Cause, Regulation and Utilization of Dye Aggregation in Dye-Sensitized Solar Cells
Abstract
1. Introduction
2. Operating Principle and Characterization Parameters of DSSCs
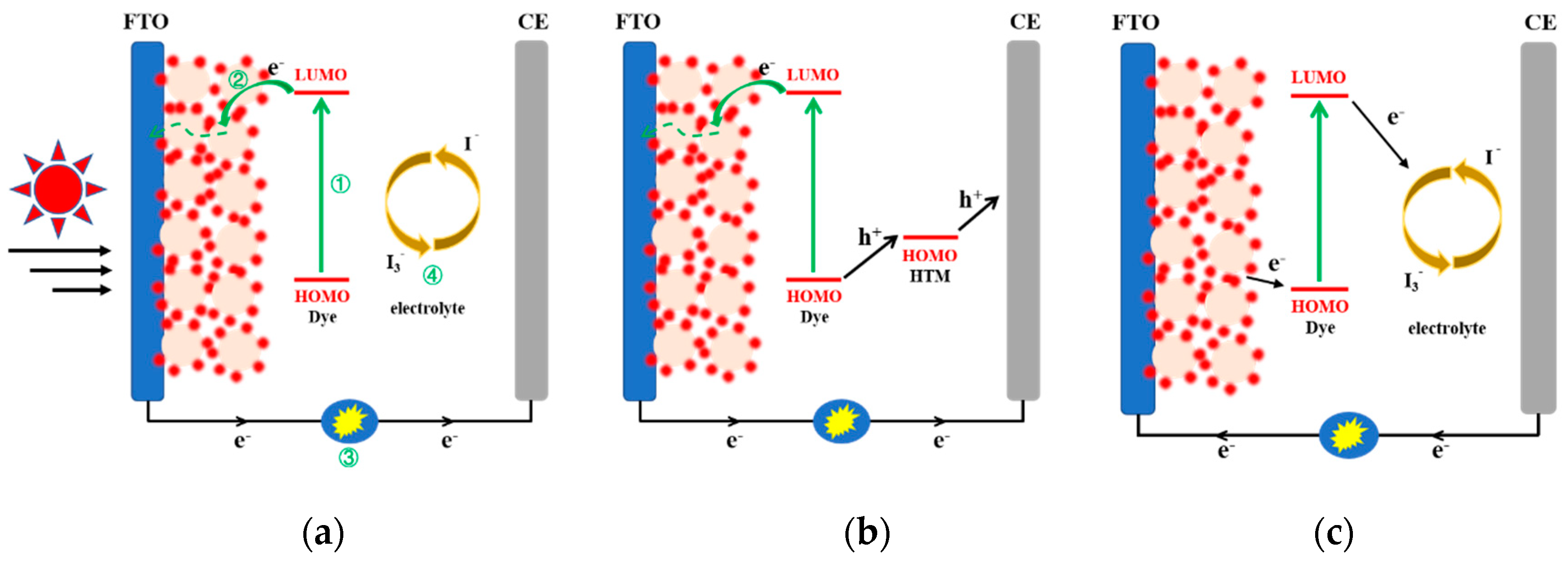
2.1. Operating Principle
- The ground-state dye molecules adsorbed on the metal oxide surface are excited by light, and the electrons transition from highest occupied molecular orbital (HOMO) to lowest unoccupied molecular orbital (LUMO).
- Excited electrons are injected into the conduction band of the metal oxide, then electrons migrate to the conductive substrate, and enter the external circuit to form a current.
- Regeneration of the oxidized dye by electron donation from the redox couple of the electrolyte.
- The oxidized species in the electrolyte receive electrons from the external circuit to complete the process.
2.2. Characterization Parameters of Cell Efficiency
3. Dye Aggregation in DSSCs
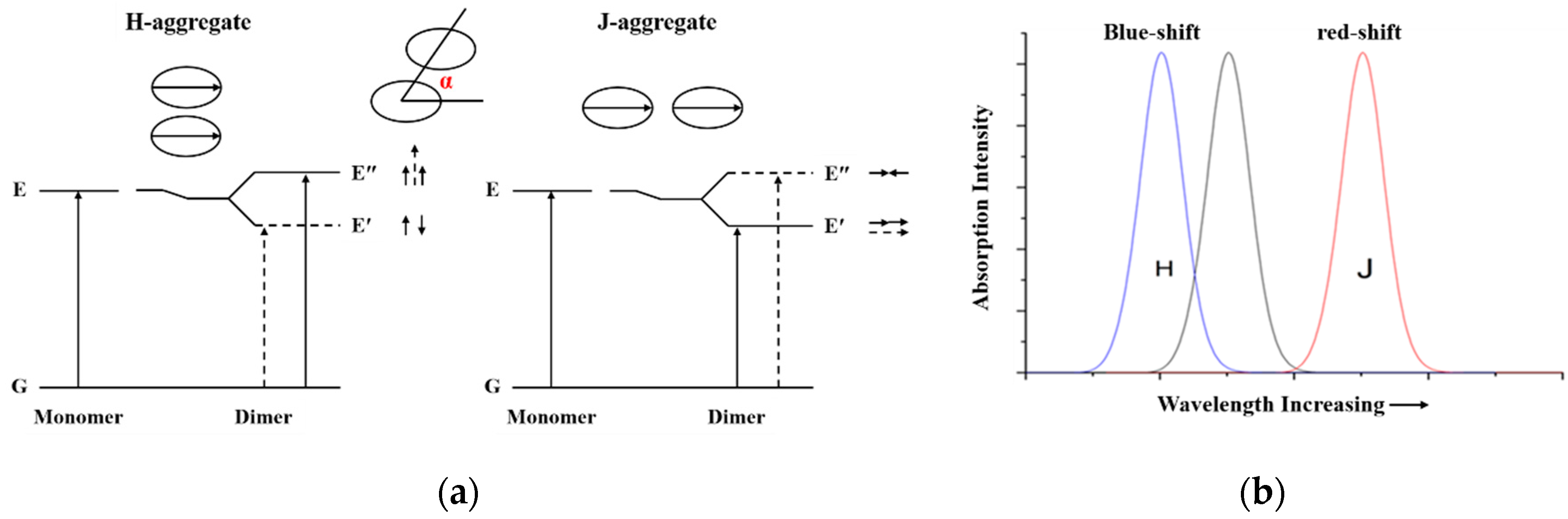
3.1. The Mechanism of Dye Aggregation
3.2. The Influence Factors of Dye Aggregation
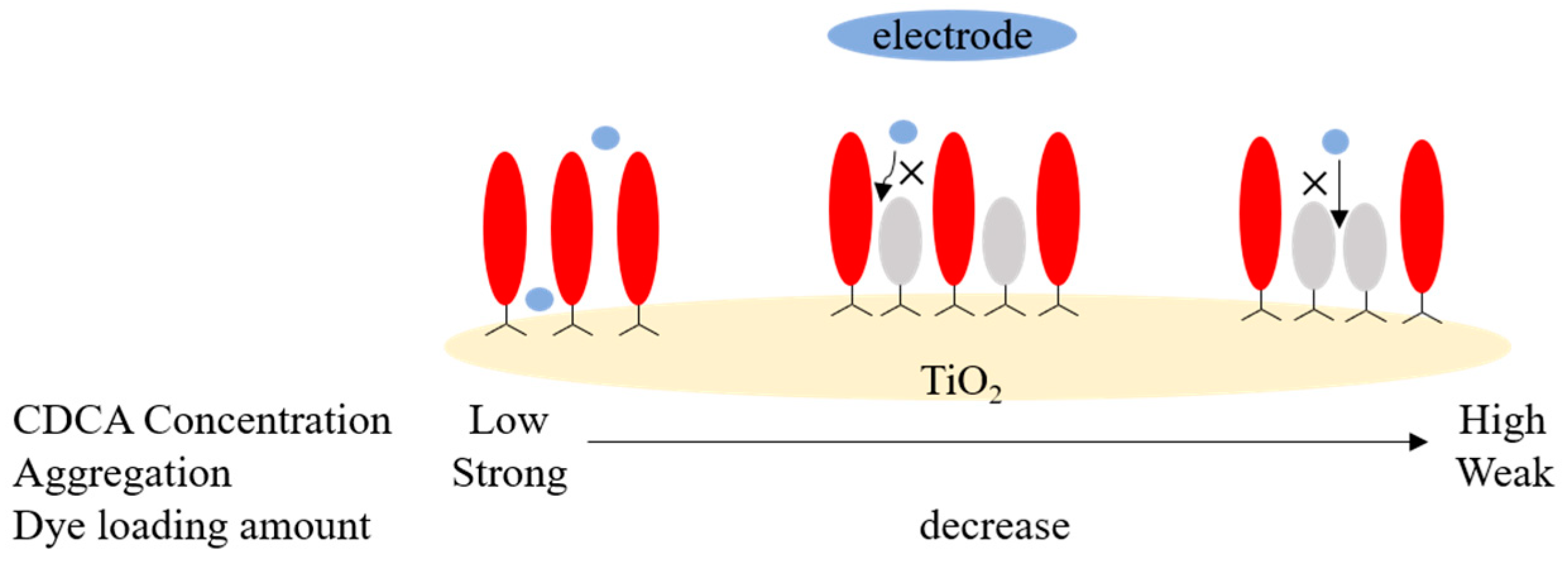
3.3. Methods for Inhibiting Dye Aggregation
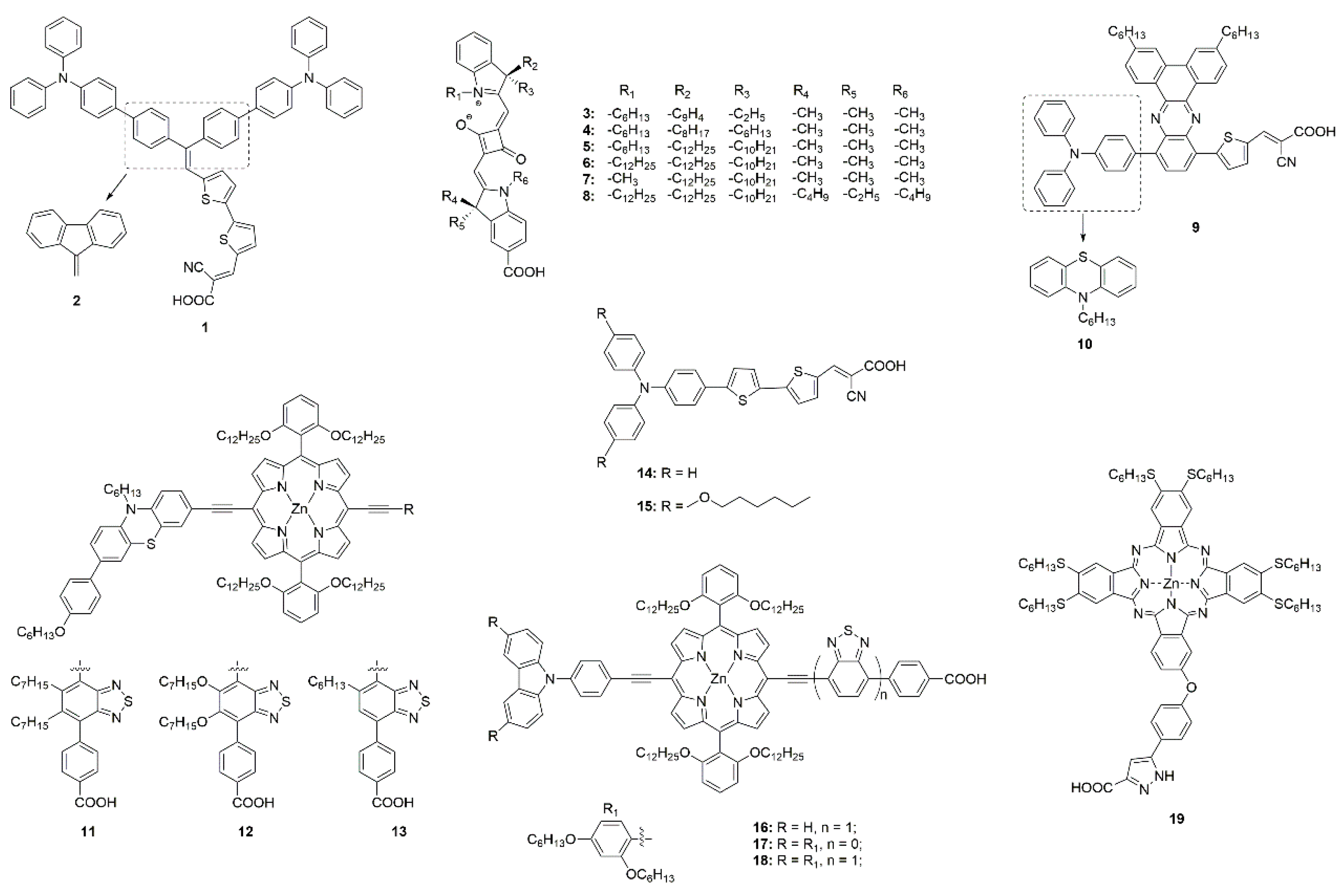
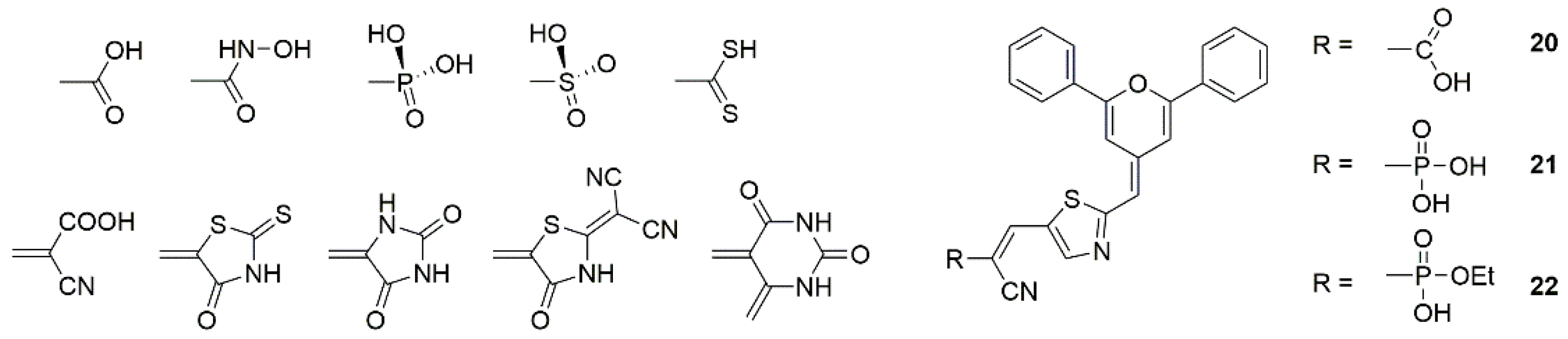
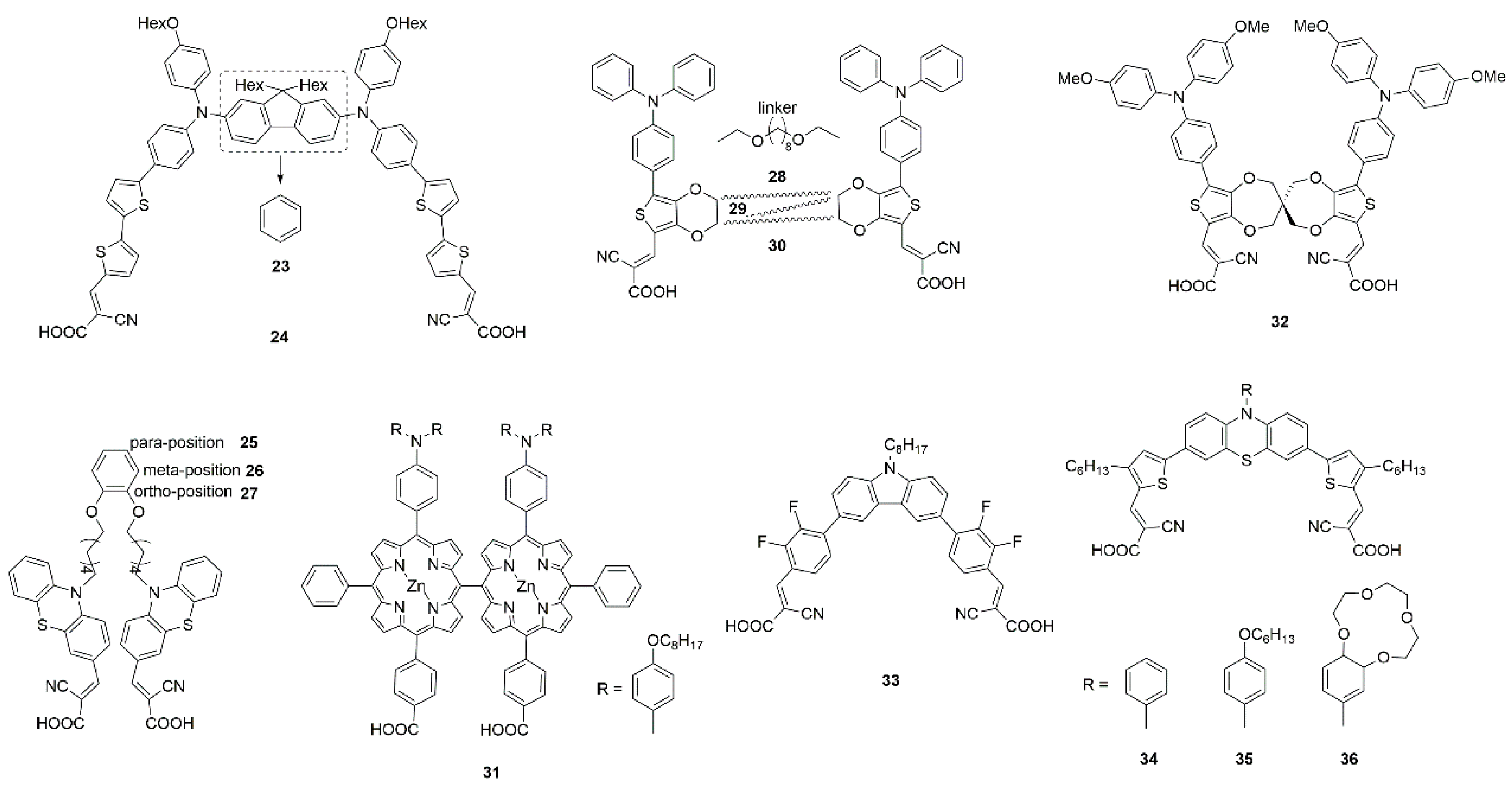
3.3.1. Molecular Engineering
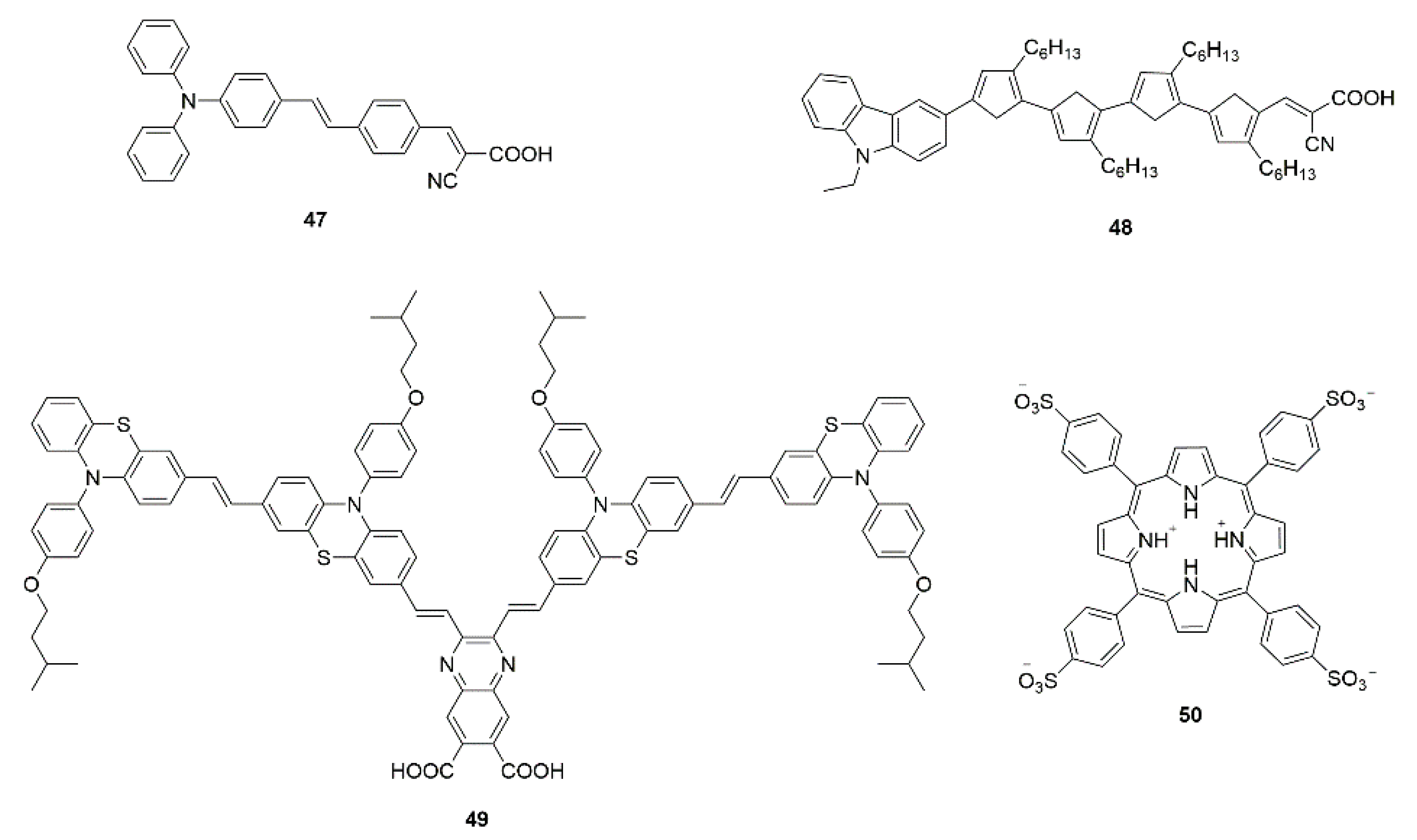
3.3.2. Co-Adsorbents
3.3.3. Sensitization Conditions
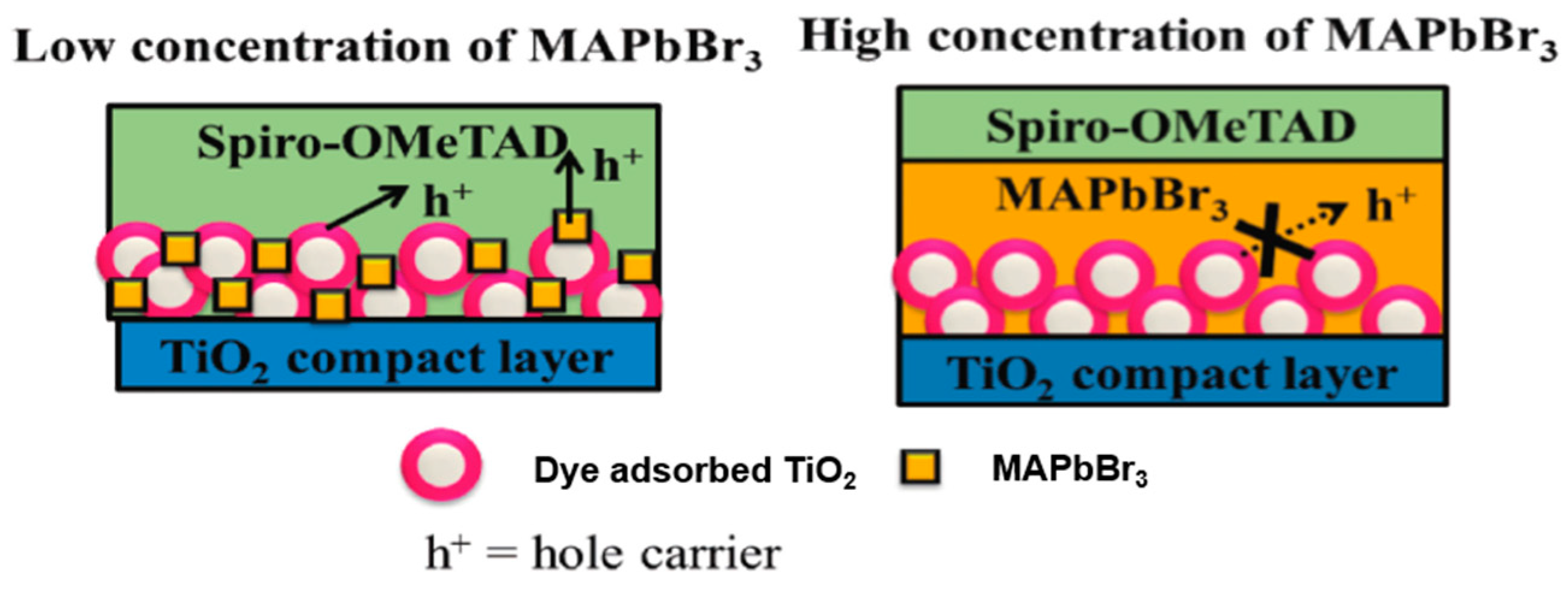
3.3.4. Other Methods
3.4. Future Prospects for Utilizing Dye Aggregation
4. Application of Quantum Computation in the Study of Dye Aggregation
4.1. Computational Methodologies
4.2. Functional Development to Accurately Describe Aggregations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Juraj, B. The effects of layered nanoparticles and their properties on the molecular aggregation of organic dyes. J. Photochem. Photobiol. C 2018, 35, 108–133. [Google Scholar]
- Luo, J.; Xie, Z.; Jacky, W.Y.L.; Cheng, L.; Chen, H.; Qiu, C.; Hoi, S.K.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef] [PubMed]
- Serap, G.; Helmut, N.; Niyazi, S.S. Gonjugated Polymer-Based Organic Solar Cell. Chem. Rev. 2007, 107, 1324–1338. [Google Scholar]
- Ragoussi, M.-E.; Torres, T. New generation solar cells: Concepts, trends and perspectives. Chem. Commun. 2015, 51, 3957–3972. [Google Scholar] [CrossRef]
- Yun, S.; Qin, Y.; Alexander, R.U.; Nick, V.; Yin, M.; Li, D.; Han, X.; Anders, H. New-generation integrated devices based on dye-sensitized and perovskite solar cells. Energy Environ. Sci. 2018, 11, 476–526. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Mathew, S.; Yella, A.; Gao, P.; Humphry-Baker, R.; Curchod, B.F.E.; Ashari-Astani, N.; Tavernelli, I.; Rothlisberger, U.; Nazeeruddin, M.K.; Grätzel, M. Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat. Chem. 2014, 6, 242–247. [Google Scholar] [CrossRef]
- Kakiage, K.; Aoyama, Y.; Yano, T.; Oya, K.; Fujisawa, J.-I.; Hanaya, M. Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes. Chem. Commun. 2015, 51, 15894–15897. [Google Scholar] [CrossRef]
- Grätzel, M. Dye-sensitized solar cells. J. Photochem. Photobiol. C 2003, 4, 145–153. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-Sensitized Solar Cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef]
- Joshi, P.; Zhang, L.; Davous, D.; Zhu, Z.; Galipeau, D.; Fong, H.; Qiao, Q. Composite of TiO2 nanofibers and nanoparticles for dye-sensitized solar cells with significantly improved efficiency. Energy Environ. Sci. 2010, 3, 1507–1510. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Lai, Y.-H.; Chen, H.-W.; Chen, J.-G.; Kung, C.-W.; Vittal, R.; Ho, K.-C. Highly efficient dye-sensitized solar cell with a ZnO nanosheet-based photoanode. Energy Environ. Sci. 2011, 4, 3448–3455. [Google Scholar] [CrossRef]
- So, S.; Hwang, I.; Schmuki, P. Hierarchical DSSC structures based on “single walled” TiO2 nanotube arrays reach a back-side illumination solar light conversion efficiency of 8%. Energy Environ. Sci. 2015, 8, 849–854. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Pandey, A.K.; Rahim, N.A. Advancements in the development of TiO2 photoanodes and its fabrication methods for dye sensitized solar cell (DSSC) applications: A review. Renew. Sust. Energ. Rev. 2017, 77, 89–108. [Google Scholar] [CrossRef]
- Kathirvel, S.; Sireesha, P.; Su, C.; Chen, B.-R.; Li, W.-R. Morphological control of TiO2 nanocrystals by solvothermal synthesis for dye-sensitized solar cell applications. Appl. Surf. Sci. 2020, 519, 146082. [Google Scholar] [CrossRef]
- Yun, S.; Hagfeldt, A.; Ma, T. Pt-Free Counter Electrode for Dye-Sensitized Solar Cells with High Efficiency. Adv. Mater. 2014, 26, 6210–6237. [Google Scholar] [CrossRef]
- Oh, W.-C.; Areerob, Y. A New Aspect for Band Gap Energy of Graphene- Mg2CuSnCoO6 -Gallic Acid as a Counter Electrode for Enhancing Dye- Sensitized Solar Cell Performance. ACS Appl. Mater. Interfaces 2019, 11, 38859–38867. [Google Scholar] [CrossRef]
- Tian, Y.-B.; Wang, Y.-Y.; Chen, S.-M.; Gu, Z.-G.; Zhang, J. Epitaxial Growth of Highly Transparent Metal−Porphyrin Framework Thin Films for Efficient Bifacial Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 1078–1083. [Google Scholar] [CrossRef]
- Memon, A.A.; Arbab, A.A.; Sahito, I.A.; Sun, K.C.; Mengal, N.; Jeong, H. Synthesis of highly photo-catalytic and electro-catalytic active textile structured carbon electrode and its application in DSSCs. Sol. Energy 2017, 150, 521–531. [Google Scholar] [CrossRef]
- Junger, I.J.; Wehlage, D.; Böttjer, R.; Grothe, T.; Juhász, L.; Grassmann, C.; Blachowicz, T.; Ehrmann, A. Dye-Sensitized Solar Cells with Electrospun Nanofiber Mat-Based Counter Electrodes. Materials 2018, 11, 1604. [Google Scholar] [CrossRef]
- Müller, S.; Wieschollek, D.; Junger, I.J.; Schwenzfeier-Hellkamp, E.; Ehrmann, A. Back electrodes of dye-sensitized solar cells on textile fabrics. Optik 2019, 198, 163243. [Google Scholar] [CrossRef]
- Mamun, A.; Trabelsi, M.; Klöcker, M.; Sabantina, L.; Großerhode, C.; Blachowicz, T.; Grötsch, G.; Cornelißen, C.; Streitenberger, A.; Ehrmann, A. Electrospun Nanofiber Mats with Embedded Non-Sintered TiO2 for Dye-Sensitized Solar Cells (DSSCs). Fibers 2019, 7, 60. [Google Scholar] [CrossRef]
- Fang, J.-K.; Xu, M.; Hu, X.; Wu, C.; Lu, S.; Yu, H.-J.; Bao, X.; Wang, Y.; Shao, G.; Liu, W. Aggregation-Free Organic Dyes Featuring Spiro[dibenzo[3,4:6,7]cyclohepta[1,2-b]quinoxaline-10,9′-fluorene] (SDBQX) for Dye-Sensitized Solar Cells. Glob. Chall. 2019, 3, 1900034. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.S.; Cui, Y.; Dan-Oh, Y.; Kasada, C.; Shinpo, A.; Hara, K. Thiophene-functionalized coumarin dye for efficient dye-sensitized solar cells: Electron lifetime improved by coadsorption of deoxycholic acid. J. Phys. Chem. C 2007, 111, 7224–7230. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Z.-E.; Hu, J.; Hong, Y. Novel rod-shaped organic sensitizers for liquid and quasi-solid-state dye-sensitized solar cells. Electrochim. Acta 2019, 295, 934–941. [Google Scholar] [CrossRef]
- Eom, Y.K.; Hong, J.Y.; Kim, J.; Kim, H.K. Triphenylamine-based organic sensitizers with π-spacer structural engineering for dye-sensitized solar cells: Synthesis, theoretical calculations, molecular spectroscopy and structure-property-performance relationships. Dyes Pigm. 2017, 136, 496–504. [Google Scholar] [CrossRef]
- Kwok, E.C.-H.; Chan, M.-Y.; Wong, K.M.-C.; Yam, V.W.-W. Molecular dyads comprising metalloporphyrin and alkynylplatinum (II) polypyridine terminal groups for use as a sensitizer in dye-sensitized solar cells. Chem. Eur. J. 2014, 20, 3142–3153. [Google Scholar] [CrossRef]
- Sakuragi, Y.; Wang, X.-F.; Miura, H.; Matsui, M.; Yoshida, T. Aggregation of indoline dyes as sensitizers for ZnO solar cells. J. Photochem. Photobiol. A 2010, 216, 1–7. [Google Scholar] [CrossRef]
- Zhang, L.; Cole, J.M. Dye aggregation in dye-sensitized solar cells. J. Mater. Chem. A 2017, 5, 19541–19559. [Google Scholar] [CrossRef]
