L-Carnitine Is Involved in Hyperbaric Oxygen-Mediated Therapeutic Effects in High Fat Diet-Induced Lipid Metabolism Dysfunction
Abstract
1. Introduction
2. Results
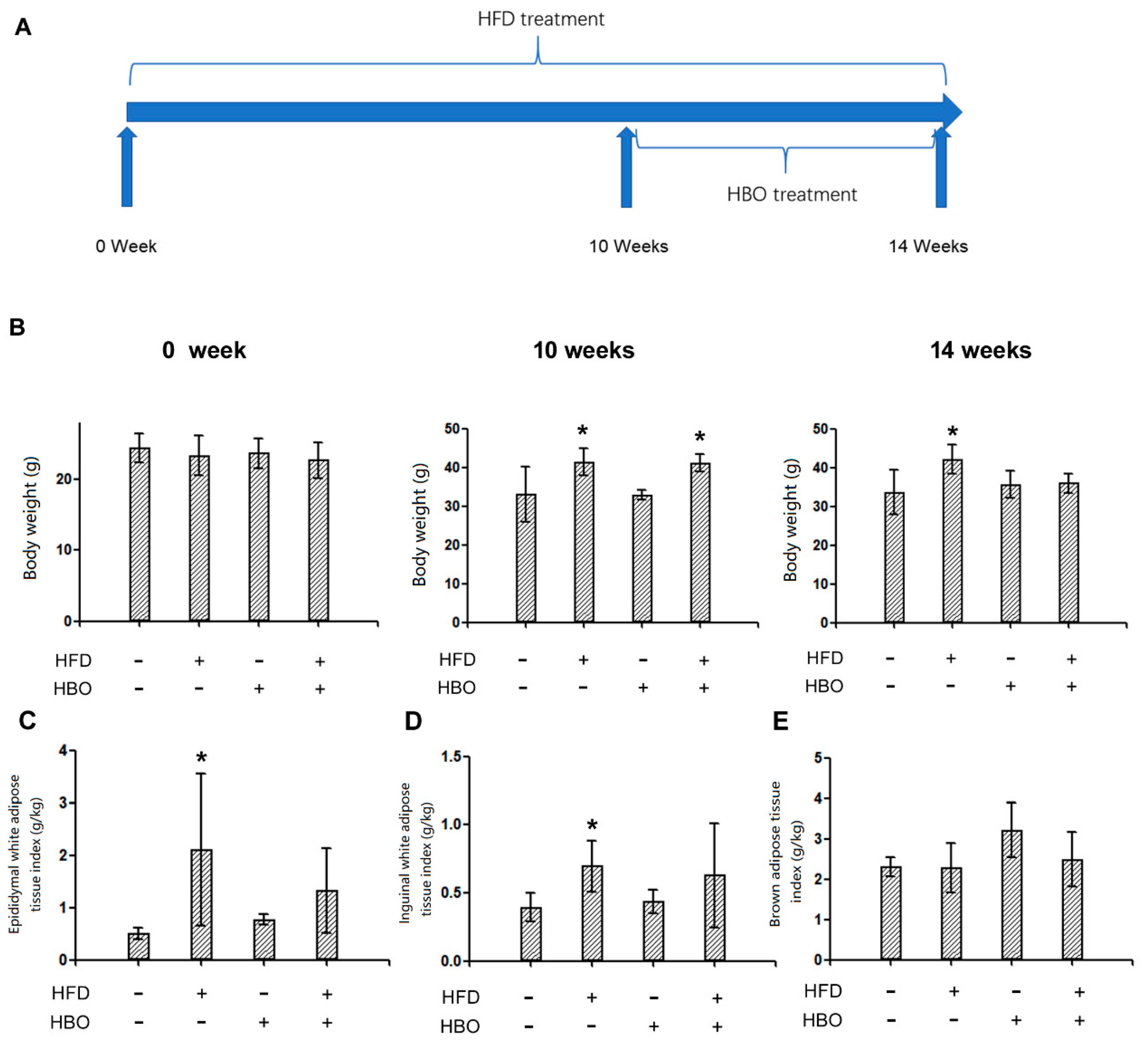
2.1. Experimental Design and General Parameters
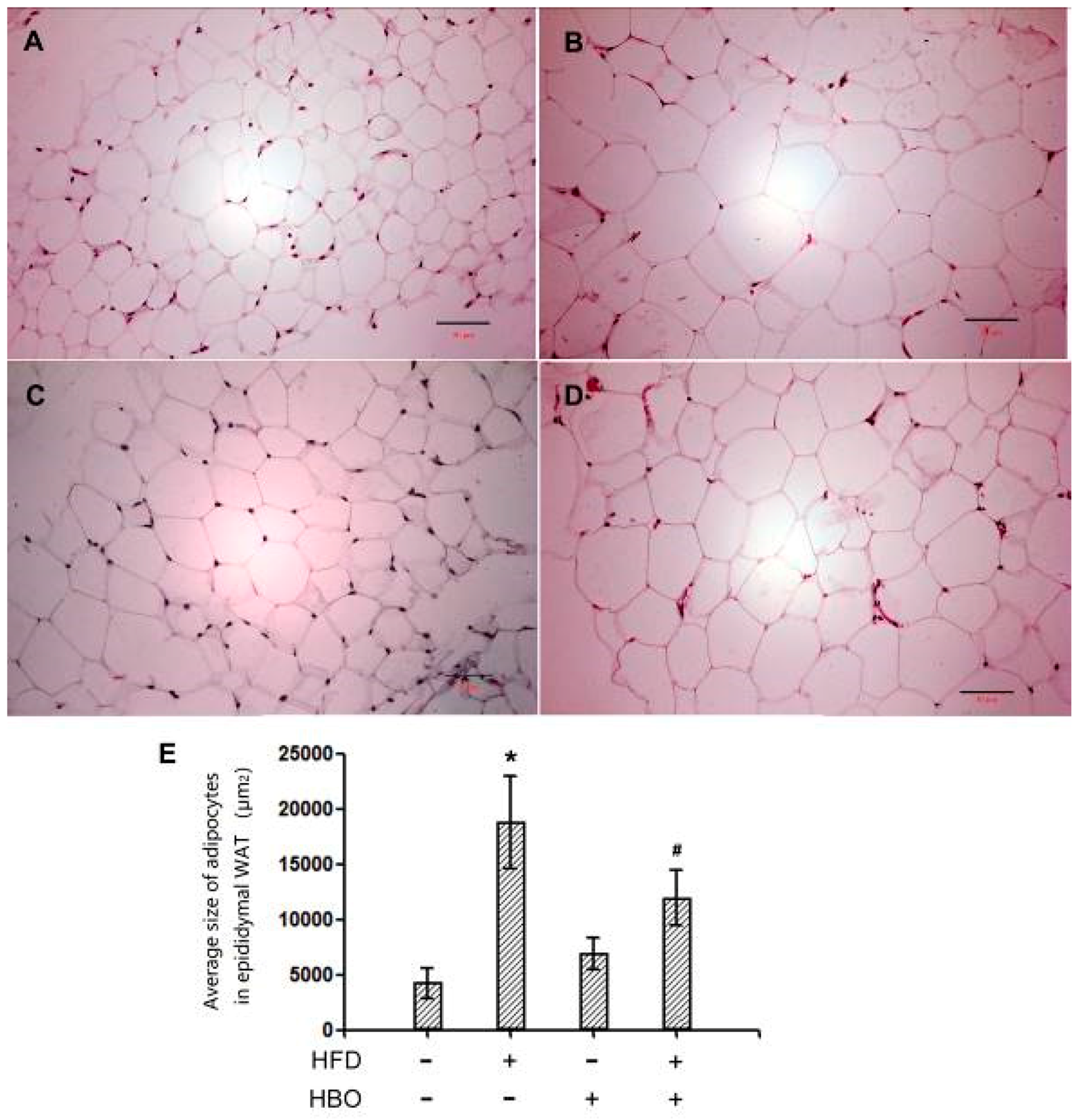
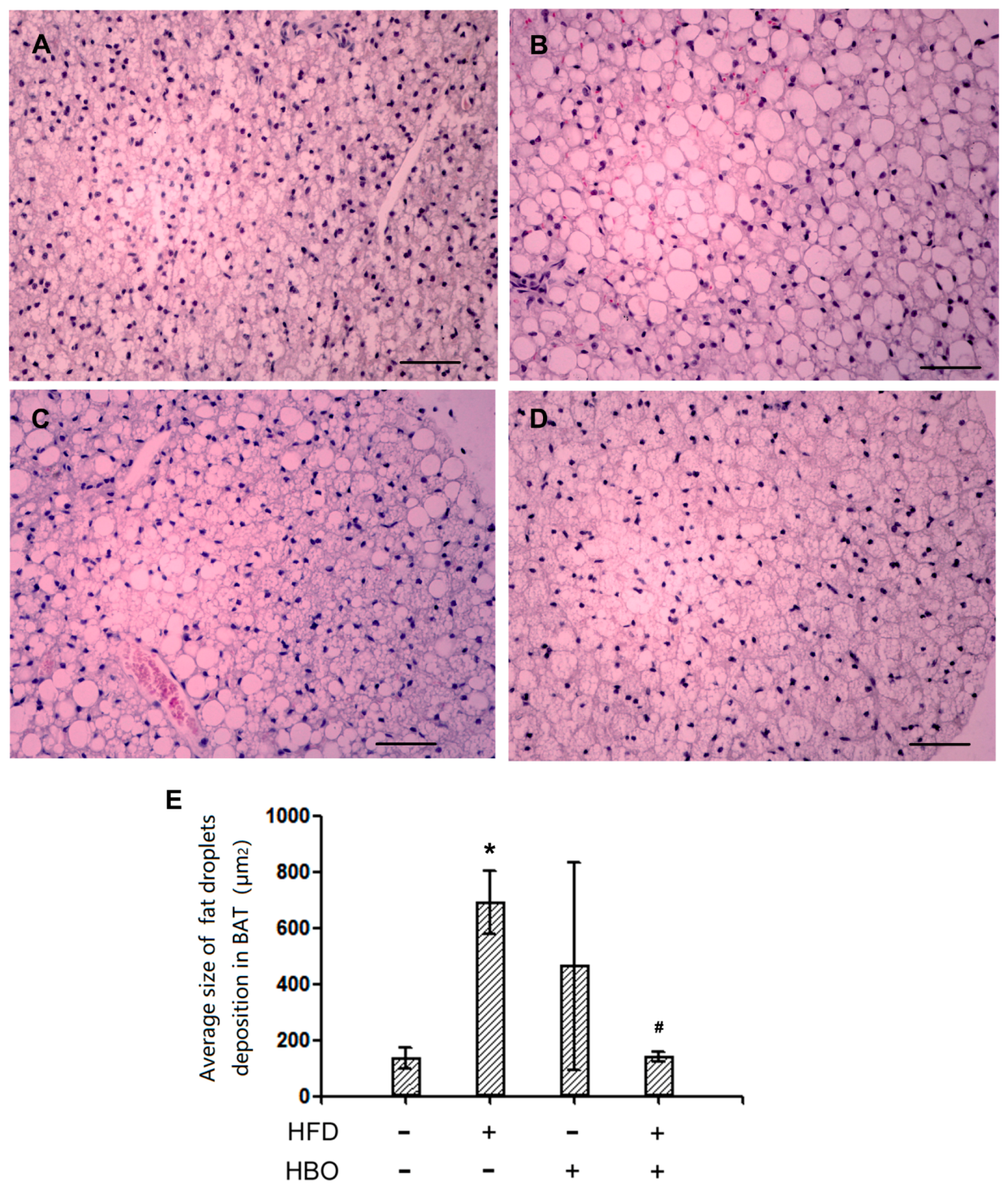
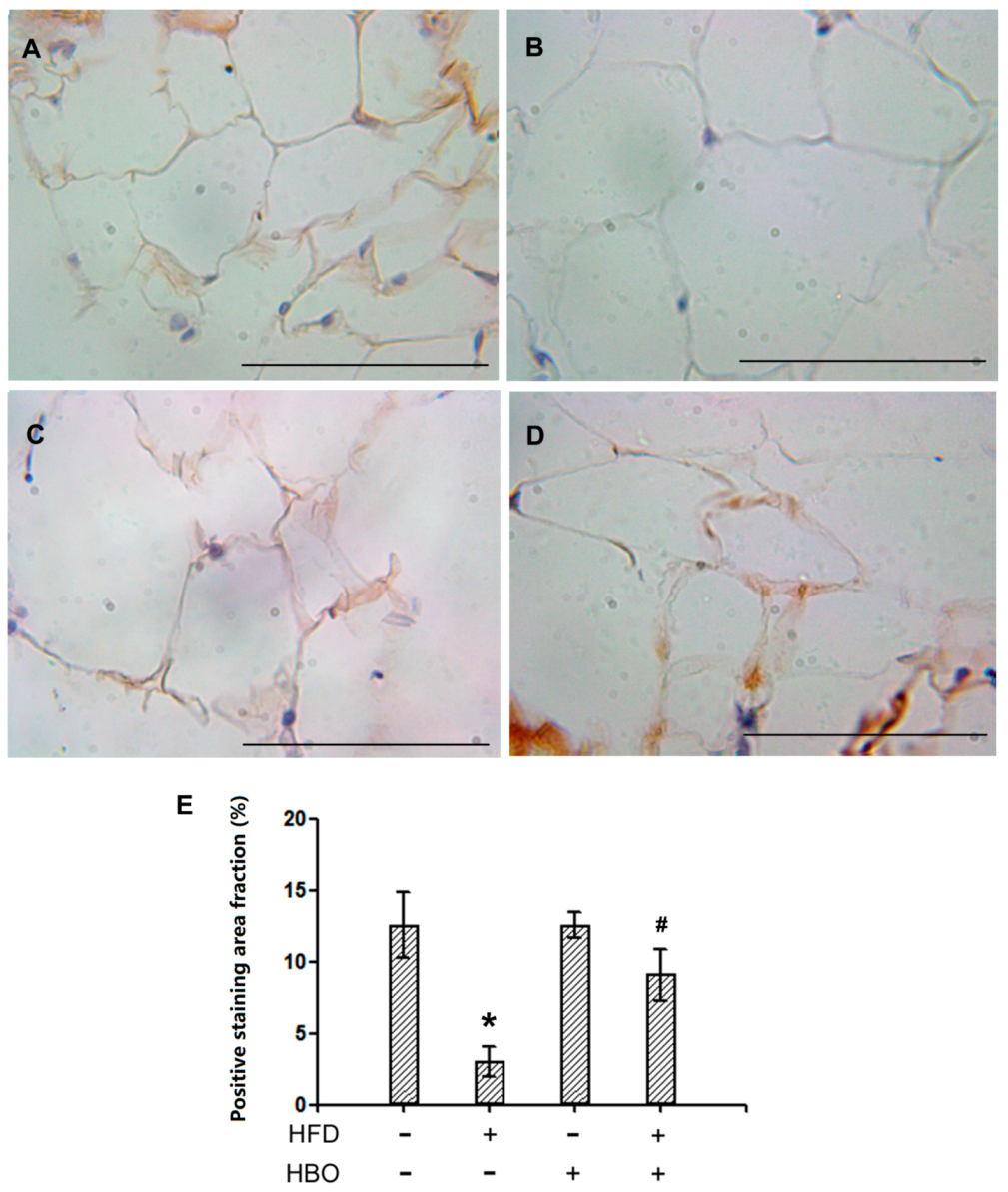
2.2. Histological Assessments
2.2.1. Hematoxylin and Eosin Staining for EWAT
2.2.2. Hematoxyin and Eosin Staining for BAT
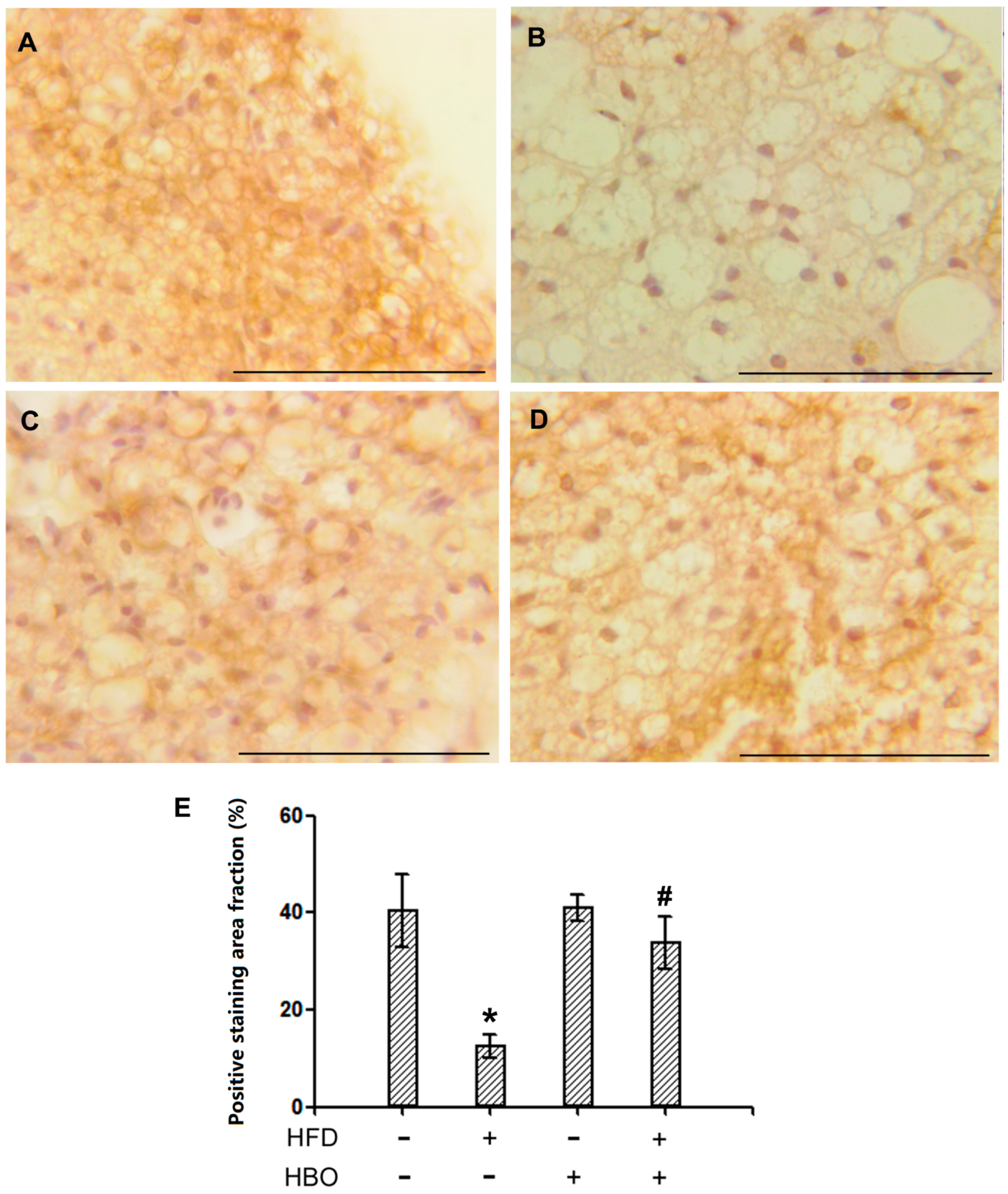
2.2.3. Immunohistochemistry for HSL in EWAT
2.2.4. Immunohistochemistry for HSL in BAT
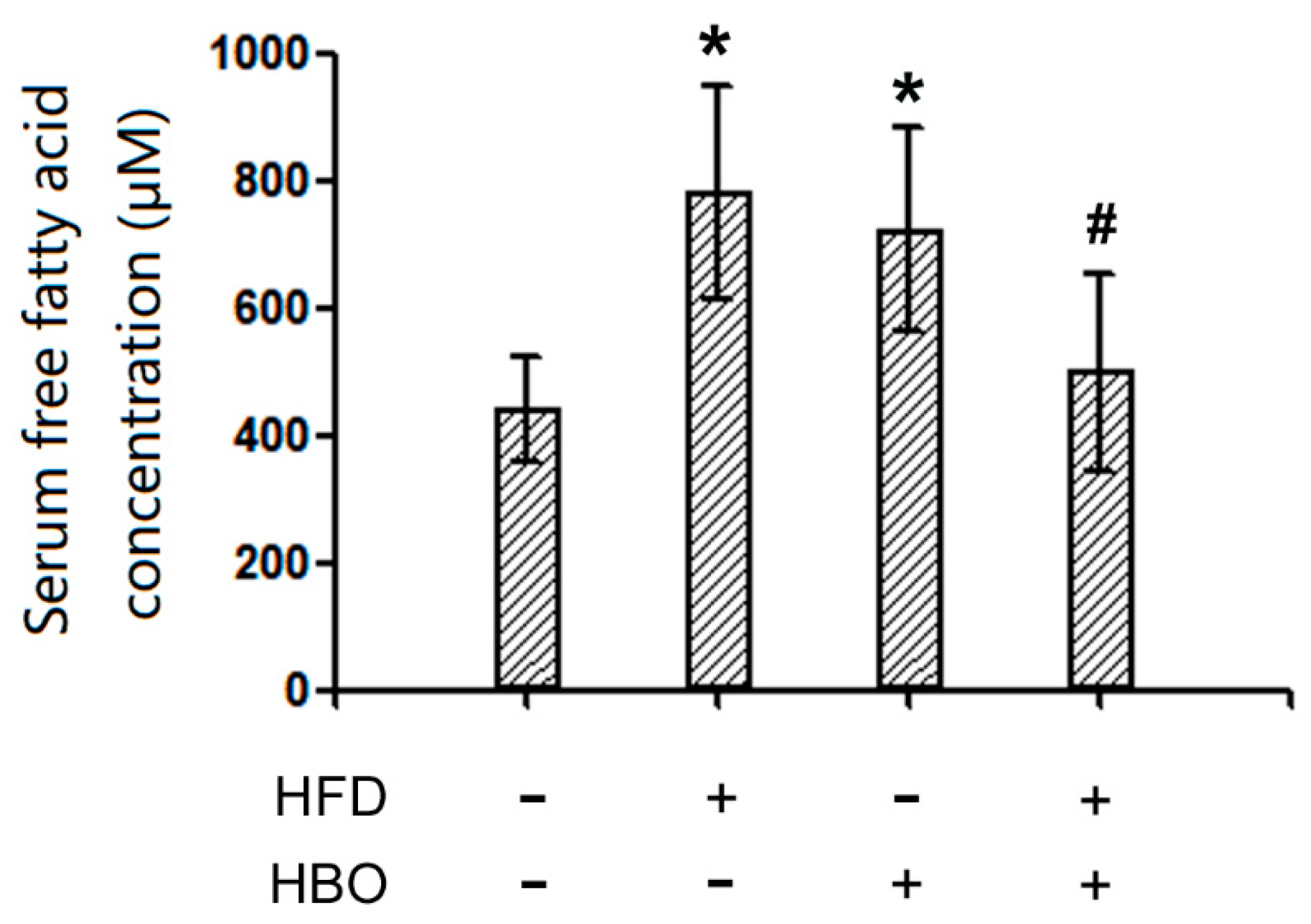
2.3. Serum FFA Levels
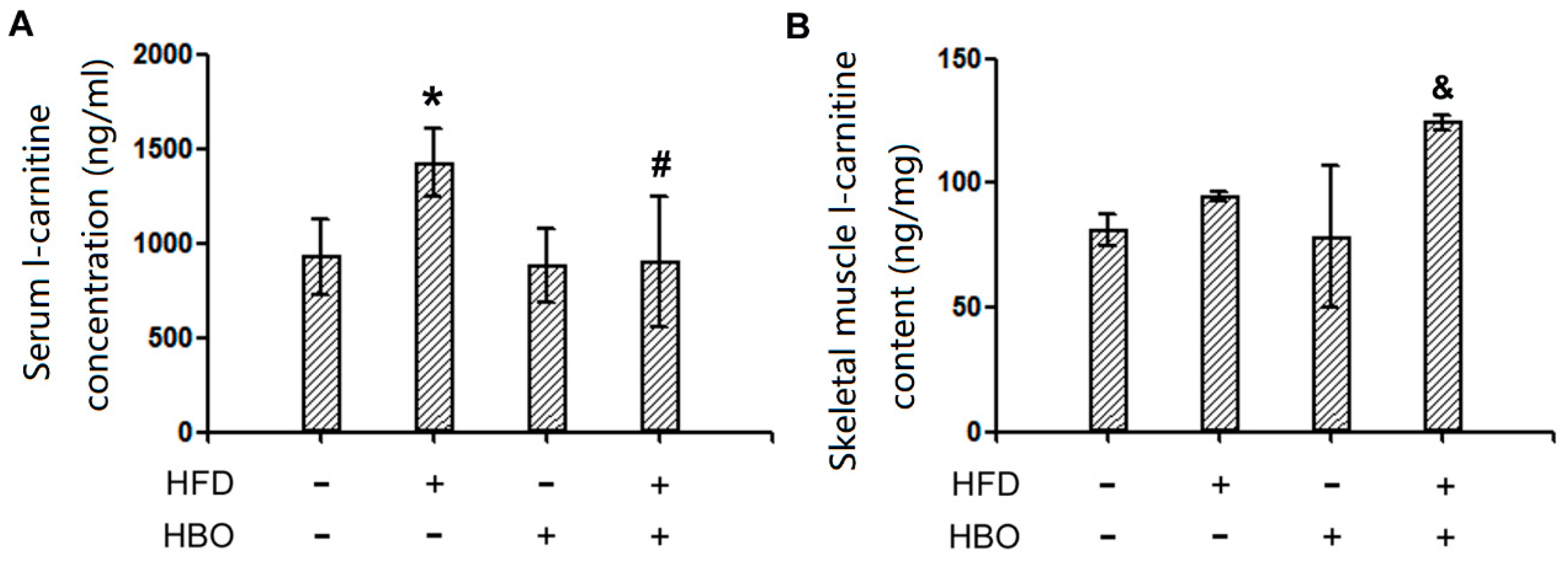
2.4. Endogenous L-Carnitine Levels in Serum and Skeletal Muscle
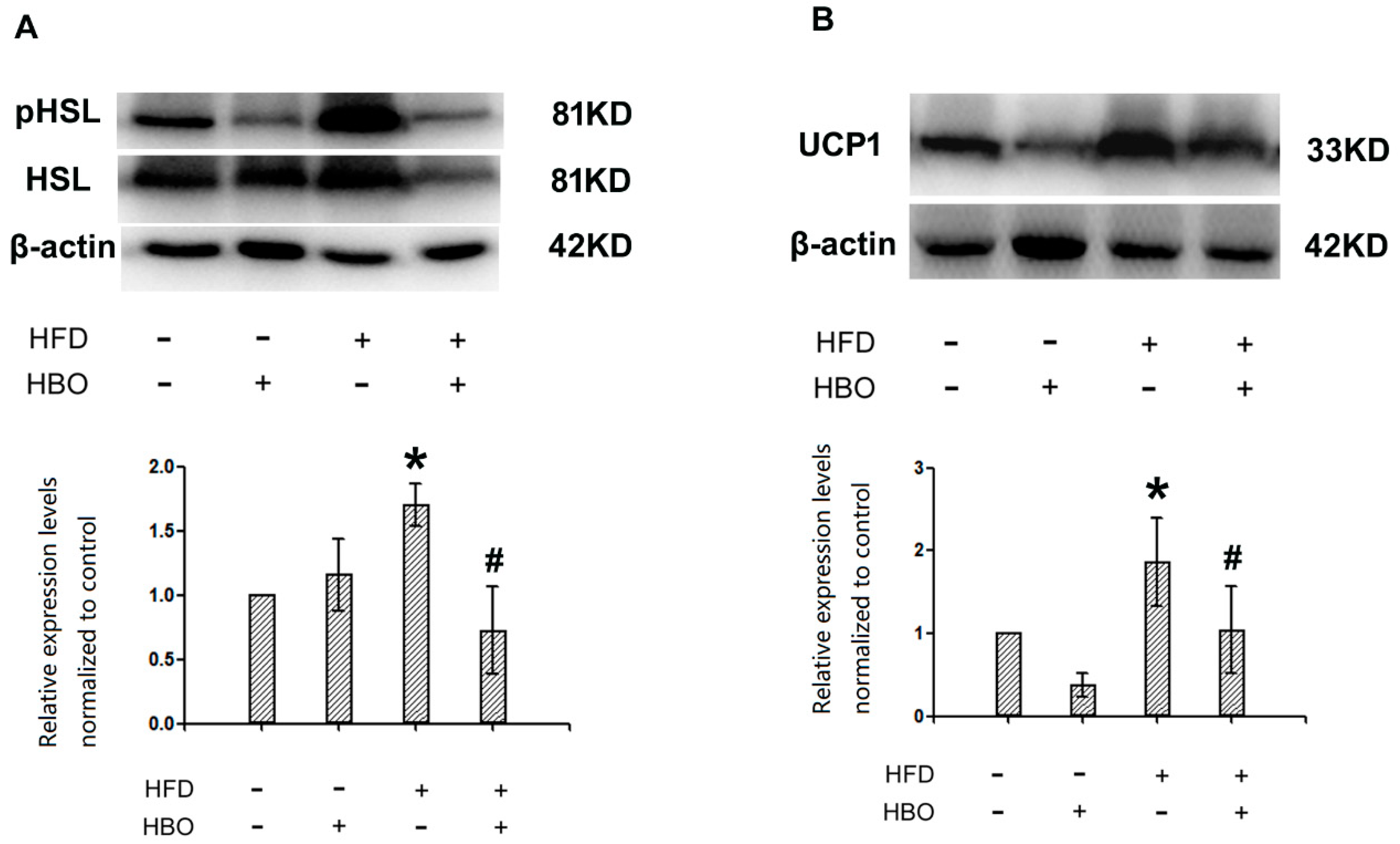
2.5. Western Blotting for pHSL/HSL and UCP1 in Brown Adipose Tissues
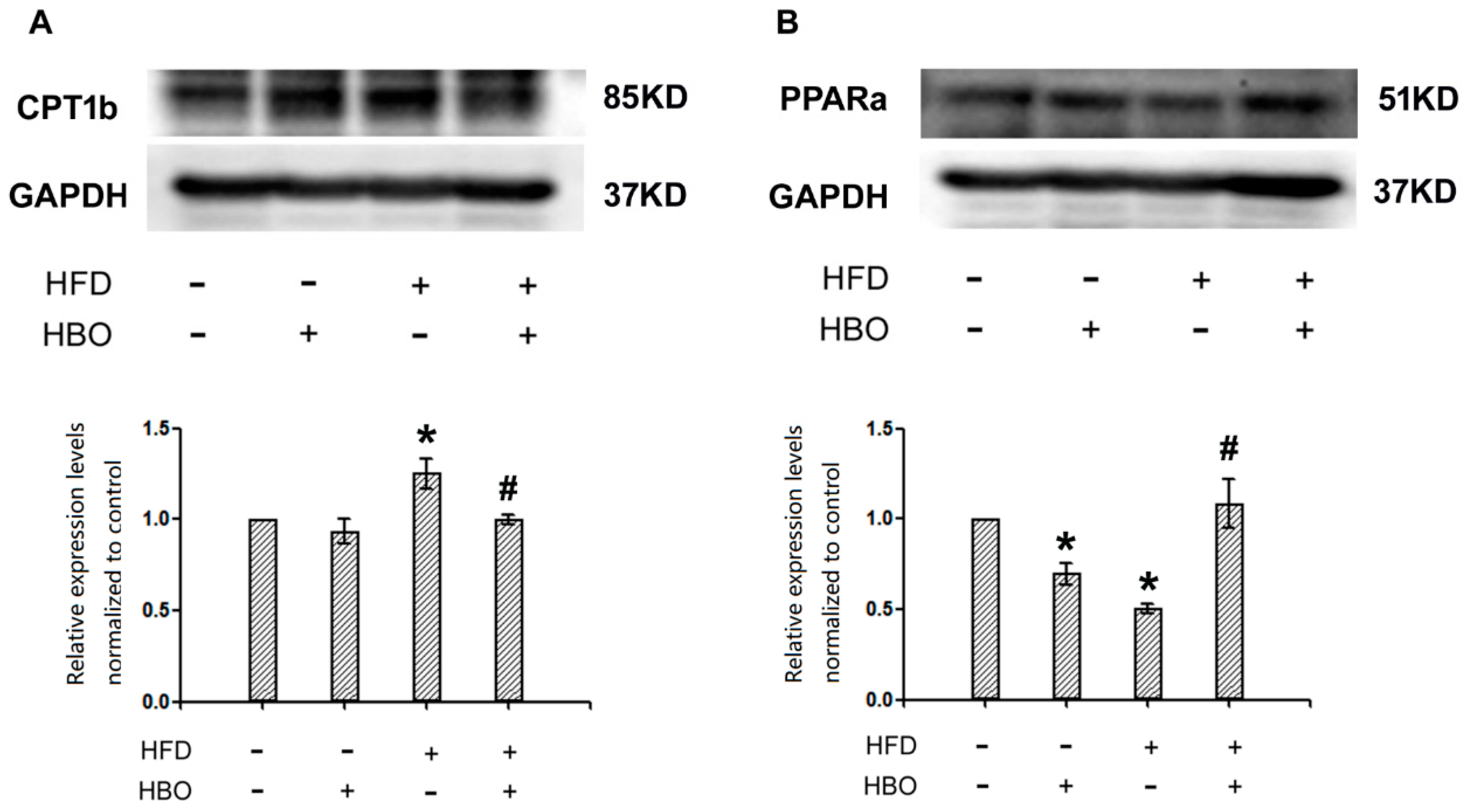
2.6. Western Blotting for CPT1b and PPARα in Skeletal Muscle
3. Discussion
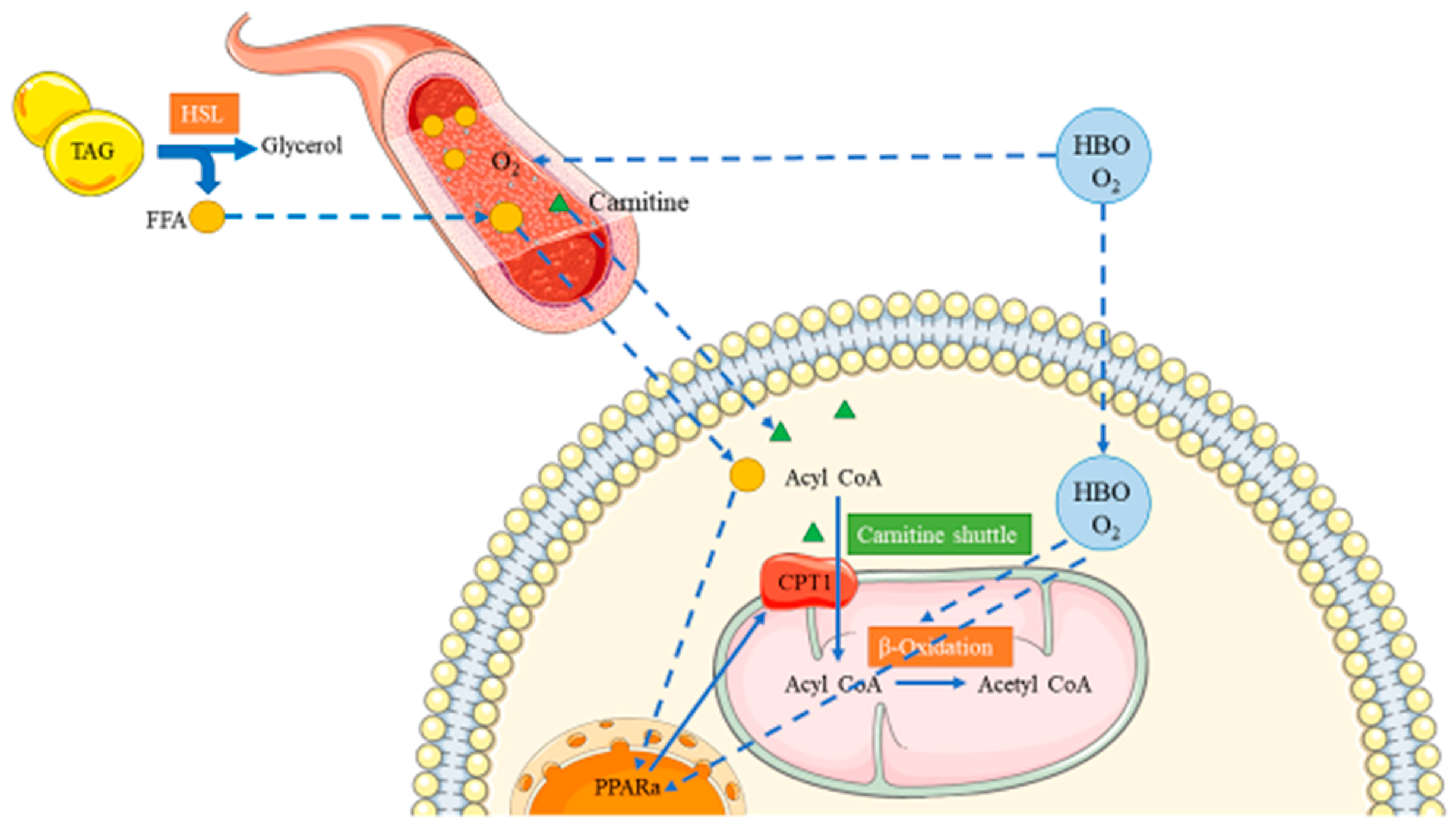
3.1. HBO and Fatty Acid Metabolism
3.2. Roles of L-Carnitine in HBO Mediated Protective Effects Against HFD-Induced Fatty Acid Metabolism Dysfunction
3.3. L-Carnitine and PPARα/CPT1b
3.4. Limitations of the Current Study and Future Directions
4. Materials and Methods
4.1. Materials
4.2. Animal Housing, Treatment and Sample Collection
4.3. Histological Methods
4.4. Serum FFA Level Measurement
4.5. Liquid Chromatography-Tandem Mass Spectrometry Measurement for Serum and Skeletal Muscle L-Carnitine Levels
4.6. Western Blotting
4.7. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mendoza, J.A.; Drewnowski, A.; Christakis, D.A. Dietary energy density is associated with obesity and the metabolic syndrome in U.S. adults. Diabetes Care 2007, 30, 974–979. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, M.; Zhao, Z.; Huang, Z.; Deng, Q.; Li, Y.; Pan, A.; Li, C.; Chen, Z.; Zhou, M.; et al. Geographic Variation in Prevalence of Adult Obesity in China: Results From the 2013-2014 National Chronic Disease and Risk Factor Surveillance. Ann. Intern. Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Boren, J.; Taskinen, M.R.; Olofsson, S.O.; Levin, M. Ectopic lipid storage and insulin resistance: A harmful relationship. J. Intern. Med. 2013, 274, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Gaidhu, M.P.; Anthony, N.M.; Patel, P.; Hawke, T.J.; Ceddia, R.B. Dysregulation of lipolysis and lipid metabolism in visceral and subcutaneous adipocytes by high-fat diet: Role of ATGL, HSL, and AMPK. Am. J. Physiol. Cell Physiol. 2010, 298, C961-71. [Google Scholar] [CrossRef] [PubMed]
- Crowley, V.E.; Yeo, G.S.; O’Rahilly, S. Obesity therapy: Altering the energy intake-and-expenditure balance sheet. Nat. Rev. Drug Discov. 2002, 1, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Rider, O.J.; Holloway, C.J.; Emmanuel, Y.; Bloch, E.; Clarke, K.; Neubauer, S. Increasing plasma free fatty acids in healthy subjects induces aortic distensibility changes seen in obesity. Circ. Cardiovasc. Imaging 2012, 5, 367–375. [Google Scholar] [CrossRef]
- Salas-Salvado, J.; Martinez-Gonzalez, M.A.; Bullo, M.; Ros, E. The role of diet in the prevention of type 2 diabetes. Nutr. Metab. Cardiovasc. Dis. 2011, 21 (Suppl. 2). [Google Scholar] [CrossRef]
- Faulds, M.H.; Dahlman-Wright, K. Metabolic diseases and cancer risk. Curr. Opin. Oncol. 2012, 24, 58–61. [Google Scholar] [CrossRef]
- Adeva-Andany, M.M.; Carneiro-Freire, N.; Seco-Filgueira, M.; Fernandez-Fernandez, C.; Mourino-Bayolo, D. Mitochondrial beta-oxidation of saturated fatty acids in humans. Mitochondrion 2019, 46, 73–90. [Google Scholar] [CrossRef]
- Schiaffino, S.; Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef]
- Li, X.; Higashida, K.; Kawamura, T.; Higuchi, M. Time Course of Decrease in Skeletal Muscle Mitochondrial Biogenesis after Discontinuation of High-Fat Diet. J. Nutr. Sci. Vitam. 2018, 64, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.; Bruce, C.R.; Beale, S.M.; Hoehn, K.L.; So, T.; Rolph, M.S.; Cooney, G.J. Excess lipid availability increases mitochondrial fatty acid oxidative capacity in muscle: Evidence against a role for reduced fatty acid oxidation in lipid-induced insulin resistance in rodents. Diabetes 2007, 56, 2085–2092. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.S. Effects of L-carnitine on obesity, diabetes, and as an ergogenic aid. Asia Pac. J. Clin. Nutr. 2008, 17 (Suppl. 1), 306–308. [Google Scholar]
