1. Introduction
Lung cancer has been the leading cause of cancer-related deaths for many years and incidence and mortality statistics vary widely worldwide [
1]. In both sexes combined, lung cancer is the most diagnosed cancer (11.6% of the total cases) and the leading cause of cancer death (18.4% of the total cancer deaths) worldwide [
2]. Tobacco consumption is a major risk factor for lung cancer. Other factors include genetic susceptibility, diet, alcohol consumption, occupational exposures, and air pollution [
3]. Indoor and outdoor air pollution create a high-risk for lung cancer in people living in several regions of Asia, including Thailand [
4,
5]. Surgical resection is the primary treatment of early stage lung cancer. For advanced disease (stage IIIB and stage IV) chemotherapy, radiotherapy, targeted therapy, and immunotherapy are the principle treatments, however, the 5-year survival rate of these standard treatments is only around 15% and metastasis is still one of the major causes of death. There are two main subtypes of lung cancer: Non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). Around 80% of all lung cancers are determined to be NSCLC. This type of lung cancer is usually diagnosed at an advanced stage of metastasis. At this stage the possibility of surgery having a curative effect is lessened greatly. [
6]. Moreover, drug resistance is a major cause for therapeutic failure in NSCLC leading to tumor recurrence and disease progression [
7].
Several anticancer agents have been found in natural products which have been investigated and developed to become effective chemotherapeutic cancer drugs [
8]. However, many of these chemotherapeutic drugs have been shown to produce significant toxic side effects and drug resistance. Thus, continued research needs to be pursued to find more effective natural products that provide fewer negative side effects [
9]. In Thailand there are various herbs that possess medicinal potential for anti-cancer treatments. In this study ethyl acetate extracts and 50% ethanolic extracts from three plant species were examined for cytotoxic activity and apoptotic induction in a human lung cancer A549 cell line and were investigated the toxicity results in primary cancer cells obtained from human lung cancer tissues. The three herbal species studied were
Bridelia ovata Decne,
Croton oblongifolius Roxb., and
Erythrophleum succirubrum Gagnep.
B. ovata and
C. oblongifolius are in the Euphorbiaceae family.
B. ovata is known as Ma-Ga in Thai and traditional medicine uses it as an expectorant, a laxative, and a medicinal astringent [
10]. There are various phytochemicals in
B. ovata that were previously identified as triterpenes and phytosterols [
11,
12]. A crude ethanolic extract of
B. ovata was recently reported to inhibit human hepatocellular carcinoma HepG2 cell invasion and migration [
13].
C. oblongifolius, or Plao-Yai in Thai, is a medicinal plant which acts as a tonic and a purgative and treats dysmenorrhea, dysentery, dyspepsia, and chronic liver enlargement. In addition, the combination of
C. oblongifolius and
C. sublyratus had been used as a treatment for gastric ulcers and gastric cancer in Thai traditional medicine [
14]. The phytochemicals of
C. oblongifolius have been reported to include megastigmane glycosides [
15], diterpenoids such as labdanes [
16,
17], clerodanes [
18,
19,
20], halimane [
21], and cembranes [
22,
23,
24]. Croblongifolin, the clerodane-type compound, exhibits cytotoxicity to human cancer cell lines, including HepG2, SW620, CHAGO, KATO3, and BT474 [
19].
E. succirubrum belongs to the Leguminosae-Caesalpinioideae family. It is known as ‘Phan-Saat’ in Thai and is used to treat fever and skin diseases in Thai traditional medicine [
25]. The cassaine diterpenoid dimers, which are isolated from the bark of
E. succirubrum, have been reported to possess anti-cancer activity by the induction of apoptosis in human gastric adenocarcinoma cells [
26]. Furthermore, the crude ethanolic extract of
E. succirubrum exhibits moderate cytotoxicity against human hepatocellular carcinoma cells (HepG2). However, the mechanism(s) of cell death is still elusive [
25].
