Molecular Recalcitrance of Hair Passing the Digestive System of a Canid
Abstract
1. Introduction
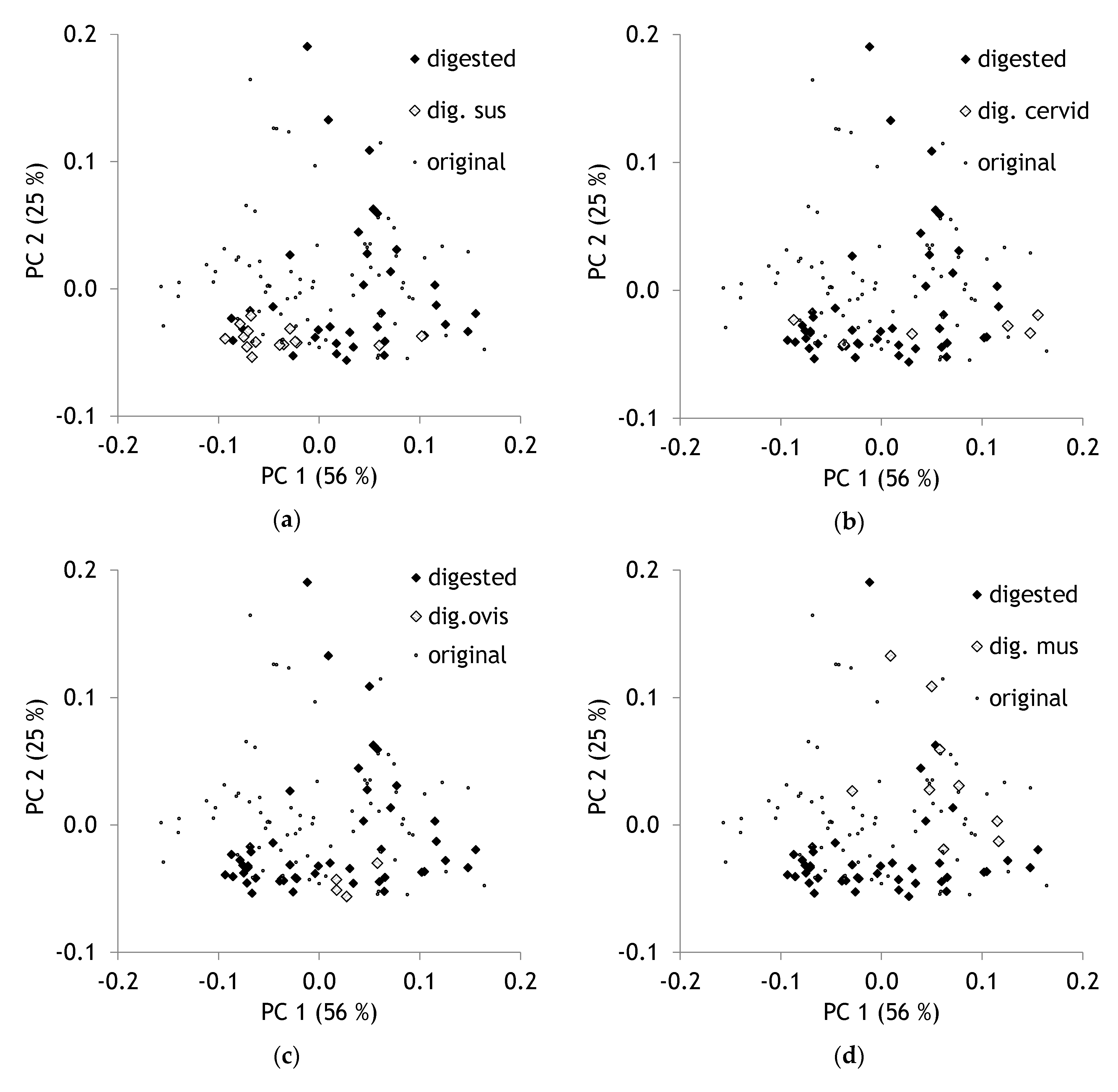
2. Results and Discussion
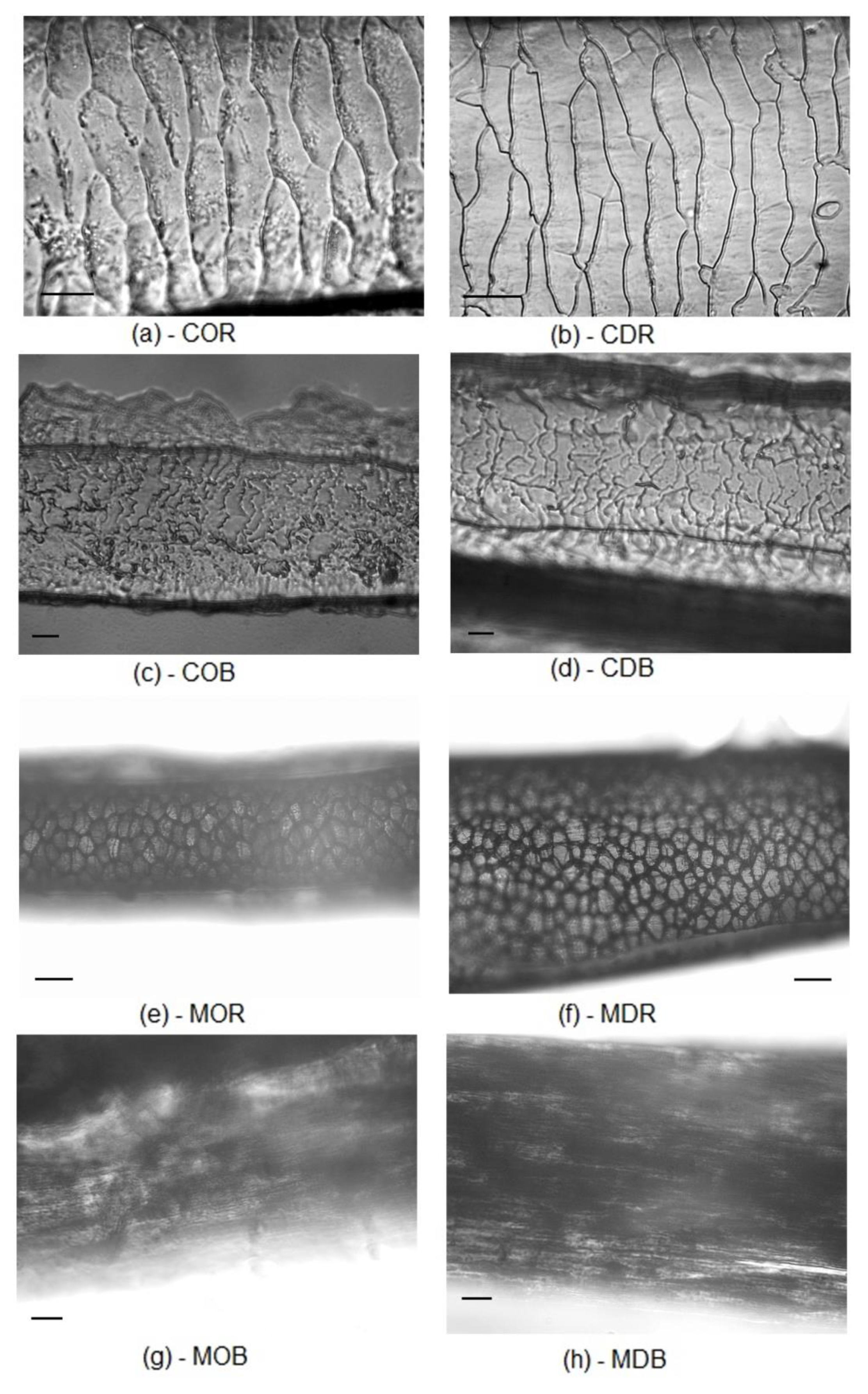
2.1. Comparison of Original, Milled and Digested Hair
2.2. Comparison of Original and Digested Hair
3. Materials and Methods
3.1. Reference Samples
3.2. Feeding Trials with Golden Jackal (Canis aureus L.)
3.3. Sample Preparation
3.4. Light Microscopic Hair Identification
3.5. Fourier Transform Infrared (FTIR) Spectroscopy and Statistical Evaluation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beltran, R.S.; Burns, J.M.; Breed, G.A. Convergence of biannual moulting strategies across birds and mammals. Proc. Biol. Sci. 2018, 285. [Google Scholar] [CrossRef]
- Yang, W.; Yu, Y.; Ritchie, R.O.; Meyers, M.A. On the Strength of Hair across Species. Matter 2020, 2, 136–149. [Google Scholar] [CrossRef]
- Lange, L.; Huang, Y.; Busk, P.K. Microbial decomposition of keratin in nature-a new hypothesis of industrial relevance. Appl. Microbiol. Biotechnol. 2016, 100, 2083–2096. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, S.; Morita, Y.; Hasan, Q.; Yokoyama, K.; Tamiya, E. Keratin degradation: A cooperative action of two enzymes from Stenotrophomonas sp. Biochem. Biophys. Res. Commun. 2002, 294, 1138–1143. [Google Scholar] [CrossRef]
- Yamamura, S.; Morita, Y.; Hasan, Q.; Rao, S.R.; Murakami, Y.; Yokoyama, K.; Tamiya, E. Characterization of a new keratin-degrading bacterium isolated from deer fur. J. Biosci. Bioeng. 2002, 93, 595–600. [Google Scholar] [CrossRef]
