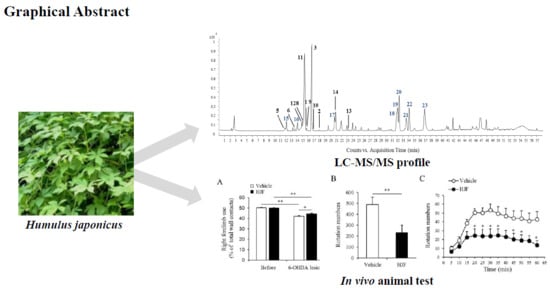
Dereplication of Components Coupled with HPLC-qTOF-MS in the Active Fraction of Humulus japonicus and It’s Protective Effects against Parkinson’s Disease Mouse Model
Abstract
1. Introduction
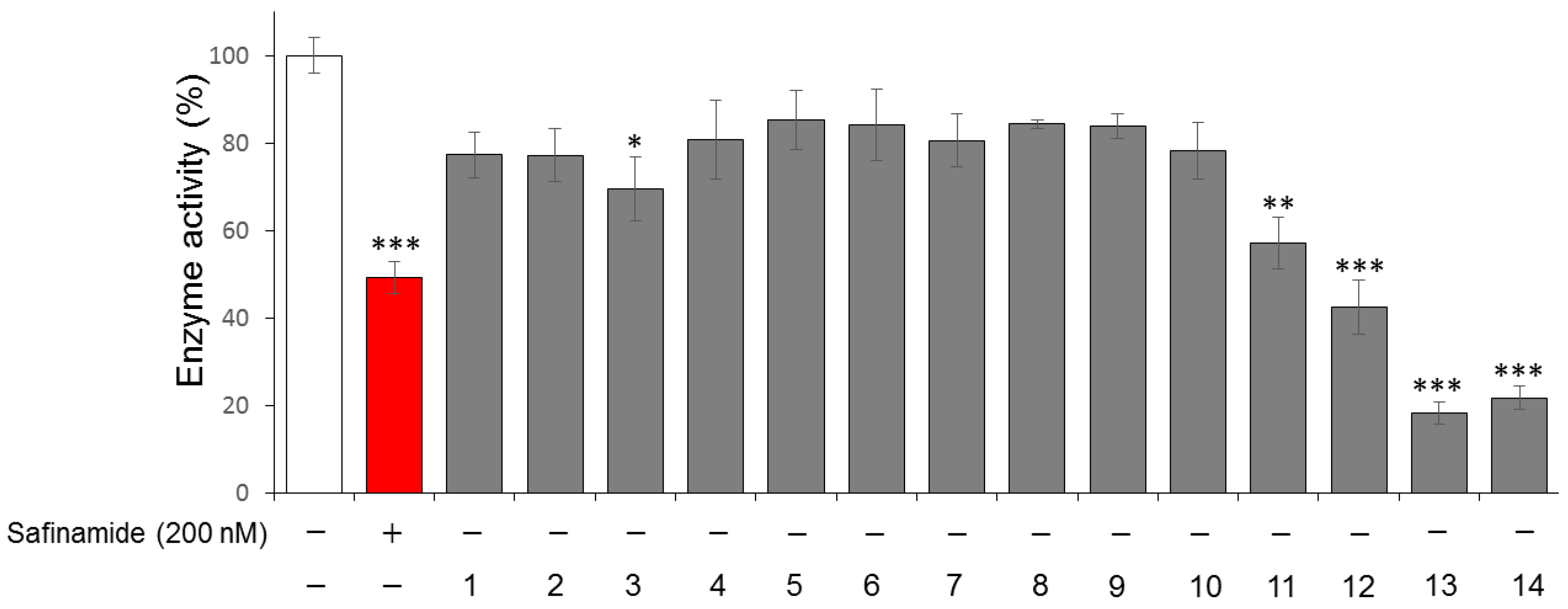
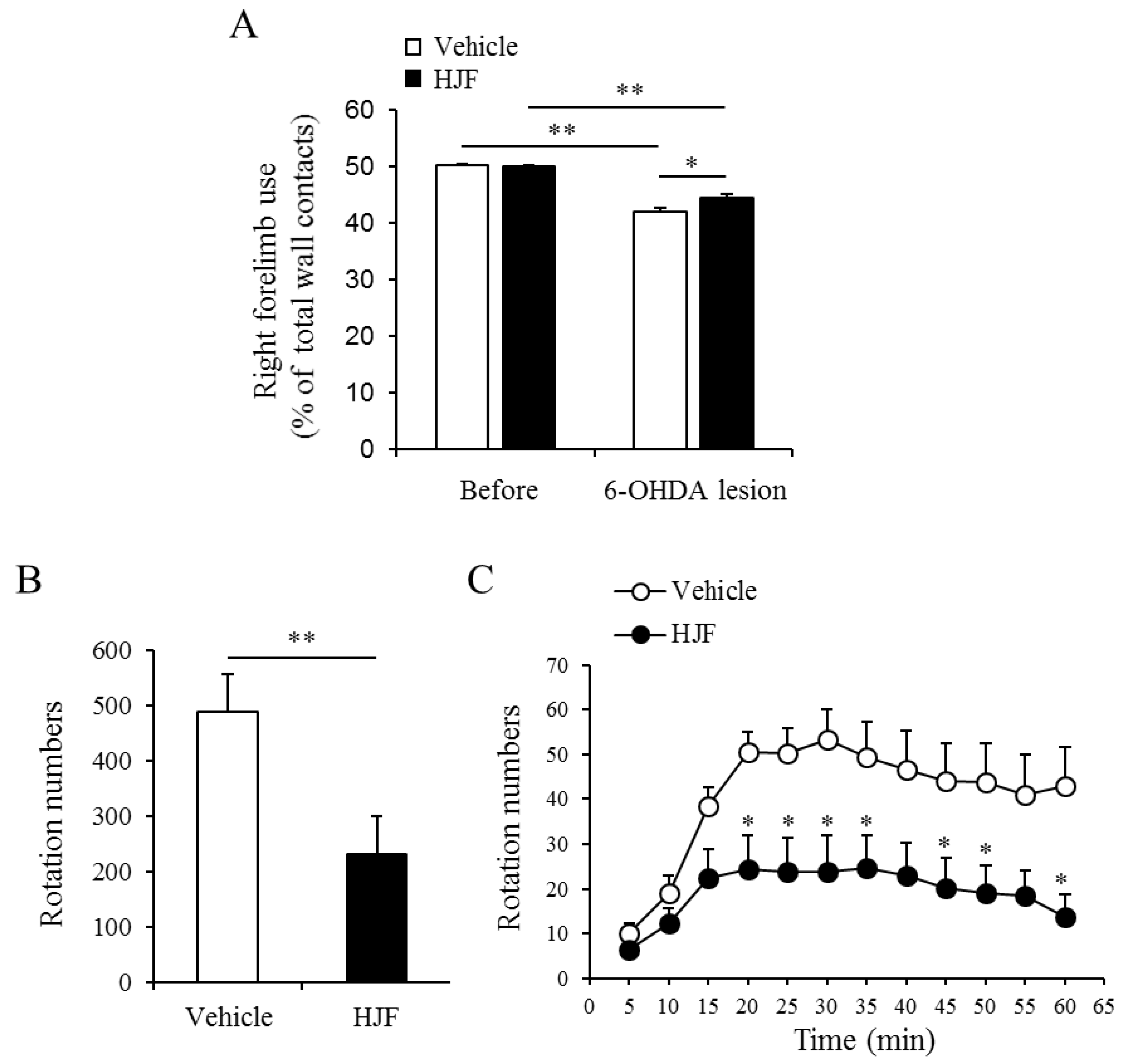
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Plant Material
3.3. MAO-B Inhibitory Activity-guided Fractionation and Isolation
3.4. HPLC-qTOF-MS/MS Analysis
3.5. In vitro MAO-B Inhibition Assay
3.6. In vivo Animal Test
3.6.1. Animals
3.6.2. 6-Hydroxydopamine (6-OHDA) Lesion
3.6.3. Cylinder Test
3.6.4. D-AMPH-induced Rotation Test
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Dauer, W.; Przedborski, S. Parkinson’s disease: Mechanisms and models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef]
- Beal, M.F. Mitochondrial dysfunction in neurodegenerative diseases. Biochim. Biophys. Acta 1998, 1366, 211–223. [Google Scholar] [CrossRef]
- Jenner, P.; Olanow, C.W. The pathogenesis of cell death in Parkinson’s disease. Neurology 2006, 66, S24–S36. [Google Scholar] [CrossRef]
- Valko, M.; Morris, H.; Cronin, M.T. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef]
- Fernstrom, J.D. Effects of dietary polyunsaturated fatty acids on neuronal function. Lipids 1999, 34, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Maeda, T.; Kume, T.; Kaneko, S.; Kochiyama, H.; Akaike, A.; Goshima, Y.; Misu, Y. Differential neurotoxicity induced by L-DOPA and dopamine in cultured striatal neurons. Brain Res. 1996, 743, 278–283. [Google Scholar] [CrossRef]