Apoptosis, the well-known cell death mechanism, is induced by many chemotherapeutic agents. Membrane blebbing, nuclear condensation, and apoptotic bodies are unique morphology characteristics of apoptotic cells that occur without cell inflammation [
27]. There are two main pathways in apoptotic signaling. The first is the intrinsic pathway which is induced by intracellular stimuli such as DNA damage or oxidative stress. The Bcl-2 family is a protein family composed of pro-apoptotic and anti-apoptotic proteins which tightly regulate the intrinsic pathway via the mitochondria. During apoptosis induction, the pro-apoptotic proteins (Noxa, Puma, Bax, and Bak) are upregulated to inhibit the function of anti-apoptotic proteins (Bcl-2, Bcl-xl, and Mcl-1), and induce the mitochondrial outer membrane premiumization (MOMP). This causes intermembranous space protein release and mitochondrial transmembrane potential loss [
28]. Then, caspase 9 and caspase 3 are activated to induce cell apoptosis. The other pathway is the extrinsic pathway which is induced by death ligand-receptor binding on the cell membrane. The oligomerization of the receptors induces the formation of a protein complex in the cytosol which activates caspase 8 and caspase 3 and then induces apoptosis [
29].
3. Discussion
Herbs are the main sources for drugs. Many herbs are used in traditional medicine and have been developed to become therapeutic drugs [
34].
B. ovata,
C. oblongifolius, and
E. succirubrum are Thai herbs in the plant genetic conservation project under the Royal Initiative of Her Royal Highness Princess Maha Chakri Sirindhorn (RSPG). This project aims to develop plant genetic resources for the maintenance of plant varieties, and for the potential to develop future advances for the farming and business sector in Thailand. In this study we investigated the effects of these Thai herbal extracts in order to analyze their potential use in future alternative treatments for lung cancer.
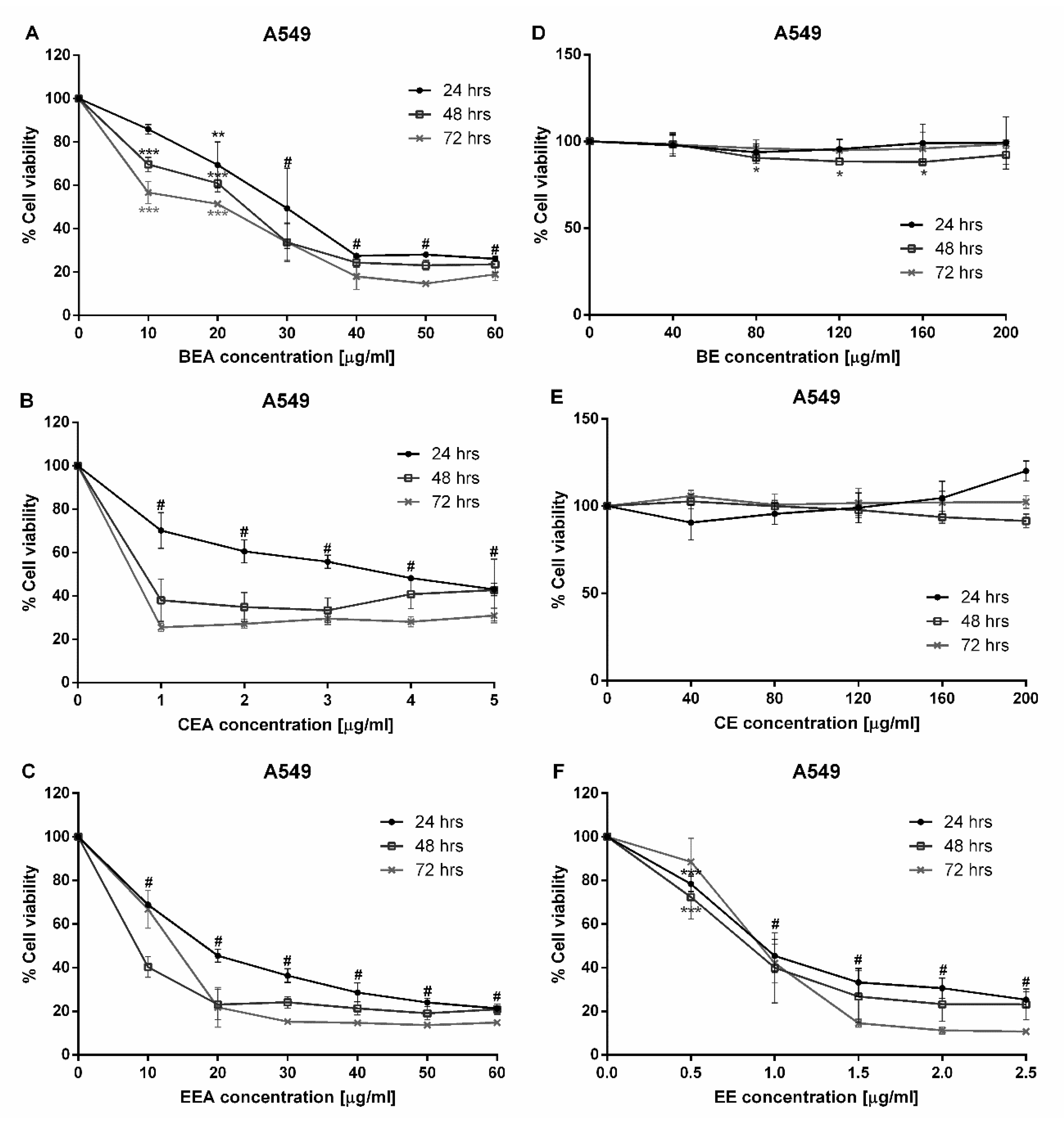
Since a major subgroup of lung cancer is NSCLC, A549 cells were used in this study. The A549 cell line is made up of adenocarcinoma from human alveolar basal epithelial cells which are commonly used as a model for the study of lung cancer and for the development of drug therapies [
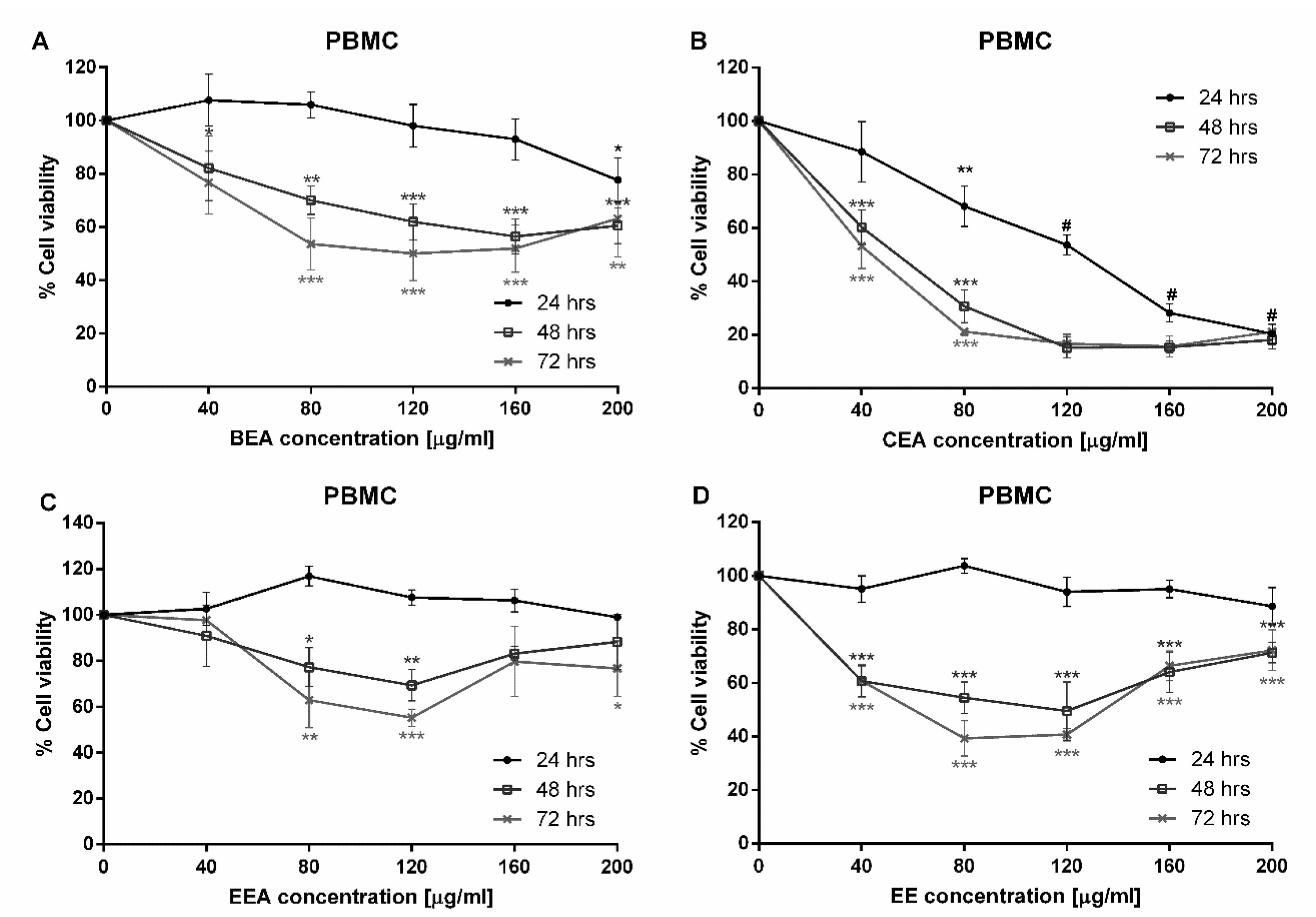
35]. This study began with a cytotoxicity test of three plants which were extracted with two different solvents, ethyl acetate and 50% ethanol. Six extracts were tested against A549 cells. The cytotoxicity results demonstrated that BEA, CEA, EEA, and EE extracts exhibited toxicity towards A549 cells at 24 h incubation. Although these four effective extracts were toxic to PBMCs, the IC
50 of PBMCs was significantly greater than that of A549 cells. Therefore, the concentrations of each effective extract used for treating cancer cells are not toxic to normal PBMCs. The SI values of these four effective extracts were more than three, which indicates a high selectivity of the effective extracts tested on the A549 cells [
36,
37].
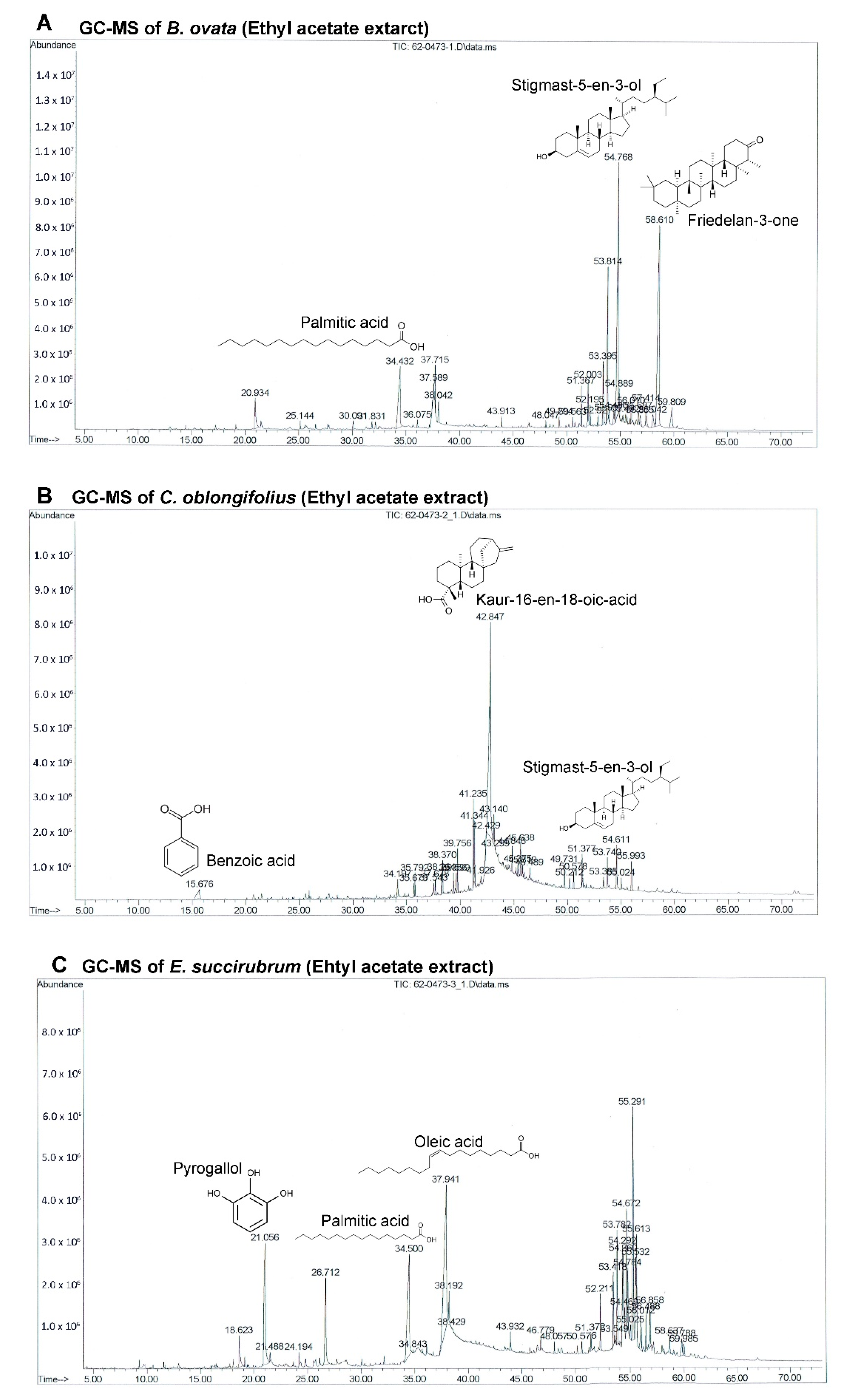
The GC-MS chromatograms of the effective extracts showed many different peaks for the active compounds. Only the structures of the three most abundant compounds were displayed in
Figure 4. Even though
B. ovata and
C. oblongifolius are plants from the same family (Euphorbiaceae) and phytosterols were identified in both BEA and CEA (campesterol, stigmasterol, and stigmast-5-en-3-ol), the main compounds in these plants are different. Friedelan-3-one (28.99%) and Stigmast-5-en-3-ol (22.52%) are the main compounds in BEA. Friedelan-3-one, or friedelin, is a triterpenoid which has been reported to have anti-tumor activities against various cancer types, including human malignant melanoma A375, human cervical tumor HeLa, human macrophage tumor THP-1, and mouse lung epithelial tumor L929 cells [
38]. The inhibitory effect of friedelan-3-one on VEGF-induced Kaposi’s sarcoma cell proliferation via apoptotic cell death induction [
39] and on breast cancer MCF-7 cell growth associated with p53 and caspase activation [
40] were also reported. Stigmast-5-en-3-ol is the second most abundant compound in both BEA (22.52%) and CEA (3.60%). The apoptotic and antiproliferative effects against human leukemia HL-60 and MCF-7 cells were also demonstrated [
41].
