Continuous Electrochemical Reduction of CO2 to Formate: Comparative Study of the Influence of the Electrode Configuration with Sn and Bi-Based Electrocatalysts
Abstract
1. Introduction
2. Comparative Study of the Electrode Configurations: GDE–CCME
2.1. Sn-Based Electrodes
2.2. Bi-Based Electrodes
3. Comparative Study of the Catalyst Nature
3.1. Sn vs. Bi in GDE Configuration
3.2. Sn vs. Bi in CCME Configuration
4. Experimental Conditions
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Overview of Greenhouse Gases: United States Environmental Protection Agency. Available online: https://www.epa.gov/ghgemissions/overview-greenhouse-gases (accessed on 16 June 2020).
- Sustainable Development Goals: United Nations. Available online: https://sustainabledevelopment.un.org/ (accessed on 16 June 2020).
- Irabien, A.; Alvarez-Guerra, M.; Albo, J.; Domínguez-Ramos, A. Electrochemical conversion of CO2 to value-added products. In Electrochememical Water Wastewater Treatment; Martínez-Huitle, C.A., Rodrigo, M.A., Scialdone, O., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 29–59. [Google Scholar]
- Qiao, J.; Liu, Y.; Zhan, J. Electrochemical Reduction of Carbon Dioxide. Fundamentals and Technologies; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9781482258257. [Google Scholar]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef]
- Sohaib, Q.; Vadillo, J.M.; Gómez-Coma, L.; Albo, J.; Druon-Bocquet, S.; Irabien, A.; Sanchez-Marcano, J. CO2 capture with room temperature ionic liquids; coupled absorption/desorption and single module absorption in membrane contactor. Chem. Eng. Sci. 2020, 223, 115719. [Google Scholar] [CrossRef]
- Nocito, F.; Dibenedetto, A. Atmospheric CO2 mitigation technologies: Carbon capture utilization and storage. Curr. Opin. Green Sustain. Chem. 2020, 21, 34–43. [Google Scholar] [CrossRef]
- Mustafa, A.; Lougou, B.G.; Shuai, Y.; Wang, Z.; Tan, H. Current technology development for CO2 utilization into solar fuels and chemicals: A review. J. Energy Chem. 2020, 49, 96–123. [Google Scholar] [CrossRef]
- Vadillo, J.M.; Gómez-Coma, L.; Garea, A.; Irabien, A. CO2 desorption performance from imidazolium ionic liquids by membrane vacuum regeneration technology. Membranes 2020, 10, 234. [Google Scholar] [CrossRef]
- Lee, C.W.; Kim, C.; Min, B.K. Theoretical insights into selective electrochemical conversion of carbon dioxide. Nano Converg. 2019, 6, 8. [Google Scholar] [CrossRef]