- Kulich, S.M.; Horbinski, C.; Patel, M.; Chu, C.T. 6-Hydroxydopamine induces mitochondrial ERK activation. Free Radic. Biol. Med. 2007, 43, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.W.; Howard, S.; LoGrasso, P.V. Blocking c-Jun N-terminal kinase (JNK) translocation to the mitochondria prevents 6-hydroxydopamine-induced toxicity in vitro and in vivo. J. Biol. Chem. 2013, 288, 1079–1087. [Google Scholar] [CrossRef]
- Shih, J.C. Molecular basis of human MAO A and B. Neuropsychopharmacology 1991, 4, 1–7. [Google Scholar]
- Saura, J.; Richards, J.G.; Mahy, N. Age-related changes on MAO in Bl/C57 mouse tissues: A quantitative radioautographic study. J. Neural. Transm. Suppl. 1994, 41, 89–94. [Google Scholar] [PubMed]
- Glover, V.; Sandler, M.; Owen, F.; Riley, G.J. Dopamine is a monoamine oxidase B substrate in man. Nature 1977, 265, 80–81. [Google Scholar] [CrossRef]
- Jellinger, K.A. Neuropathology of sporadic Parkinson’s disease: Evaluation and changes of concepts. Mov. Disord. 2012, 27, 8–30. [Google Scholar] [CrossRef]
- Aluf, Y.; Vaya, J.; Khatib, S.; Loboda, Y.; Finberg, J.P.M. Selective inhibition of monoamine oxidase A or B reduces striatal oxidative stress in rats with partial depletion of the nigro-striatal dopaminergic pathway. Neuropharmacology 2013, 65, 48–57. [Google Scholar] [CrossRef]
- Schapira, A.H.; Bezard, E.; Brotchie, J.; Calon, F.; Collingridge, G.L.; Ferger, B.; Hengerer, B.; Hirsch, E.; Jenner, P.; Le Novère, N.; et al. Novel pharmacological targets for the treatment of Parkinson’s disease. Nat. Rev. Drug Discov. 2006, 5, 845–854. [Google Scholar] [CrossRef]
- Connolly, B.S.; Lang, A.E. Pharmacological treatment of Parkinson disease: A review. JAMA 2014, 311, 1670–1683. [Google Scholar] [CrossRef]
- Park, S.W.; Woo, C.J.; Chung, S.K.; Chung, K.T. Antimicrobial and antioxidative activities of solvent fraction from Humulus japonicas. Korean J. Food Sci. Technol. 1994, 26, 464–470. [Google Scholar]