- Trivedi, J.P.; Srivastava, A.P.; Narain, K.; Chatterjee, R.C. The digestion of wool fibres in the alimentary system of Anthrenus flavipes larvae. Int. Biodeterior. 1991, 27, 327–336. [Google Scholar] [CrossRef]
- Sugiura, S.; Ikeda, H. Keratin decomposition by trogid beetles: Evidence from a feeding experiment and stable isotope analysis. Naturwissenschaften 2014, 101, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Fukatsu, T.; Koga, R.; Smith, W.A.; Tanaka, K.; Nikoh, N.; Sasaki-Fukatsu, K.; Yoshizawa, K.; Dale, C.; Clayton, D.H. Bacterial endosymbiont of the slender pigeon louse, Columbicola columbae, allied to endosymbionts of grain weevils and tsetse flies. Appl. Environ. Microbiol. 2007, 73, 6660–6668. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.; Vogler, A.P. Gene expression in the gut of keratin-feeding clothes moths (Tineola) and keratin beetles (Trox) revealed by subtracted cDNA libraries. Insect. Biochem. Mol. Biol. 2006, 36, 584–592. [Google Scholar] [CrossRef]
- Morin, D.J.; Higdon, S.D.; Lonsinger, R.C.; Gosselin, E.N.; Kelly, M.J.; Waits, L.P. Comparing methods of estimating carnivore diets with uncertainty and imperfect detection. Wildl. Soc. Bull. 2019, 43, 651–660. [Google Scholar] [CrossRef]
- Werhahn, G.; Kusi, N.; Li, X.; Chen, C.; Zhi, L.; Lázaro Martín, R.; Sillero-Zubiri, C.; Macdonald, D.W. Himalayan wolf foraging ecology and the importance of wild prey. Glob. Ecol. Conserv. 2019, 20, e00780. [Google Scholar] [CrossRef]