- Ting, L.R.L.; Yeo, B.S. Recent advances in understanding mechanisms for the electrochemical reduction of carbon dioxide. Curr. Opin. Electrochem. 2018, 8, 126–134. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, W.; Li, Y.; Chen, J.; Yu, B.; Wang, J.; Zhang, L.; Zhang, J. Energy related CO2 conversion and utilization: Advanced materials/nanomaterials, reaction mechanisms and technologies. Nano Energy 2017, 40, 512–539. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Senthil Kumar, P.; Varjani, S.J.; Saravanan, A. A review on photochemical, biochemical and electrochemical transformation of CO2 into value-added products. J. CO2 Util. 2019, 33, 131–147. [Google Scholar] [CrossRef]
- Merino-Garcia, I.; Alvarez-Guerra, E.; Albo, J.; Irabien, A. Electrochemical membrane reactors for the utilisation of carbon dioxide. Chem. Eng. J. 2016, 305, 104–120. [Google Scholar] [CrossRef]
- Castro, S.; Albo, J.; Irabien, A. Photoelectrochemical Reactors for CO2 Utilization. ACS Sustain. Chem. Eng. 2018, 6, 15877–15894. [Google Scholar] [CrossRef]
- Kumar, B.; Brian, J.P.; Atla, V.; Kumari, S.; Bertram, K.A.; White, R.T.; Spurgeon, J.M. New trends in the development of heterogeneous catalysts for electrochemical CO2 reduction. Catal. Today 2016, 270, 19–30. [Google Scholar] [CrossRef]
- Lu, Q.; Jiao, F. Electrochemical CO2 reduction: Electrocatalyst, reaction mechanism, and process engineering. Nano Energy 2016, 29, 439–456. [Google Scholar] [CrossRef]
- Endrődi, B.; Bencsik, G.; Darvas, F.; Jones, R.; Rajeshwar, K.; Janáky, C. Continuous-flow electroreduction of carbon dioxide. Prog. Energy Combust. Sci. 2017, 62, 133–154. [Google Scholar] [CrossRef]
- Gao, D.; Arán-Ais, R.M.; Jeon, H.S.; Roldan Cuenya, B. Rational catalyst and electrolyte design for CO2 electroreduction towards multicarbon products. Nat. Catal. 2019, 2, 198–210. [Google Scholar] [CrossRef]
- Ren, S.; Joulié, D.; Salvatore, D.; Torbensen, K.; Wang, M.; Robert, M.; Berlinguette, C.P. Molecular electrocatalysts can mediate fast, selective CO2 reduction in a flow cell. Science 2019, 365, 367–369. [Google Scholar] [CrossRef]
- Jouny, M.; Hutchings, G.S.; Jiao, F. Carbon monoxide electroreduction as an emerging platform for carbon utilization. Nat. Catal. 2019, 2, 1062–1070. [Google Scholar] [CrossRef]
- Du, D.; Lan, R.; Humphreys, J.; Tao, S. Progress in inorganic cathode catalysts for electrochemical conversion of carbon dioxide into formate or formic acid. J. Appl. Electrochem. 2017, 47, 661–678. [Google Scholar] [CrossRef]
- Taheri, A.; Berben, L.A. Making C-H bonds with CO2: Production of formate by molecular electrocatalysts. Chem. Commun. 2016, 52, 1768–1777. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, Z.; Lu, X.; Liang, Y.; Wang, H. Domino electroreduction of CO2 to methanol on a molecular catalyst. Nature 2019, 575, 639–642. [Google Scholar] [CrossRef]
