Heterologous Expression of a Thermostable α-Glucosidase from Geobacillus sp. Strain HTA-462 by Escherichia coli and Its Potential Application for Isomaltose–Oligosaccharide Synthesis
Abstract
1. Introduction
2. Results
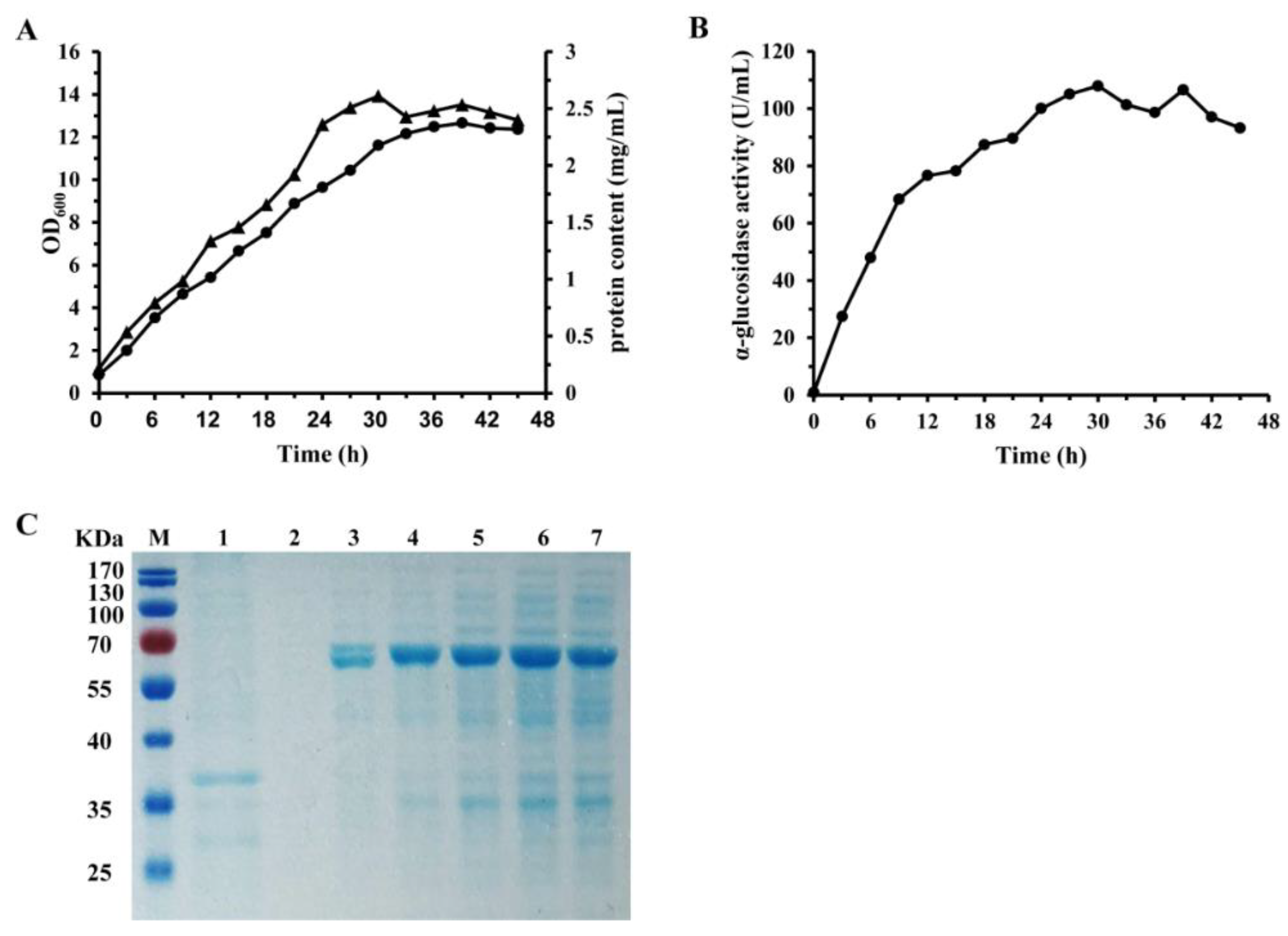
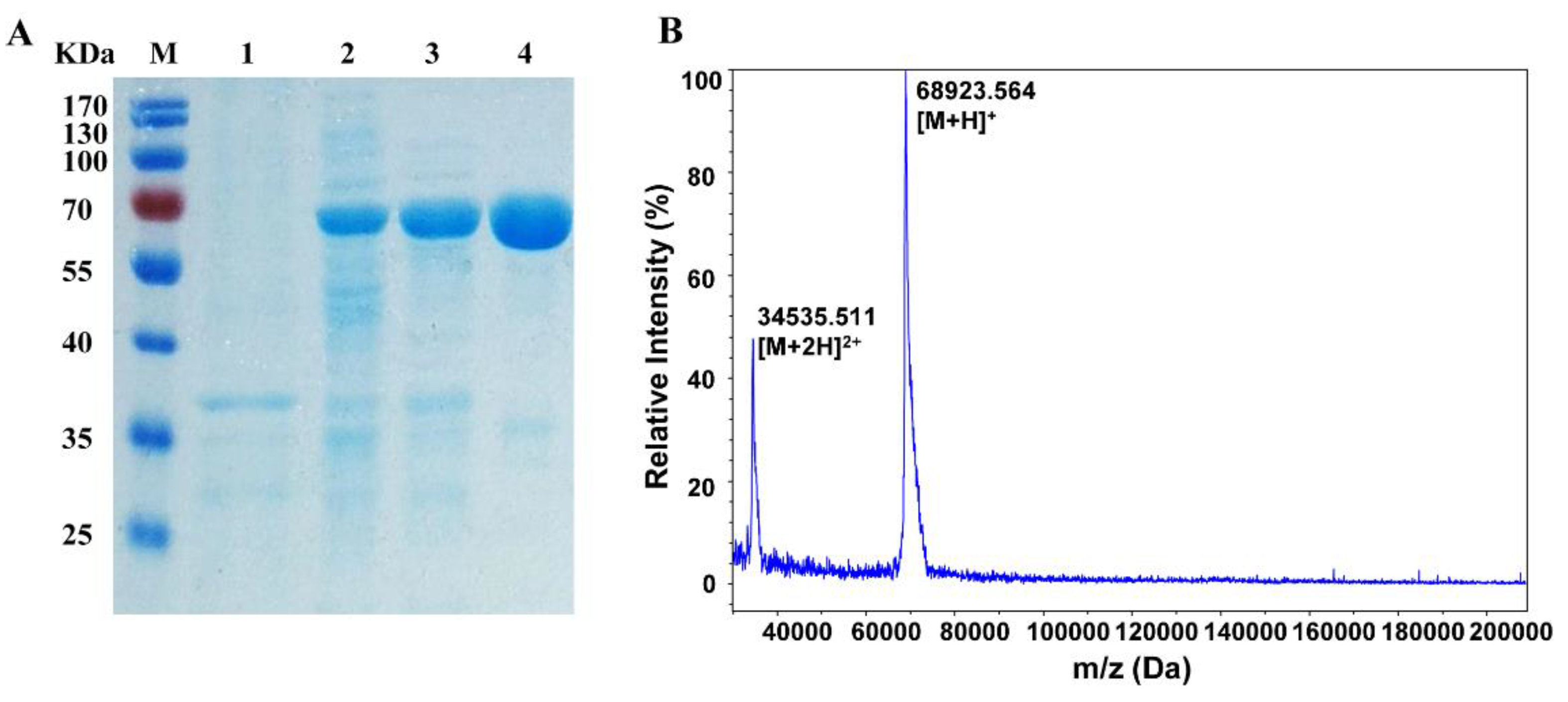
2.1. Expression and Purification of α-Glucosidase
2.2. Characterization of the Recombinant Enzymes
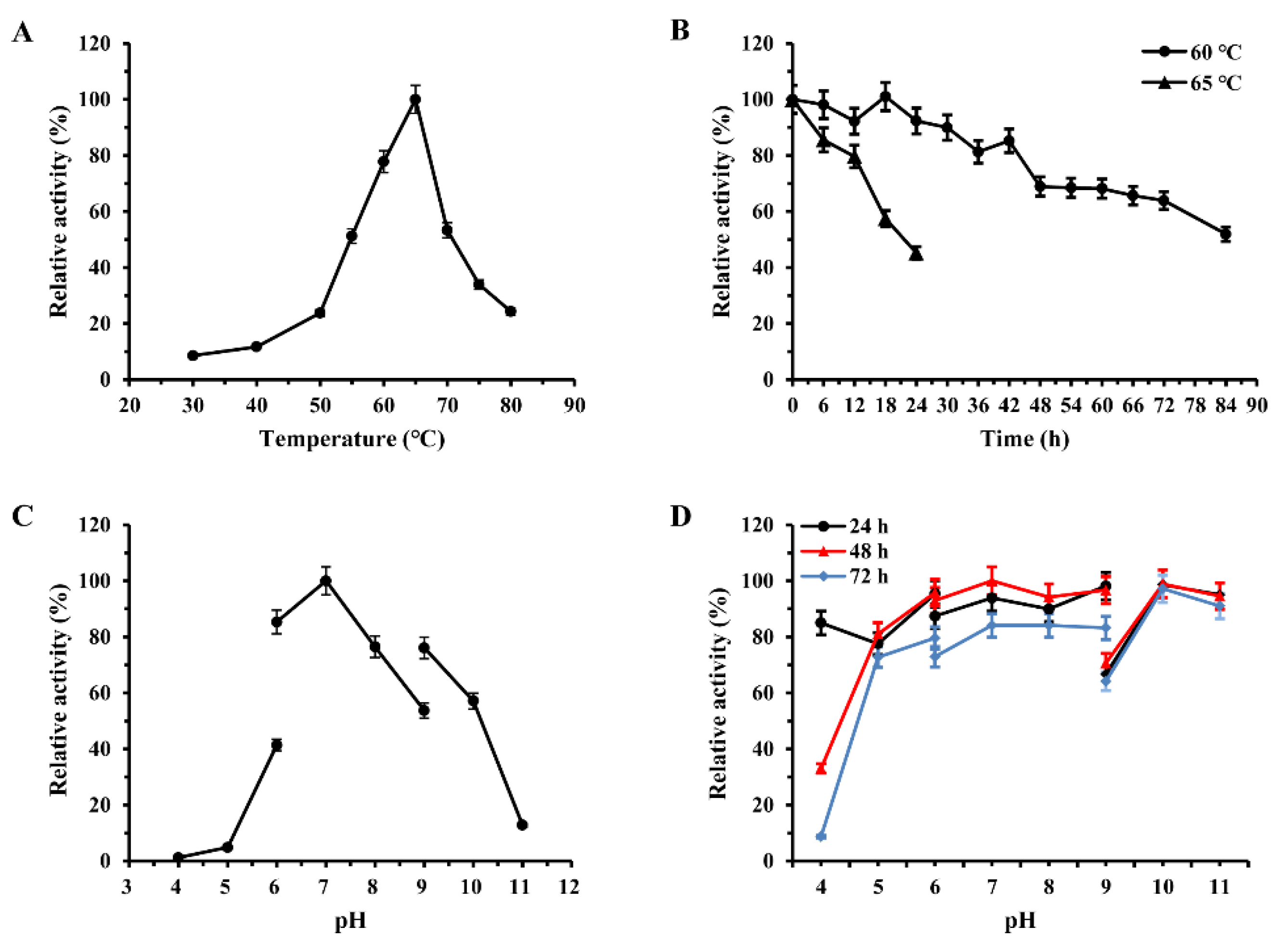
2.2.1. Temperature Optimum and Thermostability
2.2.2. pH Optimum and Stability
2.2.3. Kinetic Studies
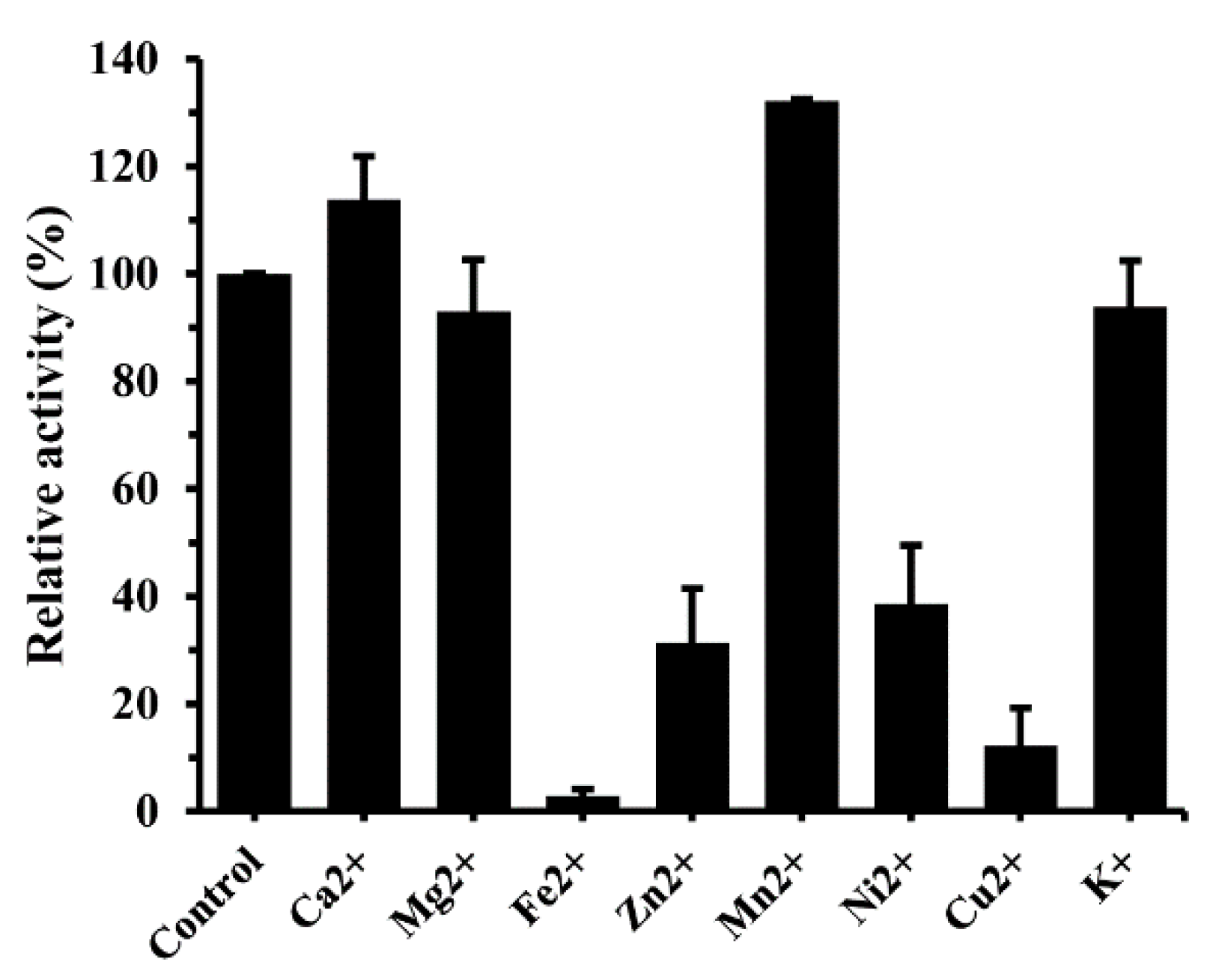
2.2.4. Metal Requirements
2.2.5. Substrate Specificity
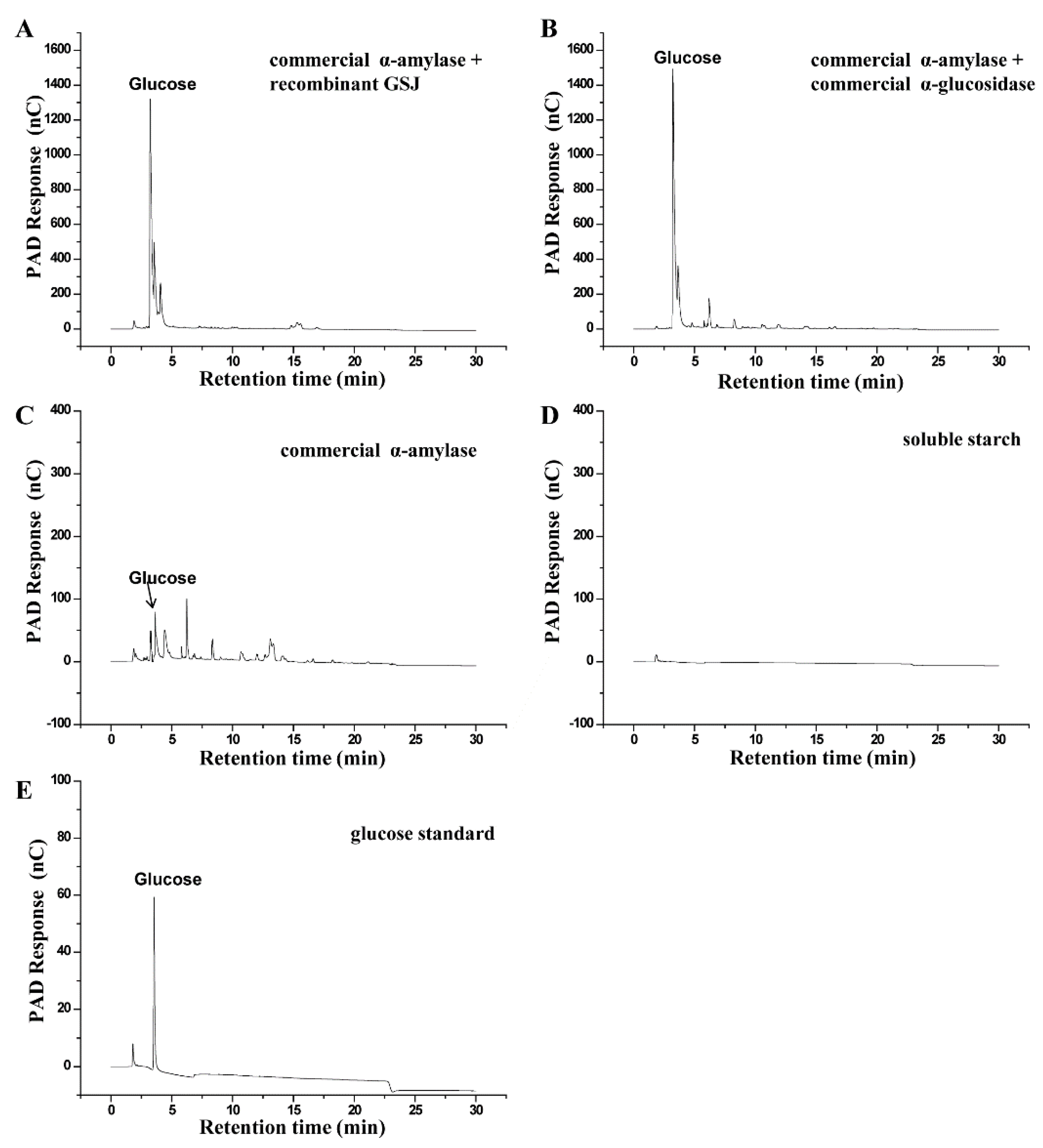
2.3. Hydrolysis Activity
2.4. Transglycosylation Activity of Recombinant α-Glucosidase
3. Discussion
4. Materials and Methods
4.1. Cloning and Expression of GSJ in E. coli BL21 (DE3)
4.2. Assay of α-Glucosidase Activity
4.3. Enzyme Preparation and Purification
4.4. Effects of Temperature and pH on the Enzyme Activity and Stability
4.5. Metal Requirement
4.6. Kinetic Parameters
4.7. Substrate Specificity
4.8. Hydrolysis Reaction
4.9. Transglycosylation Reaction
4.10. HPAEC Analysis
4.11. MALDI-TOF Mass Spectrometry