- Mann, J.R.; Gannon, M.K.; Fitzgibbons, T.C.; Detty, M.R.; Watson, D.F. Optimizing the photocurrent efficiency of dye-sensitized solar cells through the controlled aggregation of chalcogenoxanthylium dyes on nanocrystalline titania films. J. Phys. Chem. C 2008, 112, 13057–13061. [Google Scholar] [CrossRef]
- Mulhern, K.R.; Orchard, A.; Watson, D.F.; Detty, A.M.R. Influence of surface-attachment functionality on the aggregation, persistence, and electron-transfer reactivity of chalcogenorhodamine dyes on TiO2. Langmuir 2012, 28, 7071–7082. [Google Scholar] [CrossRef]
- Kawasaki, M.; Aoyama, S. High efficiency photocurrent generation by two-dimensional mixed J-aggregates of cyanine dyes. Chem. Commun. 2004, 10, 988–989. [Google Scholar] [CrossRef]
- Luo, L.; Lin, C.-J.; Tsai, C.-Y.; Wu, H.-P.; Li, L.-L.; Lo, C.-F.; Lin, C.-Y.; Diau, E.W.-G. Effects of aggregation and electron injection on photovoltaic performance of porphyrin-based solar cells with oligo(phenylethynyl) links inside TiO2 and Al2O3 nanotube arrays. Phys. Chem. Chem. Phys. 2010, 12, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-J.; Sun, L.; Jiang, L.; Zhou, Q.; Ma, Z.-B.; Yang, X.-M.; Deng, W.-Q. Molecular-scale observation of YD2-o-C8 self-assembled monolayer on TiO2 (110). Surf. Sci. 2017, 665, 103–107. [Google Scholar] [CrossRef]
- Kley, C.S.; Dette, C.; Rinke, G.; Patrick, C.E.; Čechal, J.; Jung, S.J.; Baur, M.; Dürr, M.; Rauschenbach, S.; Giustino, F.; et al. Atomic-scale observation of multiconformational binding and energy level alignment of ruthenium-based photosensitizers on TiO2 anatase. Nano Lett. 2014, 14, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Ghosh, H.N. Exciton Energy and Charge Transfer in Porphyrin Aggregate/Semiconductor (TiO2) Composites. J. Phys. Chem. Lett. 2012, 3, 1877–1884. [Google Scholar] [CrossRef]
- Cole, J.M.; Blood-Forsythe, M.A.; Lin, T.-C.; Pattison, P.; Gong, Y.; Vázquez-Mayagoitia, Á.; Waddell, P.G.; Zhang, L.; Koumura, N.; Mori, S. Discovery of s···c≡n intramolecular bonding in a thiophenylcyanoacrylate-based dye: Realizing charge transfer pathways and dye···TiO2 anchoring characteristics for dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2017, 9, 25952–25961. [Google Scholar] [CrossRef]
- Pastore, M.; Angelis, F.D. Intermolecular Interactions in Dye-Sensitized Solar Cells: A Computational Modeling Perspective. J. Phys. Chem. Lett. 2013, 4, 956–974. [Google Scholar] [CrossRef]
- Hamann, T.W.; Jensen, R.A.; Martinson, A.B.F.; Van Ryswyk, H.; Hupp, J.T. Advancing beyond current generation dye-sensitized solar cells. Energy Environ. Sci. 2008, 1, 66–78. [Google Scholar] [CrossRef]
- Karim, N.A.; Mehmood, U.; Zahid, H.F.; Asif, T. Nanostructured photoanode and counter electrode materials for efficient dye-sensitized solar cells (DSSCs). Sol. Energy 2019, 185, 165–188. [Google Scholar] [CrossRef]
- Jang, Y.J.; Thogiti, S.; Lee, K.-Y.; Kim, J.H. Long-Term Stable Solid-State Dye-Sensitized Solar Cells Assembled with Solid-State Polymerized Hole-Transporting Material. Crystals 2019, 9, 452. [Google Scholar] [CrossRef]
- Iftikhar, H.; Sonai, G.G.; Hashmi, S.G.; Nogueira, A.F.; Lund, P.D. Progress on Electrolytes Development in Dye-Sensitized Solar Cells. Materials 2019, 12, 1998. [Google Scholar] [CrossRef]
- Yum, J.-H.; Chen, P.; Grätzel, M.; Nazeeruddin, M.K. Recent Developments in Solid-State Dye-Sensitized Solar Cells. ChemSusChem 2008, 1, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, V.; Charisiadis, A.; Charalambidis, G.; Coutsolelos, A.G.; Odobel, F. Recent advances and insights in dye-sensitized NiO photocathodes for photovoltaic devices. J. Mater. Chem. A 2017, 5, 21077–21113. [Google Scholar] [CrossRef]
- Sumikura, S.; Mori, S.; Shimizu, S.; Usami, H.; Suzuki, E. Photoelectrochemical characteristics of cells with dyed and undyed nanoporous p-type semiconductor CuO electrodes. J. Photochem. Photobiol. A 2008, 194, 143–147. [Google Scholar] [CrossRef]
- Yu, M.; Draskovic, T.I.; Wu, Y. Cu(I)-based delafossite compounds as photocathodes in p-type dye-sensitized solar cells. Phys. Chem. Chem. Phys. 2014, 16, 5026–5033. [Google Scholar] [CrossRef]
- Langmar, O.; Ganivet, C.R.; Lennert, A.; Costa, R.D.; de la Torre, G.; Torres, T.; Guldi, D.M. Combining Electron-Accepting Phthalocyanines and Nanorod-like CuO Electrodes for p-Type Dye-Sensitized Solar Cells. Angew. Chem. Int. Ed. 2015, 54, 7688–7692. [Google Scholar] [CrossRef]
- Jose, R.; Kumar, A.; Thavasi, V.; Fujihara, K.; Uchida, S.; Ramakrishna, S. Relationship between the molecular orbital structure of the dyes and photocurrent density in the dye-sensitized solar cells. Appl. Phys. Lett. 2008, 93, 023125. [Google Scholar] [CrossRef]
- Fan, K.; Yu, J.; Ho, W. Improving photoanodes to obtain highly efficient dye-sensitized solar cells: A brief review. Mater. Horiz. 2017, 4, 319–344. [Google Scholar] [CrossRef]
- Angelis, F.D.; Fantacci, S.; Selloni, A.; Grätzel, M.; Nazeeruddin, M.K. Influence of the Sensitizer Adsorption Mode on the Open-Circuit Potential of Dye-Sensitized Solar Cells. Nano Lett. 2007, 7, 3189–3195. [Google Scholar] [CrossRef]
- Tian, Y.; Hu, C.; Wu, Q.; Wu, X.; Li, X.; Hashim, M. Investigation of the fill factor of dye-sensitized solar cell based on ZnO nanowire arrays. Appl. Surf. Sci. 2011, 258, 321–326. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, S.-T.; Wang, Z.-S. Effect of metal-doping in TiO2 on fill factor of dye-sensitized solar cells. Appl. Phys. Lett. 2011, 99, 113503. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, V.; Sharma, S.S. Dye-Sensitized Solar Cells: Fundamentals and Current Status. Nanoscale Res. Lett. 2018, 13, 381. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Liu, D.; Wang, T.; Lu, T.; Li, W.; Ren, S.; Hu, W.; Wang, L.; Zhou, X. Enhanced Internal Quantum Efficiency in Dye-Sinsitized Solar Cells: Effect of Long-Lived Charge-Separated State of Sensitizers. ACS Appl. Mater. Interfaces 2017, 9, 9880–9891. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Li, P.; Liu, D.; Wang, T.; Li, W.; Hu, W.; Wang, L.; Zhou, X. Tuning photophysical properties via alkoxyl groups in charge-separated triphenylamine sensitizers for dye-sensitized solar cells. J. Photochem. Photobiol. A 2019, 368, 223–241. [Google Scholar] [CrossRef]
- Sun, H.; Liu, D.; Wang, T.; Li, P.; Bridgmohan, C.N.; Li, W.; Lu, T.; Hu, W.; Wang, L.; Zhou, X. Charged-Separated Sensitizers with Enhanced Intramolecular Charge Transfer for Dye-Sensitized Solar Cells: Insight from Structure-Performance Relationship. Org. Electron. 2018, 61, 35–45. [Google Scholar] [CrossRef]
- Wang, T.; Sun, H.; Zhang, L.; Colley, N.D.; Bridgmohan, C.N.; Liu, D.; Hu, W.; Li, W.; Zhou, X.; Wang, L. Effect of photo-induced charge separated state lifetimes in donor-acceptor1-acceptor2 organic ambipolar semiconductors on their photovoltaic performances. Dyes Pigm. 2017, 139, 601–610. [Google Scholar] [CrossRef]
- Wang, T.; Sun, H.; Lu, T.; Bridgmohan, C.N.; Li, F.; Liu, D.; Hu, W.; Li, W.; Zhou, X.; Wang, L. Dissociation exists in s-triazine based donor-acceptor organic systems by photo-induced electron transfer. Dyes Pigm. 2017, 139, 264–273. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, T.; Li, D.; Lu, T.; Liu, D.; Meng, Q.; Zhang, Q.; Li, F.; Li, W.; Hu, W.; et al. Synthesis and characterization of triphenylamine modified azobenzene dyes. Dyes Pigm. 2017, 137, 256–265. [Google Scholar] [CrossRef]
- Lu, T.; Sun, H.; Colley, N.D.; Bridgmohan, C.N.; Liu, D.; Li, W.; Hu, W.; Zhou, X.; Wang, T.; Wang, L. Tuning the donors to control the lifetimes of charge separated states in triazine-based Donor-Acceptor systems. Dyes Pigm. 2017, 136, 404–415. [Google Scholar] [CrossRef]
- Wang, T.; Weerasinghe, K.C.; Sun, H.; Hu, X.; Lu, T.; Liu, D.; Hu, W.; Li, W.; Zhou, X.; Wang, L. Effect of Triplet State on the Lifetime of Charge Separation in Ambipolar D-A1-A2 Organic Semiconductors. J. Phys. Chem. C 2016, 120, 11338–11349. [Google Scholar] [CrossRef]