- Nitopi, S.; Bertheussen, E.; Scott, S.B.; Liu, X.; Engstfeld, A.K.; Horch, S.; Seger, B.; Stephens, I.E.L.; Chan, K.; Hahn, C.; et al. Progress and Perspectives of Electrochemical CO2 Reduction on Copper in Aqueous Electrolyte. Chem. Rev. 2019, 119, 7610–7672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Hu, Y.; Ma, L.; Zhu, G.; Wang, Y.; Xue, X.; Chen, R.; Yang, S.; Jin, Z. Progress and Perspective of Electrocatalytic CO2 Reduction for Renewable Carbonaceous Fuels and Chemicals. Adv. Sci. 2018, 5, 1700275. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Merino-Garcia, I.; Albo, J.; Sánchez-Sánchez, C.M. Electrochemical CO2 reduction reaction on cost-effective oxide-derived copper and transition metal–nitrogen–carbon catalysts. Curr. Opin. Electrochem. 2020, 23, 65–73. [Google Scholar] [CrossRef]
- Zhao, J.; Xue, S.; Barber, J.; Zhou, Y.; Meng, J.; Ke, X. An overview of Cu-based heterogeneous electrocatalysts for CO2 reduction. J. Mater. Chem. A 2020, 8, 4700–4734. [Google Scholar] [CrossRef]
- Agarwal, A.S.; Zhai, Y.; Hill, D.; Sridhar, N. The electrochemical reduction of carbon dioxide to formate/formic acid: Engineering and economic feasibility. ChemSusChem 2011, 4, 1301–1310. [Google Scholar] [CrossRef]
- Bienen, F.; Kopljar, D.; Löwe, A.; Aßmann, P.; Stoll, M.; Rößner, P.; Wagner, N.; Friedrich, A.; Klemm, E. Utilizing Formate as an Energy Carrier by Coupling CO2 Electrolysis with Fuel Cell Devices. Chem. Ing. Tech. 2019, 91, 872–882. [Google Scholar] [CrossRef]
- An, L.; Chen, R. Direct formate fuel cells: A review. J. Power Sources 2016, 320, 127–139. [Google Scholar] [CrossRef]
- Preuster, P.; Albert, J. Biogenic Formic Acid as a Green Hydrogen Carrier. Energy Technol. 2018, 6, 501–509. [Google Scholar] [CrossRef]
- Eppinger, J.; Huang, K.W. Formic Acid as a Hydrogen Energy Carrier. ACS Energy Lett. 2017, 2, 188–195. [Google Scholar] [CrossRef]
- Han, N.; Ding, P.; He, L.; Li, Y.; Li, Y. Promises of Main Group Metal–Based Nanostructured Materials for Electrochemical CO2 Reduction to Formate. Adv. Energy Mater. 2019, 10, 1902338. [Google Scholar] [CrossRef]
- Zhao, S.; Li, S.; Guo, T.; Zhang, S.; Wang, J.; Wu, Y.; Chen, Y. Advances in Sn-Based Catalysts for Electrochemical CO2 Reduction. Nano-Micro Lett. 2019, 11, 62. [Google Scholar] [CrossRef]
- Sen, S.; Liu, D.; Palmore, G.T.R. Electrochemical reduction of CO2 at copper nanofoams. ACS Catal. 2014, 4, 3091–3095. [Google Scholar] [CrossRef]
