Improving the High-Frequency Response of PEI-Based Earphone with Sodium Copper Chlorophyllin
Abstract
1. Introduction
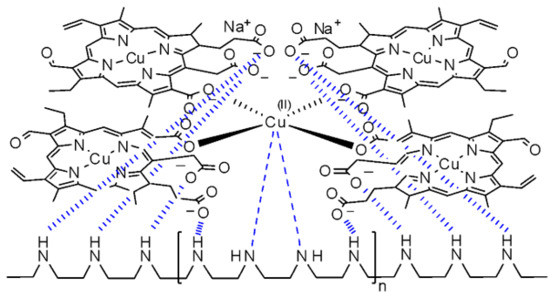
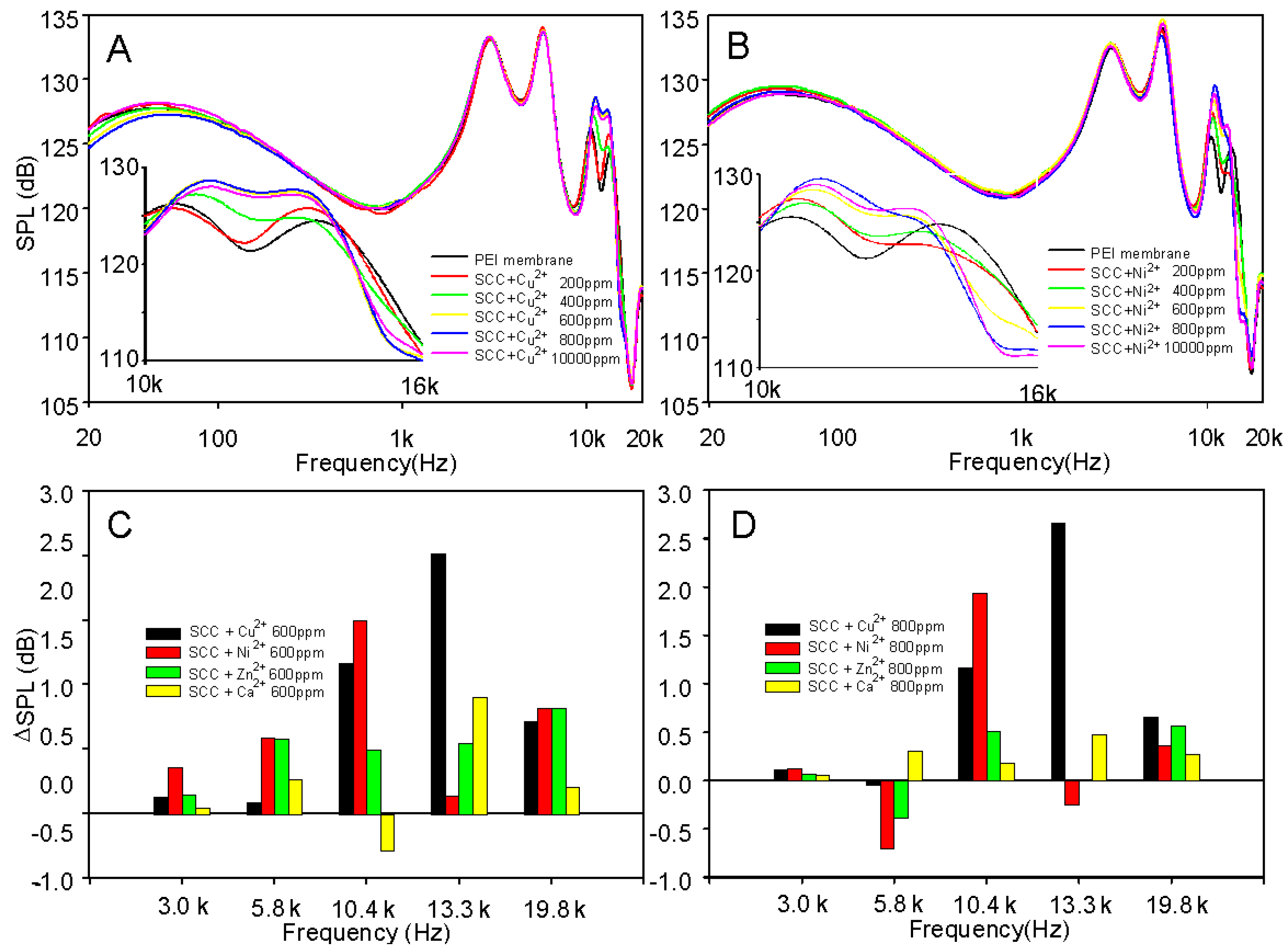
2. Result and Discussion
3. Experimental Section
3.1. General Information
3.2. Isothermal Titration Calorimetry of SCC/Cu2+, SCC/Ni2+, SCC/Zn2+, SCC/Ca2+, Cu2+/DME, SCC/DME, [SCC/Cu2+]/DME Mixtures
3.3. SCC/Metal Coating on PEI Diaphragm and Frequency Response Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gu, J.J.; Montealegre, Z.F.; Robert, D.; Engel, M.S.; Qiao, G.X.; Ren, D. Wing stridulation in a Jurassic katydid (Insecta, Orthoptera) produced low-pitched musical calls to attract females. Proc. Natl. Acad. Sci. USA 2012, 109, 3868. [Google Scholar] [CrossRef] [PubMed]
- Bennet-Clark, H.C. Resonators in insect sound production: How insects produce loud pure-tone songs. J. Exp. Biol. 1999, 202, 3347. [Google Scholar] [PubMed]
- Bennet-Clark, H.C. The mechanism and efficiency of sound production in mole crickets. J. Exp. Biol. 1970, 52, 619. [Google Scholar]
- Sanborn, A.F.; Villet, M.H.; Phillips, P.K. Hot-blooded singers: Endothermy facilitates crepuscular signaling in African platypleurine cicadas (Hemiptera: Cicadidae: Platypleura spp.). Naturwissenschaften 2003, 90, 305. [Google Scholar] [CrossRef]
- Sueur, J.; Aubin, T. Is microhabitat segregation between two cicada species (Tibicina haematodes and Cicada orni) due to calling song propagation constraints? Naturwissenschaften 2003, 90, 322. [Google Scholar] [CrossRef]
- Sueur, J. Cicada acoustic communication: Potential sound partitioning in a multispecies community from Mexico (Hemiptera: Cicadomorpha: Cicadidae). Biol. J. Linn. Soc. 2002, 75, 379. [Google Scholar] [CrossRef]
