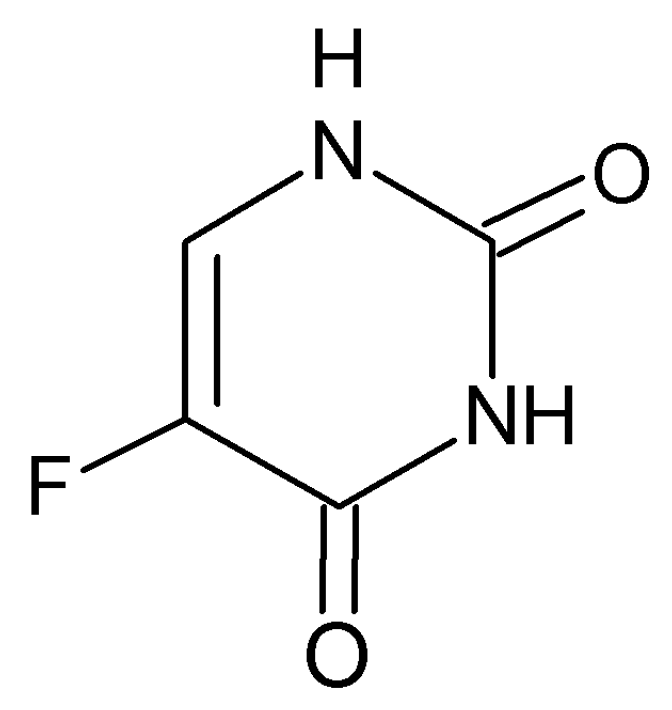
A Study of 5-Fluorouracil Desorption from Mesoporous Silica by RP-UHPLC
Abstract
:1. Introduction
2. Results and Discussion
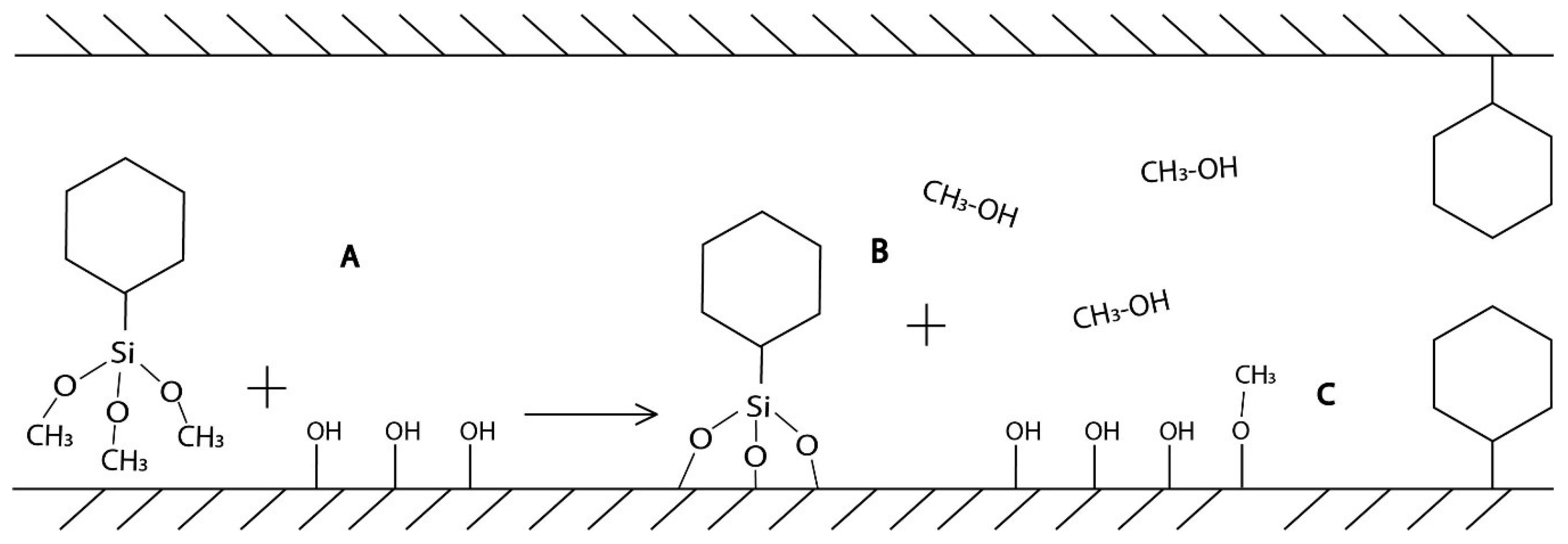
2.1. Characterization of SBA-15
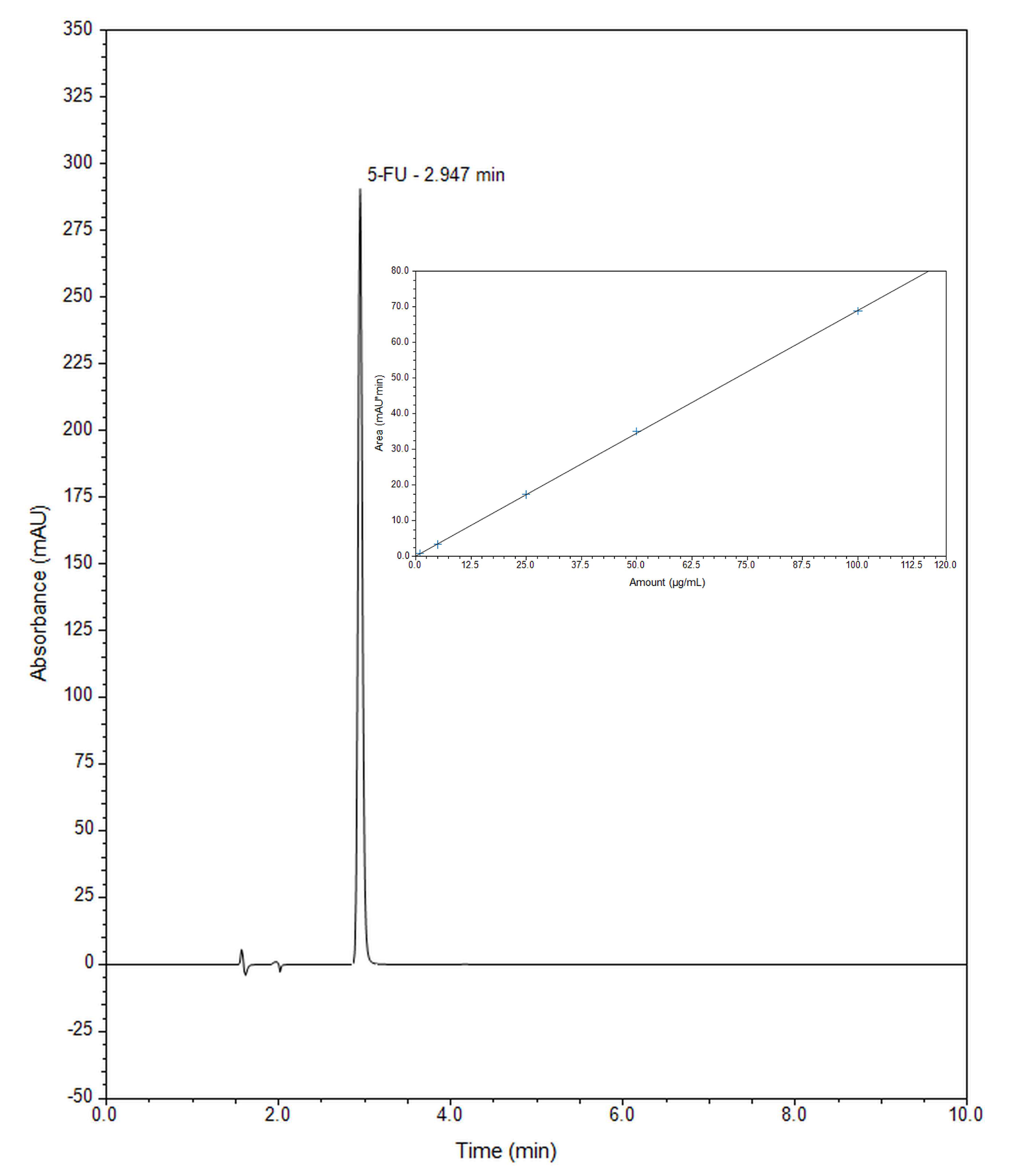
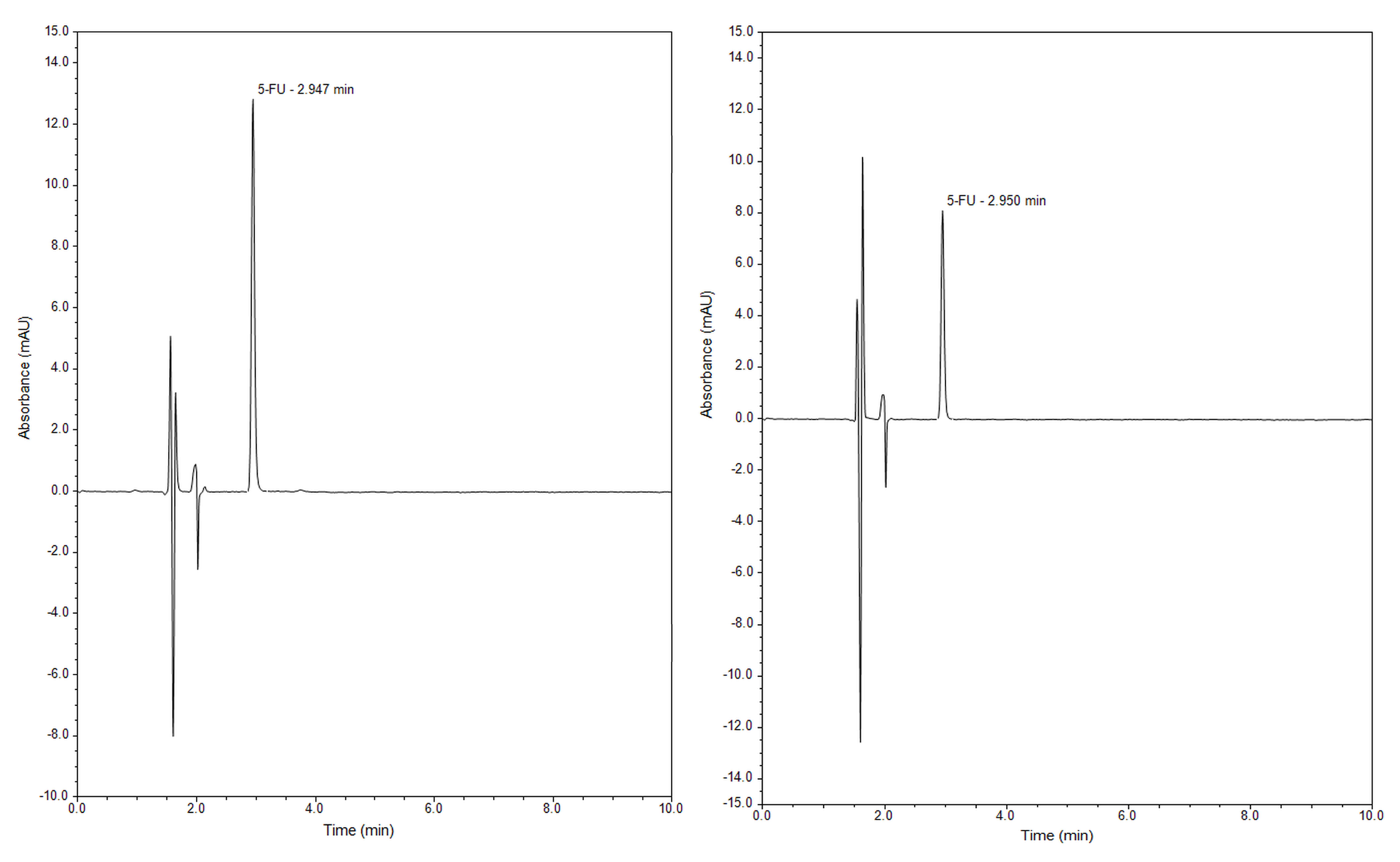
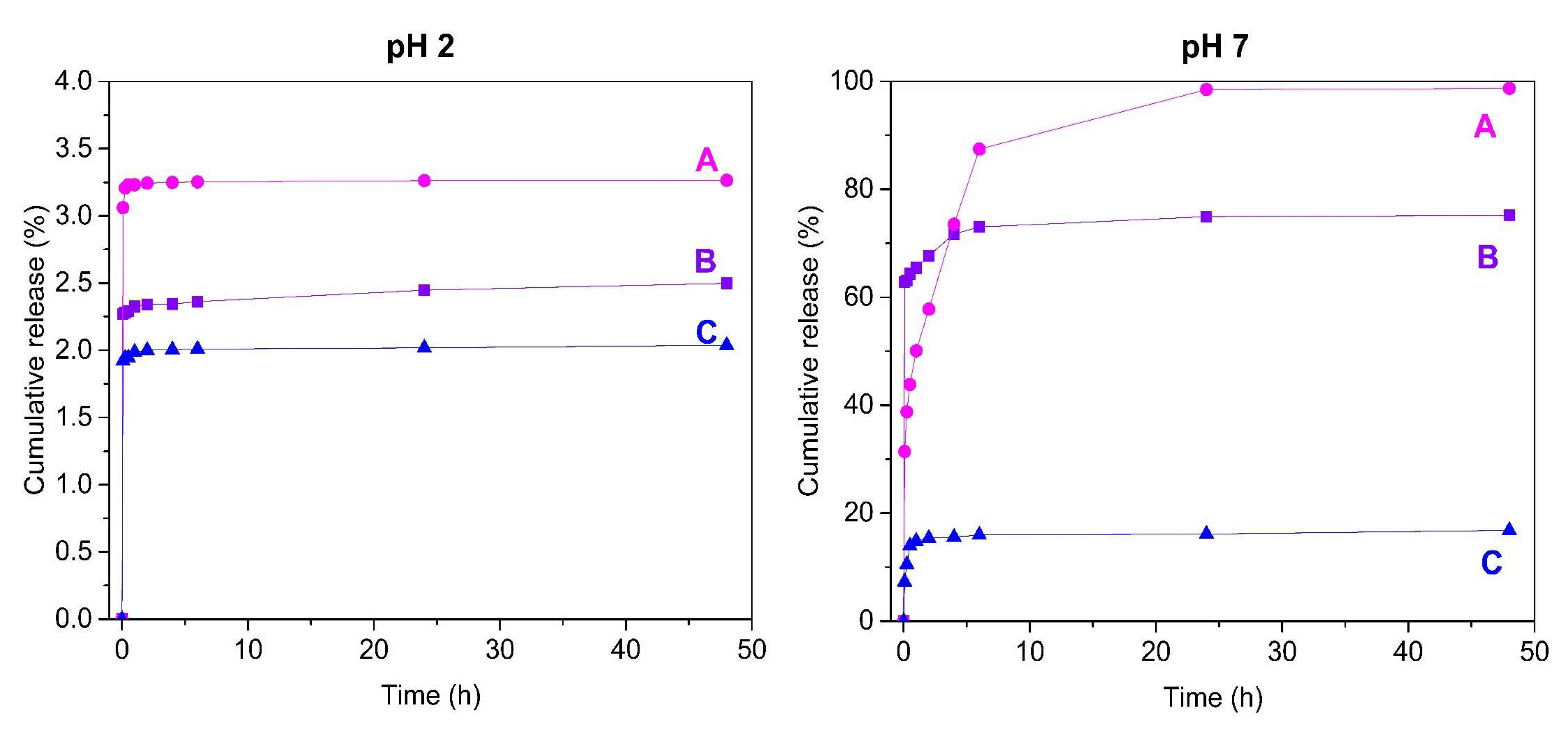
2.2. Release Study of 5-Fluorouracil from Mesoporous Silica SBA-15 by RP-UHPLC
3. Materials and Methods
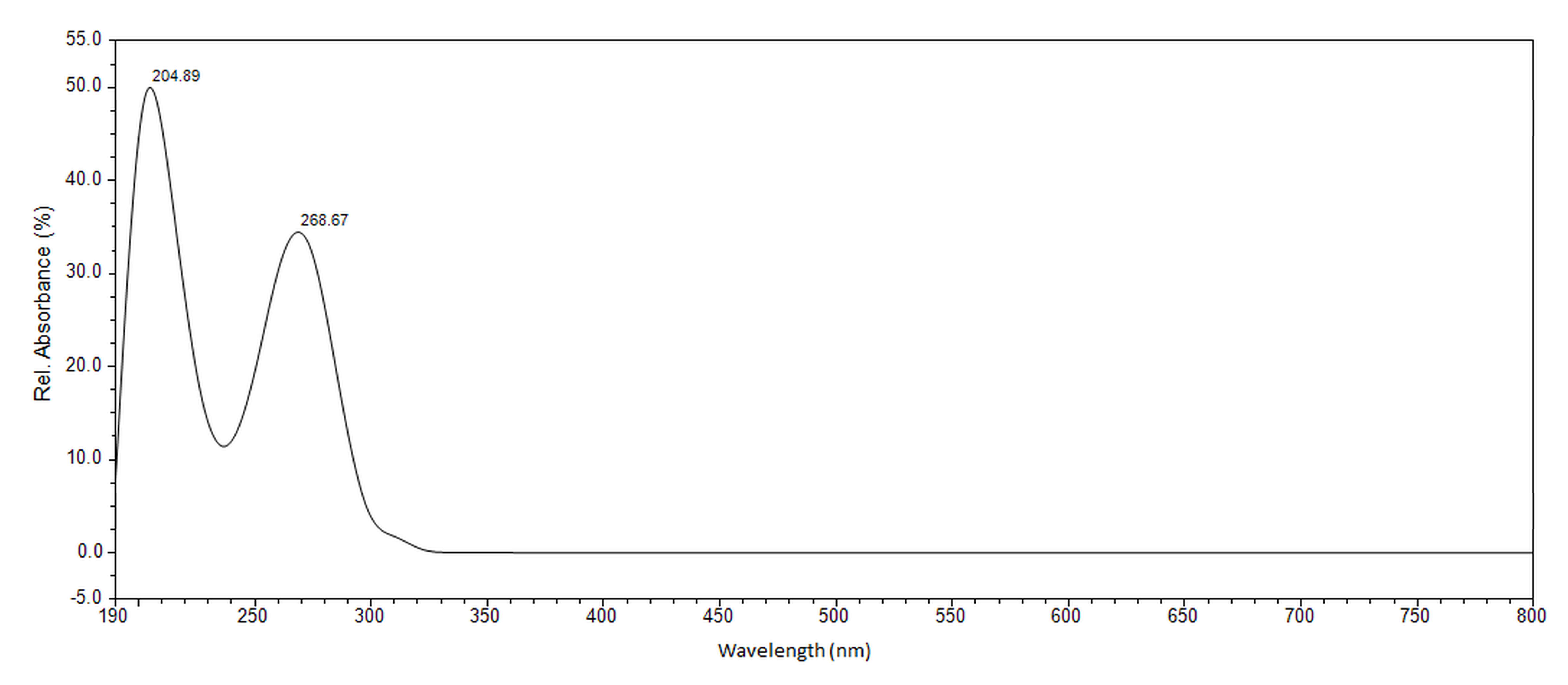
3.1. Instruments
3.2. Chemicals and Reagents
3.3. Preparation of Samples and Standard Solutions
3.4. Release Study of 5-Fluorouracil from Mesoporous Silica by RP-UHPLC and Chromatographic Conditions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yang, P.; Gai, S.; Lin, J. Functionalized mesoporous silica materials for controlled drug delivery. Chem. Soc. Rev. 2012, 41, 3679–3698. [Google Scholar] [CrossRef]
- Böttcher, H.; Slowik, P.; Süβ, W. Sol-Gel Carrier Systems for Controlled Drug Delivery. J. Sol–Gel Sci. Technol. 1998, 13, 277. [Google Scholar] [CrossRef]
- Wu, Z.; Joo, H.; Lee, T.G.; Lee, K. Controlled release of lidocaine hydrochloride from the surfactant-doped hybrid xerogels. J. Control. Release 2005, 104, 497. [Google Scholar] [CrossRef]
- Manzano, M.; Vallet-Regí, M. New developments in ordered mesoporous materials for drug delivery. J. Mater. Chem. 2010, 20, 5593–5604. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regí, M.; Rámila, A.; Real, R.P.; Pérez-Pariente, J. A new property of MCM-41: Drug delivery system. Chem. Mater. 2001, 13, 308–311. [Google Scholar] [CrossRef]