- Gao, S.; Lin, Y.; Jiao, X.; Sun, Y.; Luo, Q.; Zhang, W.; Li, D.; Yang, J.; Xie, Y. Partially oxidized atomic cobalt layers for carbon dioxide electroreduction to liquid fuel. Nature 2016, 529, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Sahin, S.; Cai, R.; Abdellaoui, S.; Hickey, D.P.; Minteer, S.D.; Milton, R.D. Creating a Low-Potential Redox Polymer for Efficient Electroenzymatic CO2 Reduction. Angew. Chem. Int. Ed. 2018, 57, 6582–6586. [Google Scholar] [CrossRef] [PubMed]
- Pander, J.E.; Lum, J.W.J.; Yeo, B.S. The importance of morphology on the activity of lead cathodes for the reduction of carbon dioxide to formate. J. Mater. Chem. A 2019, 7, 4093–4101. [Google Scholar] [CrossRef]
- Alvarez-Guerra, M.; Quintanilla, S.; Irabien, A. Conversion of carbon dioxide into formate using a continuous electrochemical reduction process in a lead cathode. Chem. Eng. J. 2012, 207–208, 278–284. [Google Scholar] [CrossRef]
- Gálvez-Vázquez, M.D.J.; Moreno-García, P.; Guo, H.; Hou, Y.; Dutta, A.; Waldvogel, S.R.; Broekmann, P. Leaded Bronze Alloy as a Catalyst for the Electroreduction of CO2. ChemElectroChem 2019, 6, 2324–2330. [Google Scholar] [CrossRef]
- Luo, W.; Xie, W.; Li, M.; Zhang, J.; Züttel, A. 3D hierarchical porous indium catalyst for highly efficient electroreduction of CO2. J. Mater. Chem. A 2019, 7, 4505–4515. [Google Scholar] [CrossRef]
- Zha, B.; Li, C.; Li, J. Efficient electrochemical reduction of CO2 into formate and acetate in polyoxometalate catholyte with indium catalyst. J. Catal. 2020, 382, 69–76. [Google Scholar] [CrossRef]
- Yuan, X.; Luo, Y.; Zhang, B.; Dong, C.; Lei, J.; Yi, F.; Duan, T.; Zhu, W.; He, R. Decoration of in nanoparticles on In2S3 nanosheets enables efficient electrochemical reduction of CO2. Chem. Commun. 2020, 56, 4212–4215. [Google Scholar] [CrossRef]
- Klinkova, A.; De Luna, P.; Dinh, C.T.; Voznyy, O.; Larin, E.M.; Kumacheva, E.; Sargent, E.H. Rational Design of Efficient Palladium Catalysts for Electroreduction of Carbon Dioxide to Formate. ACS Catal. 2016, 6, 8115–8120. [Google Scholar] [CrossRef]
- Gao, D.; Zhou, H.; Cai, F.; Wang, D.; Hu, Y.; Jiang, B.; Cai, W.-B.; Chen, X.; Si, R.; Yang, F.; et al. Switchable CO2 electroreduction via engineering active phases of Pd nanoparticles. Nano Res. 2017, 10, 2181–2191. [Google Scholar] [CrossRef]
- Lee, W.; Kim, Y.E.; Youn, M.H.; Jeong, S.K.; Park, K.T. Catholyte-Free Electrocatalytic CO2 Reduction to Formate. Angew. Chem. Int. Ed. 2018, 57, 6883–6887. [Google Scholar] [CrossRef]