However, the main compound of CEA is kaur-16-en-18-oic acid, which makes up 50.24% of the total constituents in CEA but was not found in BEA. This may be the reason that CEA possessed more effective activity comparing with BEA. Kaur-16-en-18-oic acid is a kaurane-type diterpenoid which has anticancer potential [
42]. It has been reported that kaur-16-en-18-oic acid induces apoptosis cell death in HL-60 [
43], HeLa and CaSki cervical cancer cell lines [
44], including a significant anti-cancer effect against an A549 cell line [
45].
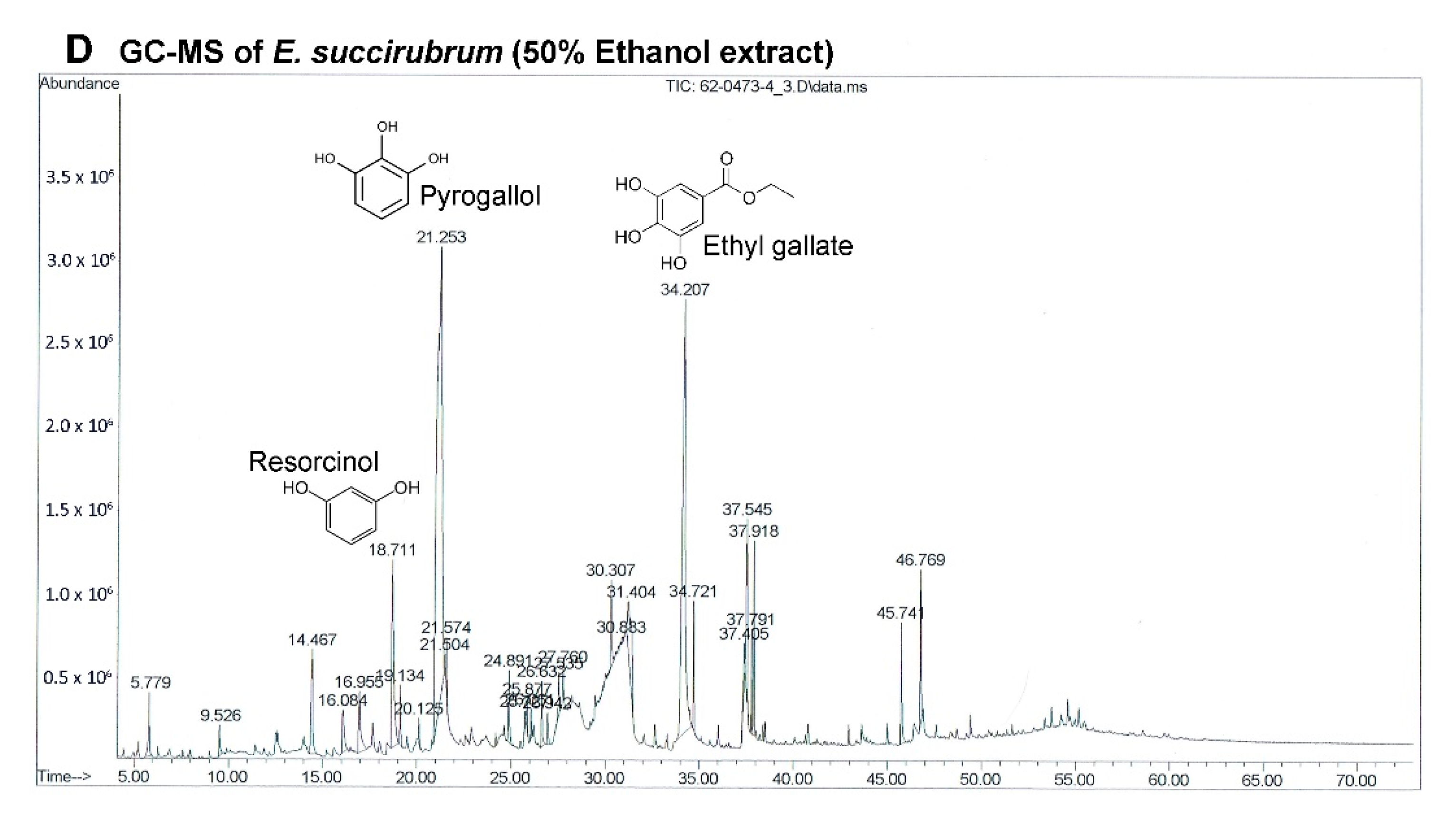
The most effective extract against A549 cells and the only extract that had a significant effect on primary lung cancer cells was EE. Although the extracts of
E. succirubrum (both ethyl acetate and ethanol) are effective on A549 cells, EE remarkably produced a more significant effect than EEA. The main compounds in EE are pyrogallol (37.82%) and ethyl gallate (21.75%). Pyrogallol, a catechin compound, is known as superoxide anion generator. It induces GSH depletion-mediated cell death in several cell types including lung cancer cells [
46]. Previous studies have reported that pyrogallol has highly cytotoxic effects on human lung cancer cell lines. Cell growth of A549, squamous cell lung carcinoma H520, and lung adenocarcinoma H441 cells were inhibited by pyrogallol via cell cycle arrest [
47,
48]. In addition, pyrogallol inhibits human lung adenocarcinoma Calu-6 cell via caspase-dependent apoptosis and cell cycle arrest [
49,
50]. Moreover, ethyl gallate, which is a phenolic compound, has been reported to exhibit anticancer activity against many cell lines such as human prostate cancer PC3, cervical cancer HeLa and CaSki, human hepatocellular carcinoma Hep3B and HepG2 cells [
51]. In previous studies ethyl gallate induces HL-60 apoptosis via the mitochondria-mediated pathway. It also suppresses proliferation and invasion of breast cancer via Akt-NF-kB signaling, and inhibits patient-derived esophageal tumor growth in an in vivo mouse model via ERK1/2 inhibition [
52,
53,
54]. Therefore, the two most abundant compounds, pyrogallol and ethyl gallate, might possess significant anticancer activity in EE.
EEA contains oleic acid (22.74%) and palmitic acid (12.89%) which are the two most abundant compounds. They are well known fatty acids which have unclear activities on cancer cells. There are some reports about oleic acid that demonstrate that they possess anti-tumor activity depending on the type of cancer, and are linked to the inhibition of angiogenesis [
55], metastasis [
56], and apoptosis induction [
57]. Alternatively, oleic acid has been reported to promote cell proliferation and migration in aggressive metastatic cancer cells via enhanced β-oxidation mediated by AMPK activation [
58]. There are similar issues in palmitic acid which has been reported to reduce and induce cancer cell growth [
59,
60]. The third most abundant compound in EEA is pyrogallol (11.73%) which is also the most abundant compound in EE.