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Huang, Z.H.; Li, Z.H.; Su, Y.J.; Zhu, Y.F.; Zeng, W.; Chen, G.G.; Liang, Z.Q. Continuous production of isomalto-oligosaccharides by thermo-inactivated cells of Aspergillus niger J2 with coarse perlite as an immobilizing material. Appl. Biochem. Biotechnol. 2018, 185, 1088–1099. [Google Scholar] [CrossRef]
- Shi, Q.; Hou, Y.X.; Juvonen, M.; Tuomainen, P.; Kajala, I.; Shukla, S.; Goyal, A.; Maaheimo, H.; Katina, K.; Tenkanen, M. Optimization of isomaltooligosaccharide size distribution by acceptor reaction of Weissella confusa dextransucrase and characterization of novel α-(1→2)-branched isomaltooligosaccharides. J. Agric. Food Chem. 2016, 64, 3276–3286. [Google Scholar] [CrossRef] [PubMed]
- Ojha, S.; Mishra, S.; Chand, S. Production of isomalto-oligosaccharides by cell bound α-glucosidase of Microbacterium sp. LWT-Food Sci. Technol. 2015, 60, 486–494. [Google Scholar] [CrossRef]
- Soto-Maldonado, C.; Concha-Olmos, J.; Cáceres-Escobar, G.; Meneses-Gómez, P. Sensory evaluation and glycaemic index of a food developed with flour from whole (pulp and peel) overripe banana (Musa cavendishii) discards. LWT-Food Sci. Technol. 2018, 92, 569–575. [Google Scholar] [CrossRef]