- Mishra, A.; Behera, R.K.; Behera, P.K.; Mishra, B.K.; Behera, G.B. Cyanines during the 1990s: A Review. Chem. Rev. 2000, 100, 1973–2011. [Google Scholar] [CrossRef] [PubMed]
- Kasha, M.; Rawls, H.R.; El-Bayoumi, M.A. The exciton model in molecular spectroscopy. Pure Appl. Chem. 1965, 11, 371–392. [Google Scholar] [CrossRef]
- Würthner, F.; Kaiser, T.E.; Saha-Möller, C.R. J-Aggregates: From Serendipitous Discovery to Supramolecular Engineering of Functional Dye Materials. Angew. Chem. Int. Ed. 2011, 50, 3376–3410. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, A.; Zeini-Isfahani, A.; Habibi, M.H. Molecular exciton theory calculations based on experimental results for Solophenyl red 3BL azo dye–surfactants interactions. Spectrochim. Acta A 2006, 64, 464–476. [Google Scholar] [CrossRef]
- Bang, S.Y.; Ko, M.J.; Kim, K.; Kim, J.H.; Jang, I.-H.; Park, N.-G. Evaluation of dye aggregation and effect of deoxycholic acid concentration on photovoltaic performance of N749-sensitized solar cell. Synth. Met. 2012, 162, 1503–1507. [Google Scholar] [CrossRef]
- Benesperi, I.; Michaels, H.; Freitag, M. The researcher’s guide to solid-state dye-sensitized solar cells. J. Mater. Chem. C 2018, 6, 11903–11942. [Google Scholar] [CrossRef]
- Matsuzaki, H.; Murakami, T.N.; Masaki, N.; Furube, A.; Kimura, M.; Mori, S. Dye Aggregation Effect on Interfacial Electron-Transfer Dynamics in Zinc Phthalocyanine-Sensitized Solar Cells. J. Phys. Chem. C 2014, 118, 17205–17212. [Google Scholar] [CrossRef]
- Lee, B.; Ezhumalai, Y.; Lee, W.; Chen, M.-C.; Yeh, C.-Y.; Marks, T.-J.; Chang, R.P.H. Cs2SnI6 -Encapsulated Multidye-Sensitized All-Solid-State Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 21424–21434. [Google Scholar] [CrossRef]
- Numata, Y.; Islam, A.; Chen, H.; Han, L. Aggregation-free branch-type organic dye with a twisted molecular architecture for dye-sensitized solar cells. Energy Environ. Sci. 2012, 5, 8548–8552. [Google Scholar] [CrossRef]
- Jadhav, M.M.; Chowdhury, T.H.; Bedja, I.; Patil, D.; Islam, A.; Sekar, N. Near IR emitting novel rhodanine-3-acetic acid based two donor-π-acceptor sensitizers for DSSC: Synthesis and application. Dyes Pigm. 2019, 165, 391–399. [Google Scholar] [CrossRef]
- Singh, A.K.; Kavungathodi, M.F.M.; Nithyanandhan, J. Alkyl-Group-Wrapped Unsymmetrical Squaraine Dyes for Dye-Sensitized Solar Cells: Branched Alkyl Chains Modulate the Aggregation of Dyes and Charge Recombination Processes. ACS Appl. Mater. Interfaces 2020, 12, 2555–2565. [Google Scholar] [CrossRef] [PubMed]
- Kabanakis, A.N.; Bidikoudi, M.; Elsenety, M.M.; Vougioukalakis, G.C.; Falaras, P. Synthesis of novel semi-squaraine derivatives and application in efficient dye-sensitized solar cells. Dyes Pigm. 2019, 165, 308–318. [Google Scholar] [CrossRef]
- Lyu, L.; Su, R.; Al-Qaradawi, S.Y.; Al-Saad, K.A.; El-Shafei, A. Three-component one-pot reaction for molecular engineering of novel cost-effective highly rigid quinoxaline-based photosensitizers for highly efficient DSSCs application: Remarkable photovoltage. Dyes Pigm. 2019, 171, 107683. [Google Scholar] [CrossRef]
- Yang, G.; Tang, Y.; Li, X.; Ågren, H.; Xie, Y. Efficient Solar Cells Based on Porphyrin Dyes with Flexible Chains Attached to the Auxiliary Benzothiadiazole Acceptor: Suppression of Dye Aggregation and the Effect of Distortion. ACS Appl. Mater. Interfaces 2017, 9, 36875–36885. [Google Scholar] [CrossRef]
- Son, H.-J.; Kim, C.H.; Kim, D.W.; Jeong, N.C.; Prasittichai, C.; Luo, L.; Wu, J.; Farha, O.K.; Wasielewski, M.R.; Hupp, J.T. Post-Assembly Atomic Layer Deposition of Ultrathin Metal-Oxide Coatings Enhances the Performance of an Organic Dye-Sensitized Solar Cell by Suppressing Dye Aggregation. ACS Appl Mater. Interfaces 2015, 7, 5150–5159. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, Y.; Song, H.; Liu, Q.; Li, X.; Cai, Y.; Xie, Y. Solar cells sensitized with porphyrin dyes with a carbazole donor: The effects of an auxiliary benzothiadiazole acceptor and bulky substituents on the donor. Dyes Pigm. 2019, 171, 107776. [Google Scholar] [CrossRef]
- Yıldız, B.; Güzel, E.; Akyüz, D.; Arslan, B.S.; Koca, A.; Şener, M.K. Unsymmetrically pyrazole-3-carboxylic acid substituted phthalocyanine-based photoanodes for use in water splitting photoelectrochemical and dye-sensitized solar cells. Sol. Energy 2019, 191, 654–662. [Google Scholar] [CrossRef]
- Seo, K.D.; You, B.S.; Choi, I.T.; Ju, M.J.; You, M.; Kang, H.S.; Kim, H.K. Dual-channel anchorable organic dyes with well-defined structures for highly efficient dye-sensitized solar cells. J. Mater. Chem. A 2013, 1, 9947–9953. [Google Scholar] [CrossRef]
- Zang, X.-F.; Zhang, T.-L.; Huang, Z.-S.; Iqbal, Z.; Kuang, D.-B.; Wang, L.; Meier, H.; Cao, D. Impact of the position isomer of the linkage in the double D-A branch-based organic dyes on the photovoltaic performance. Dyes Pigm. 2014, 104, 89–96. [Google Scholar] [CrossRef]
- Jiang, S.; Fan, S.; Lu, X.; Zhou, G.; Wang, Z.-S. Double D–π–A branched organic dye isomers for dye-sensitized solar cells. J. Mater. Chem. A 2014, 2, 17153–17164. [Google Scholar] [CrossRef]
- Zhang, T.; Qian, X.; Zhang, P.-F.; Zhu, Y.-Z.; Zheng, J.-Y. A meso–meso directly linked porphyrin dimer-based double D–p–A sensitizer for efficient dye-sensitized solar cells. Chem. Commun. 2015, 51, 3782–3785. [Google Scholar] [CrossRef] [PubMed]
- Sil, M.C.; Sudhakar, V.; Kavungathodi, M.F.M.; Punitharasu, V.; Nithyanandhan, J. Orthogonally Functionalized Donor/Acceptor Homo- and Heterodimeric Dyes for Dye-Sensitized Solar Cells: An Approach to Introduce Panchromaticity and Control the Charge Recombination. ACS Appl. Mater. Interfaces 2017, 9, 34875–34890. [Google Scholar] [CrossRef]
- Raju, T.B.; Vaghasiya, J.V.; Afroz, M.A.; Soni, S.S.; Iyer, P.K. Effect of mono- and di-anchoring dyes based on o,m-difluoro substituted phenylene spacer in liquid and solid state dye sensitized solar cells. Dyes Pigm. 2020, 174, 108021. [Google Scholar] [CrossRef]
- Li, C.-T.; Wu, F.-L.; Liang, C.-J.; Ho, K.-C.; Lin, J.-T. Effective suppression of interfacial charge recombination by a 12-crown-4 substituent on a double-anchored organic sensitizer and rotating disk electrochemical evidence. J. Mater. Chem. A 2017, 5, 7586–7594. [Google Scholar] [CrossRef]
- Ji, J.-M.; Kim, S.H.; Zhou, H.; Kim, C.H.; Kim, H.K. D–π–A-Structured Porphyrins with Extended Auxiliary π-Spacers for Highly Efficient Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 24067–24077. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhang, W.; Li, X.; Jiang, H.; Shen, C.; Zhu, W.-H. Influence of ethynyl position on benzothiadiazole based D–A–p–A dye-sensitized solar cells: Spectral response and photovoltage performance. J. Mater. Chem. C 2016, 4, 9203–9211. [Google Scholar] [CrossRef]
- Wu, Y.; Marszalek, M.; Zakeeruddin, S.M.; Zhang, Q.; Tian, H.; Grätzel, M.; Zhu, W. High-conversion-efficiency organic dye-sensitized solar cells: Molecular engineering on D–A–π-A featured organic indoline dyes. Energy Environ. Sci. 2012, 5, 8261–8272. [Google Scholar] [CrossRef]
- Schölin, R.; Quintana, M.; Johansson, E.M.J.; Hahlin, M.; Marinado, T.; Hagfeldt, A.; Rensmo, H. Preventing Dye Aggregation on ZnO by Adding Water in the Dye-Sensitization Process. J. Phys. Chem. C 2011, 115, 19274–19279. [Google Scholar] [CrossRef]
- Unny, D.; Kandregula, G.R.; Sivanadanam, J.; Ramanujam, K. Molecular engineering of pyrene carbazole dyes with a single bond and double bond as the mode of linkage. New J. Chem. 2020. [Google Scholar] [CrossRef]
- Tian, H.; Yang, X.; Chen, R.; Zhang, R.; Hagfeldt, A.; Sun, L. Effect of Different Dye Baths and Dye-Structures on the Performance of Dye-Sensitized Solar Cells Based on Triphenylamine Dyes. J. Phys. Chem. C 2008, 112, 11023–11033. [Google Scholar] [CrossRef]