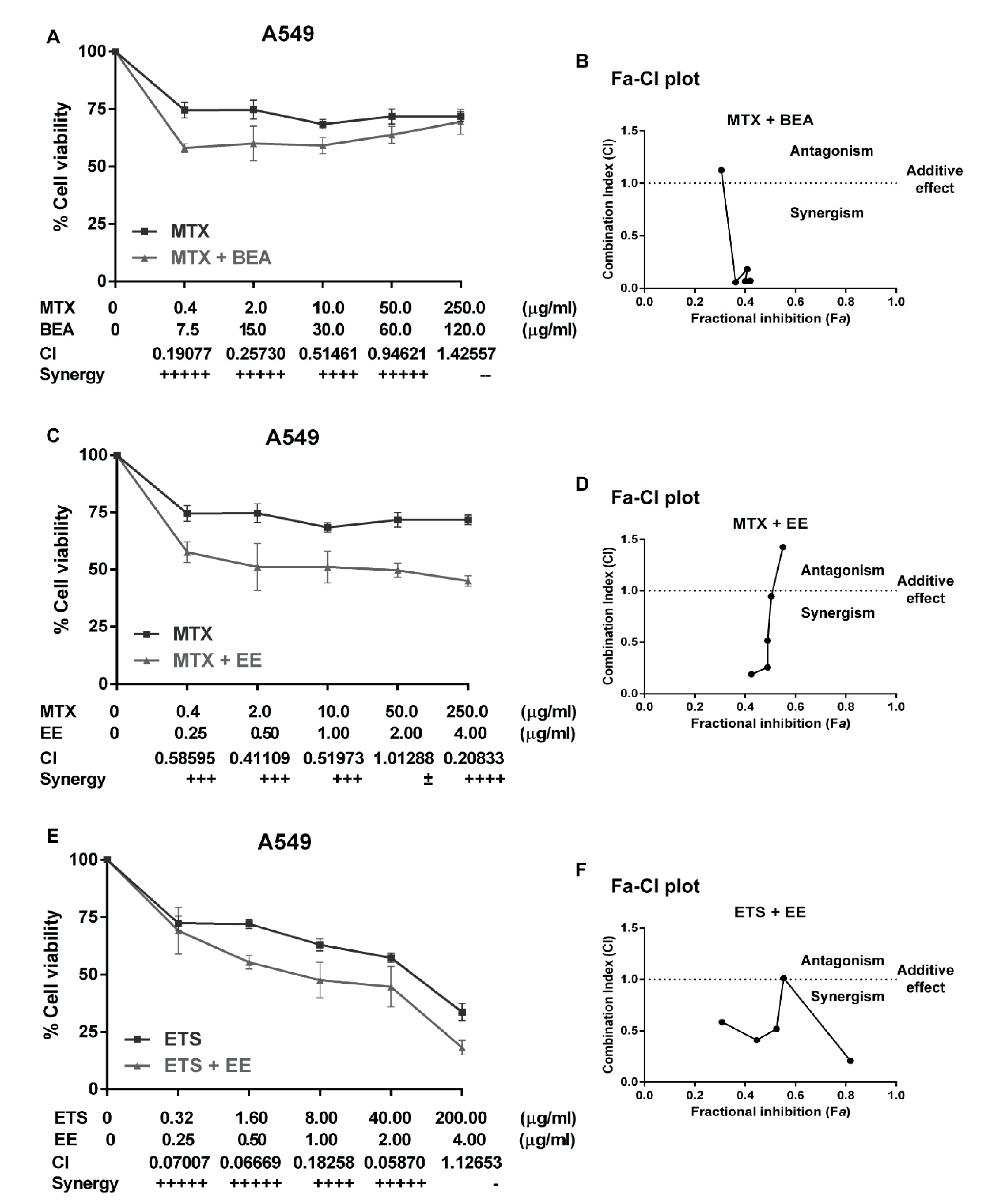
Currently, chemotherapy is the main treatment for lung cancer and is a palliative remedy for patients. However, some patients do not respond well. This generally leads to an increase in the dosage of drugs which increases the potential for adverse side effects. Moreover, some patients are resistant to chemotherapy. Therefore, a combination of chemotherapy is commonly used for cancer treatment [
61]. The main purpose of using drug combination therapies is to create a synergistic therapeutic effect that allows for a reduction of the dosage of the chemotherapy drugs. This potentially lessens adverse side effects while reversing drug resistance [
62]. The level of synergism is quantified by the drug combination index (CI) which is calculated by the Chou and Talalay’s method [
63]. The CI offers a quantitative definition for the additive effect (CI = 1), synergism (CI < 1), and antagonism (CI > 1) in drug combinations. In this study, A549 cells were treated with carboplatin, methotrexate, vinorelbine, or etoposide alone, then treated with a combination of each drug with each effective extract, but the results show only the synergistic combination. We found three synergistic combinations, which are BEA with MTX (
Figure 3A,B), EE with MTX (
Figure 3C,D), and EE with EPS (
Figure 3E,F). A549 cells did not respond well to EPS (
Figure 3E) and were resistant to MTX (
Figure 3A,C). The combinations of MTX with BEA and MTX with EE showed synergistic effects at low concentrations of MTX. Therefore, EE and BEA can sensitize A549 cells to MTX. However, a high concentration of MTX (250 µg/mL) can increase the CI value to more than one in both combinations with BEA (CI = 1.12653) and EE (CI = 1.42557). This means that the combination treatments possess a level of antagonism if used with a high dose of MTX. Therefore, it is more effective to treat A549 cells with lower concentrations of MTX combined with BEA or EE. These combination treatments can reduce the dose of MTX and feasibly decrease side effects in further clinical studies.
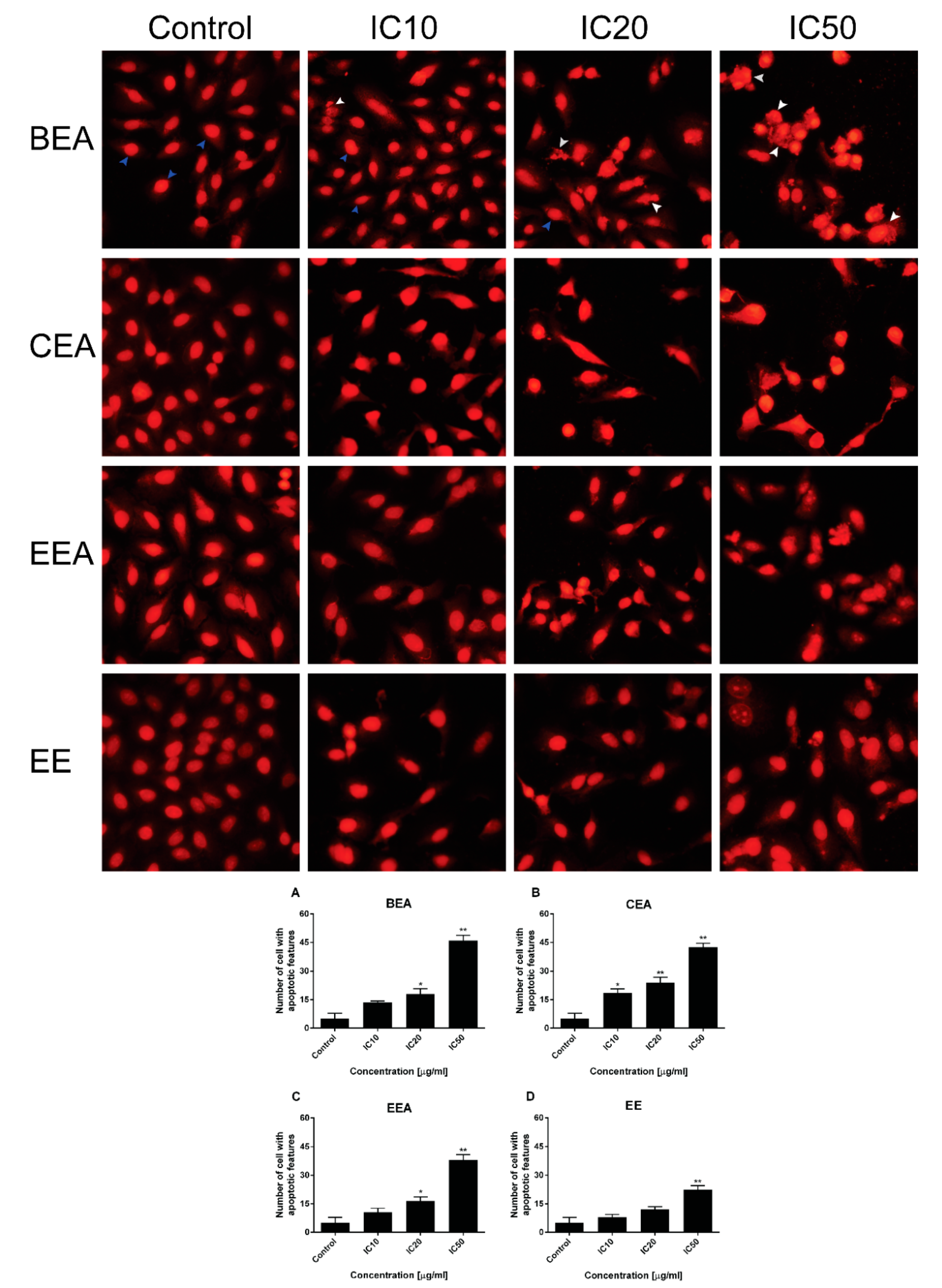
The mode of cancer cell death was determined by fluorescence microscopy and flow cytometry. Although there are a number of molecular markers for the cell death mechanism, morphological criteria is still the standard for defining the mode of cell death [
64]. PI staining was used for nuclear change observation. The results show apoptotic morphology with bright red, condensed nuclei (intact or fragmented). The formation of apoptotic bodies was compared to the control cells that are displayed as round, intact, red nuclei (
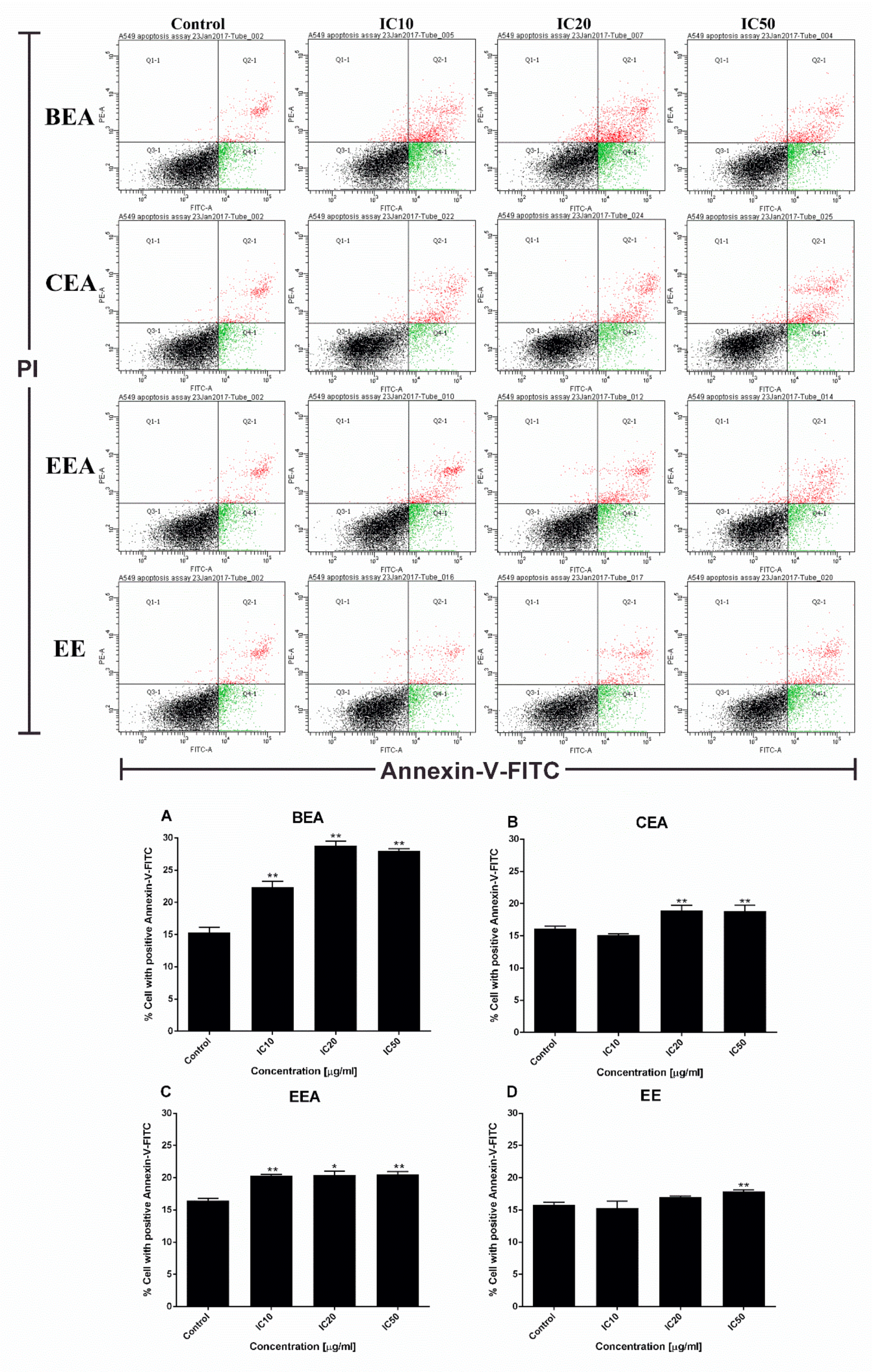
Figure 5A). Apoptotic cells and apoptotic bodies increased in a dose-dependent manner compared to the control after treating the cells with these four effective extracts. The quantification of apoptosis was obtained by flow cytometry. During apoptosis, phosphatidylserine (PS) becomes exposed on the outside of the membrane. The detection of PS by annexin V-FITC is used for the estimation of the incidence of both early (Annexin V