- Pumphrey, R.J. Upper limit of frequency for human hearing. Nature 1950, 166, 571. [Google Scholar] [CrossRef]
- Masterton, B.; Heffner, H.; Ravizza, R.J. The evolution of human hearing. Acoust. Soc. Am. 1969, 45, 966. [Google Scholar] [CrossRef]
- Lord, H.W.; Gatley, W.S.; Evensen, H.A. Noise Control for Engineers; McGraw: New York, NY, USA, 1980. [Google Scholar]
- Bodson, D. Multiplatform 3-D graphics. IEEE Veh. Technol. Mag. 2010, 5, 24. [Google Scholar] [CrossRef]
- Siciliano, P.C.; Jenks, M.A.; Ollier, L.E.; Dana, M.N. The impact of audio technology on undergraduate instruction in a study abroad course on English gardens. Hortscience 2010, 45, S246. [Google Scholar]
- Sha, J.W.; Li, Y.L.; Salvatierra, R.V.; Wang, T.; Dong, P.; Ji, Y.S.; Lee, S.K.; Zhang, C.H.; Zhang, J.B.; Smith, R.H.; et al. Three-Dimensional printed graphene foams. ACS Nano 2017, 11, 6860. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Ren, T.L.; Xie, D.; Wang, Y.F.; Zhou, C.J.; Feng, T.T.; Fu, D.; Yang, Y.; Peng, P.G.; Wang, L.G.; et al. Graphene-on-paper sound source devices. ACS Nano 2011, 5, 4878. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Chen, Z.; Feng, C.; Liu, L.; Bai, Z.Q.; Wang, Y.; Qian, L.; Zhang, Y.Y.; Li, Q.Q.; Jiang, K.L.; et al. Flexible, stretchable, transparent carbon nanotube thin film loudspeakers. Nano Lett. 2008, 8, 4539. [Google Scholar] [CrossRef] [PubMed]
- Venkatasubramanian, R. Applied physics nanothermal trumpets. Nature 2010, 463, 619. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Zettl, A. Electrostatic graphene loudspeaker. Appl. Phys. Lett. 2013, 102, 223109. [Google Scholar] [CrossRef]