- Meyer, T.S.M.; Miguel, Â.S.M.; Fernández, D.E.R.; Ortiz, G.M.D. Biotechnological production of oligosaccharides-applications in the food industry. In Food Production and Industry; IntechOpen: Rijeka, Croatia, 2015. [Google Scholar] [CrossRef]
- Yen, C.H.; Tseng, Y.H.; Kuo, Y.W.; Lee, M.C.; Chen, H.L. Long-term supplementation of isomalto-oligosaccharides improved colonic microflora profile, bowel function, and blood cholesterol levels in constipated elderly people—A placebo-controlled, diet-controlled trial. Nutrition 2011, 27, 445–450. [Google Scholar] [CrossRef]
- Madsen, L.R.; Stanley, S.; Swann, P.; Oswald, J. A Survey of Commercially Available Isomaltooligosaccharide-Based Food Ingredients. J. Food Sci. 2017, 82, 401–408. [Google Scholar] [CrossRef]
- Rudeekulthamrong, P.; Sawasdee, K.; Kaulpiboon, J. Production of long-chain isomaltooligosaccharides from maltotriose using the thermostable amylomaltase and transglucosidase enzymes. Biotechnol. Bioproc. Eng. 2013, 18, 778–786. [Google Scholar] [CrossRef]
- Zhang, L.; Su, Y.L.; Zheng, Y.; Jiang, Z.Y.; Shi, J.F.; Zhu, Y.Y.; Jiang, Y.J. Sandwich-structured enzyme membrane reactor for efficient conversion of maltose into isomaltooligosaccharides. Bioresour. Technol. 2010, 101, 9144–9149. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Zhao, G.Y.; Du, W.; Zhang, T.J. Effect of dietary isomaltooligosaccharides on nutrient digestibility and concentration of glucose, insulin, cholesterol and triglycerides in serum of growing pigs. Anim. Feed Sci. Technol. 2009, 151, 312–315. [Google Scholar] [CrossRef]
- Kaulpiboon, J.; Rudeekulthamrong, P.; Watanasatitarpa, S.; Ito, K.; Pongsawasdi, P. Synthesis of long-chain isomaltooligosaccharides from tapioca starch and an in vitro investigation of their prebiotic properties. J. Mol. Catal. B Enzym. 2015, 120, 127–135. [Google Scholar] [CrossRef]
- Sorndech, W.; Nakorn, K.N.; Tongta, S.; Blennow, A. Isomalto-oligosaccharides: Recent insights in production technology and their use for food and medical applications. LWT-Food Sci. Technol. 2018, 95, 135–142. [Google Scholar] [CrossRef]
- Kato, N.; Suyama, S.; Shirokane, M.; Kato, M.; Kobayashi, T.; Tsukagoshi, N. Novel α-glucosidase from Aspergillus nidulans with strong transglycosylation activity. Appl. Environ. Microb. 2002, 68, 1250–1256. [Google Scholar] [CrossRef]
- Casa-Villegas, M.; Marín-Navarro, J.; Polaina, J. Synthesis of isomaltooligosaccharides by Saccharomyces cerevisiae cells expressing Aspergillus niger α-glucosidase. ACS Omega 2017, 2, 8062–8068. [Google Scholar] [CrossRef]