- Balajeid Lyngdoh, S.; Habib, B.; Shrotriya, S. Dietary spectrum in Himalayan wolves: Comparative analysis of prey choice in conspecifics across high-elevation rangelands of Asia. J. Zool. 2019, 310, 24–33. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Valentini, A.; Khan, N.K.; Miquel, C.; Taberlet, P.; Swenson, J.E. Diet of the brown bear in Himalaya: Combining classical and molecular genetic techniques. PLoS ONE 2019, 14, e0225698. [Google Scholar] [CrossRef] [PubMed]
- Lanszki, J.; Kurys, A.; Szabó, L.; Nagyapáti, N.; Porter, L.B.; Heltai, M. Diet composition of the golden jackal and the sympatric red fox in an agricultural area (Hungary). Folia. Zool. 2016, 65, 310–322. [Google Scholar] [CrossRef]
- Mukherjee, S.; Goyal, S.P.; Johnsingh, A.J.T.; Leite Pitman, M.R.P. The importance of rodents in the diet of jungle cat (Felis chaus), caracal (Caracal caracal) and golden jackal (Canis aureus) in Sariska Tiger Reserve, Rajasthan, India. J. Zool. 1999, 262, 405–411. [Google Scholar] [CrossRef]
- Krofel, M.; Giannatos, G.; Ćirović, D.; Stoyanov, S.; Newsome, T.M. Golden jackal expansion in Europe: A case of mesopredator release triggered by continent-wide wolf persecution? Hystrix 2017, 9–15. [Google Scholar] [CrossRef]
- Trouwborst, A.; Krofel, M.; Linnell, J.D.C. Legal implications of range expansions in a terrestrial carnivore: The case of the golden jackal (Canis aureus) in Europe. Biodivers. Conserv. 2015, 24, 2593–2610. [Google Scholar] [CrossRef]