- Doadrio, A.L.; Sousa, E.M.B.; Doadrio, J.C.; Pérez-Pariente, J.; Izquierdo-Barba, I.; Vallet-Regí, M. Mesoporous SBA-15 HPLC evaluation for controlled gentamicin drug delivery. J. Control. Release 2004, 97, 125–132. [Google Scholar] [CrossRef]
- Vallet-Regí, M.V.; Doadrio, J.C.; Doadrio, A.L.; Barba, I.I.; Pariente, J.P. Hexagonal ordered mesoporous materials as a matrix for the controlled release of amoxicillin. Solid State Ion. 2004, 172, 435–439. [Google Scholar] [CrossRef]
- Yu, H.; Zhai, Q.Z. Mesoporous SBA- 15 molecular sieve as carrier for controlled release of nimodiphine. Micropor. Mesopor. Mater. 2009, 123, 298–305. [Google Scholar] [CrossRef]
- Halamová, D.; Zeleňák, V. NSAID naproxen in mesoporous matrix MCM-41: Drug uptake and release properties. J. Incl. Phenom. Macrocycl. Chem. 2012, 72, 15–23. [Google Scholar] [CrossRef]
- Halamová, D.; Badaničová, M.; Zeleňák, V.; Gondová, T.; Vainio, U. Naproxen drug delivery using periodic mesoporous silica SBA-15. Appl. Surf. Sci. 2010, 256, 6489–6494. [Google Scholar] [CrossRef]
- Zeleňák, V.; Halamová, D.; Almáši, M.; Žid, L.; Zeleňáková, A.; Kapusta, O. Ordered cubic nanoporous silica support MCM-48 for delivery of poorly soluble drug indomethacin. Appl. Surf. Sci. 2018, 443, 525–534. [Google Scholar] [CrossRef]