- Zhou, Q.; Zheng, J.L.; Onishi, S.; Crommie, M.F.; Zettl, A.K. Graphene electrostatic microphone and ultrasonic radio. Proc. Natl. Acad. Sci. USA 2015, 112, 8942. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.Y.; Hong, J.Y.; Jang, J. Flexible and transparent graphene films as acoustic actuator electrodes using inkjet printing. Chem. Commun. 2011, 47, 8527. [Google Scholar] [CrossRef]
- Xu, S.C.; Man, B.Y.; Jiang, S.Z.; Chen, C.S.; Yang, C.; Liu, M.; Gao, X.G.; Sun, Z.C.; Zhang, C. Flexible and transparent graphene-based loudspeakers. Appl. Phys. Lett. 2013, 102, 151902. [Google Scholar] [CrossRef]
- Solymosi, K.; Mysliwa-Kurdziel, B. Chlorophylls and their derivatives used in food industry and medicine. Mini-Rev. Med. Chem. 2017, 17, 1194. [Google Scholar] [CrossRef]
- Rafols, C.; Bosch, E.; Barbas, R.; Prohens, R. The Ca2+-EDTA chelation as standard reaction to validate isothermal titration calorimeter measurements (ITC). Talanta 2016, 154, 354. [Google Scholar] [CrossRef]
- Huynh, E.; Lovell, J.F.; Helfield, B.L.; Jeon, M.; Kim, C.; Goertz, D.E.; Wilson, B.C.; Zheng, G. Porphyrin shell microbubbles with intrinsic ultrasound and photoacoustic properties. J. Am. Chem. Soc. 2012, 134, 16464. [Google Scholar] [CrossRef] [PubMed]
- Abuteen, A.; Zanganeh, S.; Akhigbe, J.; Samankumara, L.P.; Aguirre, A.; Biswal, N.; Braune, M.; Vollertsen, A.; Roder, B.; Bruckner, C.; et al. The evaluation of NIR-absorbing porphyrin derivatives as contrast agents in photoacoustic imaging. Phys. Chem. Chem. Phys. 2013, 15, 18502. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.A.; Torres-Torres, D. Changes in wave propagation in a guitar top plate due to the fan bracing and the bridge. Rev. Int. Metod. Numer. 2015, 31, 228. [Google Scholar]
