Neutral Rhenadicarbaboranes with Re(CO)2(NO) Vertices: A Theoretical Study of Building Blocks for Rhenacarborane-Based Drug Delivery Agents
Abstract
1. Introduction
2. Results and Discussion
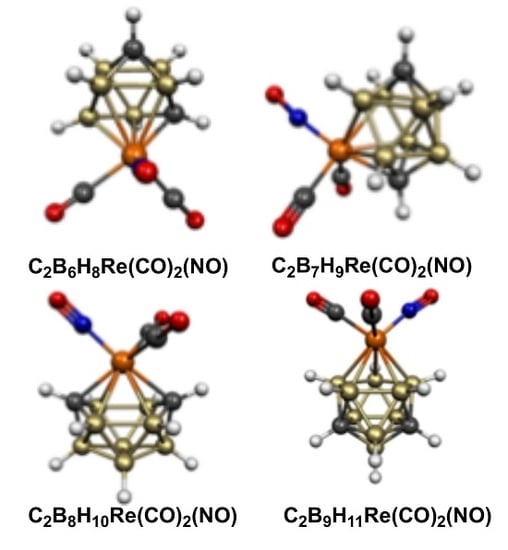
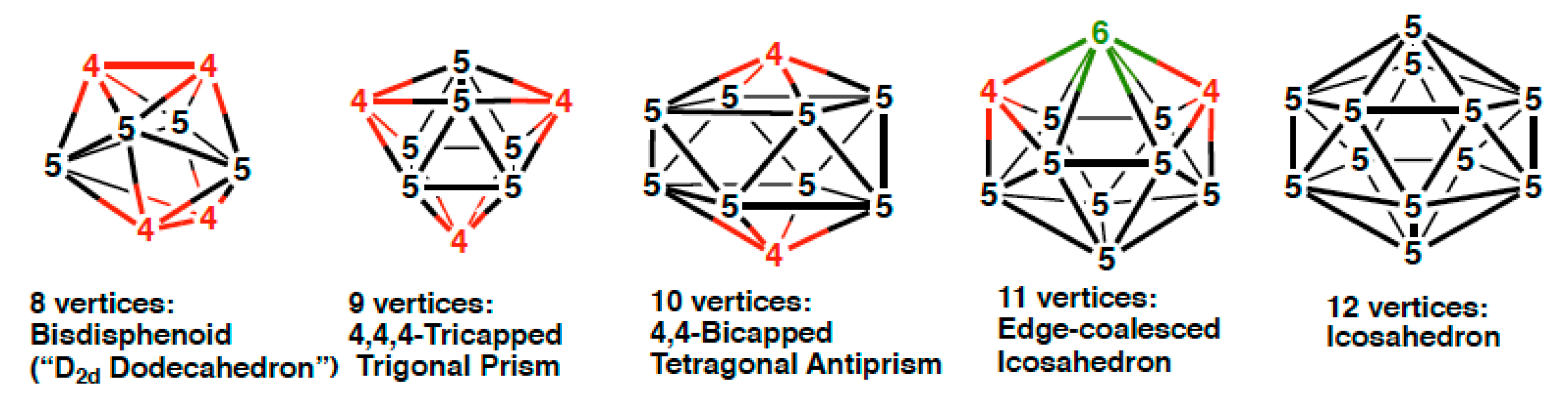
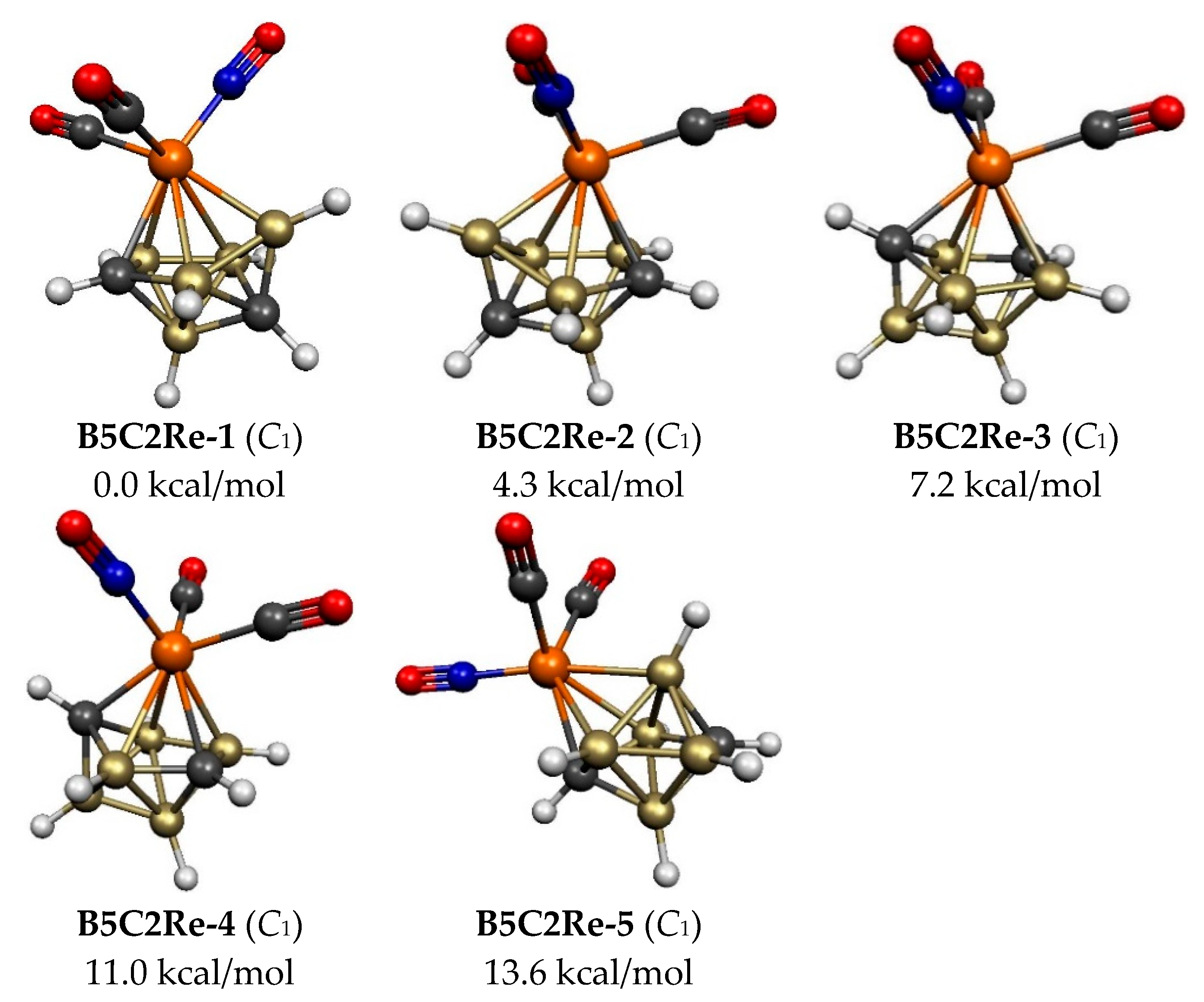
2.1. Eight-Vertex C2B5H7Re(CO)2(NO) Structures
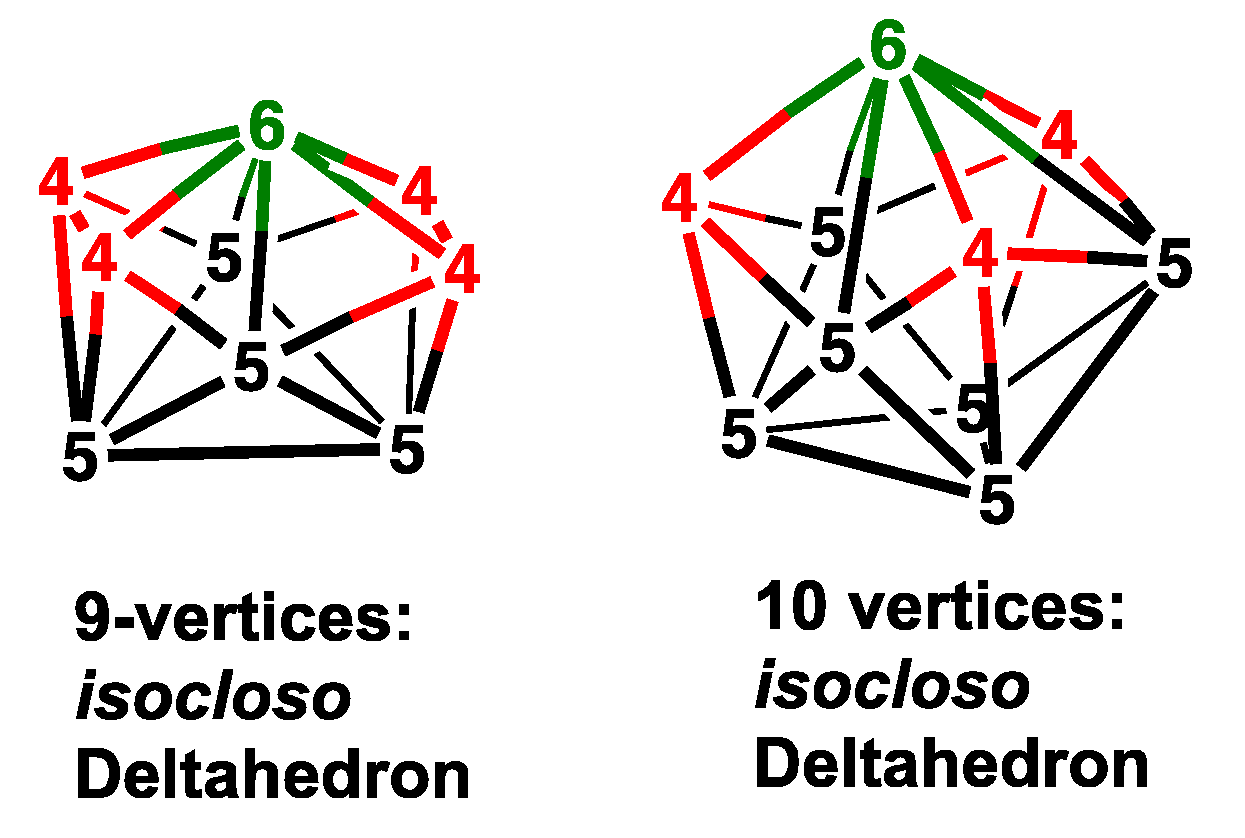
2.2. Nine-Vertex C2B6H8Re(CO)2(NO) Structures
2.3. Ten-Vertex C2B7H9Re(CO)2(NO) Structures
2.4. Eleven-Vertex C2B8H10Re(CO)2(NO) Structures
2.5. Twelve-Vertex C2B9H11Re(CO)2(NO) Structures
3. Theoretical Methods
4. Summary
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Grimes, R.N. Carboranes; Academic Press: Cambridge, MA, USA, 2016; pp. 1053–1082. [Google Scholar]
- Armstrong, A.F.; Valliant, J.F. Differences between the macroscopic and tracer level chemistry of rhenium and technetium: Contrasting cage isomerisation behaviour of Re(I) and Tc(I) carborane complexes. Dalton Trans. 2010, 39, 8128–8131. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.F.; Lebert, J.M.; Brennan, J.D.; Valliant, J.F. Functionalized carborane complexes of the [M(CO)2(NO)]2+ core (M = 99mTc, Re): A new class of organometallic probes for correlated in vitro and in vivo imaging. Organometallics 2009, 28, 2986–2992. [Google Scholar] [CrossRef]
