRef]
- Matassini, C.; Clemente, F.; Cardona, F. The double reductive amination approach to the synthesis of polyhydroxypiperidines. In Targets in Heterocyclic Systems—Chemistry and Properties (THS); Attanasi, O.A., Merino, P., Spinelli, D., Eds.; Societa’ Chimica Italiana: Rome, Italy, 2019; Volume 23, pp. 283–301. [Google Scholar] [CrossRef]
- Clemente, F.; Matassini, C.; Cardona, F. Reductive Amination Routes in the Synthesis of Piperidine IminoSugars. Eur. J. Org. Chem. 2020, 4447–4462. [Google Scholar] [CrossRef]
- Santos, C.; Stauffert, F.; Ballereau, S.; Dehoux, C.; Rodriguez, F.; Bodlenner, A.; Compain, P.; Génisson, Y. Iminosugar-based ceramide mimicry for the design of new CERT START domain ligands. Bioorganic Med. Chem. 2017, 25, 1984–1989. [Google Scholar] [CrossRef]
- Matassini, C.; Mirabella, S.; Ferhati, X.; Faggi, C.; Robina, I.; Goti, A.; Clavijo, E.M.; Moreno-Vargas, A.J.; Cardona, F. Polyhydroxyamino-Piperidine-Type Iminosugars and Pipecolic Acid Analogues from aD-Mannose-Derived Aldehyde. Eur. J. Org. Chem. 2014, 5419–5432. [Google Scholar] [CrossRef]
- Mirabella, S.; Fibbi, G.; Matassini, C.; Faggi, C.; Goti, A.; Cardona, F. Accessing 2-substituted piperidine iminosugars by organometallic addition/intramolecular reductive amination: Aldehyde vs. nitrone route. Org. Biomol. Chem. 2017, 15, 9121–9126. [Google Scholar] [CrossRef]
- Kankanamalage, A.C.G.; Kim, Y.; Damalanka, V.C.; Rathnayake, A.D.; Fehr, A.R.; Mehzabeen, N.; Battaile, K.P.; Lovell, S.; Lushington, G.H.; Perlman, S.; et al. Structure-guided design of potent and permeable inhibitors of MERS coronavirus 3CL protease that utilize a piperidine moiety as a novel design element. Eur. J. Med. Chem. 2018, 150, 334–346. [Google Scholar] [CrossRef]
- Cowan, D.O.; Mosher, H.S. Comparison of the Reactions of Grignard Reagents and Dialkylmagnesium Compounds in Addition, Reduction, and Enolization Reactions1. J. Org. Chem. 1962, 27, 1–5. [Google Scholar] [CrossRef]
- Sassian, M.; Tuulmets, A. Solvation Effects in theGrignard Reaction with Carbonyl Compounds. Helv. Chim. Acta 2003, 86, 82–90. [Google Scholar] [CrossRef]
- Engel, D.A.; Dudley, G.B. Olefination of Ketones Using a Gold(III)-Catalyzed Meyer-Schuster Rearrangement. Org. Lett. 2006, 8, 4027–4029. [Google Scholar] [CrossRef] [PubMed]
- Lamb, N.; Abrams, S.R. Synthesis of optically active cyclohexanone analogs of the plant hormone abscisic acid. Can. J. Chem. 1990, 68, 1151–1162. [Google Scholar] [CrossRef]
- Rocquet, F.; Battioni, J.-P.; Capmau, M.-L.; Chodkiewicz, W. Stereochemistry of organometallic compound addition to cyclohexanones monomethylated in the 2, 3, and 4 positions. C. R. Acad. Sci. Ser. C 1969, 268, 1449–1452. [Google Scholar]
- Ando, K.; Houk, K.N.; Busch, J.; Menassé, A.A.; Séquin, U. Experimental and Computational Studies of Nucleophilic Additions of Metal Hydrides and Organometallics to Hindered Cyclohexanones. J. Org. Chem. 1998, 63, 1761–1766. [Google Scholar] [CrossRef]