- Park, J.M.; Jung, C.Y.; Wang, Y.; Choi, H.D.; Park, S.J.; Ou, P.; Jang, W.-D.; Jaung, J.Y. Effect of additional phenothiazine donor and thiophene π-bridge on photovoltaic performance of quinoxaline cored photosensitizers. Dyes Pigm. 2019, 170, 107568. [Google Scholar] [CrossRef]
- Arai, Y.; Segawa, H. Anion-controlled Aggregation of a Porphyrin at Solid-Liquid Interfaces: A Distinguished Effect of Different Aggregates in Dye-sensitized Solar Cells. Chem. Lett. 2013, 42, 918–920. [Google Scholar] [CrossRef]
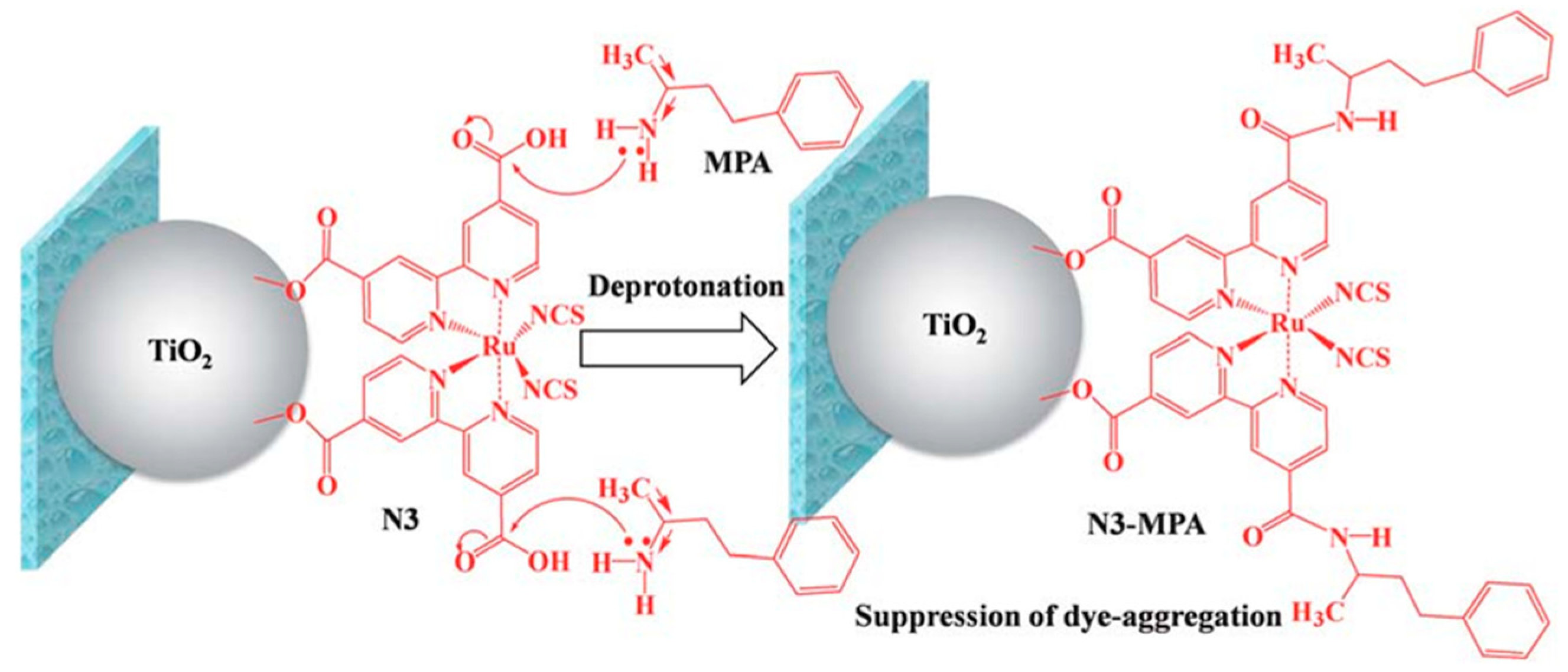
- Nath, N.C.D.; Kim, J.C.; Kim, K.P.; Yim, S.; Lee, J.-J. Deprotonation of N3 adsorbed on TiO2 for high-performance dye-sensitized solar cells (DSSCs). J. Mater. Chem. A 2013, 1, 13439–13442. [Google Scholar] [CrossRef]
- Ananth, S.; Vivek, P.; Arumanayagam, T.; Murugakoothan, P. Natural dye extract of lawsonia inermis seed as photo sensitizer for titanium dioxide based dye sensitized solar cells. Spectrochim. Acta A 2014, 128, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-L.; Jiang, K.-J.; Shao, K.-F.; Yang, L.-M. Novel organic dyes for efficient dye-sensitized solar cells. Chem. Commun. 2006, 26, 2792–2794. [Google Scholar] [CrossRef]
- Venkateswararao, A.; Thomas, K.R.J.; Lee, C.-P.; Li, C.-T.; Ho, K.-C. Organic Dyes Containing Carbazole as Donor and π-Linker: Optical, Electrochemical, and Photovoltaic Properties. ACS Appl. Mater. Interfaces 2014, 6, 2528–2539. [Google Scholar] [CrossRef]
- Yang, H.-Y.; Yen, Y.-S.; Hsu, Y.-C.; Chou, H.-H.; Lin, J.T. Organic Dyes Incorporating the Dithieno[3,2-b:2′,3′-d]thiophene Moiety for Efficient Dye-Sensitized Solar Cells. Org. Lett. 2010, 12, 16–19. [Google Scholar] [CrossRef]
- Hemavathi, B.; Jayadev, V.; Pradhan, S.C.; Gokul, G.; Jagadish, K.; Chandrashekara, G.K.; Ramamurthy, P.C.; Pai, R.K.; Unni, K.N.N.; Ahipa, T.N.; et al. Aggregation induced light harvesting of molecularly engineered D-A-π-A carbazole dyes for dye-sensitized solar cells. Sol. Energy 2018, 174, 1085–1096. [Google Scholar] [CrossRef]
- Moon, S.-J.; Yum, J.-H.; Humphry-Baker, R.; Karlsson, K.M.; Hagberg, D.P.; Marinado, T.; Hagfeldt, A.; Sun, L.; Grätzel, M.; Nazeeruddin, M.K. Highly Efficient Organic Sensitizers for Solid-State Dye-Sensitized Solar Cells. J. Phys. Chem. C 2009, 113, 16816–16820. [Google Scholar] [CrossRef]
- Abate, A.; Pérez-Tejada, R.; Wojciechowski, K.; Foster, J.M.; Sadhanala, A.; Steiner, U.; Snaith, H.J.; Franco, S.; Orduna, J. Phosphonic anchoring groups in organic dyes for solid-state solar cells. Phys. Chem. Chem. Phys. 2015, 17, 18780–18789. [Google Scholar] [CrossRef] [PubMed]
- Sirimanne, P.M.; Buddhapriya, A.N. An enhancement of photo-performance of dye sensitized solid-state cell by avoiding formation of dye aggregates. Optik 2016, 127, 3798–3802. [Google Scholar] [CrossRef]
- Pinpithak, P.; Kulkarni, A.; Chen, H.-W.; Ikegami, M.; Miyasaka, T. Solid-State Thin-Film Dye-Sensitized Solar Cell Co-Sensitized with Methylammonium Lead Bromide Perovskite. Bull. Chem. Soc. Jpn. 2018, 91, 754–760. [Google Scholar] [CrossRef]
- Liyanage, D.N.; Kumarasinghe, K.D.M.S.P.K.; Kumara, G.R.A.; Jayasundera, A.C.A.; Tennakone, K.; Onwona-Agyeman, B. Donor-π-Conjugated Spacer-Acceptor Dye-Sensitized Solid-State Solar Cell Using CuI as the Hole Collector. Int. J. Photoenergy 2019, 2019, 1–5. [Google Scholar] [CrossRef]
- Zhu, L.; Yang, H.B.; Zhong, C.; Li, C.M. Rational design of triphenylamine dyes for highly efficient p-type dye sensitized solar cells. Dyes Pigm. 2014, 105, 97–104. [Google Scholar] [CrossRef]
- Liu, Z.; Li, W.; Topa, S.; Xu, X.; Zeng, X.; Zhao, Z.; Wang, M.; Chen, W.; Wang, F.; Cheng, Y.-B.; et al. Fine Tuning of Fluorene-Based Dye Structures for High-Efficiency p-Type Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2014, 6, 10614–10622. [Google Scholar] [CrossRef]
- Li, L.-L.; Diau, E.W.-G. Porphyrin-sensitized solar cells. Chem. Soc. Rev. 2013, 42, 291–304. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, Y.; Li, X.; Ågren, H.; Zhu, W.-H.; Xie, Y. Porphyrins Containing a Triphenylamine Donor and up to Eight Alkoxy Chains for Dye-Sensitized Solar Cells: A High Efficiency of 10.9%. ACS Appl. Mater. Interfaces 2015, 7, 27976–27985. [Google Scholar] [CrossRef]
- Lu, Y.; Song, H.; Li, X.; Ågren, H.; Liu, Q.; Zhang, J.; Zhang, X.; Xie, Y. Multiply Wrapped Porphyrin Dyes with a Phenothiazine Donor: A High Efficiency of 11.7% Achieved through a Synergetic Coadsorption and Cosensitization Approach. ACS Appl. Mater. Interfaces 2019, 11, 5046–5054. [Google Scholar] [CrossRef]
- Nachimuthu, S.; Chen, W.-C.; Leggesse, E.G.; Jiang, J.-C. First principles study of organic sensitizers for dye sensitized solar cells: Effects of anchoring groups on optoelectronic properties and dye aggregation. Phys. Chem. Chem. Phys. 2016, 18, 1071–1081. [Google Scholar] [CrossRef]
- Cao, Y.; Ge, Q.; Dyer, D.J.; Wang, L. Steric Effects on the Adsorption of Alkylthiolate Self-Assembled Monolayers on Au (111). J. Phys. Chem. B 2003, 107, 3803–3807. [Google Scholar] [CrossRef]
- Wu, C.; Wang, L.; Xiao, Z.; Li, G.; Wang, L. Effects of van der Waals interactions on the dehydrogenation of n-butane on a Ni(111) surface. Chem. Phys. Lett. 2020, 746, 137299. [Google Scholar] [CrossRef]
- Kryman, M.W.; Nasca, J.N.; Watson, D.F.; Detty, M.R. Selenorhodamine Dye-Sensitized Solar Cells: Influence of Structure and Surface-Anchoring Mode on Aggregation, Persistence, and Photoelectrochemical Performance. Langmuir 2016, 32, 1521–1532. [Google Scholar] [CrossRef]
- Zhu, H.-C.; Li, C.-F.; Fu, Z.-H.; Wei, S.-S.; Zhu, X.-F.; Zhang, J. Increasing the open-circuit voltage and adsorption stability of squaraine dye binding onto the TiO2 anatase (101) surface via heterocyclic anchoring groups used for DSSC. Appl. Surf. Sci. 2018, 455, 1095–1105. [Google Scholar] [CrossRef]
- Liu, S.; Jiao, Y.; Ding, Y.; Fan, X.; Song, J.; Mi, B.; Gao, Z. Position engineering of cyanoacrylic-acid anchoring group in a dye for DSSC applications. Dyes Pigm. 2020, 180, 108470. [Google Scholar] [CrossRef]
- Chen, H.; Cole, J.M.; Stenning, G.B.G.; Yanguas-Gil, A.; Elam, J.W.; Stan, L.; Gong, Y. Imaging Dye Aggregation in MK-2, N3, N749, and SQ-2 dye···TiO2 Interfaces That Represent Dye-Sensitized Solar Cell Working Electrodes. ACS Appl. Energy Mater. 2020, 3, 3230–3241. [Google Scholar] [CrossRef]
- McCree-Grey, J.; Cole, J.M.; Holt, S.A.; Evans, P.J.; Gong, Y. Dye⋯TiO2 interfacial structure of dye-sensitised solar cell working electrodes buried under a solution of Iˉ/I3ˉ redox electrolyte. Nanoscale 2017, 9, 11793–11805. [Google Scholar] [CrossRef]
- Sirohi, R.; Kim, D.H.; Yu, S.-C.; Lee, S.H. Novel di-anchoring dye for DSSC by bridging of two mono anchoring dye molecules: A conformational approach to reduce aggregation. Dyes Pigm. 2012, 92, 1132–1137. [Google Scholar] [CrossRef]
- Meier, H.; Huang, Z.-S.; Cao, D. Double D–p–A branched dyes—A new class of metal-free organic dyes for efficient dye-sensitized solar cells. J. Mater. Chem. C 2017, 5, 9828–9837. [Google Scholar] [CrossRef]
- Heredia, D.; Natera, J.; Gervaldo, M.; Otero, L.; Fungo, F.; Lin, C.-Y.; Wong, K.-T. Spirobifluorene-Bridged Donor/Acceptor Dye for Organic Dye-Sensitized Solar Cells. Org. Lett. 2010, 12, 12–15. [Google Scholar] [CrossRef]
- Pozzi, G.; Orlandi, S.; Cavazzini, M.; Minudri, D.; Macor, L.; Otero, L.; Fungo, F. Synthesis and Photovoltaic Applications of a 4,4‘-Spirobi[cyclopenta-[2,1-b;3,4-b‘]dithiophene]-Bridged Donor/Acceptor Dye. Org. Lett. 2013, 15, 4642–4645. [Google Scholar] [CrossRef] [PubMed]
- Hung, W.-I.; Liao, Y.-Y.; Lee, T.-H.; Ting, Y.-C.; Ni, J.-S.; Kao, W.-S.; Lin, J.T.; Wei, T.-C.; Yen, Y.-S. Eugenic metal-free sensitizers with double anchors for high performance dye-sensitized solar cells. Chem. Commun. 2015, 51, 2152–2155. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-T.; Kuo, Y.-L.; Kumar, C.H.P.; Huang, P.-T.; Lin, J.T. Tetraphenylethylene tethered phenothiazine-based double-anchored sensitizers for high performance dye-sensitized solar cells. J. Mater. Chem. A 2019, 7, 23225–23233. [Google Scholar] [CrossRef]