- Causey, P.W.; Besanger, T.R.; Valliant, J.F. Synthesis and screening of mono- and di-aryl technetium and rhenium metallocarboranes. A new class of probes for the estrogen receptor. J. Med. Chem. 2008, 51, 2833–2844. [Google Scholar] [CrossRef] [PubMed]
- Louie, A.S.; Harrington, L.E.; Valliant, J.F. The preparation and characterization of functionalized carboranes and Re/Tc-metallocarboranes as platforms for developing molecular imaging probes: Structural and cage isomerism studies. Inorg. Chim. Acta 2012, 389, 159–167. [Google Scholar] [CrossRef]
- El-Zaria, M.E.; Janzen, N.; Valliant, J.F. Room-temperature synthesis of Re(I) and Tc(I) metallocarboranes. Organometallics 2012, 31, 5940–5949. [Google Scholar] [CrossRef]
- Pruitt, D.G.; Baumann, S.M.; Place, G.J.; Oyeamalu, A.N.; Sinn, E.; Jelliss, P.A. Synthesis and functionalization of nitrosyl rhenacarboranes towards their use as drug delivery vehicles. J. Organomet. Chem. 2015, 798, 60–69. [Google Scholar] [CrossRef]
- Ma, P.; Smith, T.M.; Zubieta, J.; Spencer, J.T. Synthesis of alkoxy derivatives of the 10-vertex manganadecaborane [nido-6-Mn(CO)3B9H13][NMe4]. Inorg. Chem. Commun. 2014, 46, 223–225. [Google Scholar] [CrossRef]