- Kahari, K.R.; Axelsson, A.; Hellstrom, P.A.; Zachau, G. Hearing assessment of classical orchestral musicians. Scand. Audiol. 2001, 30, 13. [Google Scholar] [CrossRef] [PubMed]
- Rensing, N.; Schemmann, H.; Zalpour, C. Musculoskeletal demands in violin and viola playing a literature review. Med. Probl. Perform. Ar. 2018, 33, 265. [Google Scholar] [CrossRef] [PubMed]
- Tai, H.C.; Li, G.C.; Huang, S.J.; Jhu, C.R.; Chung, J.H.; Wang, B.Y.; Hsu, C.S.; Brandmair, B.; Chung, D.T.; Chen, H.M.; et al. Chemical distinctions between Stradivari’s maple and modern tonewood. Proc. Natl. Acad. Sci. USA 2017, 114, 27. [Google Scholar] [CrossRef]
- Nagyvary, J.; DiVerdi, J.A.; Owen, N.L.; Tolley, H.D. Wood used by Stradivari and Guarneri. Nature 2006, 444, 565. [Google Scholar] [CrossRef]
- Tai, H.C.; Shen, Y.P.; Lin, J.H.; Chung, D.T. Acoustic evolution of old Italian violins from Amati to Stradivari. Proc. Natl. Acad. Sci. USA 2018, 115, 5926. [Google Scholar] [CrossRef]
- Ferruzzi, M.G.; Schwartz, S.J. Thermal degradation of commercial grade sodium copper chlorophyllin. J. Agric. Food Chem. 2005, 53, 7098. [Google Scholar] [CrossRef]
Sample Availability: Samples of the PEI-based earphone are available from the authors. |
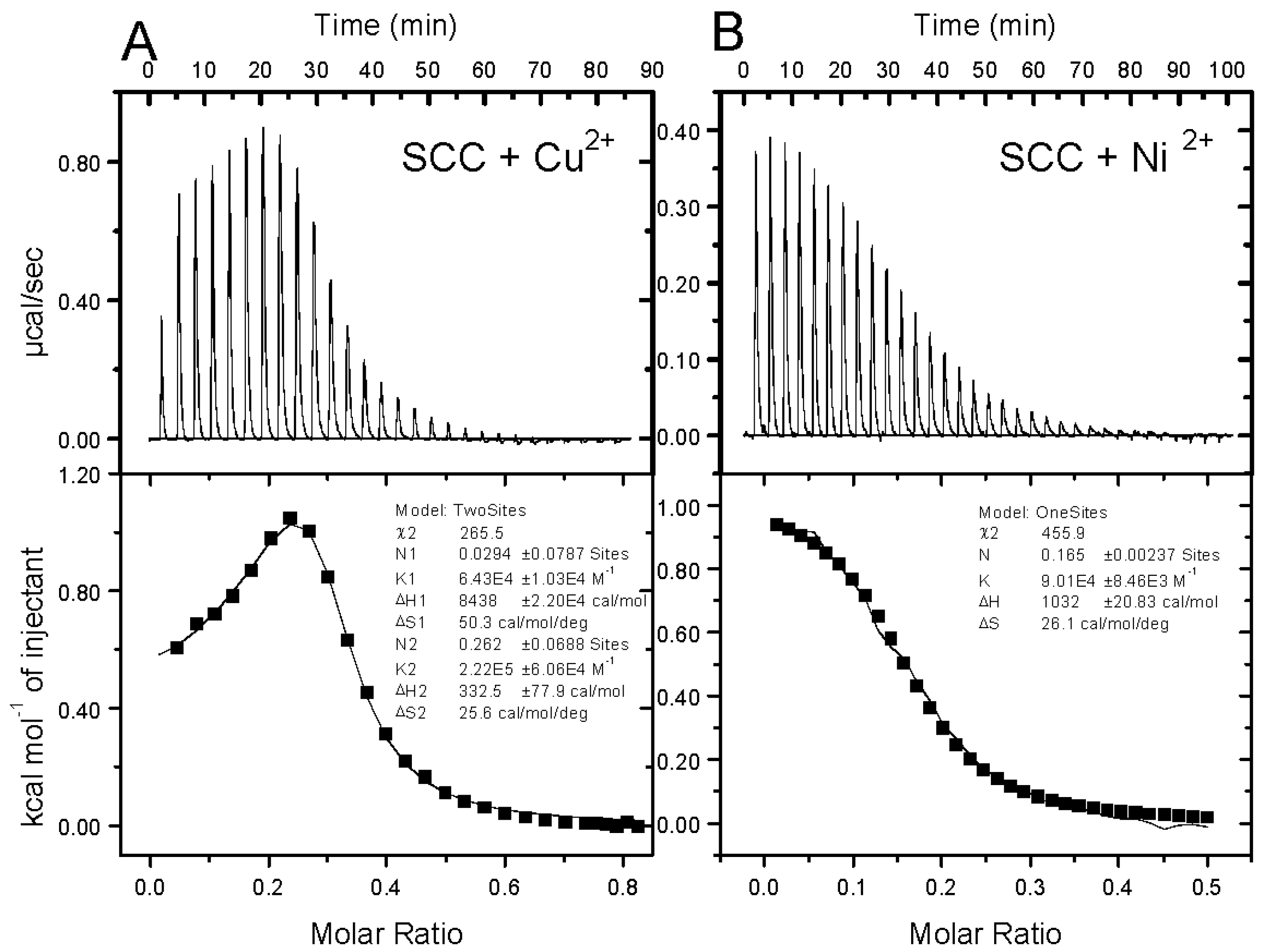


| Entry | M2+ | 1/n (SCC/M2+) | Ka/104 (M−1) | ΔH (kcal/mol) | ΔS (cal/mol/K) | ΔG (kcal/mol) |
|---|---|---|---|---|---|---|
| 1 | Cu2+ | 3.82 | 22.2 | 0.33 | 25.6 | −7.30 |
| 2 | Ni2+ | 6.06 | 9.01 | 1.03 | 26.1 | −6.75 |
| 3 a | Zn2+ | - | - | - | - | - |
| 4 a | Ca2+ | - | - | - | - | - |