- Chérest, M.; Felkin, H.; Prudent, N. Torsional strain involving partial bonds. The stereochemistry of the lithium aluminium hydride reduction of some simple open-chain ketones. Tetrahedron Lett. 1968, 9, 2199–2204. [Google Scholar] [CrossRef]
- Chérest, M.; Felkin, H. Torsional strain involving partial bonds. The steric course of the reaction between allyl magnesium bromide and 4-t-butyl-cyclohexanone. Tetrahedron Lett. 1968, 9, 2205–2208. [Google Scholar] [CrossRef]
- Anh, N.T.; Eisenstein, O. Theoretical interpretation of 1-2 asymmetric induction: The importance of antiperiplanarity. Nouv. J. Chim. 1977, 1, 61–70. [Google Scholar]
- Battioni, J.-P.; Chodkiewicz, W. cheminform abstract: Reaction of organometallics with 2-methoxycyclohexanone and cis- and trans-2-methoxy-4-tert-butylcyclohexanones. Chem. Inf. 1978, 9, 320–328. [Google Scholar] [CrossRef]
- Grabowski, G.A.; Gatt, S.; Horowitz, M. Acid β-Glucosidase: Enzymology and molecular biology of Gaucher disease. Crit. Rev. Biochem. Mol. Biol. 1990, 25, 385–414. [Google Scholar] [CrossRef]
- Advances in Gaucher Disease: Basic and Clinical Perspectives Future Medicine; Future Medicine Ltd.: London, UK, 2013; ISBN 978-1-78084-201-1. [CrossRef]
- Kato, A.; Nakagome, I.; Sato, K.; Yamamoto, A.; Adachi, I.; Nash, R.J.; Fleet, G.W.J.; Natori, Y.; Watanabe, Y.; Imahori, T.; et al. Docking study and biological evaluation of pyrrolidine-based iminosugars as pharmacological chaperones for Gaucher disease. Org. Biomol. Chem. 2016, 14, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Compain, P.; Martin, O.R.; Boucheron, C.; Godin, G.; Yu, L.; Ikeda, K.; Asano, N. Design and Synthesis of Highly Potent and Selective Pharmacological Chaperones for the Treatment of Gaucher’s disease. ChemBioChem 2006, 7, 1356–1359. [Google Scholar] [CrossRef] [PubMed]
- Oulaïdi, F.; Front-Deschamps, S.; Gallienne, E.; Lesellier, E.; Ikeda, K.; Asano, N.; Compain, P.; Martin, O.R. Second-Generation Iminoxylitol-Based Pharmacological Chaperones for the Treatment of Gaucher Disease. ChemMedChem 2011, 6, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Mellor, H.R.; Nolan, J.; Pickering, L.; Wormald, M.R.; Platt, F.M.; Dwek, R.A.; Fleet, G.W.; Butters, T.D. Preparation, biochemical characterization and biological properties of radiolabelled N-alkylated deoxynojirimycins. Biochem. J. 2002, 366, 225–233. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mellor, H.R.; Platt, F.M.; Dwek, R.A.; Butters, T.D. Membrane disruption and cytotoxicity of hydrophobic N-alkylated imino sugars is independent of the inhibition of protein and lipid glycosylation. Biochem. J. 2003, 374, 307–314. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |




| Entry a | Alkyne | Time (h) | Product | Yield (%) |
|---|---|---|---|---|
| 1 | Phenylacetylene | 2.5 | 27 | 77 |
| 2 | 1-octyne | 3 | 28 | 78 |
| 3 | 3,3-diethoxyprop-1-yne | 3 | 29 | 65 |