- Genady, A.R.; Tan, J.; El-Zaria, M.E.; Zlitni, A.; Janzen, N.; Valliant, J.F. Synthesis, characterization and radiolabeling of carborane-functionalized tetrazines for use in inverse electron demand Diels–Alder ligation reactions. J. Organomet. Chem. 2015, 791, 204–213. [Google Scholar] [CrossRef]
- Stockmann, P.; Gozzi, M.; Kuhnert, R.; Sárosi, M.B.; Hey-Hawkins, E. New keys for old locks: Carborane-containing drugs as platforms for mechanism-based therapies. Chem. Soc. Rev. 2019, 48, 3497–3512. [Google Scholar] [CrossRef]
- Issa, F.; Kassiou, M.; Rendina, L.M. Boron in drug discovery: Carboranes as unique pharmacophores in biologically active compounds. Chem. Rev. 2011, 111, 5701–5722. [Google Scholar] [CrossRef]
- Fanfrlík, J.; Hnyk, D.; Lepsík, M.; Hobza, P. Interaction of heteroboranes with biomolecules. Part 2. The effect of various metal vertices and exo-substitutions. Phys. Chem. Chem. Phys. 2007, 9, 2085–2093. [Google Scholar] [CrossRef] [PubMed]
- Cígler, P.; Kozísek, M.; Rezácová, P.; Brynda, J.; Otwinowski, Z.; Pokorná, J.; Plešek, J.; Grüner, B.; Dolecková-Maresová, L.; Mása, M.; et al. From nonpeptide toward noncarbon protease inhibitors: Metallacarboranes as specific and potent inhibitors of HIV protease. Proc. Natl. Acad. Sci. USA 2005, 102, 15394–15399. [Google Scholar] [CrossRef] [PubMed]