- Pagar, O.B.; Nagare, H.S.; Chine, Y.M.; Autade, R.R.; Narode, P.R.; Sanklecha, V.M. Mesoporous Silica: A Review. Int. J. Pharm. Drug Anal. 2018, 6, 1–12. [Google Scholar]
- Han, Y.J.; Stucky, G.D.; Butle, A. Mesoporous silicate sequestration and release of proteins. J. Am. Chem. Soc. 1999, 121, 9897–9898. [Google Scholar] [CrossRef]
- Lu, Y.; Fan, H.; Doke, N.; Loy, D.A.; Assink, R.A.; LaVan, D.A.; Brinker, C.J. Evaporation-induced self-assembly of hybrid bridged silsesquioxane film and particulate mesophases with integral organic functionality. J. Am. Chem. Soc. 2000, 122, 5258–5261. [Google Scholar] [CrossRef]
- Hoffmann, F.; Cornelius, M.; Morell, J.; Froba, M. Silica-based mesoporous organic-inorganic hybrid materials. Angew. Chem-Int. Edit. 2006, 45, 3216–3225. [Google Scholar] [CrossRef]
- Kecht, J.; Schlossbauer, A.; Bein, T. Selective functionalization of the outer and inner surfaces in mesoporous silica nanoparticles. Chem. Mater. 2008, 20, 7207–7214. [Google Scholar] [CrossRef]
- Munoz, B.; Rámila, A.; Pérez-Pariente, J.; Díaz, I.; Vallet-Regí, M. MCM-41 Organic modification as drug delivery rate regulator. Chem. Mater. 2003, 15, 500–503. [Google Scholar] [CrossRef]
- Wang, G.; Otuonye, A.N.; Blair, A.; Denton, K.; Tao, Z.; Asefa, T. Functionalized mesoporous materials for adsorption and release of different drug molecules: A comparative study. J. Solid State Chem. 2009, 182, 1649–1660. [Google Scholar] [CrossRef]