- Li, J.L.; Wang, Q.Y.; Luan, H.Y.; Kang, Z.C.; Wang, C.B. Effects of L-carnitine against oxidative stress in human hepatocytes: Involvement of peroxisome proliferator-activated receptor alpha. J. Biomed. Sci. 2012, 19, 32. [Google Scholar] [CrossRef]
- Nielsen, T.S.; Jessen, N.; Jorgensen, J.O.; Moller, N.; Lund, S. Dissecting adipose tissue lipolysis: Molecular regulation and implications for metabolic disease. J. Mol. Endocrinol. 2014, 52, R199–R222. [Google Scholar] [CrossRef]
- Wharton, S.; Bonder, R.; Jeffery, A.; Christensen, R.A.G. The safety and effectiveness of commonly-marketed natural supplements for weight loss in populations with obesity: A critical review of the literature from 2006 to 2016. Crit. Rev. Food Sci. Nutr. 2019, 1–17. [Google Scholar] [CrossRef]
- Pooyandjoo, M.; Nouhi, M.; Shab-Bidar, S.; Djafarian, K.; Olyaeemanesh, A. The effect of (L-)carnitine on weight loss in adults: A systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2016, 17, 970–976. [Google Scholar] [CrossRef]
- Anuradha, C.V. L-carnitine: Implications in the treatment of the metabolic syndrome and Type 2 diabetes. Expert Rev. Endocrinol. Metab. 2008, 3, 777–783. [Google Scholar] [CrossRef]
- Aday, A.W.; Goldfine, A.B.; Gregory, J.M.; Beckman, J.A. Impact of Acipimox Therapy on Free Fatty Acid Efflux and Endothelial Function in the Metabolic Syndrome: A Randomized Trial. Obes. (Silver Spring) 2019, 27, 1812–1819. [Google Scholar] [CrossRef]
- Hashimoto, S.; Hayashi, S.; Yoshida, A.; Naito, M. Acute effects of postprandial aerobic exercise on glucose and lipoprotein metabolism in healthy young women. J. Atheroscler. Thromb. 2013, 20, 204–213. [Google Scholar] [CrossRef]
- World Health Organization. Report of a WHO Consultation. In Obesity: Preventing and Managing the Global Epidemic; World Health Organization Technical Report Series; World Health Organization: Geneva, Switzerland, 2000; Volume 894, pp. 1–253. [Google Scholar]
- Kahle, A.C.; Cooper, J.S. Hyperbaric Physiological And Pharmacological Effects Gases; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2019. [Google Scholar]
- Ishihara, A. Mild hyperbaric oxygen: Mechanisms and effects. J. Physiol. Sci. 2019, 69, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Takemura, A.; Ishihara, A. Mild Hyperbaric Oxygen Improves Decreased Oxidative Capacity of Spinal Motoneurons Innervating the Soleus Muscle of Rats with Type 2 Diabetes. Neurochem. Res. 2016, 41, 2336–2344. [Google Scholar] [CrossRef]