- Ryu, Y.K.; Kang, Y.; Go, J.; Park, H.Y.; Noh, J.R.; Kim, Y.H.; Hwang, J.H.; Choi, D.H.; Han, S.S.; Oh, W.K.; et al. Humulus japonicus prevents dopaminergic neuron death in 6-hydroxydopamine-induced models of Parkinson’s disease. J. Med. Food 2017, 20, 116–123. [Google Scholar] [CrossRef]
- Park, T.S.; Ryu, Y.K.; Park, H.Y.; Kim, J.Y.; Go, J.; Noh, J.R.; Kim, Y.H.; Hwang, J.H.; Choi, D.H.; Oh, W.K.; et al. Humulus japonicus inhibits the progression of Alzheimer’s disease in a APP/PS1 transgenic mouse model. Int. J. Mol. Med. 2017, 39, 21–30. [Google Scholar] [CrossRef]
- Henke, M.T.; Kelleher, N.L. Modern mass spectrometry for synthetic biology and structure-based discovery of natural products. Nat. Prod. Rep. 2016, 33, 942–950. [Google Scholar] [CrossRef]
- Khan, S.H.; Mosihuzzaman, M.; Nahar, M.; Rashid, M.A.; Rokeya, B.; Azad Khan, A.K. Three megastigmane glycosides from the leaves of Pterospermum semisagittaum. Pharm. Biol. 2003, 41, 512–515. [Google Scholar] [CrossRef]
- Pabst, A.; Barron, D.; Sémon, E.; Schreier, P. Two diastereomeric 3-oxo-alpha-ionol beta-D-glucosides from raspberry fruit. Phytochemisty 1992, 31, 1649–1652. [Google Scholar] [CrossRef]
- Cho, J.Y.; Park, K.H.; Hwang, D.Y.; Chanmuang, S.; Jaiswal, L.; Park, Y.K.; Park, S.Y.; Kim, S.Y.; Kim, H.R.; Moon, J.H.; et al. Antihypertensive effects of Artemisia scoparia Waldst in spontaneously hypertensive rats and identification of angiotensin I converting enzyme inhibitors. Molecules 2015, 20, 19789–19804. [Google Scholar] [CrossRef]