- Maleki, A.; Kettiger, H.; Schoubben, A.; Rosenholm, J.M.; Ambrogi, V.; Hamidi, M. Mesoporous silica materials: From physico-chemical properties to enhanced dissolution of poorly water-soluble drugs. J. Control. Release 2017, 262, 329–347. [Google Scholar] [CrossRef]
- Zhu, K.; Ye, T.; Liu, J.; Peng, Z.; Xu, S.; Lei, J.; Deng, H.; Li, B. Nanogels fabricated by lysozyme and sodium carboxymethyl cellulose for 5-fluorouracil controlled release. Int. J. Pharm. 2013, 441, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Meyerhardt, J.A.; Mayer, R.J. Systemic therapy for colorectal cancer. N. Engl. J. Med. 2005, 352, 476–487. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lin, J.; Zhang, X.; Liu, Q.; Xu, Q.; Tan, R.-X.; Guo, Z. 5-Fluorouracil–cisplatin adducts with potential antitumor activity. J. Inorg. Biochem. 2003, 94, 186–192. [Google Scholar] [CrossRef]
- Rich, T.A.; Shepard, R.C.; Mosley, S.T. Four decades of continuing innovation with fluorouracil: Current and future approaches to fluorouracil chemoradiation therapy. J. Clin. Oncol. 2004, 22, 2214–2232. [Google Scholar] [CrossRef]
- Mundargi, R.C.; Rangaswamy, V.; Aminabhavi, T.M. A novel method to prepare 5-fluorouracil, an anti-cancer drug, loaded microspheres from poly(N-vinylcaprolactam-co-acrylamide) and controlled release studies. Des. Monomers. Polym. 2010, 13, 25–336. [Google Scholar] [CrossRef]
- Arias, J.L.; López-Viota, M.; Delgado, Á.V.; Ruiz, M.A. Iron/ethylcellulose (core/shell) nanoplatform loaded with 5-fluorouracil for cancer targeting. Colloid Surface B 2010, 77, 111–116. [Google Scholar] [CrossRef]