- Maglione, C.; Carella, A.; Centore, R.; Fusco, S.; Velardo, A.; Peluso, A.; Colonna, D.; Carlo, A.D. Tuning optical absorption in pyran derivatives for DSSC. J. Photochem. Photobiol. A 2016, 321, 79–89. [Google Scholar] [CrossRef]
- Bonomo, M.; Barbero, N.; Matteocci, F.; Carlo, A.D.; Barolo, C.; Dini, D. Beneficial Effect of Electron-Withdrawing Groups on the Sensitizing Action of Squaraines for p-Type Dye-Sensitized Solar Cells. J. Phys. Chem. C 2016, 120, 16340–16353. [Google Scholar] [CrossRef]
- Saxena, V.; Aswal, D.K. Surface modifications of photoanodes in dye sensitized solar cells: Enhanced light harvesting and reduced recombination. Semicond. Sci. Technol. 2015, 30, 064005. [Google Scholar] [CrossRef]
- Wang, X.; Bolag, A.; Yun, W.; Du, Y.; Eredun, C.; Zhang, X.; Bao, T.; Ning, J.; Alata, H.; Ojiyed, T. Enhanced performance of dye-sensitized solar cells based on a dual anchored diphenylpyranylidene dye and N719 co-sensitization. J. Mol. Struct. 2020, 1206, 127694. [Google Scholar] [CrossRef]
- Sewvandi, G.A.; Kakimoto, M.; Chen, C.; Hu, D.; Abeygunawardhana, P.K.W.; Feng, Q. Controlling dye coverage instead of addition of organic acid to reduce dye aggregation in dye-sensitized solar cells. Sol. Energy 2010, 202, 507–513. [Google Scholar] [CrossRef]
- Ito, S.; Miura, H.; Uchida, S.; Takata, M.; Sumioka, K.; Liska, P.; Comte, P.; Péchy, P.; Grätzel, M. High-conversion-efficiency organic dye-sensitized solar cells with a novel indoline dye. Chem. Commun. 2008, 44, 5194–5196. [Google Scholar] [CrossRef]
- Lin, R.Y.-Y.; Lin, H.-W.; Yen, Y.-S.; Chang, C.-H.; Chou, H.-H.; Chen, P.-W.; Hsu, C.-Y.; Chen, Y.-C.; Lin, J.T.; Ho, K.-C. 2,6-Conjugated anthracene sensitizers for high-performance dye-sensitized solar cells. Energy Environ. Sci. 2013, 6, 2477–2486. [Google Scholar]
- Lim, D.-S.; Choi, K.; Hayati, D.; Park, D.-H.; Ghifari, A.; Lee, K.M.; Ko, Y.; Jun, Y.; Suk, H.-J.; Hong, J. Blue-colored dyes featuring a diketopyrrolopyrrole spacer for translucent dye-sensitized solar cells. Dyes Pigm. 2020, 173, 107840. [Google Scholar] [CrossRef]
- Quan, Y.-Y.; Li, Q.; Wang, Z.; Ma, H.; Dong, J.; Huang, Z.-S. Effect of electron donor and acceptor in dithienopyrrolobenzothiadiazole-based organic dyes for efficient quasi-solid-state dye-sensitized solar cells. Dyes Pigm. 2020, 173, 107999. [Google Scholar] [CrossRef]
- Cole, J.M.; Pepe, G.; Bahri, O.K.A.; Cooper, C.B. Cosensitization in Dye-Sensitized Solar Cells. Chem. Rev. 2019, 119, 7279–7327. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.-L.; Chen, Y.-C.; Ji, L.; Lin, L.-X.; Guan, M.-Y.; Yang, Y. Cosensitization of porphyrin dyes with new X type organic dyes for efficient dye-sensitized solar cells. Dyes Pigm. 2019, 163, 589–593. [Google Scholar] [CrossRef]
- Sharma, G.D.; Zervaki, G.E.; Angaridis, P.A.; Vatikioti, A.; Gupta, K.S.V.; Gayathri, T.; Nagarjuna, P.; Singh, S.P.; Chandrasekharam, M.; Banthiya, A.; et al. Stepwise co-sensitization as a useful tool for enhancement of power conversion efficiency of dye-sensitized solar cells: The case of an unsymmetrical porphyrin dyad and a metal-free organic dye. Org. Electron. 2014, 15, 1324–1337. [Google Scholar] [CrossRef]
- Song, H.; Liu, Q.; Xie, Y. Porphyrin-sensitized solar cells: Systematic molecular optimization, coadsorption and cosensitization. Chem. Commun. 2018, 54, 1811–1824. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Jiang, H.; Shi, J.; Lu, B.; Cai, H.; Mao, Z.; Kong, F. In Situ Evaluation of Kinetics and Interaction Mechanism between Chenodeoxycholic Acid and N719 on Dye-Sensitized Nanofilm Surface. ACS Appl. Energy Mater. 2020, 3, 3310–3317. [Google Scholar] [CrossRef]
- Gauthier, S.; Guen, F.R.-L.; Wojcik, L.; Poul, N.L.; Planchat, A.; Pellegrin, Y.; Level, P.G.; Szuwarski, N.; Boujtita, M.; Jacquemin, D.; et al. Synthesis and properties of novel pyranylidene-based organic sensitizers for dye-sensitized solar cells. Dyes Pigm. 2019, 171, 107747. [Google Scholar] [CrossRef]
- Karthika, P.; Ganesan, S.; Kamalakannan, S.; Prakash, M. Design and Synthesis of the D−π−A-Structured Coadsorbents with the Phenanthraquinone Core and Its Application in Dye-Sensitized Solar Cells. J. Phys. Chem. C 2020, 124, 9886–9899. [Google Scholar] [CrossRef]
- Soto-Navarro, A.; Alfaro, A.; Soto-Tellini, V.H.; Moehl, T.; Barea, E.M.; Fabregat-Santiago, F.; Pineda, L.W. Co-adsorbing effect of bile acids containing bulky amide groups at 3β-position on the photovoltaic performance in dye-sensitized solar cells. Sol. Energy 2019, 189, 94–102. [Google Scholar] [CrossRef]
- Jia, H.-L.; Li, S.-S.; Gong, B.-Q.; Gu, L.; Bao, Z.-L.; Guan, M.-Y. Efficient cosensitization of new organic dyes containing bipyridine anchors with porphyrins for dye-sensitized solar cells. Sustain. Energy Fuels 2020, 4, 347–353. [Google Scholar] [CrossRef]
- Younas, M.; Harrabi, K. Performance enhancement of dye-sensitized solar cells via co-sensitization of ruthenium (II) based N749 dye and organic sensitizer RK1. Sol. Energy 2020, 203, 260–266. [Google Scholar] [CrossRef]
- Unger, E.L.; Morandeira, A.; Persson, M.; Zietz, B.; Ripaud, E.; Leriche, P.; Roncali, J.; Hagfeldt, A.; Boschloo, G. Contribution from a hole-conducting dye to the photocurrent in solid-state dye-sensitized solar cells. Phys. Chem. Chem. Phys. 2011, 13, 20172–20177. [Google Scholar] [CrossRef] [PubMed]
- Chevrier, M.; Fattori, A.; Lasser, L.; Kotras, C.; Rose, C.; Cangiotti, M.; Beljonne, D.; Mehdi, A.; Surin, M.; Lazzaroni, R.; et al. In Depth Analysis of Photovoltaic Performance of Chlorophyll Derivative-Based “All Solid-State” Dye-Sensitized Solar Cells. Molecules 2020, 25, 198. [Google Scholar] [CrossRef] [PubMed]
- Dualeh, A.; Delcamp, J.H.; Nazeeruddin, M.K.; Grätzel, M. Near-infrared sensitization of solid-state dye-sensitized solar cells with a squaraine dye. App. Phys. Lett. 2012, 100, 173512. [Google Scholar] [CrossRef]
- Asdim, I.K.; Inomata, T.; Masuda, H.; Yoshida, T. Aggregation behavior of differently substituted Ru(II)-complex dyes as sensitizers for electrodeposited ZnO solar cells. J. Photochem. Photobiol. A 2012, 242, 67–71. [Google Scholar] [CrossRef]
- Liu, X.; Cole, J.M.; Low, K.S. Molecular Origins of Dye Aggregation and Complex Formation Effects in Coumarin 343. J. Phys. Chem. C 2013, 117, 14723–14730. [Google Scholar] [CrossRef]
- Treat, N.A.; Knorr, F.J.; McHale, J.L. Templated Assembly of Betanin Chromophore on TiO2: Aggregation-Enhanced Light-Harvesting and Efficient Electron Injection in a Natural Dye-Sensitized Solar Cell. J. Phys. Chem. C 2016, 120, 9122–9131. [Google Scholar] [CrossRef]
- Chen, R.; Zhao, G.; Yang, X.; Jiang, X.; Liu, J.; Tian, H.; Gao, Y.; Liu, X.; Han, K.; Sun, M.; et al. Photoinduced intramolecular charge-transfer state in thiophene-p-conjugated donor–acceptor molecules. J. Mol. Struct. 2008, 876, 102–109. [Google Scholar] [CrossRef]
- Edwards, A.A.; Alexander, B.D. UV-Visible Absorption Spectroscopy, Organic Applications. In Encyclopedia of Spectroscopy and Spectrometry, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 511–519. [Google Scholar] [CrossRef]
- Ooyama, Y.; Kushimoto, K.; Oda, Y.; Tokita, D.; Yamaguchi, N.; Inoue, S.; Nagano, T.; Harima, Y.; Ohshita, J. Synthesis and specific solvatochromism of D-π-A type pyridinium dye. Tetrahedron 2012, 68, 8577–8580. [Google Scholar] [CrossRef]
- Zhang, L.; Cole, J.M.; Liu, X. Tuning Solvatochromism of Azo Dyes with Intramolecular Hydrogen Bonding in Solution and on Titanium Dioxide Nanoparticles. J. Phys. Chem. C 2013, 117, 26316–26323. [Google Scholar] [CrossRef]
- Venkatraman, V. Evaluation of Molecular Fingerprints for Determining Dye Aggregation on Semiconductor Surfaces. Mol. Inf. 2020, 39, 2000062. [Google Scholar] [CrossRef]