- Greca, M.D.; Ferrara, M.; Fiorentino, A.; Monaco, P.; Previtera, L. Antialgal compounds from Zantedeschia aethiopica. Phytochemisty 1998, 49, 1299–1304. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, K.H.; Lee, I.K.; Lee, K.H.; Choi, S.U.; Lee, K.R. A new flavonol glycoside from Hylomecon vernalis. Arch. Pharm. Res. 2012, 35, 415–421. [Google Scholar] [CrossRef]
- Wang, M.; Shao, Y.; Li, J.; Zhu, N.; Rangarajan, M.; LaVoie, E.J.; Ho, C.T. Antioxidative phenolic glycosides from sage (Salvia officinalis). J. Nat. Prod. 1999, 62, 454–456. [Google Scholar] [CrossRef]
- Yu, B.C.; Yang, M.C.; Lee, K.H.; Kim, K.H.; Choi, S.U.; Lee, K.R. Two new phenolic constituents of Humulus japonicus and their cytotoxicity test in vitro. Arch. Pharm. Res. 2007, 30, 1471–1475. [Google Scholar] [CrossRef]
- Burns, D.C.; Ellis, D.A.; March, R.E. A predictive tool for assessing 13 C NMR chemical shifts of flavonoids. Magn. Reson. Chem. 2007, 45, 835–845. [Google Scholar] [CrossRef]
- Rong, H.; Zhao, Y.; Lazou, K.; Keukeleire, D.D.; Milligan, S.R.; Sandra, P. Quantitation of 8-prenylnaringenin, a novel phytoestrogen in Hops (Humulus lupulus L.), hop products, and beers, by benchtop HPLC-MS using electrospray ionization. Chromatographia 2000, 51, 545–552. [Google Scholar] [CrossRef]
- Mohamed, A.F.; Andrea, P.; Jurgen, S.; Ludger, A.W. Metabolite profiling and fingerprinting of commercial cultivars of Humulus lupulu L. (hop): A comparison of MS and NMR methods in metabolomics. Metabolomics 2012, 8, 492–507. [Google Scholar]
- Binda, C.; Wang, J.; Pisani, L.; Caccia, C.; Carotti, A.; Salvati, P.; Edmondson, D.E.; Mattevi, A. Structures of human monoamine oxidase B complexes with selective noncovalent inhibitors: Safinamide and coumarin analogs. J. Med. Chem. 2007, 50, 5848–5852. [Google Scholar] [CrossRef]