- Ramkumaran, K.; Chandran, R.; Satyanarayana, C.; Chandra, K.; Shyamal, T. Density and obligatory feeding habits of an isolated Golden Jackal Canis aureus L. (Mammalia: Carnivora: Canidae) population in Pirotan Island, Gulf of Kachchh, India. J. Threat. Taxa 2017, 9, 10121. [Google Scholar] [CrossRef]
- Borkowski, J.; Zalewski, A.; Manor, R. Diet Composition of Golden Jackals in Israel. Ann. Zool. Fenn. 2011, 48, 108–118. [Google Scholar] [CrossRef]
- Lanszki, J.; Hayward, M.W.; Nagyapáti, N. Feeding responses of the golden jackal after reduction of anthropogenic food subsidies. PLoS ONE 2018, 13, e0208727. [Google Scholar] [CrossRef]
- Markov, G.; Lanszki, J. Diet composition of the golden jackal, Canis aureus in an agricultural environment. Folia. Zool. 2012, 61, 44–48. [Google Scholar] [CrossRef]
- Poché, R.M.; Evans, S.J.; Sultana, P.; Hague, M.E.; Sterner, R.; Siddique, M.A. Notes on the golden jackal (Canis aureus) in Bangladesh. Mammalia 1987, 51. [Google Scholar] [CrossRef]
- Tigabu, M.; Felton, A. Multivariate calibration of near infrared spectra for predicting nutrient concentrations of solid moose rumen contents. Silva Fenn. 2018, 52. [Google Scholar] [CrossRef]
- Steyaert, S.M.J.G.; Hütter, F.J.; Elfström, M.; Zedrosser, A.; Hackländer, K.; Lê, M.H.; Windisch, W.M.; Swenson, J.E.; Isaksson, T. Faecal spectroscopy: A practical tool to assess diet quality in an opportunistic omnivore. Wildl. Biol. 2012, 18, 431–438. [Google Scholar] [CrossRef]
- Greyling, M.D. Sex and Age Related Distinctions in the Feeding Ecology of the African Elephant, Loxodonta africana. Ph.D. Thesis, University of the Witwatersrand, Johannesburg, South Africa, 2004. [Google Scholar]