- Li, J.; Huang, Q.; Long, X.; Zhang, J.; Huang, X.; Aa, J.; Yang, H.; Chen, Z.; Xing, J. CD147 reprograms fatty acid metabolism in hepatocellular carcinoma cells through Akt/mTOR/SREBP1c and P38/PPARalpha pathways. J. Hepatol. 2015, 63, 1378–1389. [Google Scholar] [CrossRef]
- Chen, W.; Liang, X.; Nong, Z.; Li, Y.; Pan, X.; Chen, C.; Huang, L. The Multiple Applications and Possible Mechanisms of the Hyperbaric Oxygenation Therapy. Med. Chem. 2019, 15, 459–471. [Google Scholar] [CrossRef]
- Wilkinson, D.; Nolting, M.; Mahadi, M.K.; Chapman, I.; Heilbronn, L. Hyperbaric oxygen therapy increases insulin sensitivity in overweight men with and without type 2 diabetes. Diving Hyperb. Med. 2015, 45, 30–36. [Google Scholar]
- Fujita, N.; Nagatomo, F.; Murakami, S.; Kondo, H.; Ishihara, A.; Fujino, H. Effects of hyperbaric oxygen on metabolic capacity of the skeletal muscle in type 2 diabetic rats with obesity. ScientificWorldJournal 2012, 2012, 637978. [Google Scholar] [CrossRef]
- Tsuneyama, K.; Chen, Y.C.; Fujimoto, M.; Sasaki, Y.; Suzuki, W.; Shimada, T.; Iizuka, S.; Nagata, M.; Aburada, M.; Chen, S.Y. Advantages and disadvantages of hyperbaric oxygen treatment in mice with obesity hyperlipidemia and steatohepatitis. ScientificWorldJournal 2011, 11, 2124–2135. [Google Scholar] [CrossRef]
- Gonzalez-Muniesa, P.; Garcia-Gerique, L.; Quintero, P.; Arriaza, S.; Lopez-Pascual, A.; Martinez, J.A. Effects of Hyperoxia on Oxygen-Related Inflammation with a Focus on Obesity. Oxid. Med. Cell. Longev. 2015, 2015, 8957827. [Google Scholar] [CrossRef]
- Corcoran, T.; Ting, S.; Mas, E.; Phillips, M.; O’Loughlin, E.; Barden, A.; Mori, T.A. Hyperbaric oxygen therapy is not associated with oxidative stress assessed using plasma F2-isoprostanes and isofurans. Prostaglandins Leukot. Essent. Fat. Acids 2017, 127, 16–19. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Liu, Y.Y.; Liu, G.H.; Lu, H.B.; Mao, C.Y. l-Carnitine and heart disease. Life Sci. 2018, 194, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Madsen, K.L.; Preisler, N.; Rasmussen, J.; Hedermann, G.; Olesen, J.H.; Lund, A.M.; Vissing, J. L-Carnitine Improves Skeletal Muscle Fat Oxidation in Primary Carnitine Deficiency. J. Clin. Endocrinol. Metab. 