+/PI
−) and late (Annexin V
+/PI
+) apoptosis [
65]. The flow cytometry results confirmed that the effective extracts induced A549 cell apoptosis (
Figure 6). Therefore, apoptosis cell death was the mode of cell death that was triggered by these four effective extracts.
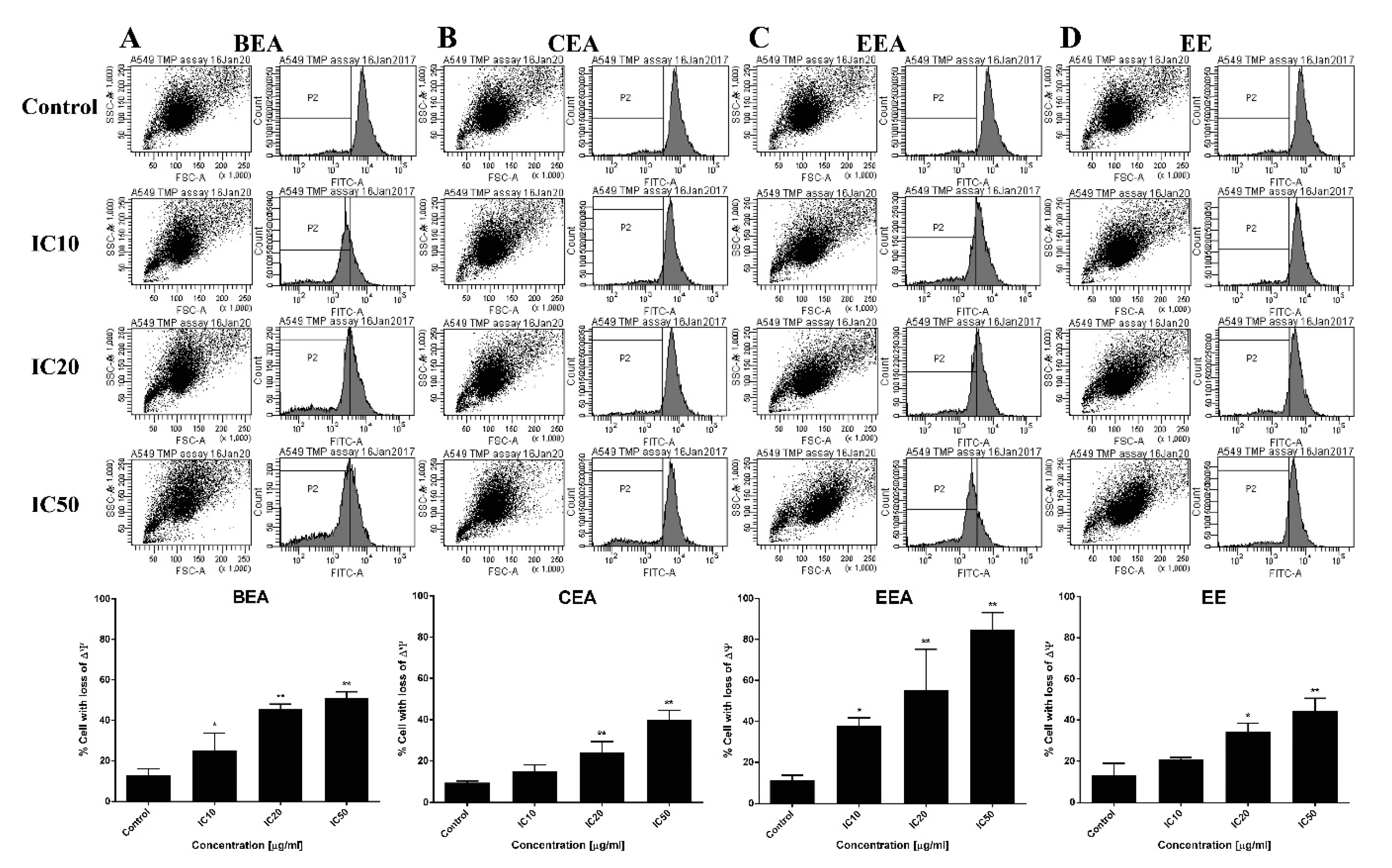
Mitochondrial disturbances occurred during apoptosis in several ways, including through a loss of ∆Ψm, a release of apoptotic proteins from the intermembranous space into the cytosol, and a generation of ROS [
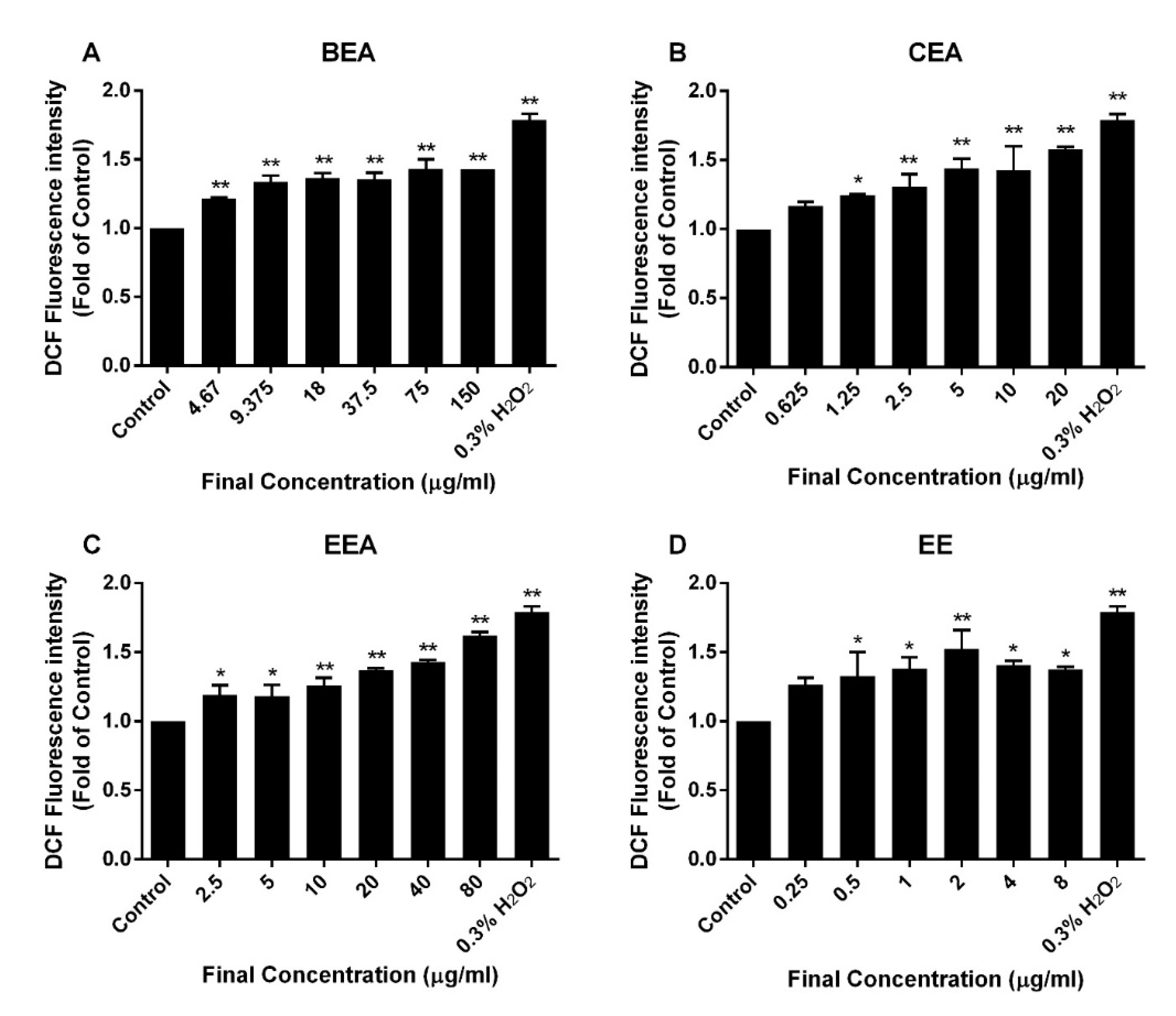
66]. A reduction of ∆Ψm was determined and it was found that the percentage of cells with a loss of ∆Ψm increased after treatment of A549 with the effective extracts. Moreover, ROS generation increased in extract-treated cells compared to the control cells. This suggests that the effective extracts induced A549 apoptosis, which involved a disruption of the mitochondria and intracellular oxidative stress.
Apoptotic pathways require several protein functions. The Bcl-2 family proteins play important roles in the apoptosis pathway. The two main types of these proteins are pro-apoptotic and anti-apoptotic. There are two different subgroups of pro-apoptotic protein, activator and effector. The BH3-only activators promote the multidomain effector forming of pores on the mitochondrial outer membrane leading to ∆Ψm loss and initiating the apoptotic programmed cell death [
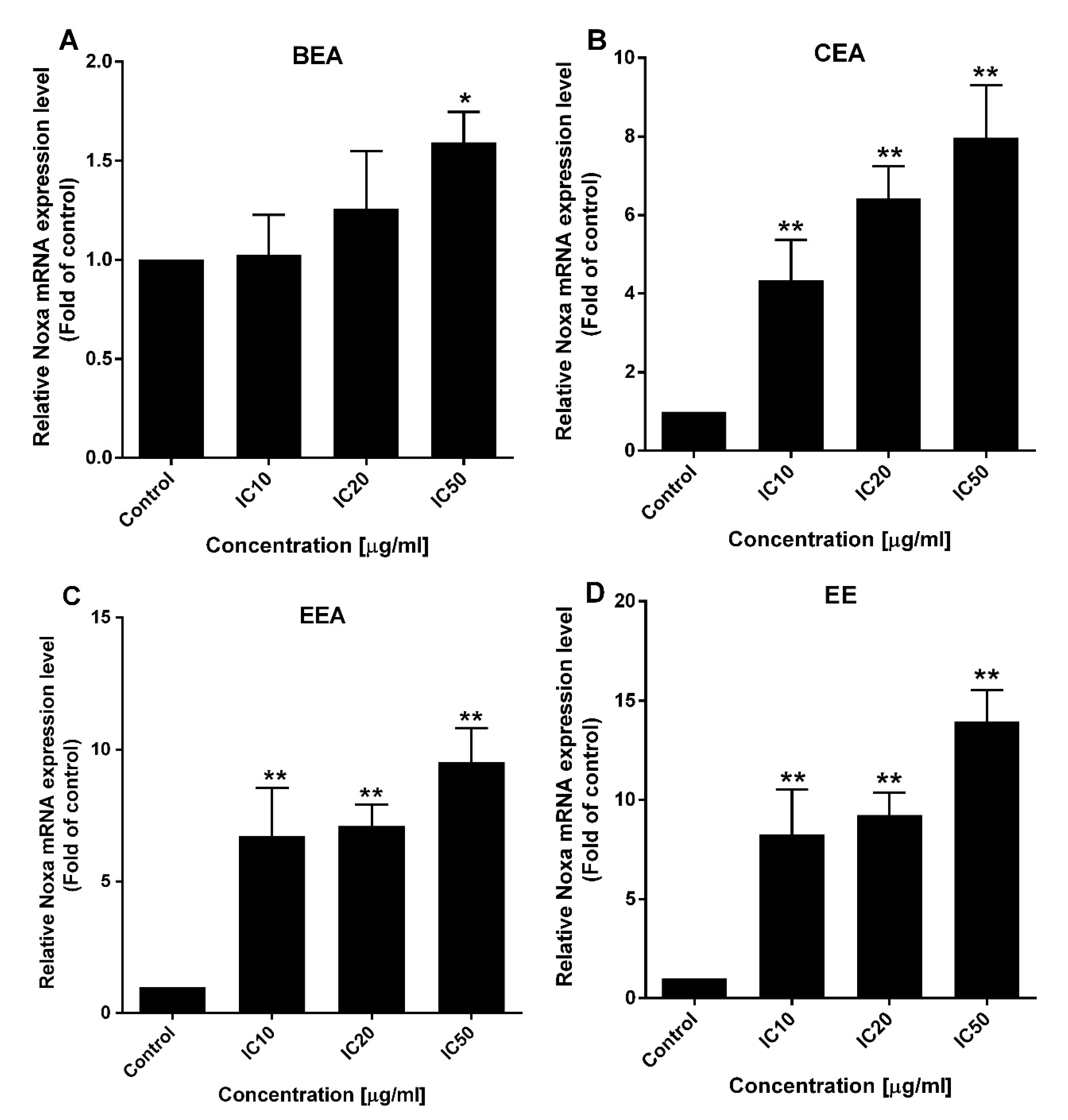
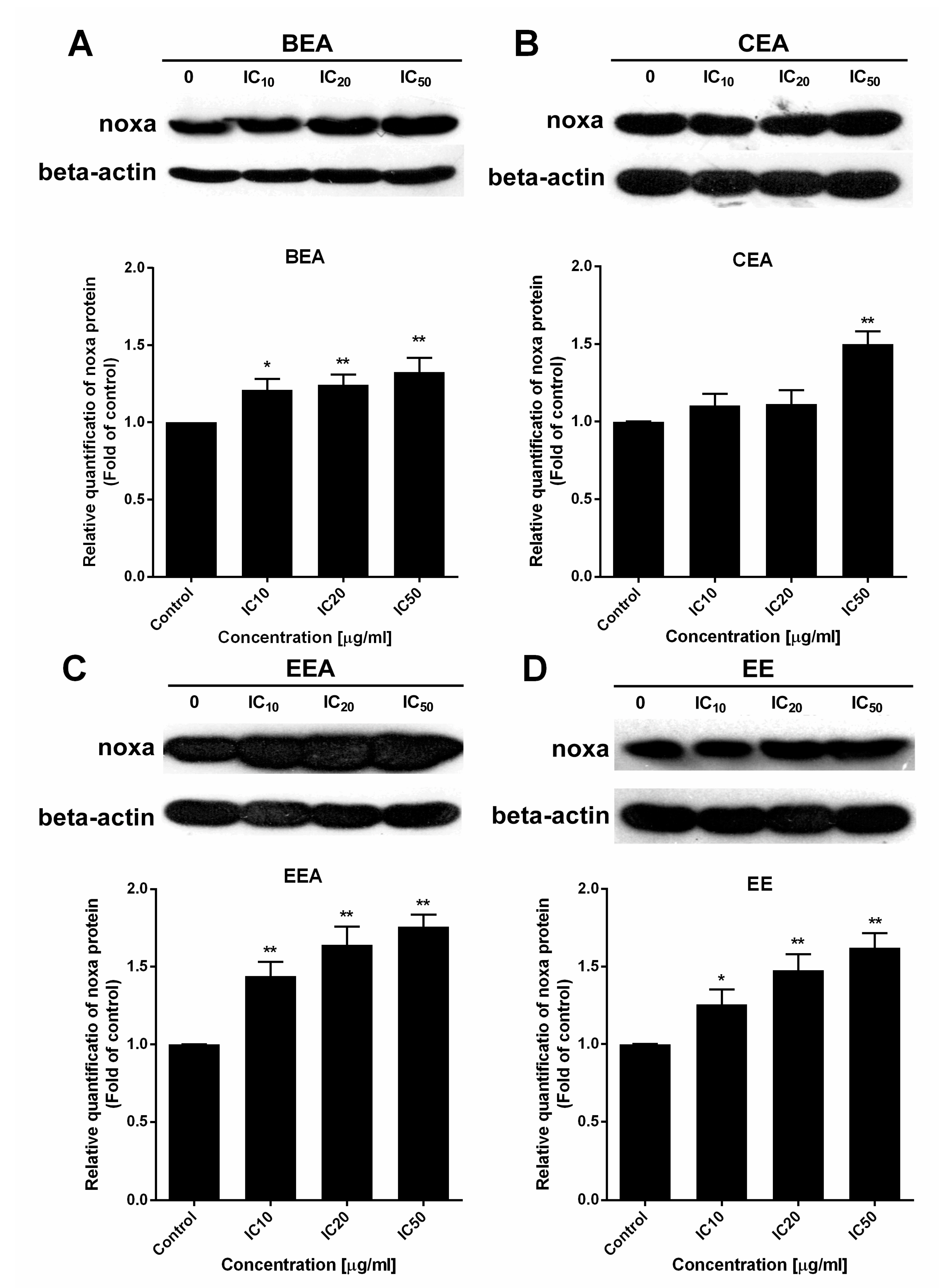
67]. Noxa, a BH3-only activator, was examined for the upregulation of both gene and protein expression levels to indicate the ∆Ψm loss and cell apoptosis resulting from the function of the Bcl-2 family. The effective extracts induced ROS generation and Noxa upregulation in A549 cells leading to a loss of ∆Ψm and apoptotic cell death via the mitochondrial pathway.
Human cancer-derived cells are elementarily used as a model to study cancer biology and to analyze the efficacy of therapeutic anti-cancer agents. These cells are effective because they can be easily cultured and they have a long lifespan [
68]. However, cancer cells possess a high heterogeneity. Cancer cell lines may have a limitation in representing these complicated cancer diseases [
69]. Primary cancer cells are used to mimic the in vivo cancer cells better than cancer cell lines. These compounds have more predictive values and can be more useful in new drug discovery [
70,
71]. In this study we established both primary lung cancer cells and normal lung cells were obtained from the same patients in a cultured model in the mimicry of in vivo. The normal histology and cancer tissue pathology were proved by a pathologist from the Department of Pathology, Maharaj Nakorn Chiang Mai Hospital, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand. The patients’ characteristics are shown in