- Park, H.Y.; Kang, Y.M.; Kang, Y.; Park, T.S.; Ryu, Y.K.; Hwang, J.H.; Kim, Y.H.; Chung, B.H.; Nam, K.H.; Kim, M.R.; et al. Inhibition of adenylyl cyclase type 5 prevents L-DOPA-induced dyskinesia in an animal model of Parkinson’s disease. J. Neurosci. 2014, 34, 11744–11753. [Google Scholar] [CrossRef]
- Matsumoto, T.; Nakamura, S.; Nakashima, S.; Ohta, T.; Ogawa, K.; Fukaya, M.; Tsukioka, J.; Hasei, T.; Watanabe, T.; Matsuda, H. Neolignan and megastigmane glucosides from the aerial parts of Isodon japonicus with cell protective effects on BaP-induced cytotoxicity. Phytochemistry 2017, 137, 101–108. [Google Scholar] [CrossRef]
- Rezai-Zadeh, K.; Ehrhart, J.; Bai, Y.; Sanberg, P.R.; Bickford, P.; Tan, J.; Shytle, R.D. Apigenin and luteolin modulate microglial activation via inhibition of STAT1-induced CD40 expression. J. Neuroinflamm. 2008, 5. [Google Scholar] [CrossRef]
- Sawmiller, D.; Li, S.; Shahaduzzaman, M.; Smith, A.J.; Obregon, D.; Giunta, B.; Borlongan, C.V.; Sanberg, P.R.; Tan, J. Luteolin reduces Alzheimer’s disease pathologies induced by traumatic brain injury. Int. J. Mol. Sci. 2014, 15, 895–904. [Google Scholar] [CrossRef]
- Day, A.J.; Cañada, F.J.; Díaz, J.C.; Kroon, P.A.; Mclauchlan, R.; Faulds, C.B.; Plumb, G.W.; Morgan, M.R.; Williamson, G. Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Lett. 2000, 468, 166–170. [Google Scholar] [CrossRef]
- Lin, L.L.; Pai, Y.F.; Tasi, T.H. Isolation of luteolin and luteolin-7-O-glucoside from Dendranthema morifolium Ramat Tzvel and their pharmacokinetics in rats. J. Agric. Food Chem. 2015, 63, 7700–7706. [Google Scholar] [CrossRef]
- Németh, K.; Plumb, G.W.; Berrin, J.G.; Juge, N.; Jacob, R.; Naim, H.Y.; Williamson, G.; Swallow, D.M.; Kroon, P.A. Deglycosylation by small intestinal epithelial cell beta-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur. J. Nutr. 2003, 2, 29–42. [Google Scholar] [CrossRef]