- Valliant, J.F.; Schaffer, P. A new approach for the synthesis of isonitrile carborane derivatives. Ligands for metal based boron neutron capture therapy (BNCT) and boron neutron capture synovectomy (BNCS) agents. J. Inorg. Biochem. 2001, 85, 43–51. [Google Scholar] [CrossRef]
- Valliant, J.F.; Sogbein, O.O.; Morel, P.; Schaffer, P.; Guenther, K.J.; Bain, A.D. Synthesis, NMR, and X-ray crystallographic analysis of C-hydrazino-C-carboxycarboranes: Versatile ligands for the preparation of BNCT and BNCS agents and (99m) Tc radiopharmaceuticals. Inorg. Chem. 2002, 41, 2731–2737. [Google Scholar] [CrossRef] [PubMed]
- Hawthorne, M.F.; Andrews, T.D. Carbametallic boron hydride derivatives. II. apparent analogs of π-C5H5Mn(CO)3 and π-C5H5Re(CO)3. J. Am. Chem. Soc. 1965, 87, 2496. [Google Scholar] [CrossRef]
- Andrews, T.D.; Hawthorne, M.F.; Howe, D.V.; Pilling, R.L.; Pitts, D.; Reintjes, M.; Warren, L.F.; Wegner, P.A.; Young, D.C. π-Dicarbollyl derivatives of the transition metals. Metallocene analogs. J. Am. Chem. Soc. 1968, 90, 879–896. [Google Scholar]
- Ellis, D.D.; Jelliss, P.A.; Stone, F.G.A. Rhenacarborane complexes with nitrosyl and alkylidene ligands. Chem. Commun. 1999, 23, 2385–2386. [Google Scholar] [CrossRef]
- Gozzi, M.; Schwarze, B.; Hey-Hawkins, E. Half- and mixed-sandwich metallacarboranes for potential applications in medicine. Pure Appl. Chem. 2019, 91, 563–573. [Google Scholar] [CrossRef]