Table 7. The molecular test of EGFR mutation predicted the response rates of tyrosine kinase inhibitors (TKIs) treatment in the lung cancer patients. The overexpression of EGFR has been reported in many cancers including NSCLC, which leads to cell proliferation and anti-apoptosis [
72]. Therefore, the studies of a subset of NSCLC patients with EGFR mutant tumors, and initial therapy with TKIs appears to be a significant survival advantage [
73]. Greater than 90% of EGFR mutation in NSCLC occurs as short in frame shift deletions in exon 19 or as point mutations in exon 21 [
74]. In this study there were three patients with EGFR wild types, three patients with EGFR mutations and another three with an unknown EGFR status as shown in
Table 7.
The primary cells were collected and were determined as either sensitive or resistant samples towards the inhibitory growth effect of lung cancers and normal lung cells. This indicated the effectiveness of each substance at IC
50. The number of sensitive samples showed that CEA and EE were effective against cancer samples from every patient, but no sample was sensitive to BEA and EEA. The IC
50 values in cancer cells showed that EE was the most effective extract (lowest IC
50) in primary lung cancer cells, which corresponded to the A549 cell line result in this study. The IC
50 of the substances against primary cells have a wide range of SD because the primary cells possessed various considerable heterogeneous properties, such as the expression levels of oncogenes, tumor suppressor genes, and multi-drug resistance genes. Moreover, comparing IC
50 values of cancer and normal samples using the
p-value in
Table 8 demonstrated that the IC
50 of EE against cancer samples was significantly different from that of the normal samples (
p < 0.05). However, the data in
Table 8 is a univariate analysis without confounding variable control and univariate analyses can yield misleading results. In this case, the multivariate analysis was more appropriate [
75]. The multi-level random effects model was used for calculating the mean difference of IC
50 comparing cancer samples and normal samples. Mean differences of IC
50 for EE, and Doxorubicin and Vinblastine against cancer were less than those of normal samples (negative values) but only the
p-value of EE (
p-value = 0.04) exhibited a significant result (
p < 0.05). There are many factors in the primary culture which made the results hard to be interpreted and repeated. The primary cell culture is difficult to establish without contamination. It is hard to obtain and has a limitation for cultured cell passages. Hence, there was no further examination for the modes of cell death in the primary cancer cells or in the normal lung cells due to such limiting factors.