- Nopp-Mayr, U.; Zohmann-Neuberger, M.; Tintner, J.; Kriechbaum, M.; Rosenberger, R.; Nopp, H.; Bosa, A.; Smidt, E. From plants to feces: Pilot applications of FTIR spectroscopy for studies on the foraging ecology of an avian herbivore. J. Ornithol. 2020, 161, 203–215. [Google Scholar] [CrossRef]
- Tintner, J.; Rennhofer, H.; Kennedy, C.J.; Revie, W.A.; Weber, H.; Pavlik, C.; Lanszki, J. Recalcitrance of hair in historical plasters. Poly. Degr. Stab. 2020, 109333. [Google Scholar] [CrossRef]
- Schwanninger, M.; Rodrigues, J.C.; Pereira, H.; Hinterstoisser, B. Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vibr. Spectr. 2004, 36, 23–40. [Google Scholar] [CrossRef]
- Kennedy, C.J.; Revie, W.A.; Troalen, L.; Wade, M.; Wess, T.J. Studies of hair for use in lime plaster: Implications for conservation and new work. Poly. Degr. Stab. 2013, 98, 894–898. [Google Scholar] [CrossRef]
- Cardamone, J.M.; Nuñez, A.; Garcia, R.A.; Aldema-Ramos, M. Characterizing Wool Keratin. Res. Lett. Mater. Sci. 2009, 2009, 1–5. [Google Scholar] [CrossRef]
- Sionkowska, A.; Skopinska-Wiśniewska, J.; Kozłowska, J.; Płanecka, A.; Kurzawa, M. Photochemical behaviour of hydrolysed keratin. Int. J. Cosmet. Sci. 2011, 33, 503–508. [Google Scholar] [CrossRef]
- Smidt, E.; Lechner, P.; Schwanninger, M.; Haberhauer, G.; Gerzabek, M.H. Characterization of Waste Organic Matter by FT-IR Spectroscopy: Application in Waste Science. Appl. Spectrosc. 2016, 56, 1170–1175. [Google Scholar] [CrossRef]