- Li, D.; Wu, J.; Liu, T.; Liu, J.; Yan, Z.; Zhen, L.; Feng, Y. Tuning the pore structure of porous tin foam electrodes for enhanced electrochemical reduction of carbon dioxide to formate. Chem. Eng. J. 2019, 375, 122024. [Google Scholar] [CrossRef]
- He, G.; Tang, H.; Wang, H.; Bian, Z. Highly Selective and Active Pd-In/three-dimensional Graphene with Special Structure for Electroreduction CO2 to Formate. Electroanalysis 2018, 30, 84–93. [Google Scholar] [CrossRef]
- Proietto, F.; Schiavo, B.; Galia, A.; Scialdone, O. Electrochemical conversion of CO2 to HCOOH at tin cathode in a pressurized undivided filter-press cell. Electrochim. Acta 2018, 277, 30–40. [Google Scholar] [CrossRef]
- Alvarez-Guerra, M.; Del Castillo, A.; Irabien, A. Continuous electrochemical reduction of carbon dioxide into formate using a tin cathode: Comparison with lead cathode. Chem. Eng. Res. Des. 2014, 92, 692–701. [Google Scholar] [CrossRef]
- Del Castillo, A.; Alvarez-Guerra, M.; Irabien, A. Continuous electroreduction of CO2 to formate using Sn gas diffusion electrodes. AIChE J. 2014, 60, 3557–3564. [Google Scholar] [CrossRef]
- Del Castillo, A.; Alvarez-Guerra, M.; Solla-Gullón, J.; Sáez, A.; Montiel, V.; Irabien, A. Electrocatalytic reduction of CO2 to formate using particulate Sn electrodes: Effect of metal loading and particle size. Appl. Energy 2015, 157, 165–173. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, T.; Zheng, W.; Lei, C.; Yang, B.; Chen, J.; Li, Z.; Lei, L.; Yuan, C.; Hou, Y. Nanoconfined Tin Oxide within N-Doped Nanocarbon Supported on Electrochemically Exfoliated Graphene for Efficient Electroreduction of CO2 to Formate and C1 Products. ACS Appl. Mater. Interf. 2020, 12, 16178–16185. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, X.; Guo, S.-X.; Bond, A.M.; Zhang, J. Formation of lattice-dislocated bismuth nanowires on copper foam for enhanced electrocatalytic CO2 reduction at low overpotential. Energy Environ. Sci. 2019, 12, 1334–1340. [Google Scholar] [CrossRef]
- Wu, D.; Huo, G.; Chen, W.Y.; Fu, X.Z.; Luo, J.L. Boosting formate production at high current density from CO2 electroreduction on defect-rich hierarchical mesoporous Bi/Bi2O3 junction nanosheets. Appl. Catal. B Environ. 2020, 271, 118957. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, C.; Wu, C.; Yu, H. Direct synthesis of bismuth nanosheets on a gas diffusion layer as a high-performance cathode for a coupled electrochemical system capable of electroreduction of CO2 to formate with simultaneous degradation of organic pollutants. Electrochim. Acta 2019, 319, 138–147. [Google Scholar] [CrossRef]