- Kevadiya, B.D.; Patel, T.A.; Jhala, D.D.; Thumbar, R.P.; Brahmbhatt, H.; Pandya, M.P.; Rajkumar, S.; Jena, P.K.; Joshi, G.V.; Gadhia, P.K.; et al. Layered inorganic nanocomposites: A promising carrier for 5-fluorouracil (5-FU). Eur. J. Pharm. Biopharm. 2012, 81, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Vilaça, N.; Machado, A.F.; Morais-Santos, F.; Amorim, R.; Neto, A.P.; Logodin, E.; Pereira, M.F.R.; Sardo, M.; Rocha, J.; Parpot, P.; et al. Comparison of different silica microporous structures as drug delivery systems for in vitro models of solid tumors. RSC Adv. 2017, 7, 13104–13111. [Google Scholar]
- Gârea, S.; Mihai, A.; Ghebaur, A.; Nistor, C.; Sârbu, A. Porous clay heterostructures: A new inorganic host for 5-fluorouracil encapsulation. Int. J. Pharm. 2015, 491, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Faria, H.A.M.; de Queiroz, A.A.A. A novel drug delivery of 5-fluorouracil device based on TiO2/ZnS nanotubes. Mater. Sci. Eng. C. 2015, 56, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Zhao, Q.; Luan, Y.; Jiang, Y.; Shi, J.; Shao, W. Polymer fibrous materials composed of sodium carboxymethylcellulose and poly(vinyl alcohol) for a hydrophilic anticancer drug release. J. Disper. Sci. Technol. 2012, 33, 835–839. [Google Scholar] [CrossRef]