| Entry | Complex | n (Complex/DME) | Ka/104 (M−1) | ΔH (kcal/mol) | ΔS (cal/mol/K) | ΔG (kcal/mol) |
|---|---|---|---|---|---|---|
| 1 a | Cu2+ | 1.88 | 1.16 | −9.45 | −13.10 | −5.54 |
| 2 b | SCC | 1.01 | 1.38 | −5.64 | 0.03 | −5.65 |
| 3 c | SCC/Cu2+ (4/1) | 1.16 (n1) 3.97 (n2) | 942 3.50 | −9.23 −4.08 | −0.95 7.10 | −8.95 −6.20 |
| Entry | Sample | δsample without Cu2+ | δsample with Cu2+ | δsample with Cu2++DME |
|---|---|---|---|---|
| 1 | SCC | 1568(S) | 1554(M) | 1571(M) b |
| 2 | sodium benzoate (SB) | 1588(M), 1552(S) | 1595(M), 1550(S) | 1595(M), 1550(S) c |
| 3 | sodium citrate (SC) | 1596(M), 1554(S) | 1596(M), 1557(M) | 1567(M) d |
| Entry | PEI Composite | E (GPa) |
|---|---|---|
| 1 | PEI polymer | 0.96 ± 0.15 |
| 2 | 400 ppm SCC/Cu2+ | 0.97 ± 0.18 |
| 3 | 600 ppm SCC/Cu2+ | 1.08 ± 0.16 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.-Z.; Wu, J.-J.; Lee, W.-J.; Chen, C.-S. Improving the High-Frequency Response of PEI-Based Earphone with Sodium Copper Chlorophyllin. Molecules 2020, 25, 219. https://doi.org/10.3390/molecules25010219
Li H-Z, Wu J-J, Lee W-J, Chen C-S. Improving the High-Frequency Response of PEI-Based Earphone with Sodium Copper Chlorophyllin. Molecules. 2020; 25(1):219. https://doi.org/10.3390/molecules25010219
Chicago/Turabian StyleLi, Hao-Zhi, Jun-Jie Wu, Wei-Jen Lee, and Chien-Sheng Chen. 2020. "Improving the High-Frequency Response of PEI-Based Earphone with Sodium Copper Chlorophyllin" Molecules 25, no. 1: 219. https://doi.org/10.3390/molecules25010219
APA StyleLi, H.-Z., Wu, J.-J., Lee, W.-J., & Chen, C.-S. (2020). Improving the High-Frequency Response of PEI-Based Earphone with Sodium Copper Chlorophyllin. Molecules, 25(1), 219. https://doi.org/10.3390/molecules25010219