- Rozenberg, M.; Shoham, G. FTIR spectra of solid poly-l-lysine in the stretching NH mode range. Biophys. Chem. 2007, 125, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Meissl, K.; Smidt, E.; Schwanninger, M.; Tintner, J. Determination of Humic Acids Content in Composts by Means of Near- and Mid-Infrared Spectroscopy and Partial Least Squares Regression Models. Appl. Spectrosc. 2008, 62, 873–880. [Google Scholar] [CrossRef]
- Smith, B.C. Infrared Spectral Interpretation. A Systematic Approach; CRC Press: Boca Raton, FL, USA, 1999; ISBN 0849324637. [Google Scholar]
- Espinoza, E.O.; Baker, B.W.; Moores, T.D.; Voin, D. Forensic identification of elephant and giraffe hair artifacts using HATR FTIR spectroscopy and discriminant analysis. Endang. Species. Res. 2010, 9, 239–246. [Google Scholar] [CrossRef]
- de Beus, A.M.; Fabry, T.L.; Lacker, H.M. A gastric acid secretion model. Biophys. J. 1993, 65, 362–378. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Matsunaga, Y.; Yamamoto, O.; Ueki, S.; Haga, N.; Mizusawa, F.; Mizumoto, A.; Sano, I.; Itoh, Z. Inhibition of phase III activity by acid in canine stomach. Regul. Peptides 1994, 52, 61–72. [Google Scholar] [CrossRef]
- Istrate, D.; Popescu, C.; Rafik, M.E.; Möller, M. The effect of pH on the thermal stability of fibrous hard alpha-keratins. Poly. Degr. Stab. 2013, 98, 542–549. [Google Scholar] [CrossRef]
- Mondini, M. Carnivore Taphonomy and the Early Human Occupations in the Andes. J. Archaeol. Sci. 2002, 29, 791–801. [Google Scholar] [CrossRef]
- Mondini, M.; Rodríguez, M. Taphonomic analysis of Plant Remains Contained in Carnivore Scats in Andean South America. J. Taphon. 2006, 223–235. [Google Scholar]