- Yang, F.; Elnabawy, A.O.; Schimmenti, R.; Song, P.; Wang, J.; Peng, Z.; Yao, S.; Deng, R.; Song, S.; Lin, Y.; et al. Bismuthene for highly efficient carbon dioxide electroreduction reaction. Nat. Commun. 2020, 11, 1088. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Wang, Q.; Wu, C. Rapid and scalable synthesis of bismuth dendrites on copper mesh as a high-performance cathode for electroreduction of CO2 to formate. J. CO2 Util. 2020, 36, 96–104. [Google Scholar] [CrossRef]
- Tran-Phu, T.; Daiyan, R.; Fusco, Z.; Ma, Z.; Amal, R.; Tricoli, A. Nanostructured β-Bi2O3 Fractals on Carbon Fibers for Highly Selective CO2 Electroreduction to Formate. Adv. Funct. Mater. 2020, 30, 1–8. [Google Scholar] [CrossRef]
- Lu, P.; Gao, D.; He, H.; Wang, Q.; Liu, Z.; Dipazir, S.; Yuan, M.; Zu, W.; Zhang, G. Facile synthesis of a bismuth nanostructure with enhanced selectivity for electrochemical conversion of CO2 to formate. Nanoscale 2019, 11, 7805–7812. [Google Scholar] [CrossRef]
- García de Arquer, F.P.; Bushuyev, O.S.; De Luna, P.; Dinh, C.T.; Seifitokaldani, A.; Saidaminov, M.I.; Tan, C.S.; Quan, L.N.; Proppe, A.; Kibria, M.G.; et al. 2D Metal Oxyhalide-Derived Catalysts for Efficient CO2 Electroreduction. Adv. Mater. 2018, 30, 6–11. [Google Scholar] [CrossRef]
- Kumawat, A.S.; Sarkar, A. Comparative Study of Carbon Supported Pb, Bi and Sn Catalysts for Electroreduction of Carbon Dioxide in Alkaline Medium. J. Electrochem. Soc. 2017, 164, H1112–H1120. [Google Scholar] [CrossRef]
- Vennekoetter, J.B.; Sengpiel, R.; Wessling, M. Beyond the catalyst: How electrode and reactor design determine the product spectrum during electrochemical CO2 reduction. Chem. Eng. J. 2019, 364, 89–101. [Google Scholar] [CrossRef]
- Vennekötter, J.B.; Scheuermann, T.; Sengpiel, R.; Wessling, M. The electrolyte matters: Stable systems for high rate electrochemical CO2 reduction. J. CO2 Util. 2019, 32, 202–213. [Google Scholar]
- Del Castillo, A.; Alvarez-Guerra, M.; Solla-Gullón, J.; Sáez, A.; Montiel, V.; Irabien, A. Sn nanoparticles on gas diffusion electrodes: Synthesis, characterization and use for continuous CO2 electroreduction to formate. J. CO2 Util. 2017, 18, 222–228. [Google Scholar] [CrossRef]
- Díaz-Sainz, G.; Alvarez-Guerra, M.; Solla-Gullón, J.; García-Cruz, L.; Montiel, V.; Irabien, A. Catalyst coated membrane electrodes for the gas phase CO2 electroreduction to formate. Catal. Today 2020, 346, 58–64. [Google Scholar] [CrossRef]
- Díaz-Sainz, G.; Alvarez-Guerra, M.; Solla-Gullón, J.; García-Cruz, L.; Montiel, V.; Irabien, A. CO2 electroreduction to formate: Continuous single-pass operation in a filter-press reactor at high current densities using Bi gas diffusion electrodes. J. CO2 Util. 2019, 34, 12–19. [Google Scholar]