In this study, EE exhibited the highest effect according to the lowest IC50 against A549 cell line and primary lung cancer cells. Also, it induced the synergistic effect in combined treatment with ETS or MTX. However, the effect of EE in apoptosis induction and ∆Ψm reduction was less effective than the other three effective extracts, whereas EE induced ROS generated around 1.5-fold of control which was the same as the other three extracts. This suggests the cytotoxicity of EE may be the result of EE induced in other mechanism(s) over mitochondrial apoptosis. Although, Noxa gene expression was at different levels between each extract, the protein which is the functional molecule, was increased in the similar level in each extract. This indicates Noxa might not be the key different effect of these four effective extracts. Therefore, more details of the mechanisms inducing lung cancer cell death by each extract need to be investigated in further studies.
4. Experimental Sections
4.1. Plant Materials
Twigs of the three herbs were collected and authenticated by Professor Dr. Bungorn Sripanidkulchai, Center for Research and Development of Herbal Health Products, Department of Pharmaceutical Chemistry, Faculty of Pharmaceutical Sciences, Khon Kaen University, Khon Kaen, Thailand. The voucher numbers are TT-OC-SK-1253, TT-OC-SK- 1215, and TT-OC-SK-1082 for B. ovata, C. oblongifolius, and E. succirubrum, respectively. The herbs were washed, cut, dried, and mashed. The herb powders were macerated with 50% ethanol or ethyl acetate at room temperature for 72 h. Then, the plant macerations were filtered and centrifuged at 500× g for 10 min and the supernatant was collected. The supernatant extracts were concentrated under rotary evaporator.
4.2. Reagents
Dulbecco’s Modified Eagle Medium (DMEM), Roswell Park Memorial Institute (RPMI)-1640, fetal bovine serum (FBS), phosphate-buffered saline (PBS), trypsin-EDTA solution, penicillin, and streptomycin were purchased from GIBCO-Invitrogen (Carlsbad, CA, USA). Small Airway Epithelial Cell Growth Medium (SAGM) and its supplement; bovine pituitary extract (BPE), insulin, hydrocortisone, gentamicin-amphotericin (GA)-1000, retinoic acid, bovine serum albumin-fatty acid free (BSA-FAF), transferrin, triiodothyronine, epinephrine, and human epidermal growth factor (hEGF) were purchased from Lonza (Walkersville, MD, USA). Collagenase and elastase were obtained from Worthington Biochemical (Lakewood, NJ, USA). Histopaque-1077, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT), 3,3′-dihexyloxacarbocyanine iodide (DiOC6), propidium iodide (PI), and 2′,7′-dichlorodihydrofluoresceine diacetate (DCFH-DA) were purchased from Sigma Chemica (St. Louis, MO, USA). Annexin V-FITC/PI kit was obtained from Roche, Mannheim, Germany. Tiangen RNA prep pure kit was purchased from Tiangen Biotech (Beijing, China). SensiFAST SYBR Lo-ROX kit from Bioline (Taunton, MA, USA). RevertAid first-strand cDNA synthesis kit and primers were obtained from Thermo Fisher Scientific (Waltham, MA, USA). Antibodies against Noxa and β-Actin, and HRP-conjugated secondary antibody were purchased from Abcam (Cambridge, UK). SuperSignal West Pico Chemiluminecent Substrate was obtained from Pierce (Rockford, IL, USA).