- Nüesch, F.; Grätzel, M. H-aggregation and correlated absorption and emission of a merocyanine dye in solution, at the surface and in the solid state. A link between crystal structure and photophysical properties. Chem. Phys. 1995, 193, 1–17. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Y.; Wagner, W.; Stepanenko, V.; Ren, X.; Ogi, S.; Würthner, F. Near-IR Absorbing J-Aggregate of an Amphiphilic BF2-Azadipyrromethene Dye by Kinetic Cooperative Self-Assembly. Angew. Chem. Int. Ed. 2017, 56, 5729–5733. [Google Scholar] [CrossRef]
- Gräf, K.; Rahim, M.A.; Das, S.; Thelakkat, M. Complementary co-sensitization of an aggregating squaraine dye in solid-state dye-sensitized solar cells. Dyes Pigm. 2013, 99, 1101–1106. [Google Scholar] [CrossRef]
- Kubát, P.; Lang, K.; Janda, P.; Anzenbacher, P. Interaction of Porphyrins with a Dendrimer Template: Self-Aggregation Controlled by pH. Langmuir 2005, 21, 9714–9720. [Google Scholar] [CrossRef]
- Ma, B.; Gao, R.; Wang, L.; Luo, F.; Zhan, C.; Li, J.; Qiu, Y. Alternating assembly structure of the same dye and modification material in quasi-solid state dye-sensitized solar cell. J. Photoch. Photobiol. A 2009, 202, 33–38. [Google Scholar] [CrossRef]
- Bian, Z.; Tachikawa, T.; Cui, S.-C.; Fujitsuka, M.; Majima, T. Single-molecule charge transfer dynamics in dye-sensitized p-type NiO solar cells: Influences of insulating Al2O3 layers. Chem. Sci. 2012, 3, 370–379. [Google Scholar] [CrossRef]
- Flynn, C.J.; McCullough, S.M.; Oh, E.; Li, L.; Mercado, C.C.; Farnum, B.H.; Li, W.; Donley, C.L.; You, W.; Nozik, A.J.; et al. Site-Selective Passivation of Defects in NiO Solar Photocathodes by Targeted Atomic Deposition. ACS Appl Mater. Interfaces 2016, 8, 4754–4761. [Google Scholar] [CrossRef]
- Wang, Z.-S.; Yamaguchi, T.; Sugihara, H.; Arakawa, H. Significant Efficiency Improvement of the Black Dye-Sensitized Solar Cell through Protonation of TiO2 Films. Langmuir 2005, 21, 4272–4276. [Google Scholar] [CrossRef] [PubMed]
- D’Amario, L.; Jiang, R.; Cappel, U.B.; Gubson, E.A.; Boschloo, G.; Rensmo, H.; Sun, L.; Hammarström, L.; Tian, H. Chemical and Physical Reduction of High Valence Ni States in Mesoporous NiO Film for Solar Cell Application. ACS Appl Mater. Interfaces 2017, 9, 33470–33477. [Google Scholar] [CrossRef] [PubMed]
- Sayama, K.; Hara, K.; Mori, N.; Satsuki, M.; Suga, S.; Tsukagoshi, S.; Abe, Y.; Sugihara, H.; Arakawa, H. Photosensitization of a porous TiO2 electrode with merocyanine dyes containing a carboxyl group and a long alkyl chain. Chem. Commun. 2000, 1173–1174. [Google Scholar] [CrossRef]
- Chen, C.; Cheng, M.; Ding, X.; Li, H.; Qiao, F.; Xu, L.; Li, H.; Li, H. Molecular engineering of triphenylamine functionalized phenoxazine sensitizers for highly efficient solid-state dye sensitized solar cells. Dyes Pigm. 2019, 162, 606–610. [Google Scholar] [CrossRef]
- Obotowo, I.N.; Obot, I.B.; Ekpe, U.J. Organic sensitizers for dye-sensitized solar cell (DSSC): Properties from computation, progress and future perspectives. J. Mol. Struct. 2016, 1122, 80–87. [Google Scholar] [CrossRef]
- Amao, Y.; Yamada, Y. Near-IR Light-Sensitized Voltaic Conversion System Using Nanocrystalline TiO2 Film by Zn Chlorophyll Derivative Aggregate. Langmuir 2005, 21, 3008–3012. [Google Scholar] [CrossRef]
- Feng, S.; Li, Q.-S.; Sun, P.-P.; Niehaus, T.A.; Li, Z.-S. Dynamic Characteristics of Aggregation Effects of Organic Dyes in Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 22504–22514. [Google Scholar] [CrossRef]
- Anathem, A.; Fairos Mk, M.; Vellimalai, P.; Sil, M.C.; Nithyanandhan, J. Effect of Out-of-Plane Alkyl Group’s Position in Dye-Sensitized Solar Cell Efficiency: A Structure-Property Relationship Utilizing Indoline-Based Unsymmetrical Squaraine Dyes. ACS Appl. Mater. Interfaces 2016, 8, 35353–35367. [Google Scholar]
- Ni, J.-S.; Chiu, T.-Y.; Kao, W.-S.; Chou, H.-J.; Su, C.-C.; Lin, J.T. Organic Photosensitizers Incorporating Rigidified Dithieno[3,2-f:2′,3′-h]quinoxaline Segment Tethered with Thiophene Substitutes for Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2016, 8, 23066–23073. [Google Scholar] [CrossRef]
- Konno, A.; Kumara, G.R.A.K.; Kaneko, S.; Onwona-Agyeman, B.; Tennakone, K. Solid-state Solar Cells Sensitized with Indoline Dye. Chem. Lett. 2007, 36, 716. [Google Scholar] [CrossRef]
- Schmidt-Mende, L.; Zakeeruddin, S.M.; Grätzel, M. Efficiency improvement in solid-state-dye-sensitized photovoltaics with an amphiphilic Ruthenium-dye. Appl. Phys. Lett. 2005, 86, 013504. [Google Scholar] [CrossRef]
- Karlsson, M.; Yang, L.; Karlsson, M.K.; Sun, L.; Boschloo, G.; Hagfeldt, A. Phenoxazine dyes in solid-state dye-sensitized solar cells. J. Photochem. Photobiol. A 2012, 239, 55–59. [Google Scholar] [CrossRef]
- Martsinovich, N.; Troisi, A. Theoretical studies of dye-sensitised solar cells: From electronic structure to elementary processes. Energy Environ. Sci. 2011, 4, 4473–4495. [Google Scholar] [CrossRef]
- Curutchet, C.; Mennucci, B. Quantum Chemical Studies of Light Harvesting. Chem. Rev. 2017, 117, 294–343. [Google Scholar] [CrossRef] [PubMed]
- Roy, J.K.; Kar, S.; Leszczynski, J. Insight into the optoelectronic properties of designed solar cells efcient tetrahydroquinoline dyesensitizers on TiO2(101) surface: Frst principles approach. Sci. Rep 2018, 8, 10997. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Govind, N.; Isborn, C.; DePrince, A.E., III; Lopata, K. Real-Time Time-Dependent Electronic Structure Theory. Chem. Rev. 2020. [Google Scholar] [CrossRef]
- Feng, S.; Li, Q.-S.; Niehaus, T.A.; Li, Z.-S. Effects of different electron donating groups on dye regeneration and aggregation in phenothiazine-based dye-sensitized solar cells. Org. Electron. 2017, 42, 234–243. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.; Rao, W.; Li, J. Multilayer Dye Aggregation at Dye/TiO2 Interface via π-π Stacking and Hydrogen Bond and Its Impact on Solar Cell Performance: A DFT Analysis. Sci. Rep. 2016, 6, 35893. [Google Scholar] [CrossRef]
- Hu, Z.; Metiu, H. Choice of U for DFT+U Calculations for Titanium Oxides. J. Phys. Chem. C 2011, 115, 5841–5845. [Google Scholar] [CrossRef]
- Xu, H.; Miao, B.; Zhang, M.; Chen, Y.; Wang, L. Mechanism of C–C and C–H bond cleavage in ethanol oxidation reaction on Cu2O(111): A DFT-D and DFT+U study. Phys. Chem. Chem. Phys. 2017, 19, 26210–26220. [Google Scholar] [CrossRef]
- Marcal, C.-C.; Zbigniew, Ł.; Núria, L. Performance of DFT+U Approaches in the Study of Catalytic Materials. ACS Catal. 2016, 6, 8370–8379. [Google Scholar]
- Feng, S.; Li, Q.-S.; Yang, L.-N.; Sun, Z.-Z.; Niehaus, T.A.; Li, Z.-S. Insights into aggregation effects on optical property and electronic coupling of organic dyes in dye sensitized solar cells. J. Power Sources 2015, 273, 282–289. [Google Scholar] [CrossRef]
- Marotta, G.; Reddy, M.A.; Singh, S.P.; Islam, A.; Han, L.; Angelis, F.D.; Pastore, M.; Chandrasekharam, M. Novel Carbazole-Phenothiazine Dyads for Dye-Sensitized Solar Cells: A Combined Experimental and Theoretical Study. ACS Appl. Mater. Interfaces 2013, 5, 9635–9647. [Google Scholar] [CrossRef]
- Ding, W.-L.; Li, Q.-S.; Li, Z.-S. Anti-aggregation and intra-type Forster resonance energy transfer in bulky indoline sensitizers for dye sensitized solar cells: A combined DFT/TDDFT and molecular dynamics study. J. Mater. Chem. A 2015, 3, 19948–19959. [Google Scholar] [CrossRef]
- Pastore, M.; Angelis, F.D. Aggregation of Organic Dyes on TiO2 in Dye-Sensitized Solar Cells Models: An ab Initio Investigation. ACS Nano 2010, 4, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cole, J.M. Adsorption properties of p-methyl red monomeric-to-pentameric dye aggregates on anatase (101) titania surfaces: First-principles calculations of dye/TiO2 photoanode interfaces for dye-sensitized solar cells. ACS Appl Mater Interfaces 2014, 6, 15760–15766. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Leijtens, T.; Ronca, E.; Pastore, M.; Snaith, H.; Angelis, F.D. Modeling the effect of ionic additives on the optical and electronic properties of a dye-sensitized TiO2 heterointerface: Absorption, charge injection and aggregation. J. Mater. Chem. A 2013, 1, 14675–14685. [Google Scholar] [CrossRef]