- Chen, P.; Xu, R.X.; Wang, J.H.; Wu, Z.R.; Yan, L.; Zhao, W.B.; Liu, Y.H.; Ma, W.T.; Shi, X.F.; Li, H.Y. Starch biotransformation into isomaltooligosaccharides using thermostable alpha-glucosidase from Geobacillus stearothermophilus. PeerJ 2018, 6, e5086. [Google Scholar] [CrossRef]
- Fernández-Arrojo, L.; Marin, D.; De Segura, A.G.; Linde, D.; Alcalde, M.; Gutiérrez-Alonso, P.; Ghazi, I.; Plou, F.J.; Fernández-Lobato, M.; Ballesteros, A. Transformation of maltose into prebiotic isomaltooligosaccharides by a novel α-glucosidase from Xantophyllomyces dendrorhous. Process Biochem. 2007, 42, 1530–1536. [Google Scholar] [CrossRef]
- Goffin, D.; Delzenne, N.; Blecker, C.; Hanon, E.; Deroanne, C.; Paquot, M. Will isomalto-oligosaccharides, a well-established functional food in Asia, break through the European and American market? The status of knowledge on these prebiotics. Crit. Rev. Food Sci. Nutr. 2011, 51, 394–409. [Google Scholar] [CrossRef]
- Chen, D.L.; Tong, X.; Chen, S.W.; Chen, S.; Wu, D.; Fang, S.G.; Wu, J.; Chen, J. Heterologous expression and biochemical characterization of α-glucosidase from Aspergillus niger by Pichia pastroris. J. Agric. Food Chem. 2010, 58, 4819–4824. [Google Scholar] [CrossRef]