- Wade, K. The structural significance of the number of skeletal bonding electron-pairs in carboranes, the higher boranes and borane anions, and various transition-metal carbonyl cluster compounds. Chem. Commun. 1971, 15, 792–793. [Google Scholar] [CrossRef]
- Mingos, D.M.P. A general theory for cluster and ring compounds of the main group and transition elements. Nat. Phys. Sci. 1972, 236, 99–102. [Google Scholar] [CrossRef]
- Mingos, D.M.P. Polyhedral skeletal electron pair approach. Acc. Chem. Res. 1984, 17, 311–319. [Google Scholar] [CrossRef]
- Attia, A.A.A.; Lupan, A.; King, R.B. Deviations from the most spherical deltahedra in rhenatricarbaboranes having 2n + 2 wadean skeletal electrons. Inorg. Chem. 2017, 56, 15015–15025. [Google Scholar] [CrossRef] [PubMed]
- Bould, J.; Kennedy, J.D.; Thornton-Pett, M. Ten-vertex metallaborane chemistry. Aspects of the iridadecaborane closo⟶isonido⟶isocloso structural continuum. J. Chem. Soc. Dalton Trans. 1992, 4, 563–576. [Google Scholar] [CrossRef]
- Kennedy, J.D. The Borane-Carborane-Carbocation Continuum; Casanova, J., Ed.; Wiley: New York, NY, USA, 1998; Chapter 3; pp. 85–116. [Google Scholar]