2018, 103, 4580–4588. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.W.; Song, M.; Li, Y.L.; Liu, Y.P.; Liu, C.; Han, L.; Wang, Z.H.; Zhang, W.; Xing, Y.Q.; Zhong, M. L-Carnitine inhibits the senescence-associated secretory phenotype of aging adipose tissue by JNK/p53 pathway. Biogerontology 2019, 20, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Li, Q.; Zhong, W.; Dong, J.; Wang, Z.; Wang, C. L-carnitine ameliorated fatty liver in high-calorie diet/STZ-induced type 2 diabetic mice by improving mitochondrial function. Diabetol. Metab. Syndr. 2011, 3, 31. [Google Scholar] [CrossRef]
- Yamazaki, N. Identification of muscle-type carnitine palmitoyltransferase I and characterization of its atypical gene structure. Biol. Pharm. Bull. 2004, 27, 1707–1716. [Google Scholar] [CrossRef]
- Salic, K.; Gart, E.; Seidel, F.; Verschuren, L.; Caspers, M.; van Duyvenvoorde, W.; Wong, K.E.; Keijer, J.; Bobeldijk-Pastorova, I.; Wielinga, P.Y.; et al. Combined Treatment with L-Carnitine and Nicotinamide Riboside Improves Hepatic Metabolism and Attenuates Obesity and Liver Steatosis. Int. J. Mol. Sci. 2019, 20, 4359. [Google Scholar] [CrossRef]
- Stephens, F.B.; Galloway, S.D. Carnitine and fat oxidation. Nestle Nutr. Inst. Workshop Ser. 2013, 76, 13–23. [Google Scholar] [CrossRef]
- Gao, X.; Tian, Y.; Randell, E.; Zhou, H.; Sun, G. Unfavorable Associations Between Serum Trimethylamine N-Oxide and L-Carnitine Levels With Components of Metabolic Syndrome in the Newfoundland Population. Front. Endocrinol. 2019, 10, 168. [Google Scholar] [CrossRef]
- Noland, R.C.; Koves, T.R.; Seiler, S.E.; Lum, H.; Lust, R.M.; Ilkayeva, O.; Stevens, R.D.; Hegardt, F.G.; Muoio, D.M. Carnitine insufficiency caused by aging and overnutrition compromises mitochondrial performance and metabolic control. J. Biol. Chem. 2009, 284, 22840–22852. [Google Scholar] [CrossRef]
- Takemura, A.; Ishihara, A. Mild Hyperbaric Oxygen Inhibits Growth-related Decrease in Muscle Oxidative Capacity of Rats with Metabolic Syndrome. J. Atheroscler. Thromb. 2017, 24, 26–38. [Google Scholar] [CrossRef]