- Zhao, N.N.; Xu, Y.S.; Wang, K.; Zheng, S. Synthesis of isomalto-oligosaccharides by Pichia pastoris displaying the Aspergillus niger α-glucosidase. J. Agric. Food Chem. 2017, 65, 9468–9474. [Google Scholar] [CrossRef]
- Hung, V.S.; Hatada, Y.J.; Goda, S.; Lu, J.; Hidaka, Y.K.; Li, Z.J.; Akita, M.; Ohta, Y.; Watanabe, K.; Matsui, H.; et al. α-Glucosidase from a strain of deep-sea Geobacillus: A potential enzyme for the biosynthesis of complex carbohydrates. Appl. Microbial. Biot. 2005, 68, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Raasch, C.; Streit, W.; Schanzer, J.; Bibel, M.; Gosslar, U.; Liebl, W. Thermotoga maritima AglA, an extremely thermostable NAD+-, Mn2+-, and thiol-dependent α-glucosidase. Extremophiles 2000, 4, 189–200. [Google Scholar] [CrossRef]
- Olusanya, O.; Olutiola, P.O. Characterisation of maltase from enteropathogenic Escherichia coli. FEMS Microbial. Lett. 1986, 36, 239–244. [Google Scholar] [CrossRef][Green Version]
- Yamasaki, Y.; Suzuki, Y.; Ozawa, J. Certain properties of α-glucosidase from Mucor racemosus. Agric. Biol. Chem. 1977, 41, 1559–1565. [Google Scholar] [CrossRef]
- Gutiérrez-Alonso, P.; Gimeno-Pérez, M.; Ramírez-Escudero, M.; Plou, F.J.; Sanz-Aparicio, J.; Fernández-Lobato, M. Molecular characterization and heterologous expression of a Xanthophyllomyces dendrorhous α-glucosidase with potential for prebiotics production. Appl. Microbial. Biot. 2016, 100, 3125–3135. [Google Scholar] [CrossRef]