- Štibr, B.; Kennedy, J.D.; Drdáková, E.; Thornton-Pett, M. Nine-vertex polyhedral iridamonocarbaborane chemistry. Products of thermolysis of [(CO)(PPh3)2IrCB7H8] and emerging alternative cluster-geometry patterns. J. Chem. Soc. Dalton 1994, 2, 229–236. [Google Scholar] [CrossRef]
- Gaussian, Inc. Gaussian09 (Revision E.01); Gaussian, Inc.: Wallingford, CT, USA, 2009; The Complete Reference Is Given in the Supporting Information. [Google Scholar]

| Vertex Degrees | Re–C | C–C | ||||
|---|---|---|---|---|---|---|
| Structure | ∆E | Re | C | Edges | Distance, Å | Comments |
| B5C2Re-1 | 0.0 | 5 | 4,4 | 1 | 2.60 | bisdisphenoid |
| B5C2Re-2 | 4.3 | 5 | 4,4 | 1 | 2.59 | bisdisphenoid |
| B5C2Re-3 | 7.2 | 5 | 4,4 | 2 | 2.70 | bisdisphenoid |
| B5C2Re-4 | 11.0 | 5 | 4,4 | 2 | 2.70 | bisdisphenoid |
| B5C2Re-5 | 13.6 | 4 | 4,4 | 1 | 2.59 | bisdisphenoid |
| Structure | Vertex Degrees | Re–C | C–C | |||
|---|---|---|---|---|---|---|
| (Symmetry) | ∆E | Re | C | Edges | Distance, Å | Comments |
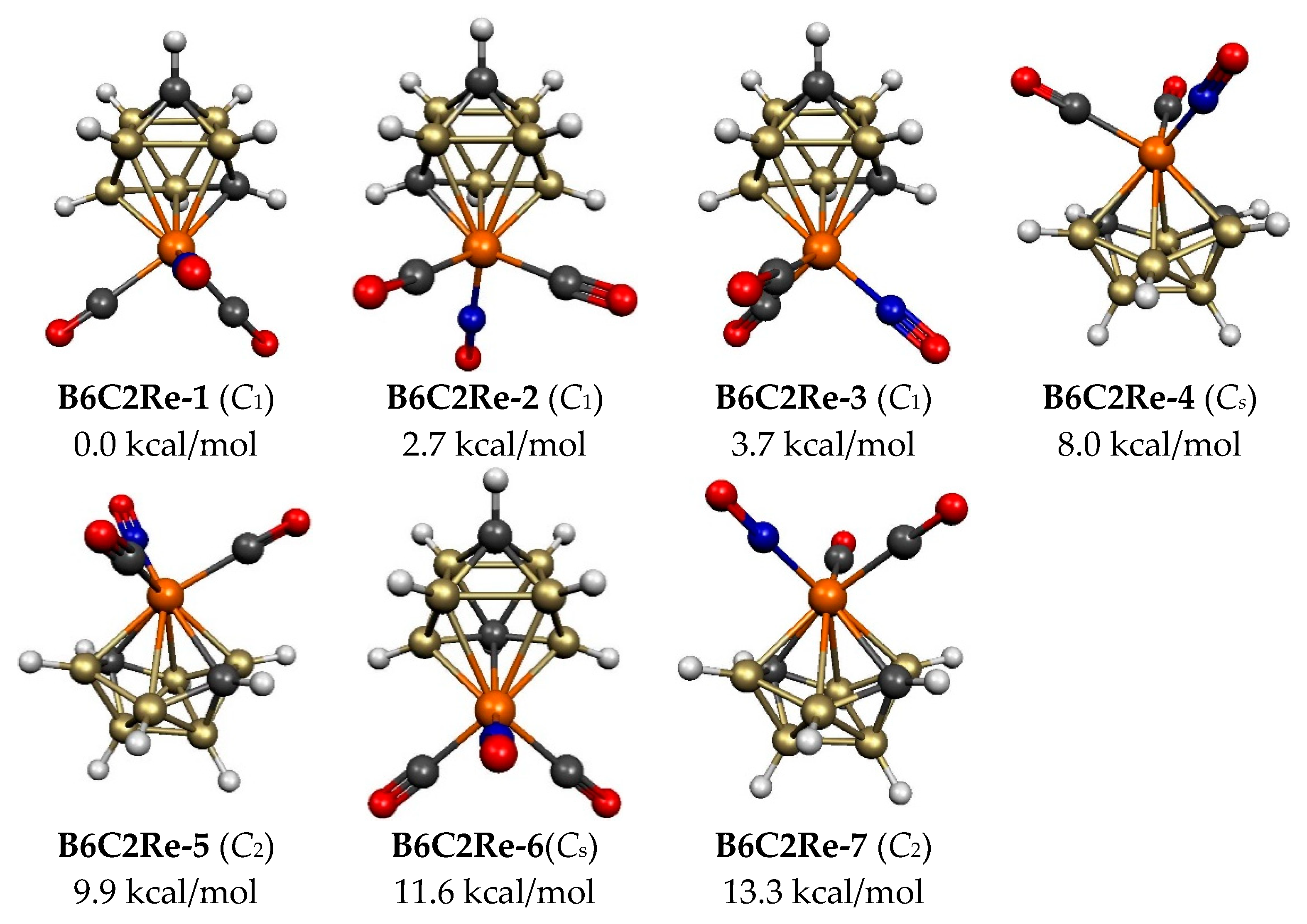
| B6C2Re-1 (C1) | 0.0 | 5 | 4.4 | 1 | 2.56 | tricap trig prism |
| B6C2Re-2 (C1) | 2.7 | 5 | 4,4 | 1 | 2.57 | tricap trig prism |
| B6C2Re-3 (C1) | 3.7 | 5 | 4,4 | 1 | 2.55 | tricap trig prism |
| B6C2Re-4 (Cs) | 8.0 | 6 | 4,4 | 2 | 2.77(m) | 9-vertex isocloso |
| B6C2Re-5 (C2) | 9.9 | 6 | 4,4 | 2 | 3.17(p) | 9-vertex isocloso |