- Hanske, L.; Loh, G.; Sczesny, S.; Blaut, M.; Braune, A. The bioavailability of apigenin-7-glucoside is influenced by human intestinal microbiota in rats. J. Nutr. 2009, 139, 1095–1102. [Google Scholar] [CrossRef]
- Naoto, Y.; Keiko, S.; Mitsunori, O. In vitro evaluation of antibacterial, anticollagenase, and antioxidant activities of hop components (Humulus lupulus) addressing acne vulgaris. Phytomedicine 2009, 16, 369–376. [Google Scholar]
- Boix, J.; Padel, T.; Paul, G. A partial lesion model of Parkinson’s disease in mice-- characterization of a 6-OHDA-induced medial forebrain bundle lesion. Behav. Brain Res. 2015, 284, 196–206. [Google Scholar] [CrossRef]
- Morinan, A.; Garratt, H.M. An improved fluorimetric assay for brain monoamine oxidase. J. Pharmacol. Methods 1985, 13, 213–223. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. A voucher specimen (No 2014-04) has been deposited at the Herbarium of the Korea Bioactive Natural Material Bank, Seoul, Korea. |
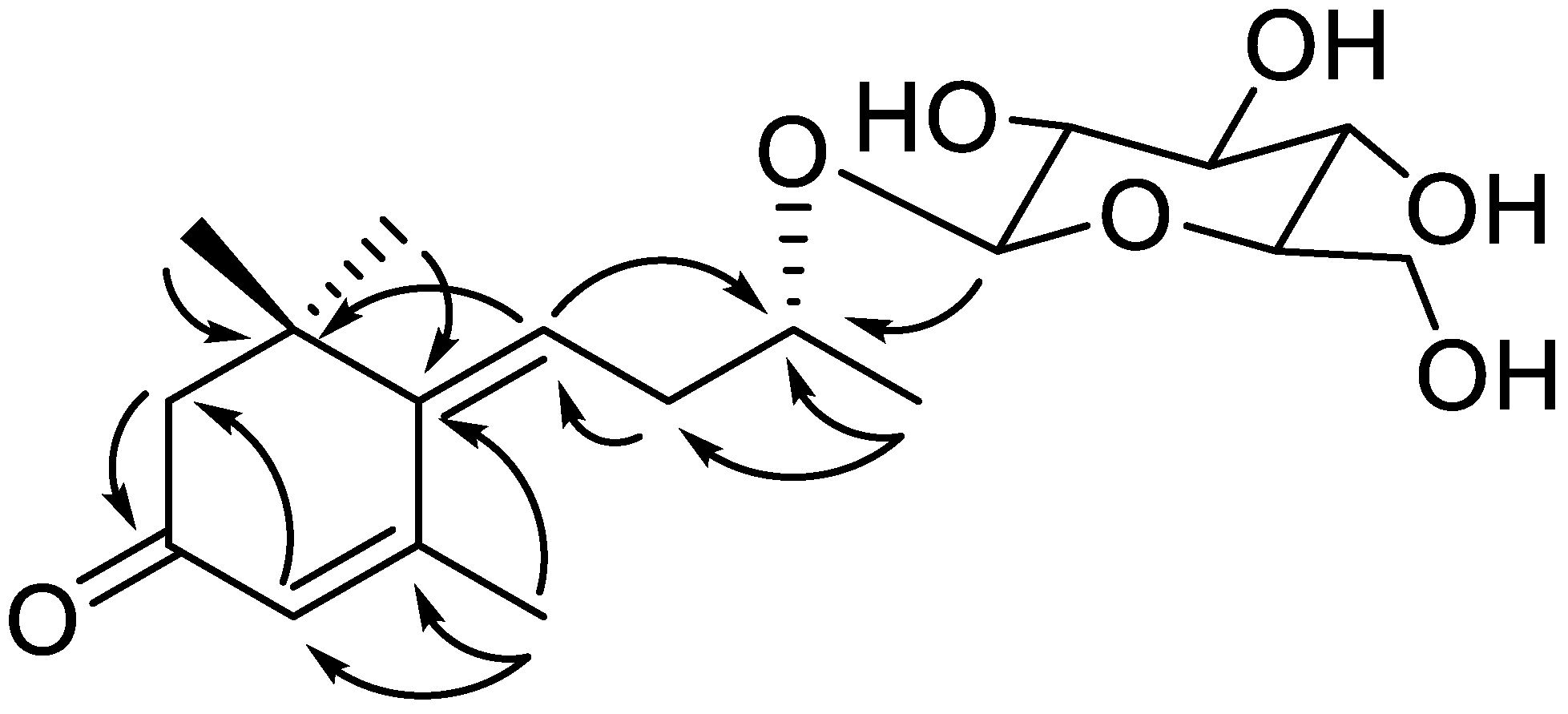
| Position | 1 a | |
|---|---|---|
| δH | δC | |
| 1 | 201.8, C | |
| 2 | 5.91, br s | 129.5, CH |
| 3 | 159.6, C | |
| 4 | 144.8, C | |
| 5 | 42.0, C | |
| 6 | 2.31, d (6.0) | 53.7, CH2 |
| 7 | 1.20, s | 28.2, CH3 |
| 8 | 1.20, s | 28.3, CH3 |
| 9 | 2.28, br s | 25.1, CH3 |
| 1′ | 5.97, t (7.50) | 129.2, CH |
| 2′ | 2.55, m 2.64, m | 38.0, CH2 |
| 3′ | 4.01, m | 77.9, CH |
| 4′ | 1.29, d (6.0) | 22.0, CH3 |
| 1″ | 4.39, d (7.8) | 103.9, CH |
| 2″ | 3.18, t (8.0) | 75.3, CH |
| 3″ | 3.50–3.54, overlap | 78.2, CH |
| 4″ | 3.44, m | 71.7, CH |
| 5″ | 3.50–3.54, overlap | 78.0, CH |
| 6″ | 3.88, br d (11.8) 3.67, dd (11.8, 6.0) | 62.8, CH2 |