- Afridi, H.I.; Kazi, T.G.; Jamali, M.K.; Kazi, G.H.; Arain, M.B.; Jalbani, N.; Shar, G.Q. Analysis of Heavy Metals in Scalp Hair Samples of Hypertensive Patients by Conventional and Microwave Digestion Methods. Spectrosc. Lett. 2006, 39, 203–214. [Google Scholar] [CrossRef]
- Rahman, L.; Corns, W.T.; Bryce, D.W.; Stockwell, P.B. Determination of mercury, selenium, bismuth, arsenic and antimony in human hair by microwave digestion atomic fluorescence spectrometry. Talanta 2000, 52, 833–843. [Google Scholar] [CrossRef]
- Brombach, C.-C.; Gajdosechova, Z.; Chen, B.; Brownlow, A.; Corns, W.T.; Feldmann, J.; Krupp, E.M. Direct online HPLC-CV-AFS method for traces of methylmercury without derivatisation: A matrix-independent method for urine, sediment and biological tissue samples. Anal. Bioanal. Chem. 2015, 407, 973–981. [Google Scholar] [CrossRef]
- Teerink, B.J. Hair of West.-European Mammals. Atlas and Identification Key, 1st ed.; Cambridge University Press: Cambridge, UK, 1991; ISBN 0521545773. [Google Scholar]
- Senthilkumar, S.; Gnanadevi, R.; Kannan, T.A.; Arunaman, C.S.; Ramesh, G. Microanatomical observations of hair in domestic animals: A comparative study. J. Entomol. Zool. Stud. 2018, 6, 1925–1929. [Google Scholar]
- MicrolabNW Photomicrograph Gallery. Photographic Gallery of Hair. Available online: http://www.microlabgallery.com/hair.aspx (accessed on 7 June 2020).
Sample Availability: Hair samples are available from the authors upon request. |
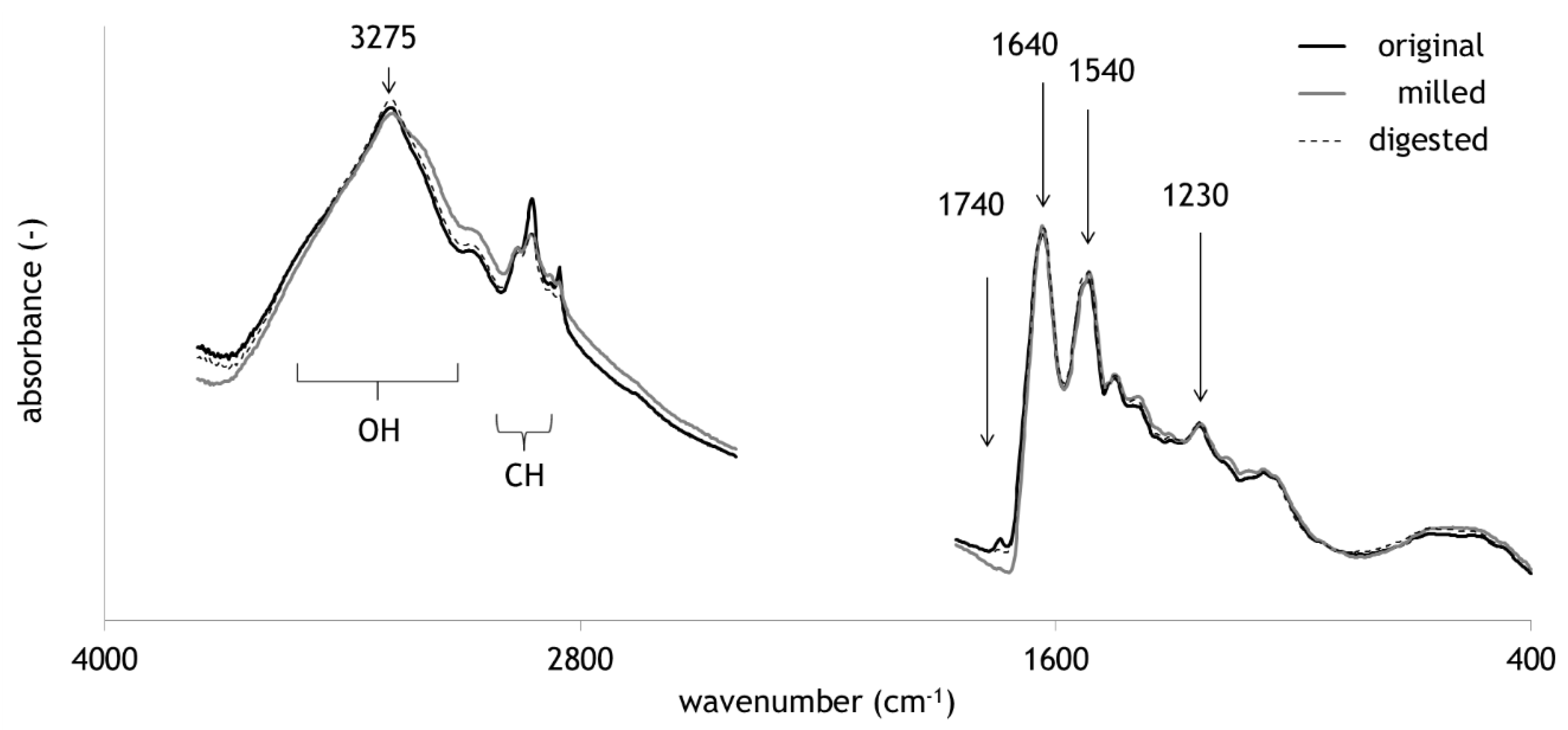