- Díaz-Sainz, G.; Alvarez-Guerra, M.; Solla-Gullón, J.; García-Cruz, L.; Montiel, V.; Irabien, A. Gas–liquid–solid reaction system for CO2 electroreduction to formate without using supporting electrolyte. AIChE J. 2020, 66, e16299. [Google Scholar]
- Xia, C.; Zhu, P.; Jiang, Q.; Pan, Y.; Liang, W.; Stavitsk, E. Continuous production of pure liquid fuel solutions via electrocatalytic CO2 reduction using solid-electrolyte devices. Nat. Energy 2019, 5, 776–785. [Google Scholar] [CrossRef]
- Díaz-Sainz, G.; Alvarez-Guerra, M.; Ávila-Bolívar, B.; Solla-Gullón, J.; Montiel, V.; Irabien, A. Improving trade-offs in the figures of merit of gas-phase single-pass continuous CO2 electrocatalytic reduction to formate. Chem. Eng. J. 2020, 405, 126965. [Google Scholar] [CrossRef]
- Chen, Y.; Vise, A.; Klein, W.E.; Cetinbas, F.C.; Myers, D.J.; Smith, W.A.; Deutsch, T.G.; Neyerlin, K.C. A Robust, Scalable Platform for the Electrochemical Conversion of CO2 to Formate: Identifying Pathways to Higher Energy Efficiencies. ACS Energy Lett. 2020, 5, 1825–1833. [Google Scholar] [CrossRef]
- Marcos-Madrazo, A.; Casado-Coterillo, C.; Irabien, Á. Sustainable Membrane-Coated Electrodes for CO2 Electroreduction to Methanol in Alkaline Media. ChemElectroChem 2019, 6, 5273–5282. [Google Scholar] [CrossRef]
- Merino-Garcia, I.; Albo, J.; Krzywda, P.; Mul, G.; Irabien, A. Bimetallic Cu-based hollow fibre electrodes for CO2 electroreduction. Catal. Today 2020, 346, 34–39. [Google Scholar] [CrossRef]
- Hosseini, M.G.; Mahmoodi, R.; Daneshvari-Esfahlan, V. Ni@Pd core-shell nanostructure supported on multi-walled carbon nanotubes as efficient anode nanocatalysts for direct methanol fuel cells with membrane electrode assembly prepared by catalyst coated membrane method. Energy 2018, 161, 1074–1084. [Google Scholar] [CrossRef]
- Ávila-Bolívar, B.; García-Cruz, L.; Montiel, V.; Solla-Gullón, J. Electrochemical Reduction of CO2 to Formate on Easily Prepared Carbon-Supported Bi Nanoparticles. Molecules 2019, 24, 2032. [Google Scholar]

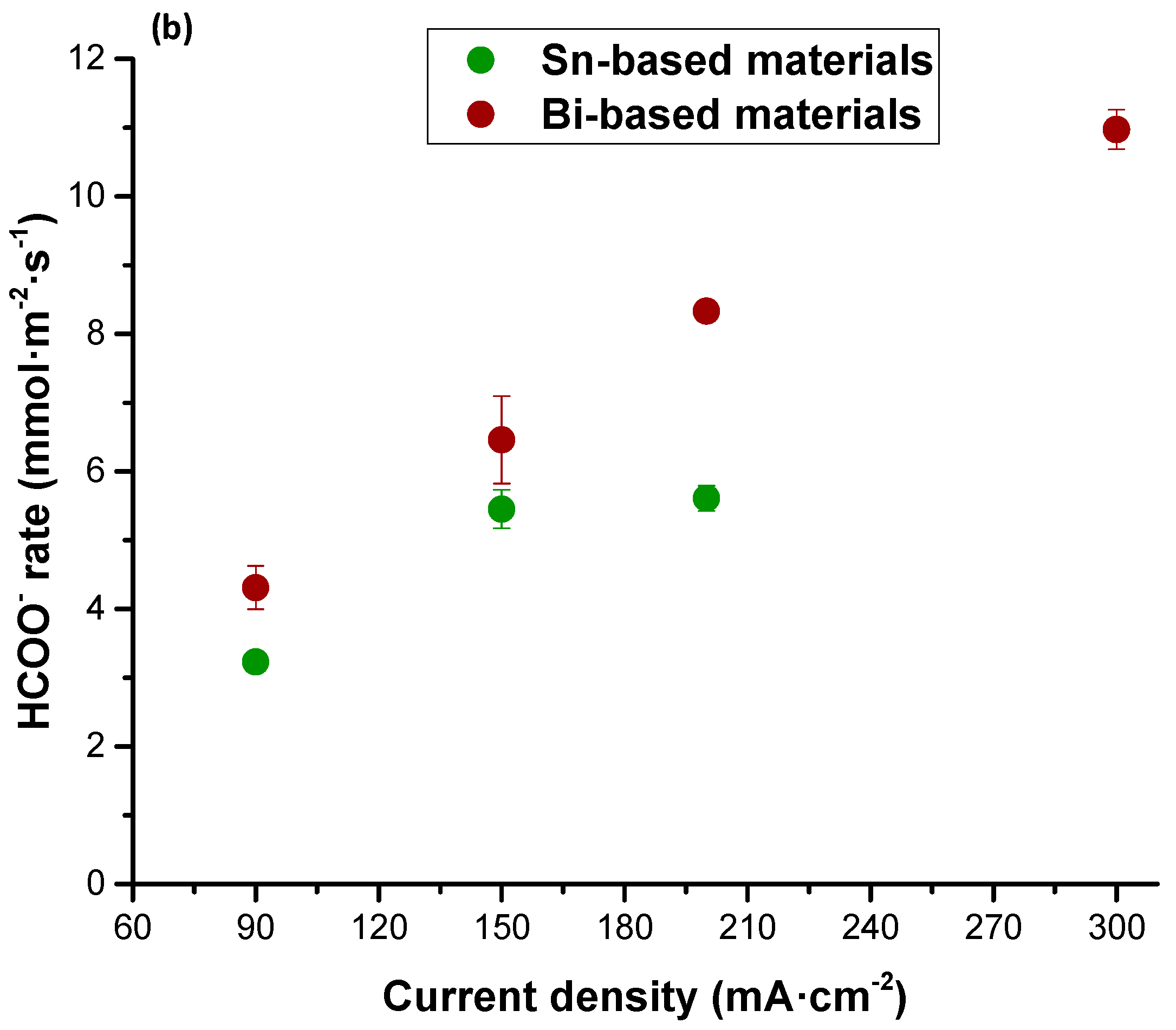

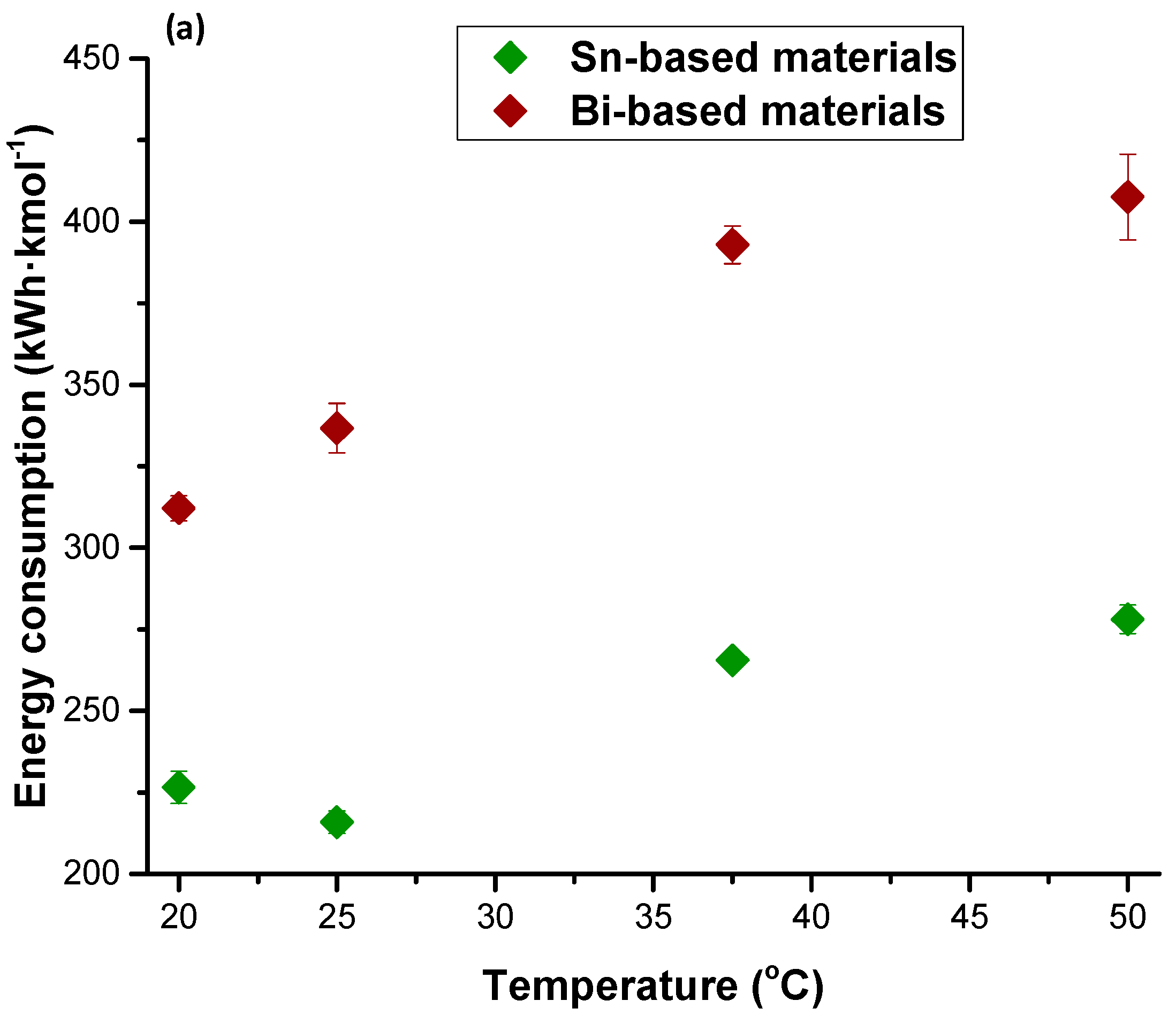

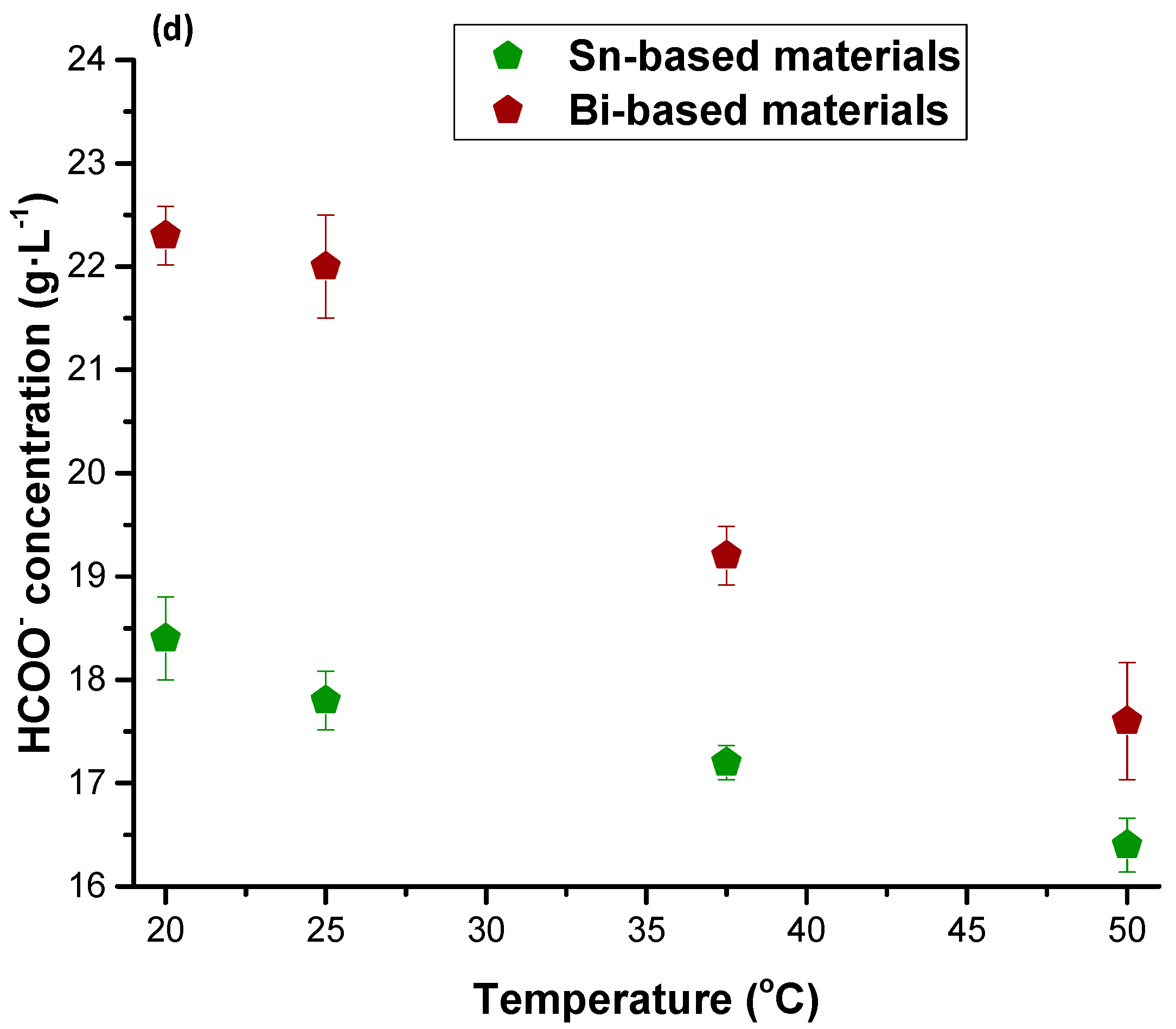
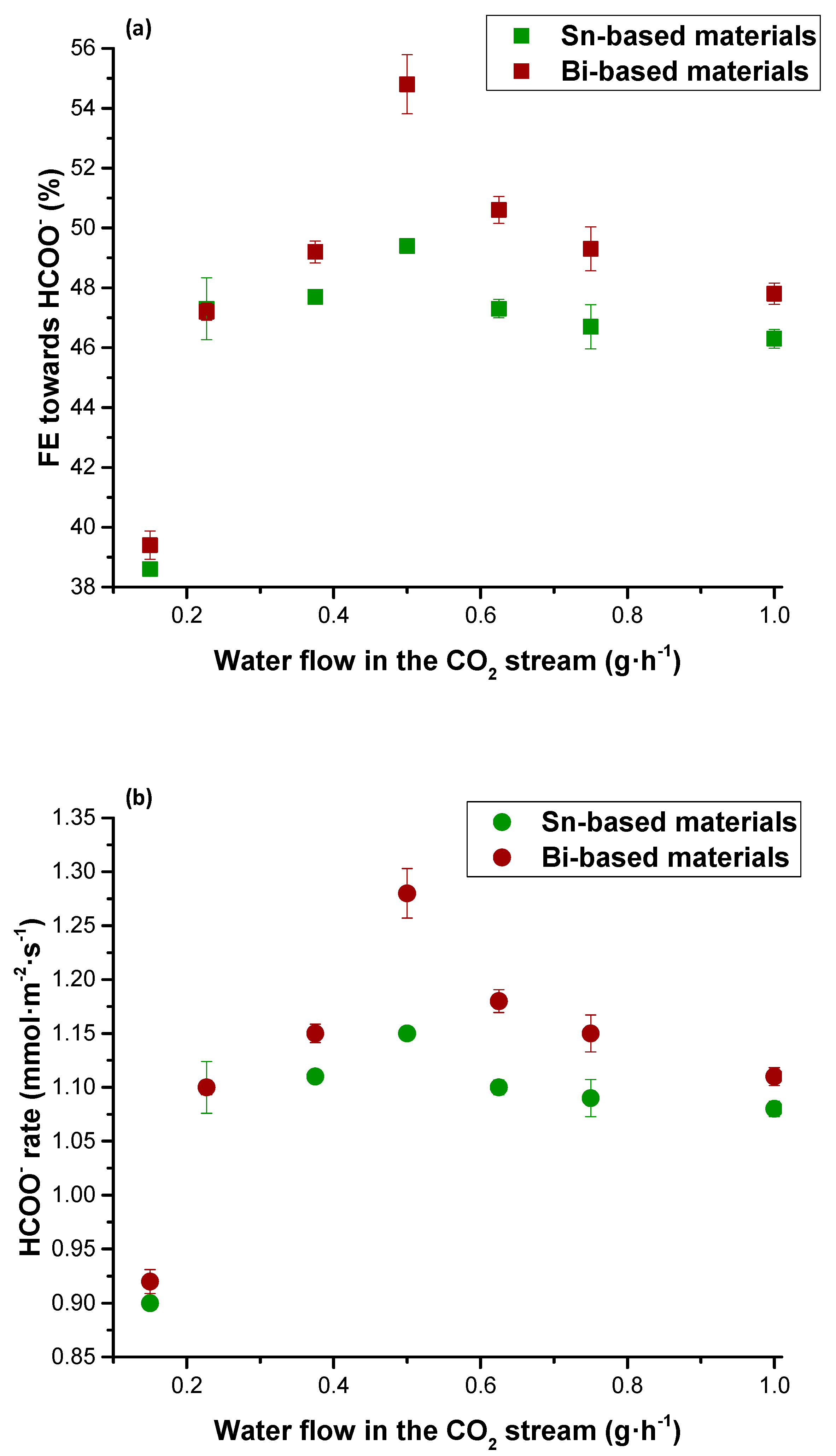


| Figures of Merit | Configuration | ||||
|---|---|---|---|---|---|
| Sn/C-GDEs [66] | Sn/C-CCMEs [67] | ||||
| Flow in the cathode side | 5.7 | 5.7 | 5.7 | 0.7 | ~0.008 (0.5 g·h−1) |
| (mL·min−1) | |||||
| Current density | 90 | 150 | 200 | 200 | 45 |
| (mA·cm−2) | |||||