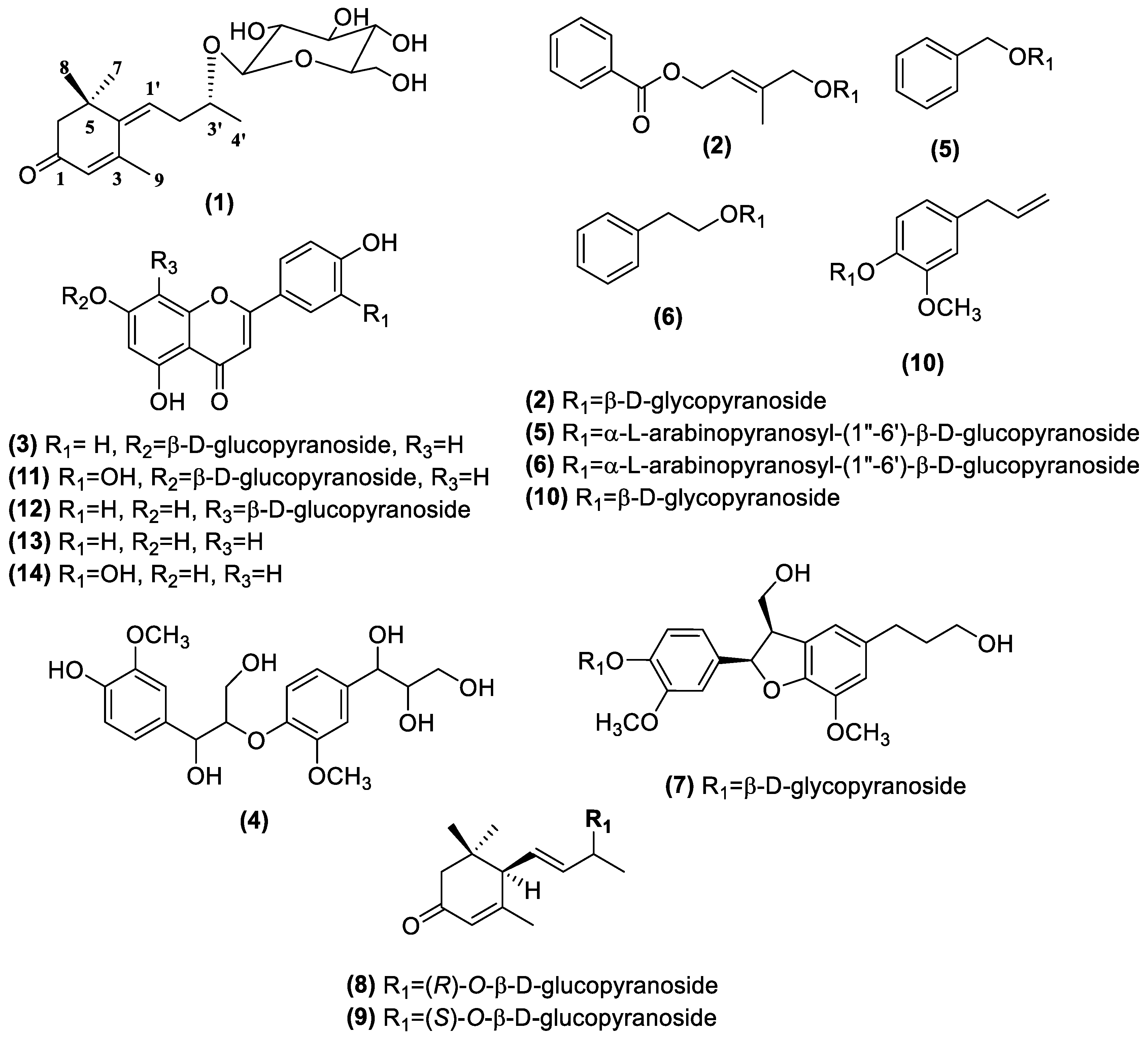
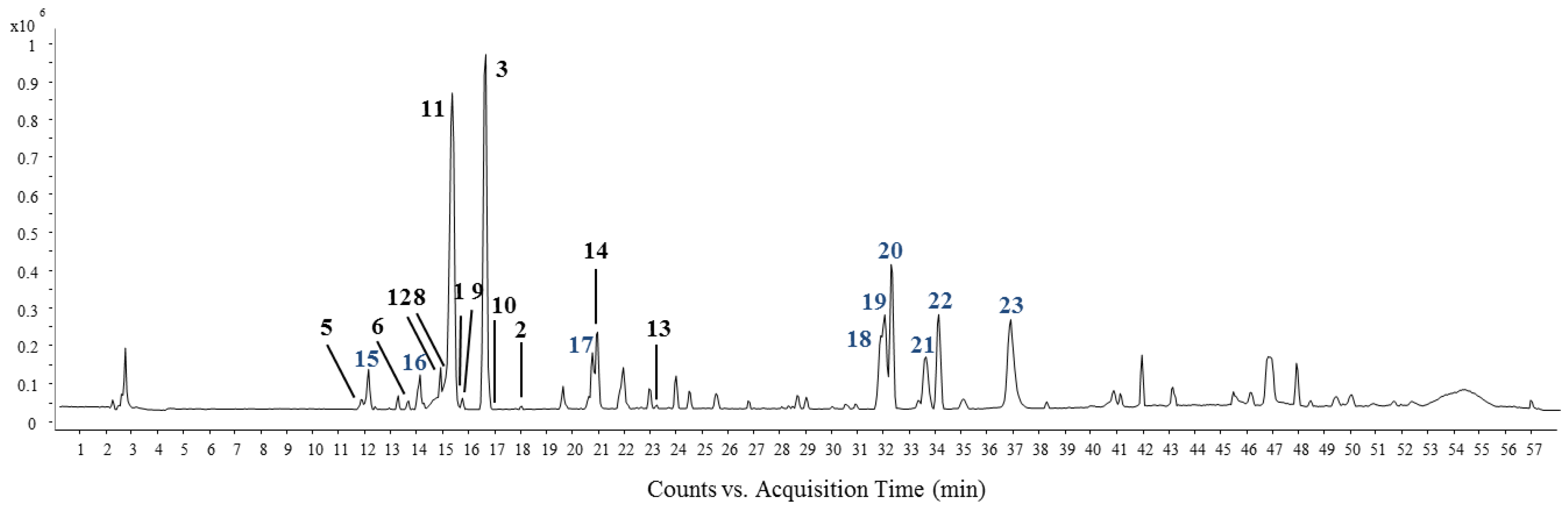
| Comp NO a | tR (min) | Molecular Formula | [M − H]−/[M + HCOO]− | Fragment Ion | Identification |
|---|---|---|---|---|---|
| 1 | 15.352 | C19H30O7 | 415.1975 | 339.1388, 271.1549, 223.0263 | (Z)-6-[9-(β-d-glucopyranosyloxy)butylidene]-5,1,1-trimethyl-4-cyclohexen-3-one |
| 2 | 17.885 | C18H24O8 | 413.1446 | 343.2107, 299.1835 | (2E)-4-[benzoyl-oxy]-3-methyl-2-buten-1-yl-β-d-glucopyranoside, |
| 3 | 16.495 | C21H20O10 | 431.1030 | 268.0363, 176.0077 | Apigenin-7-O-β-d-glucopyranoside |
| 5 | 11.876 | C18H26O10 | 447.1689 | 315.0884, 269.0979, 191.0557 | Benzyl-α-l-arabinopyranosyl-(1″→6′)-β-d-glucopyranoside |
| 6 | 13.515 | C19H28O10 | 461.1689 | 415.1607 | Phenylethyl-α-l-arabinopyranosyl-(1″→6′)-β-d-glucopyranoside |
| 8 | 14.955 | C19H30O7 | 415.1964 | 284.0315, 223.0289, 130.9644 | (6R,9R)-3-Oxo-α-ionol-β-d-glucopyranoside |
| 9 | 15.601 | C19H30O7 | 415.1989 | 369.1907, 223.0259, 119.0389 | (6R,9S)-3-Oxo-α-ionol-β-d-glucopyranoside |
| 10 | 16.931 | C16H22O7 | 371.1354 | 163.0738 | Eugenyl-β-d-glucopyranoside |
| 11 | 15.203 | C21H20O11 | 447.0990 | 377.1665, 284.0322, 151.0022 | Luteolin-7-O-β-d-glucopyranoside |
| 12 | 14.756 | C21H20O10 | 431.0996 | 311.0547, 283.1125 | Vitexin |
| 13 | 23.100 | C15H10O5 | 269.0463 | 227.1268, 183.1374 | Apigenin |
| 14 | 20.816 | C15H10O6 | 285.0412 | 211.1314, 171.1022 | Luteolin |
| 15 | 11.975 | 431.1953 | 385.1836, 264.1225 | Unknown | |
| 16 | 13.962 | C27H30O16 | 609.1506 | 447.0973, 285.0291 | Flavonoid diglucosides |
| 17 | 20.617 | 327.2200 | 211.1321, 119.0367 | Unknown | |
| 18 | 31.742 | C21H30O6 | 361.2007 | 293.2150, 265.1484 | Humulone |