- Marotta, G.; Lobello, M.G.; Anselmi, C.; Consiglio, G.B.; Calamante, M.; Mordini, A.; Pastore, M.; Angelis, F.D. An Integrated Experimental and Theoretical Approach to the Spectroscopy of Organic-Dye-Sensitized TiO2 Heterointerfaces: Disentangling the Effects of Aggregation, Solvation, and Surface Protonation. ChemPhysChem 2014, 15, 1116–1125. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Risko, C.; Marder, S.R.; Brédas, J.-L. Polymethine dyes for all-optical switching applications: A quantum-chemical characterization of counter-ion and aggregation effects on the third-order nonlinear optical response. Chem. Sci. 2012, 3, 3103–3112. [Google Scholar] [CrossRef]
- Laura, A.G.; David, B.C.; Alexandra, M.Z.S.; Denis, J.; Mark, I.O.; Massimiliano, M.; Eli, Z.-C. Analyzing the Relation between Structure and Aggregation Induced Emission (AIE) Properties of Iridium(III) Complexes through Modification of Non-Chromophoric Ancillary Ligands. Eur. J. Inorg. Chem. 2019, 2019, 152–163. [Google Scholar]
- Grimme, S. Density functional theory with London dispersion corrections. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2011, 1, 211–228. [Google Scholar] [CrossRef]
- Ehrlich, S.; Moellmann, J.; Grimme, S. Dispersion-corrected density functional theory for aromatic interactions in complex systems. Acc. Chem. Res. 2013, 46, 916–926. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.J.; Mori-Sanchez, P.; Yang, W. Challenges for density functional theory. Chem. Rev. 2012, 112, 289–320. [Google Scholar] [CrossRef]
- Ruzsinszky, A.; Perdew, J.P. Twelve outstanding problems in ground-state density functional theory: A bouquet of puzzles. Comput. Theor. Chem. 2011, 963, 2–6. [Google Scholar] [CrossRef]
- Singh, R.; Giussani, E.; Mróz, M.M.; Fonzo, F.D.; Fazzi, D.; Cabanillas-González, J.; Oldridge, L.; Vaenas, N.; Kontos, A.G.; Falaras, P.; et al. On the role of aggregation effects in the performance of perylene-diimide based solar cells. Org. Electron. 2014, 15, 1347–1361. [Google Scholar] [CrossRef]
- Tennakone, K.; Pitigala, P.K.D.D.P.; Perera, A.G.U. Exciton transport and electron mobility of organized aggregates of cationic dye thiocyanates. RSC Adv. 2013, 3, 2770–2775. [Google Scholar] [CrossRef]
- Spano, F.C.; Silva, C. H- and J-Aggregate Behavior in Polymeric Semiconductors. Annu. Rev. Phys. Chem. 2014, 65, 477–500. [Google Scholar] [CrossRef]
- Hestand, N.J.; Spano, F.C. Molecular Aggregate Photophysics beyond the Kasha Model: Novel Design Principles for Organic Materials. Acc. Chem. Res. 2017, 50, 341–350. [Google Scholar] [CrossRef]
- Hestand, N.J.; Spano, F.C. Expanded Theory of H- and J-Molecular Aggregates: The Effects of Vibronic Coupling and Intermolecular Charge Transfer. Chem. Rev. 2018, 118, 7069–7163. [Google Scholar] [CrossRef]
- Felter, K.M.; Caselli, V.M.; Günbaş, D.D.; Savenije, T.J.; Grozema, F.C. Interplay between Charge Carrier Mobility, Exciton Diffusion, Crystal Packing, and Charge Separation in Perylene Diimide-Based Heterojunctions. ACS Appl. Energy Mater. 2019, 2, 8010–8021. [Google Scholar] [CrossRef]
- Nakano, K.; Suzuki, K.; Chen, Y.; Tajima, K. Roles of Energy/Charge Cascades and Intermixed Layers at Donor/Acceptor Interfaces in Organic Solar Cells. Sci. Rep. 2016, 6, 29529. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, O.T.; Deinert, J.-C.; Xu, Y.J.; Rinke, P.; Stähler, J.; Wolf, M.; Scheffler, M. Large work function reduction by adsorption of a molecule with a negative electron affinity: Pyridine on ZnO(1010). J. Chem. Phys. 2013, 139, 174701. [Google Scholar] [CrossRef] [PubMed]
- Polkehn, M.; Tamura, H.; Eisenbrandt, P.; Haacke, S.; Méry, S.; Burghardt, I. Molecular Packing Determines Charge Separation in a Liquid Crystalline Bisthiophene-Perylene Diimide Donor-Acceptor Material. J. Phys. Chem. Lett. 2016, 7, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Burns, L.A.; Vázquez-Mayagoitia, Á.; Sumpter, B.G.; Sherrill, C.D. Density-functional approaches to noncovalent interactions: A comparison of dispersion corrections (DFT-D), exchange-hole dipole moment (XDM) theory, and specialized functionals. J. Chem. Phy. 2011, 134, 084107. [Google Scholar] [CrossRef]
- Peverati, R.; Truhlar, D.G. Quest for a universal density functional: The accuracy of density functionals across a broad spectrum of databases in chemistry and physics. Phil. Trans. R. Soc. A 2014, 372, 20120476. [Google Scholar] [CrossRef]
- Klimes, J.; Michaelides, A. Perspective: Advances and challenges in treating van der Waals dispersion forces in density functional theory. J. Chem. Phys. 2012, 137, 120901. [Google Scholar] [CrossRef]
- Steinmann, S.N.; Corminboeuf, C. A System-Dependent Density-Based Dispersion Correction. J. Chem. Theory Comput. 2010, 6, 1990–2001. [Google Scholar] [CrossRef]
- Austin, A.; Petersson, G.A.; Frisch, M.J.; Dobek, F.J.; Scalmani, G.; Throssell, K. A Density Functional with Spherical Atom Dispersion Terms. J. Chem. Theory Comput. 2012, 8, 4989–5007. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2007, 120, 215–241. [Google Scholar]
- Goerigk, L. A Comprehensive Overview of the DFT-D3 London-Dispersion Correction. In Non-Covalent Interactions in Quantum Chemistry and Physics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 195–219. [Google Scholar] [CrossRef]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef]
- Raghavachari, K. Perspective on “Density functional thermochemistry. III. The role of exact exchange”. Theor. Chem. Acc. 2000, 103, 361–363. [Google Scholar] [CrossRef]
- Van de Walle, A.; Ceder, G. Correcting overbinding in local-density-approximation calculations. Phys. Rev. B Condens. Matter 1999, 59, 14992–15001. [Google Scholar] [CrossRef]
- Pérez-Jordá, J.; Becke, A.D. A density-functional study of van der Waals forces: Rare gas diatomics. Chem. Phys. Lett. 1995, 233, 134–137. [Google Scholar] [CrossRef]
- Dilabio, G.A.; Otero-de-la-Roze, A. Noncovalent Interactions in Density Functional Theory. In Reviews in Computational Chemistry; Parrill, A.L., Lipkowitz, K.B., Eds.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2016; Volume 29, pp. 1–97. [Google Scholar]
- Whittleton, S.R.; Otero-de-la-Roza, A.; Johnson, E.R. Exchange-Hole Dipole Dispersion Model for Accurate Energy Ranking in Molecular Crystal Structure Prediction. J. Chem. Theory Comput. 2017, 13, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Goerigk, L.; Hansen, A.; Bauer, C.; Ehrlich, S.; Najibi, A.; Grimme, S. A look at the density functional theory zoo with the advanced GMTKN55 database for general main group thermochemistry, kinetics and noncovalent interactions. Phys. Chem. Chem. Phys. 2017, 19, 32184–32215. [Google Scholar] [CrossRef]
- Rehak, F.R.; Piccini, G.; Alessio, M.; Saure, J. Including dispersion in density functional theory for adsorption on flat oxide surfaces, in metal-organic frameworks and in acidic zeolites. Phys. Chem. Chem. Phys. 2020, 22, 7577–7585. [Google Scholar] [CrossRef]
- Grimme, S.; Hansen, A.; Brandenburg, J.G.; Bannwarth, C. Dispersion-Corrected Mean-Field Electronic Structure Methods. Chem. Rev. 2016, 116, 5105–5154. [Google Scholar] [CrossRef]
- Walker, M.; Harvey, A.J.A.; Sen, A.; Dessent, C.E.H. Performance of M06, M06-2X, and M06-HF density functionals for conformationally flexible anionic clusters: M06 functionals perform better than B3LYP for a model system with dispersion and ionic hydrogen-bonding interactions. J. Phys. Chem. A 2013, 117, 12590–12600. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, X.; Yu, H.S.; Truhlar, D.G.; He, X. Revised M06-L functional for improved accuracy on chemical reaction barrier heights, noncovalent interactions, and solid-state physics. Proc. Natl. Acad. Sci. USA 2017, 114, 8487–8492. [Google Scholar] [CrossRef]