- Bayramgil, N.P. Synthesis, characterization and drug release behavior of poly(1-vinyl 1,2,4-triazole) hydrogels prepared by gamma irradiation. Colloid Surface B 2012, 97, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, M.S.; Park, S.S.; Fuping, D.; Hong, S.H.; Selvaraj, M.; Ha, C.S. Step-up synthesis of amidoxime-functionalised periodic mesoporous organosilicas with an amphoteric ligand in the framework for drug delivery. J. Mater. Chem. 2012, 22, 9100–9108. [Google Scholar] [CrossRef]
- El-Ghannam, A.; Ricci, K.; Malkawi, A.; Jahed, K.; Vedantham, K.; Wyan, H.; Allen, L.D.; Dréau, D. A ceramic-based anticancer drug delivery system to treat breast cancer. J. Mater. Sci. Mater. Med. 2010, 21, 2701–2710. [Google Scholar] [CrossRef] [PubMed]
- Larsen, S.C. Nanocrystalline zeolites and zeolite structures: Synthesis, characterization, and applications. J. Phys. Chem. C 2007, 111, 18464–18474. [Google Scholar] [CrossRef]
- Petushkov, A.; Intra, J.; Graham, J.B.; Larsen, S.C.; Salem, A.K. Effect of crystal size and surface functionalization on the cytotoxicity of silicalite-1 nanoparticles. Chem. Res. Toxicol. 2009, 22, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- Murugan, B.; Ramana, L.N.; Gandhi, S.; Sethuraman, S.; Krishnan, U.M. Engineered chemoswitchable mesoporous silica for tumor-specific cytotoxicity. J. Mater. Chem. B 2013, 1, 3494–3505. [Google Scholar] [CrossRef]
- Hodali, H.A.; Marzouqa, D.M.; Tekfa, F.Z. Evaluation of mesoporous silicate nanoparticles for the sustained release of the anticancer drugs: 5-fluorouracil and 7-hydroxycoumarin. J. Sol-Gel Sci. Technol. 2016, 80, 417–425. [Google Scholar] [CrossRef]