| 19 | 31.891 | C21H30O6 | 361.2006 | 293.2149, 275.2023 | Adhumulone |
| 20 | 32.139 | C21H30O6 | 361.2003 | 293.21516, 246.1214 | Adprehunumolne |
| 21 | 33.480 | 687.31692 | 623.2733, 555.2895 | Unknown | |
| 22 | 33.977 | C26H38O4 | 431.2019 | 363.2112, 295.2307 | Lupulone |
| 23 | 36.758 | 389.2071 | 321.2218, 293.1807 | Unknown |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.J.; Dhodary, B.; Lee, J.Y.; An, J.-P.; Ryu, Y.-K.; Kim, K.-S.; Lee, C.-H.; Oh, W.K. Dereplication of Components Coupled with HPLC-qTOF-MS in the Active Fraction of Humulus japonicus and It’s Protective Effects against Parkinson’s Disease Mouse Model. Molecules 2019, 24, 1435. https://doi.org/10.3390/molecules24071435
Lee HJ, Dhodary B, Lee JY, An J-P, Ryu Y-K, Kim K-S, Lee C-H, Oh WK. Dereplication of Components Coupled with HPLC-qTOF-MS in the Active Fraction of Humulus japonicus and It’s Protective Effects against Parkinson’s Disease Mouse Model. Molecules. 2019; 24(7):1435. https://doi.org/10.3390/molecules24071435
Chicago/Turabian StyleLee, Hee Ju, Basanta Dhodary, Ju Yong Lee, Jin-Pyo An, Young-Kyoung Ryu, Kyoung-Shim Kim, Chul-Ho Lee, and Won Keun Oh. 2019. "Dereplication of Components Coupled with HPLC-qTOF-MS in the Active Fraction of Humulus japonicus and It’s Protective Effects against Parkinson’s Disease Mouse Model" Molecules 24, no. 7: 1435. https://doi.org/10.3390/molecules24071435
APA StyleLee, H. J., Dhodary, B., Lee, J. Y., An, J.-P., Ryu, Y.-K., Kim, K.-S., Lee, C.-H., & Oh, W. K. (2019). Dereplication of Components Coupled with HPLC-qTOF-MS in the Active Fraction of Humulus japonicus and It’s Protective Effects against Parkinson’s Disease Mouse Model. Molecules, 24(7), 1435. https://doi.org/10.3390/molecules24071435