| B6C2Re-6 (Cs) | 11.6 | 5 | 5,4 | 1 | 2.61 | tricap trig prism |
| B6C2Re-7 (C2) | 13.3 | 6 | 4,4 | 2 | 3.18(p) | 9-vertex isocloso |
| Structure | Vertex Degrees | Re–C | C–C | |||
|---|---|---|---|---|---|---|
| (Symmetry) | ∆E | Re | C | Edges | Distance, Å | Comments |
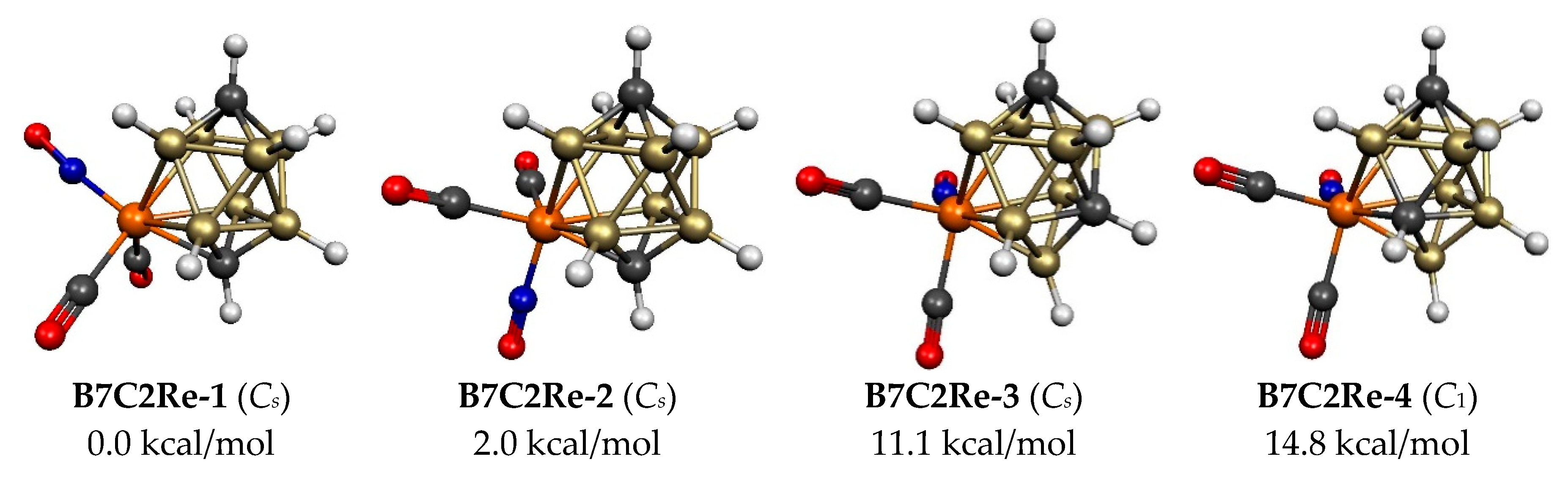
| B7C2Re-1 (Cs) | 0.0 | 5 | 4,4 | 1 | 3.42 | Bicap tetrag antipr |
| B7C2Re-2 (Cs) | 2.0 | 5 | 4,4 | 1 | 3.42 | Bicap tetrag antipr |
| B7C2Re-3 (Cs) | 11.1 | 5 | 5,4 | 0 | 2.60 | Bicap tetrag antipr |
| B7C2Re-4 (C1) | 14.8 | 5 | 5,4 | 1 | 2.64 | Bicap tetrag antipr |
| Structure | Vertex Degrees | Re–C | C–C | |||
|---|---|---|---|---|---|---|
| (Symmetry) | ∆E | Re | C | Edges | Distance, Å | Comments |
| B8C2Re-1 (C2v) | 0.0 | 6 | 4.4 | 2 | 3.36 | 11-vertex closo |
| B8C2Re-2 (C1) | 6.6 | 6 | 5.4 | 1 | 2.60 | 11-vertex closo |
| B8C2Re-3 (Cs) | 7.1 | 5 | 4,4 | 1 | 3.04 | 11-vertex closo |
| B8C2Re-4 (C1) | 10.7 | 6 | 5,4 | 1 | 2.85 | 11-vertex closo |
| B8C2Re-5 (Cs) | 11.3 | 5 | 4,4 | 0 | 3.01 | 11-vertex closo |
| B8C2Re-6 (C1) | 12.3 | 5 | 4,4 | 1 | 3.02 | 11-vertex closo |
| Re–C | C–C | ||
|---|---|---|---|
| Structure (Symmetry) | ∆E | Edges | Distance, Å |
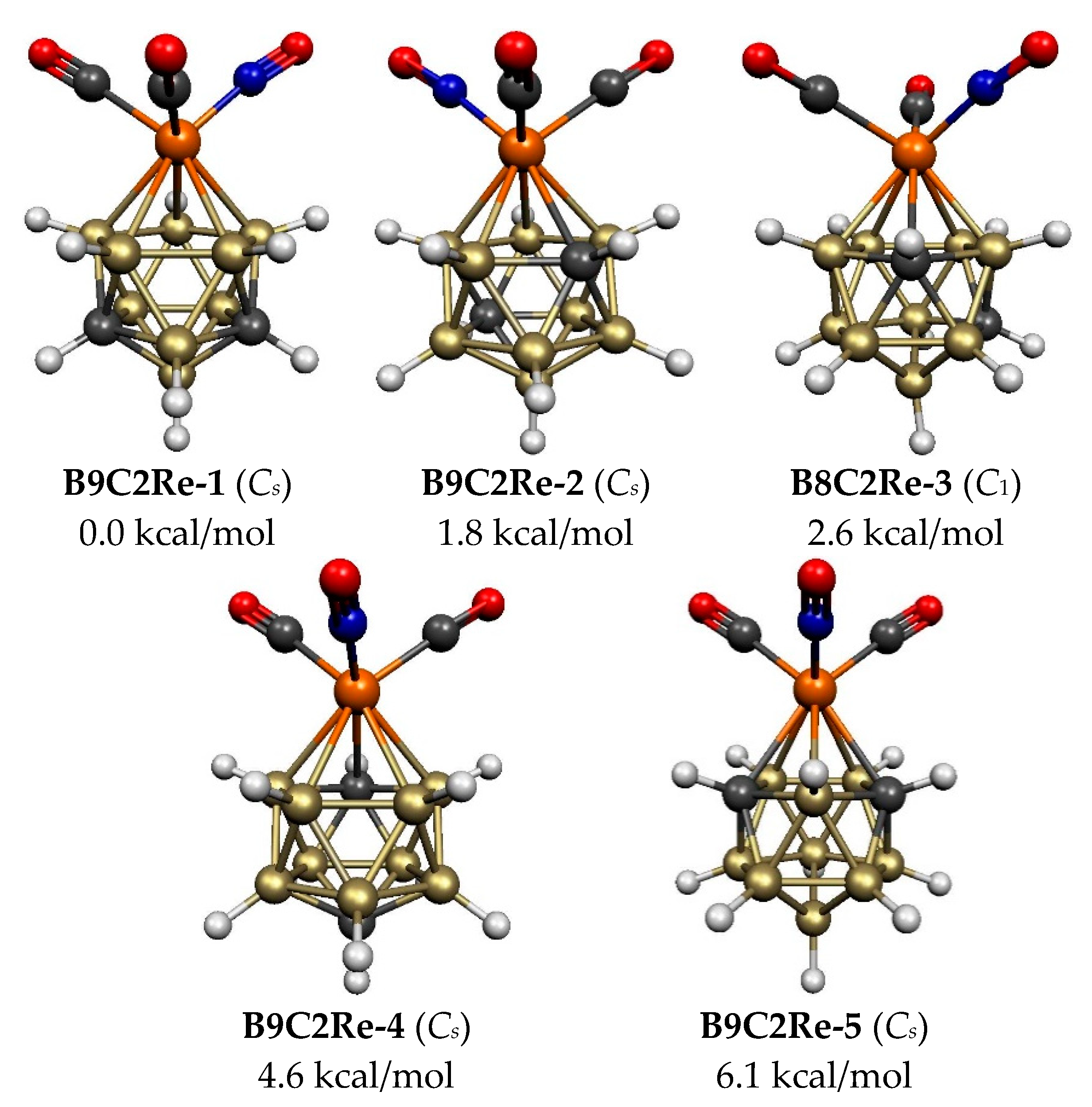
| B9C2Re-1 (C1) | 0.0 | 0 | 2.58 |
| B9C2Re-2 (C1) | 1.8 | 1 | 3.05 |