- Prakash, O.; Jaiswal, N. α-Amylase: An ideal representative of thermostable enzymes. Appl. Biochem. Biotech. 2010, 160, 2401–2414. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]


| Purification | Total Protein (mg) | Total Activity (U) | Specific Activity (U/mg) | Purification Fold | Yield (%) |
|---|---|---|---|---|---|
| Crude enzyme | 90.90 | 3876.1 | 42.64 | 1.0 | 100.0 |
| Heat treatment | 25.05 | 3206.9 | 128.0 | 3.0 | 82.7 |
| Ni-Sepharose | 10.08 | 2856.0 | 283.3 | 6.6 | 73.7 |
| Substrate | Km (mM) | Vmax (U mg−1) | kcat (s−1) | kcat/Km (mM−1s−1) |
|---|---|---|---|---|
| pNP-α-D-glucopyranoside | 2.321 | 306.3 | 352 | 151.6 |
| Substrate | Relative Activity (%) |
|---|---|
| pNP-α-D-glucopyranoside | 100.0 |
| pNP-β-D-glucopyranoside | 4.1 |
| pNP-α-L-arabinofuranoside | 6.6 |
| pNP-α-L-arabinopyranoside | 0.6 |
| pNP-α-D-galactopyranoside | 2.7 |
| pNP-β-D-galactopyranoside | 0.0 |
| pNP-α-L-rhamnopyranoside | 6.8 |
| pNP-β-D-mannopyranoside | 0.0 |