| Absolute cell potential | 3.1 | 3.7 | 4.0 | 4.3 | 2.2 |
| (V) | |||||
| HCOO− concentration | 1.5 | 2.5 | 2.7 | 16.9 | 19.2 |
| (g·L−1) | |||||
| HCOO− rate | 3.23 | 5.45 | 5.61 | 4.38 | 1.15 |
| (mmol·m−2·s−1) | |||||
| FE for HCOO− | 69.4 | 70 | 54.1 | 42.3 | 49.4 |
| (%) | |||||
| Energy consumption | 239 | 282 | 396 | 513 | 244 |
| (kWh·kmol−1) | |||||
| Figures of Merit | Configuration | |||||
|---|---|---|---|---|---|---|
| Bi/C-GDEs [68] | Bi/C-CCMEs [69] | |||||
| Flow in the cathode side | 5.7 | 5.7 | 5.7 | 5.7 | 0.7 | ~0.008 (0.5 g·h−1) |
| (mL·min−1) | ||||||
| Current density | 90 | 150 | 200 | 300 | 200 | 45 |
| (mA·cm−2) | ||||||
| Absolute cell potential | 3.1 | 3.7 | 4.2 | 5.4 | 4.5 | 2.7 |
| (V) | ||||||
| HCOO− concentration | 2.0 | 3.06 | 3.9 | 5.2 | 18.0 | 25.9 |
| (g·L−1) | ||||||
| HCOO− rate | 4.31 | 6.46 | 8.33 | 10.97 | 4.67 | 1.28 |
| (mmol·m−2·s−1) | ||||||
| FE for HCOO− | 92.4 | 83.1 | 80.4 | 70.6 | 45.1 | 54.8 |
| (%) | ||||||
| Energy consumption | 177 | 240 | 277 | 410 | 535 | 266 |
| (kWh·kmol−1) | ||||||
| Operating Condition | Energy Consumption (kWh·kmol−1 of HCOO−) | ||
|---|---|---|---|
| Current Density (mA·cm−2) | Catholyte Flow (mL·min−1) | Sn/C-GDEs [66] | Bi/C-GDEs [68] |
| 90 | 5.7 | 239 | 177 |
| 150 | 282 | 240 | |
| 200 | 396 | 277 | |
| 90 | 1.5 | 267 | 186 |
| 200 | 395 | 364 | |
| 0.7 | 544 | 535 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Sainz, G.; Alvarez-Guerra, M.; Irabien, A. Continuous Electrochemical Reduction of CO2 to Formate: Comparative Study of the Influence of the Electrode Configuration with Sn and Bi-Based Electrocatalysts. Molecules 2020, 25, 4457. https://doi.org/10.3390/molecules25194457
Díaz-Sainz G, Alvarez-Guerra M, Irabien A. Continuous Electrochemical Reduction of CO2 to Formate: Comparative Study of the Influence of the Electrode Configuration with Sn and Bi-Based Electrocatalysts. Molecules. 2020; 25(19):4457. https://doi.org/10.3390/molecules25194457
Chicago/Turabian StyleDíaz-Sainz, Guillermo, Manuel Alvarez-Guerra, and Angel Irabien. 2020. "Continuous Electrochemical Reduction of CO2 to Formate: Comparative Study of the Influence of the Electrode Configuration with Sn and Bi-Based Electrocatalysts" Molecules 25, no. 19: 4457. https://doi.org/10.3390/molecules25194457
APA StyleDíaz-Sainz, G., Alvarez-Guerra, M., & Irabien, A. (2020). Continuous Electrochemical Reduction of CO2 to Formate: Comparative Study of the Influence of the Electrode Configuration with Sn and Bi-Based Electrocatalysts. Molecules, 25(19), 4457. https://doi.org/10.3390/molecules25194457