- Egodawatte, S.; Dominguez, S., Jr.; Larsen, S.C. Solvent effects in the development of a drug delivery system for 5-fluorouracil using magnetic mesoporous silica nanoparticles. Micropor. Mesopor. Mater. 2017, 237, 108–116. [Google Scholar] [CrossRef]
- Yang, L.; Chu, J.S.; Fix, J.A. Colon-specific drug delivery: New approaches and in vitro/in vivo evaluation. Int. J. Pharm. 2002, 235, 1–15. [Google Scholar] [CrossRef]
- Anderson, D.; Kerr, D.J.; Blesing, C.; Seymour, L.W. Simultaneous gas chromatographic-mass spectrophotometric determination of alpha-fluoro-beta-alanine and 5-fluorouracil in plasma. J. Chromatogr. B Biomed. Sci. Appl. 1997, 688, 87–93. [Google Scholar] [CrossRef]
- Barberi-Heyob, M.; Merlin, J.L.; Weber, B. Analysis of 5-fluorouracil in plasma and urine by high-performance liquid chromatography. J. Chromatogr. 1992, 581, 281–286. [Google Scholar] [CrossRef]
- Iwamoto, M.; Yoshida, S.; Hirose, S. Fluorescence determination of 5-fluorouracil and 1-(tetrahydro-2-furanyl)-5-fluorouracil in blood serum by high-performance liquid chromatography. J. Chromatogr. 1984, 310, 151–157. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Björklund, S.; Kocherbitov, V. Alcohols react with MCM-41 at room temperature and chemically modify mesoporous silica. Sci. Rep. 2017, 7, 9960. [Google Scholar] [CrossRef] [PubMed]
- Beňová, E.; Zeleňák, V.; Halamová, D.; Almáši, M.; Petruľová, V.; Psotka, M.; Zeleňáková, A.; Bačkor, M.; Hornebecq, V. A drug delivery system based on switchable photo-controlled p-coumaric acid derivatives anchored on mesoporous silica. J. Mater. Chem. B 2017, 5, 817–825. [Google Scholar] [CrossRef]