- Hughes, M.L.; Liu, B.; Halls, M.L.; Wagstaff, K.M.; Patil, R.; Velkov, T.; Jans, D.A.; Bunnett, N.W.; Scanlon, M.J.; Porter, C.J. Fatty Acid-binding Proteins 1 and 2 Differentially Modulate the Activation of Peroxisome Proliferator-activated Receptor alpha in a Ligand-selective Manner. J. Biol. Chem. 2015, 290, 13895–13906. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, P.J.; Chiou, H.C.; Jiang, H.J.; Lee, M.Y.; Hsieh, T.J.; Kuo, K.K. Pioglitazone Enhances Cytosolic Lipolysis, beta-oxidation and Autophagy to Ameliorate Hepatic Steatosis. Sci. Rep. 2017, 7, 9030. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Xue, B.; Ahmed, R.Z.; Ding, G.; Li, Z. Fine particles cause the abnormality of cardiac ATP levels via PPARa-mediated utilization of fatty acid and glucose using in vivo and in vitro models. Env. Pollut. 2019, 249, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, M.D.; Hwang, S.K.; Pauli, C.; Murphy, C.J.; Cheng, Z.; Hopkins, B.D.; Wu, D.; Loughran, R.M.; Emerling, B.M.; Zhang, G.; et al. Fenofibrate prevents skeletal muscle loss in mice with lung cancer. Proc. Natl. Acad. Sci. USA 2018, 115, E743–E752. [Google Scholar] [CrossRef]
- Burri, L.; Thoresen, G.H.; Berge, R.K. The Role of PPARalpha Activation in Liver and Muscle. Ppar Res. 2010, 2010. [Google Scholar] [CrossRef]
- Faiola, B.; Falls, J.G.; Peterson, R.A.; Bordelon, N.R.; Brodie, T.A.; Cummings, C.A.; Romach, E.H.; Miller, R.T. PPAR alpha, more than PPAR delta, mediates the hepatic and skeletal muscle alterations induced by the PPAR agonist GW0742. Toxicol. Sci. 2008, 105, 384–394. [Google Scholar] [CrossRef]
- Strand, E.; Lysne, V.; Grinna, M.L.; Bohov, P.; Svardal, A.; Nygard, O.; Berge, R.K.; Bjorndal, B. Short-Term Activation of Peroxisome Proliferator-Activated Receptors alpha and gamma Induces Tissue-Specific Effects on Lipid Metabolism and Fatty Acid Composition in Male Wistar Rats. PPAR Res. 2019, 2019, 8047627. [Google Scholar] [CrossRef]
- Zambrano, S.; Blanca, A.J.; Ruiz-Armenta, M.V.; Miguel-Carrasco, J.L.; Revilla, E.; Santa-Maria, C.; Mate, A.; Vazquez, C.M. The renoprotective effect of L-carnitine in hypertensive rats is mediated by modulation of oxidative stress-related gene expression. Eur. J. Nutr. 2013, 52, 1649–1659. [Google Scholar] [CrossRef]