| pNP-β-D-xylopyranoside | 3.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Wang, W.; Bah, F.B.M.; Song, C.; Zhou, Y.; Ji, L.; Yuan, Y. Heterologous Expression of a Thermostable α-Glucosidase from Geobacillus sp. Strain HTA-462 by Escherichia coli and Its Potential Application for Isomaltose–Oligosaccharide Synthesis. Molecules 2019, 24, 1413. https://doi.org/10.3390/molecules24071413
Zhang F, Wang W, Bah FBM, Song C, Zhou Y, Ji L, Yuan Y. Heterologous Expression of a Thermostable α-Glucosidase from Geobacillus sp. Strain HTA-462 by Escherichia coli and Its Potential Application for Isomaltose–Oligosaccharide Synthesis. Molecules. 2019; 24(7):1413. https://doi.org/10.3390/molecules24071413
Chicago/Turabian StyleZhang, Fan, Weiyang Wang, Fatoumata Binta Maci Bah, Chengcheng Song, Yifa Zhou, Li Ji, and Ye Yuan. 2019. "Heterologous Expression of a Thermostable α-Glucosidase from Geobacillus sp. Strain HTA-462 by Escherichia coli and Its Potential Application for Isomaltose–Oligosaccharide Synthesis" Molecules 24, no. 7: 1413. https://doi.org/10.3390/molecules24071413
APA StyleZhang, F., Wang, W., Bah, F. B. M., Song, C., Zhou, Y., Ji, L., & Yuan, Y. (2019). Heterologous Expression of a Thermostable α-Glucosidase from Geobacillus sp. Strain HTA-462 by Escherichia coli and Its Potential Application for Isomaltose–Oligosaccharide Synthesis. Molecules, 24(7), 1413. https://doi.org/10.3390/molecules24071413