| 4 | 3-ethynylthiophene | 4 | 30 | 88 |
| 5 | 4-ethynyl-N,N-dimethylaniline | 4 | 31 | 83 |
 | H-4 | H-5 | H-6a | H-6b |
|---|---|---|---|---|
| δ (ppm) | δ (ppm) | δ (ppm) | δ (ppm) | |
| R = vinyl, 26 | 4.07 (d, J = 6.8 Hz) | 4.33 (br s) | 3.95–3.69 (m) | 3.53–3.32 (m) |
R =  , 35 , 35 | 3.98 (d, J = 6.4 Hz) | 4.30 (br s) | 3.68–3.55 (m) | 3.43–3.10 (m) |
 | H-4 | H-5 | H-6a | H-6b |
|---|---|---|---|---|
| δ (ppm) | δ (ppm) | δ (ppm) | δ (ppm) | |
R =  , 32 , 32 | 3.89 (br s) | 3.98–3.91 (m) | 2.86–2.76 (m) | |
R =  , 11 , 11 | 3.51 (br d, J = 2.2 Hz) | 3.81 (br s) | 2.96 (dd, J = 13.7, 4.0 Hz) | 2.76–2.70 (m) |
| R = octyl, 12 | 3.44 (br s) | 3.77 (br s) | 2.92 (d, J = 12.6 Hz) | 2.68 (d, J = 12.6 Hz) |
| R = ethyl, 13 | 3.47 (d, J = 3.2 Hz) | 3.81 (br s) | 2.96 (dd, J = 13.6, 3.4 Hz) | 2.72 (dd, J = 13.7, 2.4 Hz) |
R =  , 14 , 14 | 3.53 (br d, J = 2.7 Hz) | 3.86 (br s) | 3.02 (dd, J = 13.6, 3.8 Hz) | 2.79 (br d, J = 13.6 Hz) |
R =  , 15 , 15 | 3.49 (br s) | 3.80 (br s) | 2.95 (d, J = 13.2 Hz) | 2.70 (d, J = 13.8 Hz) |
| Entry | Compound | β-Gal | GCase | |
|---|---|---|---|---|
| Inhibition (%) a | Inhibition (%) a | IC50 (µM) b | ||
| 1 |  | 22 | 98 | 12 ± 6 |
| 2 |  | 0 | 100 | 6.4 ± 0.7 |
| 3 |  | 0 | 36 | n.d. |
| 4 |  | 0 | 93 | 60 ± 23 |
| 5 |  | 3 | 30 | n.d. |
| 6 |  | 16 | 9 | n.d. |
| 7 |  | 6 | 6 | n.d. |
| 8 |  | 0 | 100 | 130 ± 13 |
| 9 |  | 0 | 25 | n.d. |
| 10 |  | 4 | 65 | n.d. |
| 11 |  | 15 | 93 c | 40 ± 3 c |
| 12 |  | 14 d | 100 d | 29 ± 2 d |
| 13 |  | 5 d | 100 d | 1.5 ± 0.1 d |
| 14 |  | 31 d | 80 d | 94 ± 5 d |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davighi, M.G.; Clemente, F.; Matassini, C.; Morrone, A.; Goti, A.; Martínez-Bailén, M.; Cardona, F. Synthesis of “All-Cis” Trihydroxypiperidines from a Carbohydrate-Derived Ketone: Hints for the Design of New β-Gal and GCase Inhibitors. Molecules 2020, 25, 4526. https://doi.org/10.3390/molecules25194526
Davighi MG, Clemente F, Matassini C, Morrone A, Goti A, Martínez-Bailén M, Cardona F. Synthesis of “All-Cis” Trihydroxypiperidines from a Carbohydrate-Derived Ketone: Hints for the Design of New β-Gal and GCase Inhibitors. Molecules. 2020; 25(19):4526. https://doi.org/10.3390/molecules25194526
Chicago/Turabian StyleDavighi, Maria Giulia, Francesca Clemente, Camilla Matassini, Amelia Morrone, Andrea Goti, Macarena Martínez-Bailén, and Francesca Cardona. 2020. "Synthesis of “All-Cis” Trihydroxypiperidines from a Carbohydrate-Derived Ketone: Hints for the Design of New β-Gal and GCase Inhibitors" Molecules 25, no. 19: 4526. https://doi.org/10.3390/molecules25194526
APA StyleDavighi, M. G., Clemente, F., Matassini, C., Morrone, A., Goti, A., Martínez-Bailén, M., & Cardona, F. (2020). Synthesis of “All-Cis” Trihydroxypiperidines from a Carbohydrate-Derived Ketone: Hints for the Design of New β-Gal and GCase Inhibitors. Molecules, 25(19), 4526. https://doi.org/10.3390/molecules25194526