| B9C2Re-3 (C1) | 2.6 | 1 | 2.60 |
| B9C2Re-4 (C1) | 4.6 | 1 | 2.60 |
| B9C2Re-5 (C1) | 6.1 | 2 | 2.67 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attia, A.A.A.; Lupan, A.; Silaghi-Dumitrescu, R.; King, R.B. Neutral Rhenadicarbaboranes with Re(CO)2(NO) Vertices: A Theoretical Study of Building Blocks for Rhenacarborane-Based Drug Delivery Agents. Molecules 2020, 25, 110. https://doi.org/10.3390/molecules25010110
Attia AAA, Lupan A, Silaghi-Dumitrescu R, King RB. Neutral Rhenadicarbaboranes with Re(CO)2(NO) Vertices: A Theoretical Study of Building Blocks for Rhenacarborane-Based Drug Delivery Agents. Molecules. 2020; 25(1):110. https://doi.org/10.3390/molecules25010110
Chicago/Turabian StyleAttia, Amr A. A., Alexandru Lupan, Radu Silaghi-Dumitrescu, and R. Bruce King. 2020. "Neutral Rhenadicarbaboranes with Re(CO)2(NO) Vertices: A Theoretical Study of Building Blocks for Rhenacarborane-Based Drug Delivery Agents" Molecules 25, no. 1: 110. https://doi.org/10.3390/molecules25010110
APA StyleAttia, A. A. A., Lupan, A., Silaghi-Dumitrescu, R., & King, R. B. (2020). Neutral Rhenadicarbaboranes with Re(CO)2(NO) Vertices: A Theoretical Study of Building Blocks for Rhenacarborane-Based Drug Delivery Agents. Molecules, 25(1), 110. https://doi.org/10.3390/molecules25010110