- Kienesberger, K.; Pordes, A.G.; Volk, T.G.; Hofbauer, R. L-carnitine and PPARalpha-agonist fenofibrate are involved in the regulation of Carnitine Acetyltransferase (CrAT) mRNA levels in murine liver cells. Bmc Genom. 2014, 15, 514. [Google Scholar] [CrossRef]
- Jung, T.W.; Kim, H.C.; Abd El-Aty, A.M.; Jeong, J.H. Protectin DX ameliorates palmitate- or high-fat diet-induced insulin resistance and inflammation through an AMPK-PPARalpha-dependent pathway in mice. Sci. Rep. 2017, 7, 1397. [Google Scholar] [CrossRef]
- Chacinska, M.; Zabielski, P.; Ksiazek, M.; Szalaj, P.; Jarzabek, K.; Kojta, I.; Chabowski, A.; Blachnio-Zabielska, A.U. The Impact of OMEGA-3 Fatty Acids Supplementation on Insulin Resistance and Content of Adipocytokines and Biologically Active Lipids in Adipose Tissue of High-Fat Diet Fed Rats. Nutrients 2019, 11, 835. [Google Scholar] [CrossRef] [PubMed]
- Gille, A.; Stojnic, B.; Derwenskus, F.; Trautmann, A.; Schmid-Staiger, U.; Posten, C.; Briviba, K.; Palou, A.; Bonet, M.L.; Ribot, J. A Lipophilic Fucoxanthin-Rich Phaeodactylum tricornutum Extract Ameliorates Effects of Diet-Induced Obesity in C57BL/6J Mice. Nutrients 2019, 11, 796. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, H.; Batista, R.; Alfradique, V.A.P.; Pinto, P.H.N.; Ribeiro, L.S.; Oliveira, C.S.; Souza-Fabjan, J.M.G.; Camargo, L.S.A.; Fonseca, J.F.; Brandao, F.Z. l-carnitine supplementation during vitrification or warming of in vivo-produced ovine embryos does not affect embryonic survival rates, but alters CrAT and PRDX1 expression. Theriogenology 2018, 105, 150–157. [Google Scholar] [CrossRef]
- Leduc-Gaudet, J.P.; Reynaud, O.; Chabot, F.; Mercier, J.; Andrich, D.E.; St-Pierre, D.H.; Gouspillou, G. The impact of a short-term high-fat diet on mitochondrial respiration, reactive oxygen species production, and dynamics in oxidative and glycolytic skeletal muscles of young rats. Physiol. Rep. 2018, 6, e13548. [Google Scholar] [CrossRef] [PubMed]
- Frias, F.T.; Rocha, K.C.E.; de Mendonca, M.; Murata, G.M.; Araujo, H.N.; de Sousa, L.G.O.; de Sousa, E.; Hirabara, S.M.; Leite, N.C.; Carneiro, E.M.; et al. Fenofibrate reverses changes induced by high-fat diet on metabolism in mice muscle and visceral adipocytes. J. Cell. Physiol. 2018, 233, 3515–3528. [Google Scholar] [CrossRef] [PubMed]