4.3. Cells Culture
A549 is the human adenocarcinoma cell line that was obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). The cells were cultured in DMEM supplemented with 10% FBS, 100 Units/mL penicillin, and 100 µg/mL streptomycin. Cells were grown at 37 °C in a 5% CO2 atmosphere.
PBMCs were obtained from a buffy coat bag from volunteers at Blood Bank Unit, with the informed consent signed, whereas the approval was available from the Ethic Committee of the Maharaj Nakorn Chiang Mai Hospital, Faculty of Medicine, Chiang Mai University as the project code BIO-2558-03332. The cells in the buffy coat were separated by histopaque-1077 following density gradient centrifugation standard protocol. The PBMCs were cultured in RPMI-1640 medium and supplemented with 10% FBS, 2 mM glutamine, 100 Units/mL penicillin, and 100 µg/mL streptomycin at 37 °C in a 5% CO
2 atmosphere [
76].
4.4. Cytotoxicity Test
The cytotoxicity of the extracts and chemotherapy drugs was determined to analyze their inhibitory effects on A549 cells and PBMCs growth by MTT assay as previously described [
77]. The cells were treated with the extracts and/or chemotherapy drugs at various concentrations for 24 h, and more 48- and 72-h treatment for the cytotoxicity screening of the extracts. The treatments used a final concentration of DMSO of less than 0.2% to avoid DMSO vehicle toxicity. The percentages of reduction in cell viability were compared to untreated control cells and then the inhibitory concentration (IC) values were calculated. The combined effects of the extracts and chemotherapeutic drugs were determined by the Chou-Talalay method on CompuSyn software using the cell viability results from MTT assay [
62].
4.5. GC-MS Analysis
The GC-MS analysis of the effective extracts (BEA, CEA, EEA, and EE) were performed with Agilent technology GC 7890A coupled to Agilent technology MSD 5975C (EI) (Agilent, Santa Clara, CA, USA). The extracts were separated in a DB-5MS fused silica capillary column (30 m × 0.25 mm, 0.25 µm film thickness). The conditions of the GC were maintained operating at a temperature held at 50 °C for 3 min, and increased from 50 to 300 °C at a rate of 5 °C/min. The final isothermal was held for 20 min. The injector temperature was set at 250 °C with a 1.0 µL injection volume in 1:1 split mode. The detector temperature was set at 280 °C. Helium was used as a carrier gas with a 1 mL/min flow rate. The mass spectrometer was operated in electron impact mode at 70 eV and scanned from 50 to 550 amu. The compounds were identified by a comparison of the mass spectra and retention times through computer matching to the National Institute Standard and Technology (NIST) library (Gaithersburg, MD, USA) as well as with literature data.
4.6. Detection of Apoptotic Cell Morphology
Cell morphology was determined by PI staining, which stains the nuclei to observe the condensed nuclei and fragmented or apoptotic bodies. The cells were cultured on a coverslip and then treated with the effective extracts at IC
10, IC
20, and IC
50 for 24 h. The cells were stained with the PI method as previously described [
78]. Then, the cells were examined under a fluorescence microscope (Olympus, Japan). A total of 200 cells (condensed nuclei and fragmented cells) per slide were recorded for apoptotic cells.
4.7. Apoptosis Determination Method
The Annexin V-FITC/PI assay is used to investigate cells which undergo apoptosis. Apoptosis cells are positive for annexin V (early apoptosis) or annexin V with PI (late apoptosis/necrosis) and are detected by a flow cytometer. After the cells were treated with the effective extracts at IC
10, IC
20, and IC
50 for 24 h, they were stained with annexin V-FITC and PI for 15 min. The stained cells were measured by a flow cytometer (Becton Dickinson, Frankin Lakes, NJ, USA) and analyzed with Cell Quest software program [
79].
4.8. Assessment of Mitochondrial Depolarization
Depolarization of the ∆Ψm occurs during apoptosis. The changing of ∆Ψm is determined by DiOC
6, which is a cationic fluorescence dye. The cells were treated with the effective extracts at IC
10, IC
20, and IC
50 for 24 h and then stained with DiOC
6 at a final concentration of 40 nM for 15 min. Then, the cells were washed and analyzed by a flow cytometer (Becton Dickinson, Frankin Lakes, NJ, USA) [
80].
4.9. Measurement of Intracellular ROS Generation
The intracellular ROS levels were measured by a fluorescence dye called DCFH-DA. After treatment with the effective extracts at IC
10, IC
20, and IC
50 and 0.3% of H
2O
2 (positive control) for 4 h, the cells were stained with DCFH-DA at a final concentration of 2 µM for 30 min. Then, fluorescence was detected using a fluorescence microplate reader, and was measured at 485 nm excitation and 525 nm emission wavelengths [
81].
4.10. Gene Expression Analysis
After treatment of the cells with the effective extracts at IC
10, IC
20, and IC
50 for 24 h, the total RNA was extracted using Tiangen RNAprep pure kit and then reverse transcribed to cDNA using a RevertAid first-strand cDNA synthesis kit. The mRNA expressions of
Noxa were quantified by SensiFAST SYBR Lo-ROX on a 7500 Fast Real-time PCR system. The relative gene expression level was analyzed by 2
−∆∆Ct, using
GAPDH as a housekeeping gene. The primers of
Noxa that were used: F-5′GCTGGAAGTCGAGTGTGCTA3′ and R-5′CCTGAGCAGAAGAGTTTGGA3′; and
GAPDH: F-5′TGCACCACCAACTGCTTAGC3′ and R-5′GGCATGGACTGTGGTCATGAG3′ [
82].
4.11. Protein Expression Analysis
The cells were treated with the effective extracts at IC
10, IC
20, and IC
50 for 24 h. Then, the proteins were determined at the concentration previously described [
82]. Western blot analysis was conducted by the method described previously [
78]. Briefly, protein extracts were subjected to 15% SDS-PAGE and blotted onto 0.45 µM nitrocellulose membranes which were incubated with Noxa antibody overnight. Then, after incubation with HRP-conjugated secondary antibody, protein bands were developed with chemiluminescence substrate and X-ray film exposure. The protein band intensity was analyzed by ImageJ software and β-actin was normalized and used as a protein loading control.
4.12. Lung Sample Processing
Normal lung tissue (n = 7) and cancer lung tissues (n = 9) were collected by Assistant Professor Apichat Tantraworasin, M.D., and his team of surgeons, General Thoracic Surgery Unit, Department of Surgery, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand. The patients were informed and consent forms were signed before lung cancer tissues and normal tissues were obtained at the Operation Room, Department of Surgery, Maharaj Nakorn Chiang Mai Hospital, Faculty of Medicine, Chiang Mai University with the approval of the ethics committee referenced with the ethics number REC-25600901-11296 and project code BIO-2558-03332.
The lung tissues were immediately put into RPMI-1640 media and supplemented with 100 U/mL penicillin G and 100 µg/mL streptomycin. They were then transported to the lab. The blood clots, blood vessels and connective tissue were removed from the tissues, and then washed with sterile PBS twice. Next, they were cut into small pieces (~1 mm
3) using a scalpel. The small pieces of tissues were incubated with collagenase at 50 units/mL and elastase at 10 units/mL at 37 °C and gently shaken every 15 min for 2 h. The digested tissues were passed through a 38 µm mesh sieve to collect the cells. Then, the cell suspension was washed twice by PBS and centrifuged at 300×
g for 10 min. The cells were cultured in a Small Airway Epithelial Cell Growth Medium (SAGM) supplemented with bovine pituitary extract 2.0 mL, insulin 0.5 mL, hydrocortisone 0.5 mL, GA-1000 0.5 mL, retinoic acid 0.5 mL, bovine serum albumin-fatty acid free 5 mL, transferrin 0.5 mL, triiodothyronine 0.5 mL, epinephrine 0.5 mL, and hEGF 0.5 mL. The cultures were maintained at 37 °C in a humidified incubator with 5% CO
2 [
83,
84]. The primary cells were cultured until passage 3 then the cells were treated with the effective extracted and chemotherapeutics drugs for 24 h and tested the cytotoxicity by MTT assay.
4.13. Statistical Analysis
The data was presented as a mean ± SD from three independent experiments. Statistical differences between the control (non-treatment) and treatment groups were determined by one-way ANOVA (Dunnett’s multiple comparisons test). The statistical significance is expressed as * p < 0.05, ** p < 0.01, *** p < 0.001. For primary cells the data in normal distribution was reported as a mean ± SD and was compared to the difference between cancer groups and normal groups by an unpaired t-test. Alternatively, non-normal distribution data was reported in median (interquartile range) and the statistical difference between groups was determined by a Wilcoxon rank-sum test. Lung cancer tissue and normal tissue were collected from the same patients. Therefore, the data was comparably correlated. The mean difference of the correlated data was analyzed by a multi-level random effects model which adjusted the IC50 by age, gender, cell type, and tumor size.