- Mardirossian, N.; Head-Gordon, M. How Accurate Are the Minnesota Density Functionals for Noncovalent Interactions, Isomerization Energies, Thermochemistry, and Barrier Heights Involving Molecules Composed of Main-Group Elements? J. Chem. Theory Comput. 2016, 12, 4303–4325. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, L. The effect of cluster thickness on the adsorption of CH4 on Pdn. Comput. Theor. Chem. 2011, 963, 236–244. [Google Scholar] [CrossRef]





| DSSCs | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Dye | Dye-Bath Solvent 1 (Co-Adsorbents) | λmax/nm 2 | λmax/nm 3 | Dye Loading Amount (10−7 mol cm−2) | Jsc/mA cm−2 | Voc/V | FF | η/% | Ref. |
| 1 | CHCl3/MeOH | 307, 344, 485 | 441 | - | 10.36 | 0.715 | 0.722 | 5.35 | [71] |
| CHCl3/MeOH (DCA) | - | 441 | - | 10.02 | 0.714 | 0.712 | 5.09 | ||
| 2 | CHCl3/MeOH | 306, 368, 468 | 454 | - | 6.87 | 0.687 | 0.678 | 3.20 | |
| CHCl3/MeOH (DCA) | - | 450 | - | 7.48 | 0.683 | 0.734 | 3.75 | ||
| 3 | ACN/CHCl3 | 643 | - | 2.16 | 7.64 | 0.668 | 0.710 | 3.62 | [73] |
| 4 | ACN/CHCl3 | 643 | - | 2.29 | 8.89 | 0.683 | 0.770 | 4.67 | |
| 5 | ACN/CHCl3 | 643 | - | 2.00 | 10.95 | 0.706 | 0.750 | 5.80 | |
| 6 | ACN/CHCl3 | 643 | - | 1.96 | 11.55 | 0.715 | 0.700 | 5.78 | |
| 7 | ACN/CHCl3 | 642 | - | 2.47 | 8.78 | 0.671 | 0.770 | 4.53 | |
| 8 | ACN/CHCl3 | 650 | - | 1.63 | 11.95 | 0.717 | 0.710 | 6.08 | |
| 9 | ACN/TBA/DMSO | 378, 496 | - | 14.11 | 0.660 | 0.653 | 6.08 | [75] | |
| 10 | ACN/TBA/DMSO | 384, 494 | - | 14.32 | 0.910 | 0.539 | 7.04 | ||
| 11 | Toluene/EtOH | 465, 620, 675 | - | 1.55 | 10.51 | 0.700 | 0.719 | 5.19 | [76] |
| 12 | Toluene/EtOH | 465, 621, 678 | - | 1.62 | 12.79 | 0.701 | 0.716 | 6.42 | |
| 13 | Toluene/EtOH | 465, 623, 683 | - | 1.67 | 17.93 | 0.711 | 0.715 | 9.12 | |
| 14 | THF/EtOH | - | - | 0.782 | 8.60 | 0.604 | 0.690 | 3.60 | [77] |
| THF/EtOH (CDCA) | - | - | 0.537 | 8.90 | 0.610 | 0.680 | 3.70 | ||
| THF/EtOH 4 | - | - | 0.776 | 10.70 | 0.650 | 0.700 | 4.90 | ||
| 16 | CHCl3/EtOH | 432, 459, 580, 646 | 634 | 0.525 | 11.60 | 0.760 | 0.710 | 6.26 | [78] |
| 17 | CHCl3/EtOH | 446, 575, 628 | 626 | 0.358 | 11.47 | 0.860 | 0.670 | 6.60 | |
| 18 | CHCl3/EtOH | 431, 457, 585, 646 | 640 | 0.402 | 12.50 | 0.781 | 0.720 | 7.03 | |
| 19 | EtOH/ACN | 697 | 705 | 0.307 | 3.98 | 0.601 | 0.700 | 1.67 | [79] |
| EtOH/ACN (CDCA) | - | - | 0.276 | 4.27 | 0.611 | 0.720 | 1.89 | ||
| 23 | THF/EtOH | 458 | - | - | 2.50 | 0.520 | 0.780 | 1.00 | [80] |
| THF/EtOH (DCA) | - | - | - | 8.90 | 0.600 | 0.760 | 4.05 | ||
| 24 | THF/EtOH | 494 | - | - | 9.25 | 0.630 | 0.610 | 3.56 | |
| THF/EtOH (DCA) | - | - | - | 10.8 | 0.650 | 0.600 | 4.20 | ||
| 25 | CH2Cl2/MeOH | 305, 424 | 422 | 8.12 | 12.26 | 0.756 | 0.660 | 6.14 | [81] |
| 26 | CH2Cl2/MeOH | 305, 414 | 412 | 9.48 | 11.92 | 0.740 | 0.660 | 5.85 | |
| 27 | CH2Cl2/MeOH | 305, 420 | 422 | 10.57 | 10.92 | 0.705 | 0.680 | 5.25 | |
| 28 | Toluene | 501 | 419 | - | 14.83 | 0.755 | 0.720 | 8.10 | [82] |
| 29 | Toluene | 494 | 416 | - | 13.21 | 0.756 | 0.750 | 7.50 | |
| 30 | Toluene | 488 | 414 | - | 12.00 | 0.752 | 0.730 | 6.60 | |
| 31 | THF | 422, 464, 569, 615 | - | - | 13.20 | 0.650 | 0.620 | 5.33 | [83] |
| 32 | DMSO | 437 | 464 | - | 14.00 | 0.570 | 0.690 | 5.51 | [84] |
| 33 | N/A | 253, 290, 401 | 453 | - | 10.20 | 0.707 | 0.592 | 5.20 | [85] |
| 34 | ACN/TBA | 477 | - | 2.88 | 15.64 | 0.667 | 0.670 | 7.02 | [86] |
| 35 | ACN/TBA | 488 | - | 2.26 | 18.16 | 0.680 | 0.650 | 7.99 | |
| 36 | ACN/TBA | 487 | - | 2.38 | 18.19 | 0.706 | 0.690 | 8.82 | |
| 37 | THF/EtOH (CDCA) | - | - | 0.238 | 13.59 | 0.759 | 0.772 | 8.30 | [87] |
| THF/EtOH (HC-A1) | - | - | 0.220 | 15.62 | 0.759 | 0.762 | 9.05 | ||
| 38 | THF/EtOH (CDCA) | - | - | 0.208 | 15.58 | 0.858 | 0.738 | 9.87 | |
| THF/EtOH (HC-A1) | - | - | 0.204 | 16.42 | 0.846 | 0.769 | 10.69 | ||
| 39 | THF/EtOH (CDCA) | - | - | 0.199 | 15.82 | 0.858 | 0.731 | 9.94 | |
| THF/EtOH (HC-A1) | - | - | 0.186 | 16.50 | 0.846 | 0.772 | 10.80 | ||
| 40 | CHCl3/EtOH | 508 | 474 | 2.33 | 13.44 | 0.786 | 0.675 | 7.13 | [88] |
| CHCl3/EtOH (CDCA) | - | - | 2.32 | 15.06 | 0.775 | 0.704 | 8.21 | ||
| CHCl3/EtOH (dye 41) | - | - | 1.87 + 0.45 | 18.30 | 0.737 | 0.729 | 9.83 | ||
| 41 | CH2Cl2 | 318, 405, 546 | - | - | 8.46 | 0.601 | 0.760 | 3.86 | [89] |
| CH2Cl2 (CDCA) | - | - | - | 17.50 | 0.657 | 0.740 | 8.56 | ||
| CHCl3 | - | - | - | 16.25 | 0.618 | 0.720 | 7.22 | ||
| CHCl3 (CDCA) | - | - | - | 17.82 | 0.646 | 0.720 | 8.29 | ||
| N719 | EtOH | - | - | - | 0.22 | 0.600 | 0.230 | 0.03 | [90] |
| EtOH/H2O | - | - | - | 4.15 | 0.650 | 0.570 | 1.50 | ||
| 45 | TBA/ACN | 8.07 | 0.800 | 0.760 | 4.90 | [91] | |||
| TBA/ACN (CDCA) | 8.08 | 0.790 | 0.680 | 4.34 | |||||
| 46 | TBA/ACN | 11.57 | 0.810 | 0.590 | 5.57 | ||||
| TBA/ACN (CDCA) | 11.59 | 0.800 | 0.680 | 6.30 | |||||
| 47 | CH2Cl2 | 438 | 423 | 4.46 | 9.70 | 0.760 | 0.720 | 5.33 | [92] |
| ACN | - | - | 4.39 | 9.40 | 0.720 | 0.680 | 4.59 | ||
| EtOH | - | - | 3.36 | 9.10 | 0.709 | 0.660 | 4.23 | ||
| THF | - | - | 3.25 | 8.20 | 0.663 | 0.670 | 3.61 | ||
| DMF | - | - | 0.69 | 5.60 | 0.579 | 0.620 | 2.00 | ||
| 49 | THF 5 | - | - | 1.11 | 12.73 | 0.650 | 0.680 | 5.60 | [93] |
| THF 6 | - | - | 1.25 | 11.83 | 0.640 | 0.700 | 5.29 | ||
| 50 | ACN/H2O 7 | - | - | - | 0.48 | 0.220 | 0.460 | 0.05 | [94] |
| ACN/H2O 8 | - | - | - | 2.83 | 0.290 | 0.610 | 0.50 | ||
| 51 | N/A | - | - | - | 14.37 | 0.740 | 0.685 | 7.23 | [95] |
| N/A 9 | - | - | - | 14.91 | 0.780 | 0.715 | 8.28 | ||
| 52 | N/A | - | - | - | 1.60 | 0.480 | 0.643 | 1.00 | [96] |
| N/A 10 | - | - | - | 2.99 | 0.500 | 0.669 | 1.47 | ||
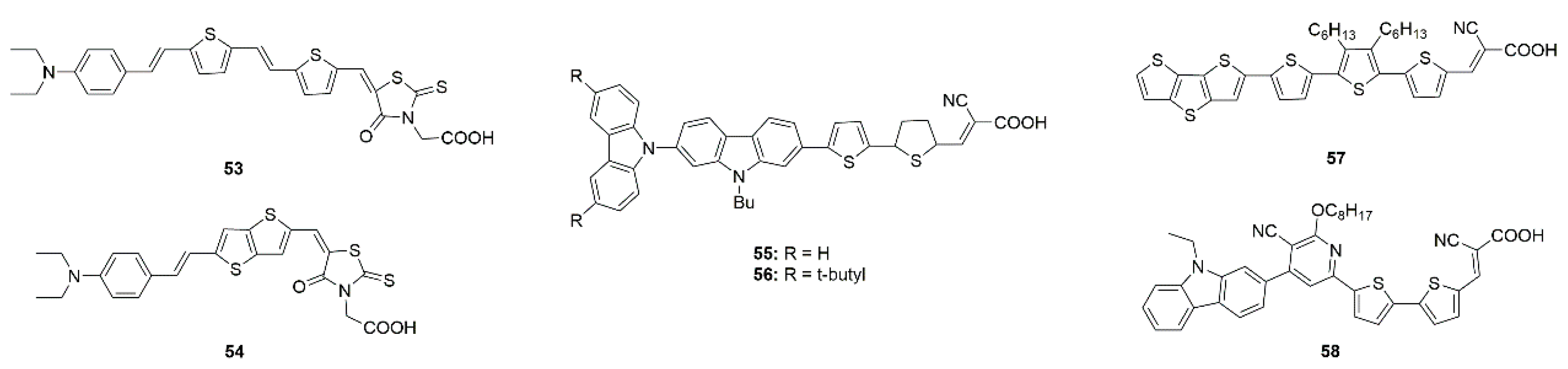
| 53 | THF | 513 | - | - | 10.64 | 0.520 | 0.700 | 3.87 | [97] |
| 54 | THF | 488 | - | - | 15.23 | 0.560 | 0.730 | 6.23 | |
| 55 | ACN/TBA/DMSO | 262, 294, 345, 476 | - | - | 15.78 | 0.601 | 0.640 | 6.04 | [98] |
| 56 | ACN/TBA/DMSO | 258, 298, 351, 478 | - | - | 14.00 | 0.612 | 0.640 | 5.48 | |
| 57 | ACN/TBA | 314, 429 | - | 3.69 | 12.09 | 0.620 | 0.670 | 5.02 | [99] |
| 58 | ACN | 285, 405, 475 | - | 1.92 | 6.56 | 0.540 | 0.689 | 2.44 | [100] |
| ACN (CDCA) | - | - | 2.04 | 5.87 | 0.560 | 0.686 | 2.25 | ||
| ssDSSCs | |||||||||
| 14 | N/A 11 | 438 | - | - | 0.65 | 0.719 | 0.780 | 3.89 | [101] |
| 15 | N/A 11 | 458 | - | - | 0.78 | 0.740 | 0.730 | 4.51 | |
| 20 | ACN/TBA | - | - | 0.28 | 6.40 | 0.710 | 0.570 | 2.60 | [102] |
| 21 | ACN/TBA | - | - | - | 6.80 | 0.790 | 0.430 | 2.30 | |
| 22 | ACN/TBA | - | - | 0.19 | 7.10 | 0.790 | 0.460 | 2.60 | |
| 44 | ACN | - | - | - | 12.90 | 0.550 | 0.483 | 3.40 | [103] |
| ACN (CA) | - | - | - | 13.45 | 0.552 | 0.506 | 3.60 | ||
| 44 | TBA/ACN | - | - | 0.066 | 4.70 | 0.760 | 0.727 | 2.60 | [104] |
| TBA/ACN (MAPbBr3) | - | - | 0.052 | 5.40 | 0.810 | 0.704 | 3.10 | ||
| 48 | ACN/TBA | 439 | - | - | 16.14 | 0.496 | 0.420 | 3.33 | [105] |
| Toluene | 484 | - | - | 13.42 | 0.453 | 0.390 | 2.40 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, F.; Testoff, T.T.; Wang, L.; Zhou, X. Cause, Regulation and Utilization of Dye Aggregation in Dye-Sensitized Solar Cells. Molecules 2020, 25, 4478. https://doi.org/10.3390/molecules25194478
Xu F, Testoff TT, Wang L, Zhou X. Cause, Regulation and Utilization of Dye Aggregation in Dye-Sensitized Solar Cells. Molecules. 2020; 25(19):4478. https://doi.org/10.3390/molecules25194478
Chicago/Turabian StyleXu, Fang, Thomas T. Testoff, Lichang Wang, and Xueqin Zhou. 2020. "Cause, Regulation and Utilization of Dye Aggregation in Dye-Sensitized Solar Cells" Molecules 25, no. 19: 4478. https://doi.org/10.3390/molecules25194478
APA StyleXu, F., Testoff, T. T., Wang, L., & Zhou, X. (2020). Cause, Regulation and Utilization of Dye Aggregation in Dye-Sensitized Solar Cells. Molecules, 25(19), 4478. https://doi.org/10.3390/molecules25194478