- Faleo, G.; Fotino, C.; Bocca, N.; Molano, R.D.; Zahr-Akrawi, E.; Molina, J.; Villate, S.; Umland, O.; Skyler, J.S.; Bayer, A.L.; et al. Prevention of autoimmune diabetes and induction of beta-cell proliferation in NOD mice by hyperbaric oxygen therapy. Diabetes 2012, 61, 1769–1778. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lv, N.; Zhao, M.; Han, Y.; Cui, L.; Zhong, W.; Wang, C.; Jiang, Q. The roles of bone morphogenetic protein 2 in perfluorooctanoic acid induced developmental cardiotoxicity and l-carnitine mediated protection. Toxicol. Appl. Pharm. 2018, 352, 68–76. [Google Scholar] [CrossRef]
- Lv, N.; Yuan, J.; Ji, A.; Shi, L.; Gao, M.; Cui, L.; Jiang, Q. Perfluorooctanoic acid-induced toxicities in chicken embryo primary cardiomyocytes: Roles of PPAR alpha and Wnt5a/Frizzled2. Toxicol. Appl. Pharm. 2019, 381, 114716. [Google Scholar] [CrossRef]
Sample Availability: Samples of the adipose tissues and protein samples from skeletal muscle are available from the corresponding authors (QJ or JD). |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, J.; Jiang, Q.; Song, L.; Liu, Y.; Li, M.; Lin, Q.; Li, Y.; Su, K.; Ma, Z.; Wang, Y.; et al. L-Carnitine Is Involved in Hyperbaric Oxygen-Mediated Therapeutic Effects in High Fat Diet-Induced Lipid Metabolism Dysfunction. Molecules 2020, 25, 176. https://doi.org/10.3390/molecules25010176
Yuan J, Jiang Q, Song L, Liu Y, Li M, Lin Q, Li Y, Su K, Ma Z, Wang Y, et al. L-Carnitine Is Involved in Hyperbaric Oxygen-Mediated Therapeutic Effects in High Fat Diet-Induced Lipid Metabolism Dysfunction. Molecules. 2020; 25(1):176. https://doi.org/10.3390/molecules25010176
Chicago/Turabian StyleYuan, Junhua, Qixiao Jiang, Limin Song, Yuan Liu, Manwen Li, Qian Lin, Yanrun Li, Kaizhen Su, Zhengye Ma, Yifei Wang, and et al. 2020. "L-Carnitine Is Involved in Hyperbaric Oxygen-Mediated Therapeutic Effects in High Fat Diet-Induced Lipid Metabolism Dysfunction" Molecules 25, no. 1: 176. https://doi.org/10.3390/molecules25010176
APA StyleYuan, J., Jiang, Q., Song, L., Liu, Y., Li, M., Lin, Q., Li, Y., Su, K., Ma, Z., Wang, Y., Liu, D., & Dong, J. (2020). L-Carnitine Is Involved in Hyperbaric Oxygen-Mediated Therapeutic Effects in High Fat Diet-Induced Lipid Metabolism Dysfunction. Molecules, 25(1), 176. https://doi.org/10.3390/molecules25010176