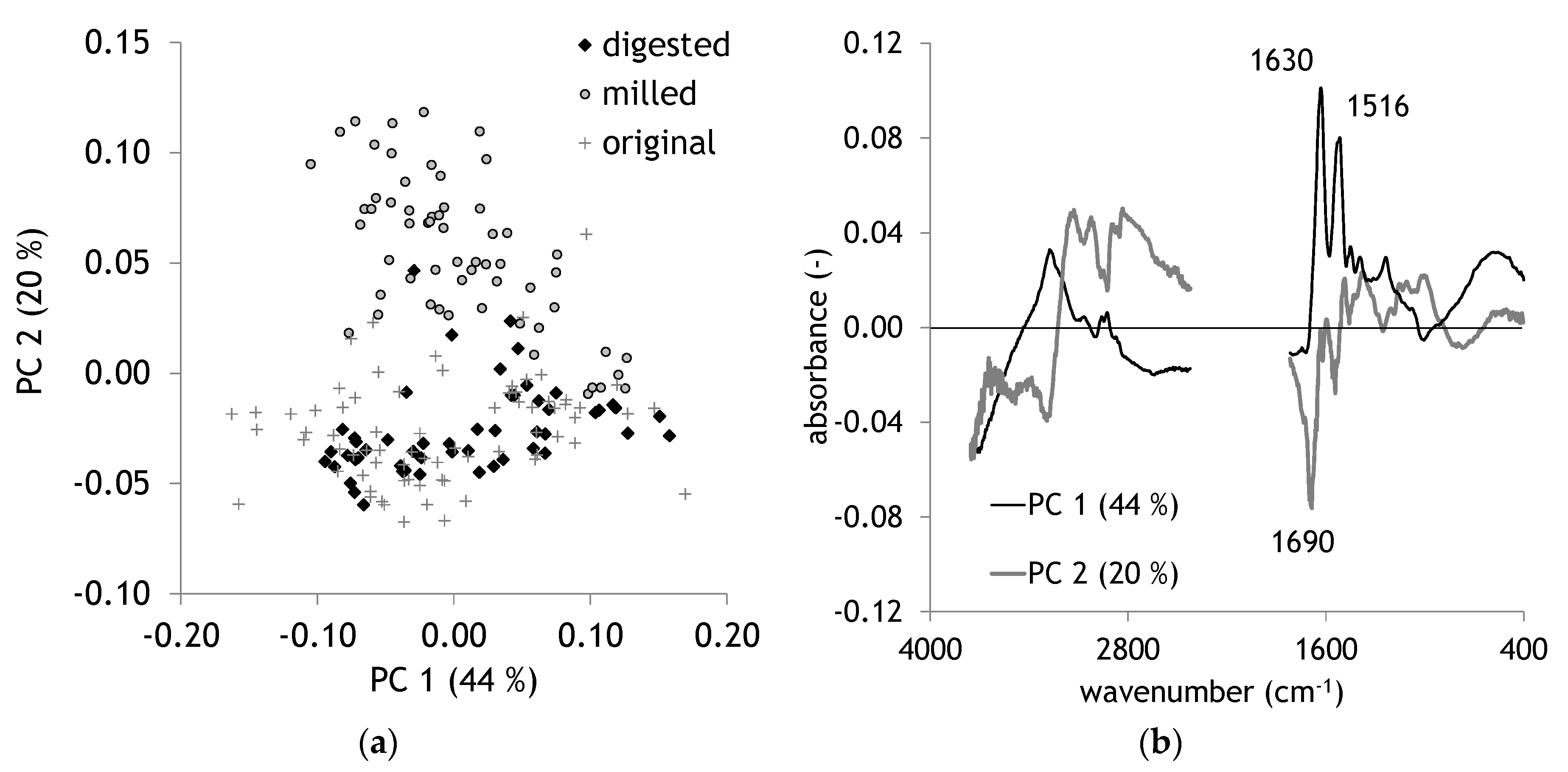
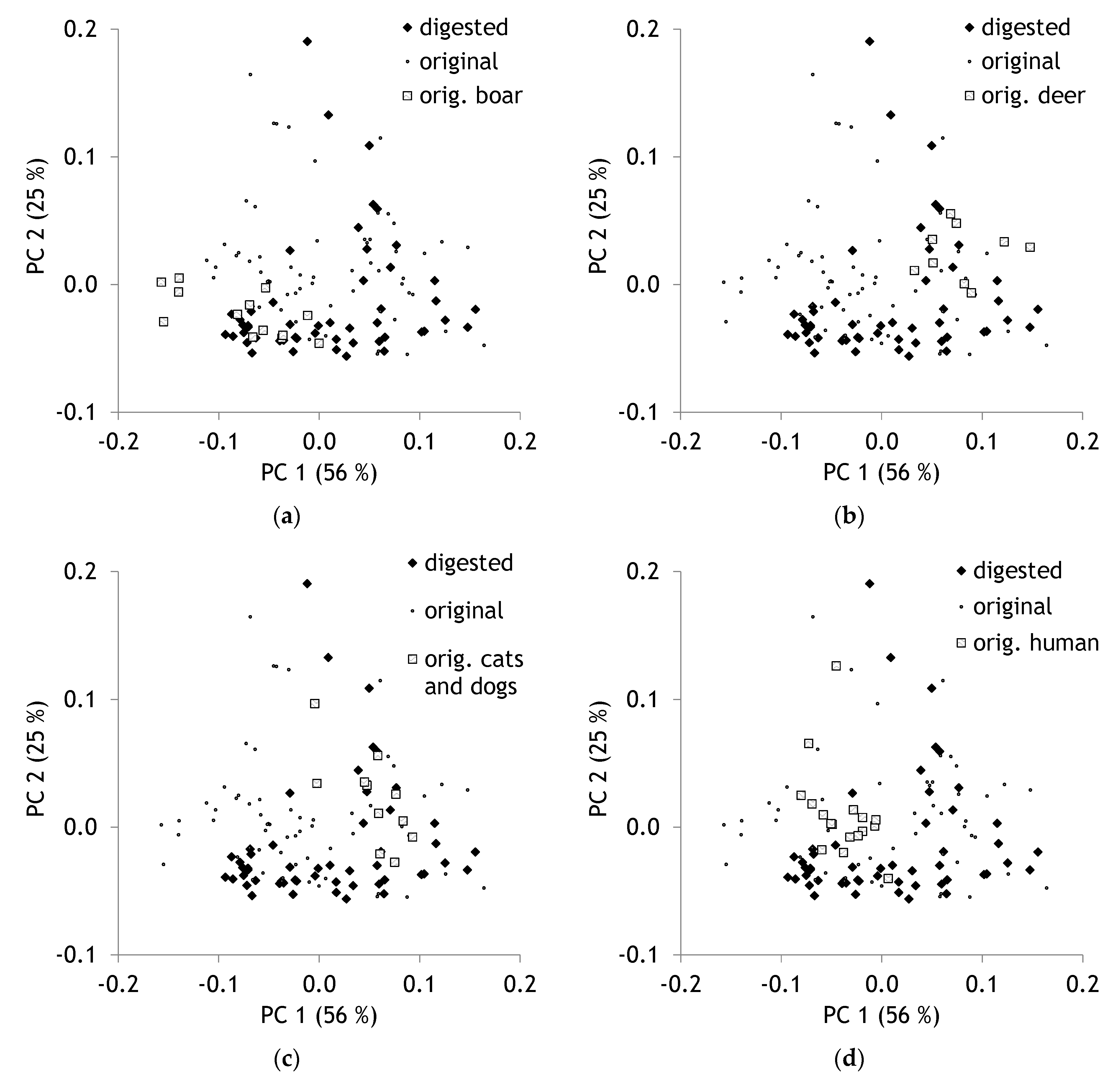
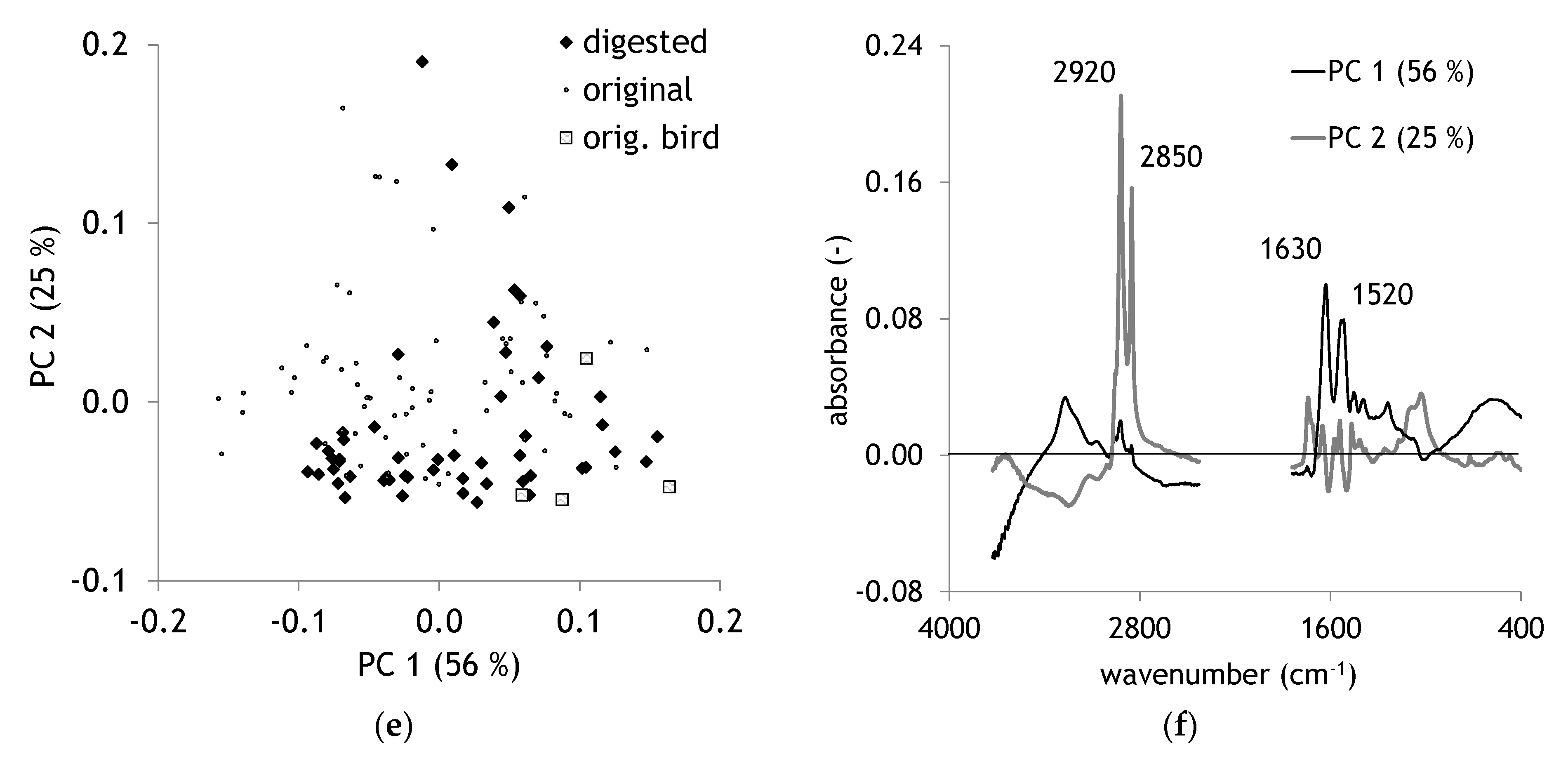
| Band Region. | Assignment | Reference |
|---|---|---|
| 3600–2600 | OH stretch | [32] |
| 3300–3250 | NH stretch | [33] |
| 3000–2800 | CH stretch | [34] |
| 1740–1710 | C=O stretch | [28] |
| 1640–1620 | Amide I | [35] |
| 1540–1515 | Amide II | [35] |
| 1230–1220 | Amide III | [35] |
| Species | Milled | Original |
|---|---|---|
| human, male | 14 | 7 |
| human, female | 20 | 10 |
| goat | 1 | 0 |
| dog | 8 | 6 |
| cat | 5 | 4 |
| horse | 5 | 10 |
| donkey | 2 | 1 |
| cow | 1 | 5 |
| boar | 1 | 13 |
| deer | 2 | 9 |
| rat | 0 | 2 |
| badger | 0 | 1 |
| bird | 0 | 4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tintner, J.; Hatlauf, J.; Weber, H.; Lanszki, J. Molecular Recalcitrance of Hair Passing the Digestive System of a Canid. Molecules 2020, 25, 4404. https://doi.org/10.3390/molecules25194404
Tintner J, Hatlauf J, Weber H, Lanszki J. Molecular Recalcitrance of Hair Passing the Digestive System of a Canid. Molecules. 2020; 25(19):4404. https://doi.org/10.3390/molecules25194404
Chicago/Turabian StyleTintner, Johannes, Jennifer Hatlauf, Heidi Weber, and József Lanszki. 2020. "Molecular Recalcitrance of Hair Passing the Digestive System of a Canid" Molecules 25, no. 19: 4404. https://doi.org/10.3390/molecules25194404
APA StyleTintner, J., Hatlauf, J., Weber, H., & Lanszki, J. (2020). Molecular Recalcitrance of Hair Passing the Digestive System of a Canid. Molecules, 25(19), 4404. https://doi.org/10.3390/molecules25194404