- Zeleňák, V.; Beňová, E.; Almáši, M.; Halamová, D.; Hornebecq, V.; Hronský, V. Photo-switchable nanoporous silica supports for controlled drug delivery. New J. Chem. 2018, 42, 13263–13271. [Google Scholar] [CrossRef]
- Zeleňák, V.; Halamová, D.; Zeleňáková, A.; Girman, V. Periodic 3D nanoporous silica modified by amine or SPION nanoparticles as NSAID delivery system. J. Porous Mater. 2016, 23, 1633–1645. [Google Scholar] [CrossRef]
- Zeleňák, V.; Hornebecq, V.; Llewellyn, P. Zinc(II)-benzoato complexes immobilised in mesoporous silica host. Micropor. Mesopor. Mater. 2005, 83, 125–135. [Google Scholar] [CrossRef]
- Tzankov, B.; Voycheva, C.; Aluani, D.; Yordanov, Y.; Avramova, K.; Tzankova, V.; Spassova, I.; Kovacheva, D.; Yoncheva, K. Improvement of dissolution of poorly soluble glimepiride by loading on two types of mesoporous silica carriers. J. Solid State Chem. 2019, 271, 253–259. [Google Scholar] [CrossRef]
- Shishu; Gupta, N.; Aggarwal, N. Stomach-specific drug delivery of 5-fluorouracil using floating alginate beads. AAPS Pharm. Sci. Tech. 2007, 8, E143–E149. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples nSBA-15, cSBA-15, 5-FU-SBA-15, 5-FU-nSBA-15 and 5-FU-cSBA-15 are available from the authors. |
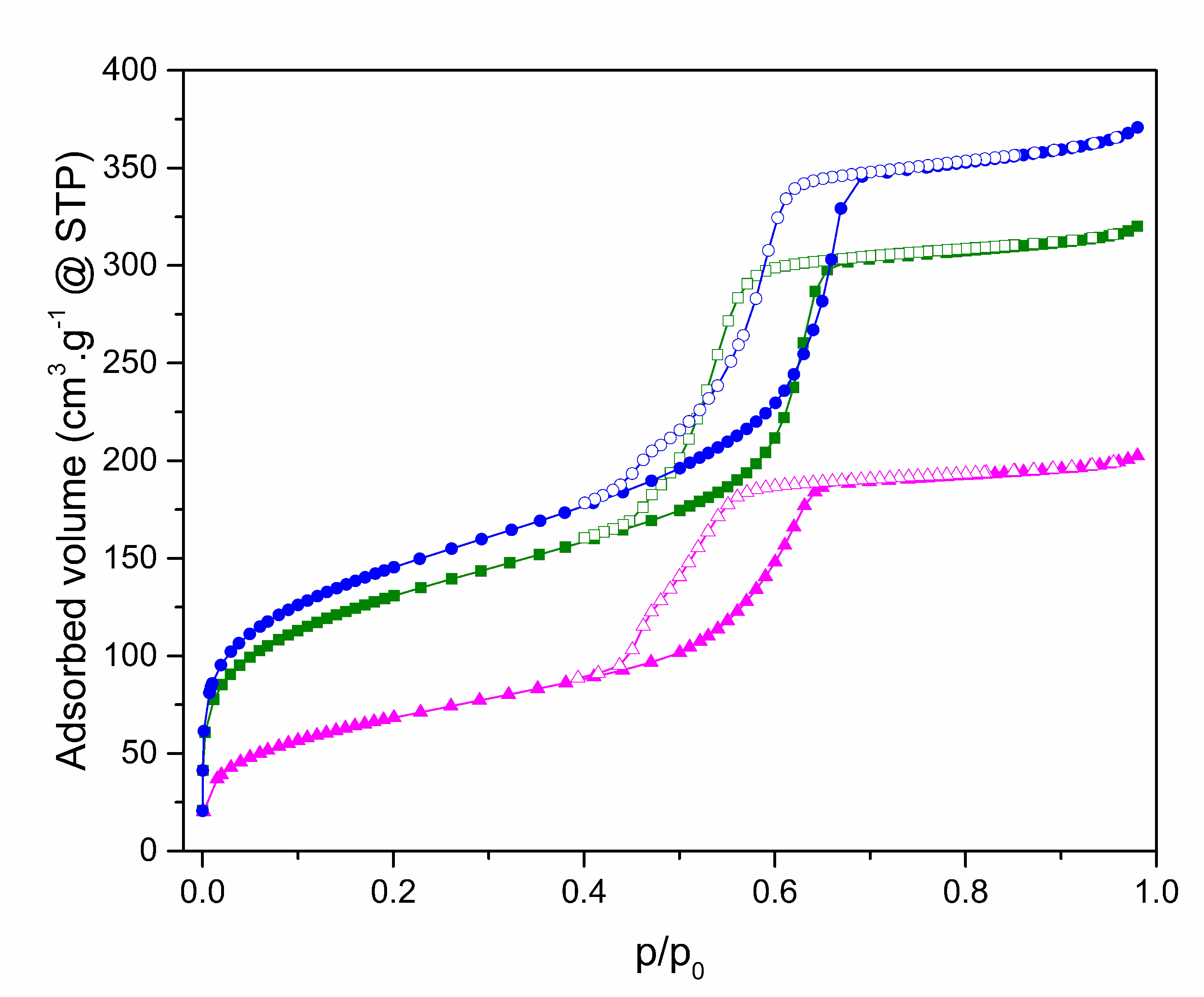
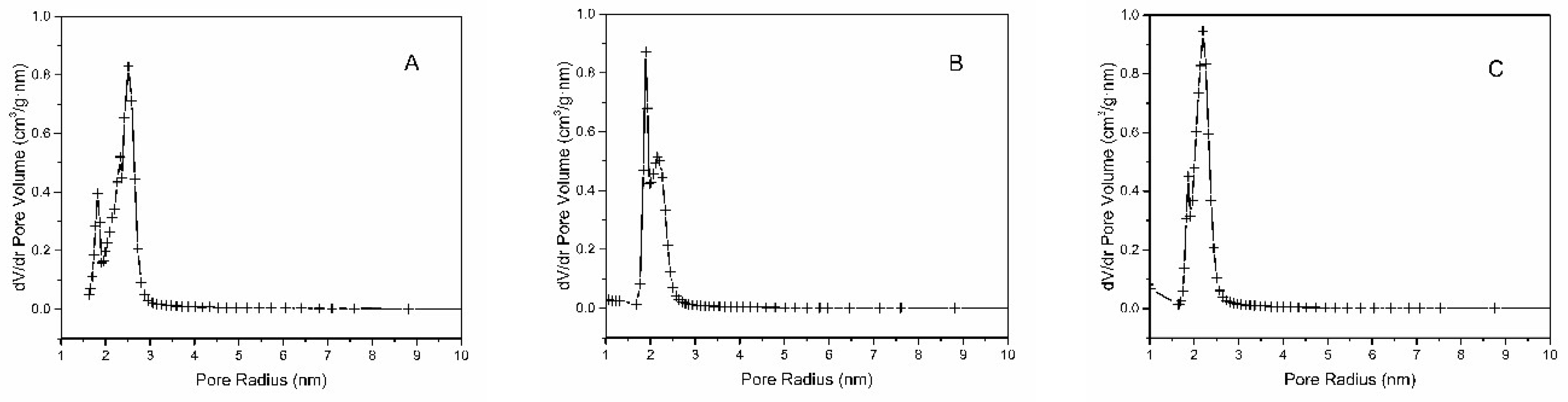
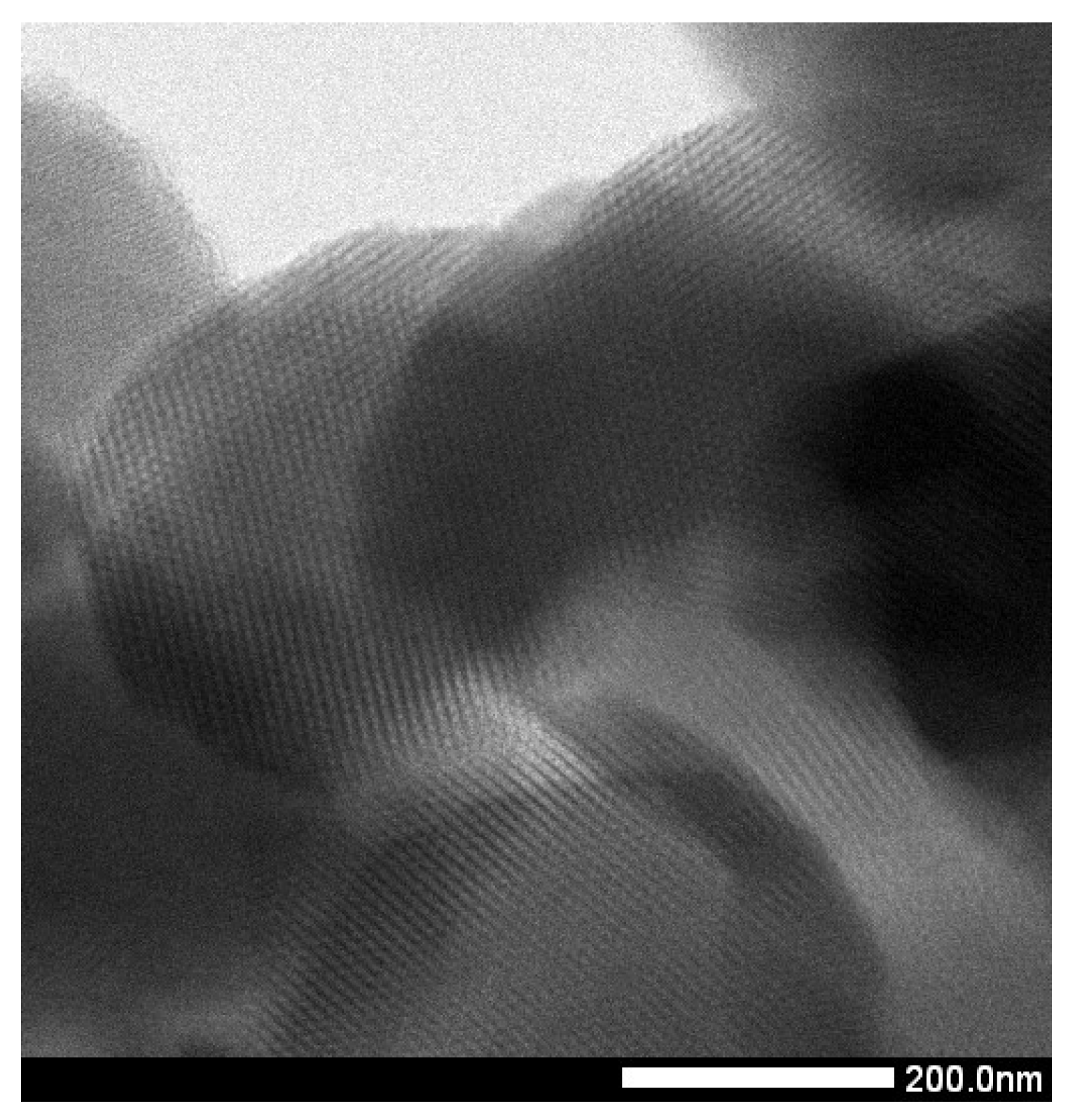
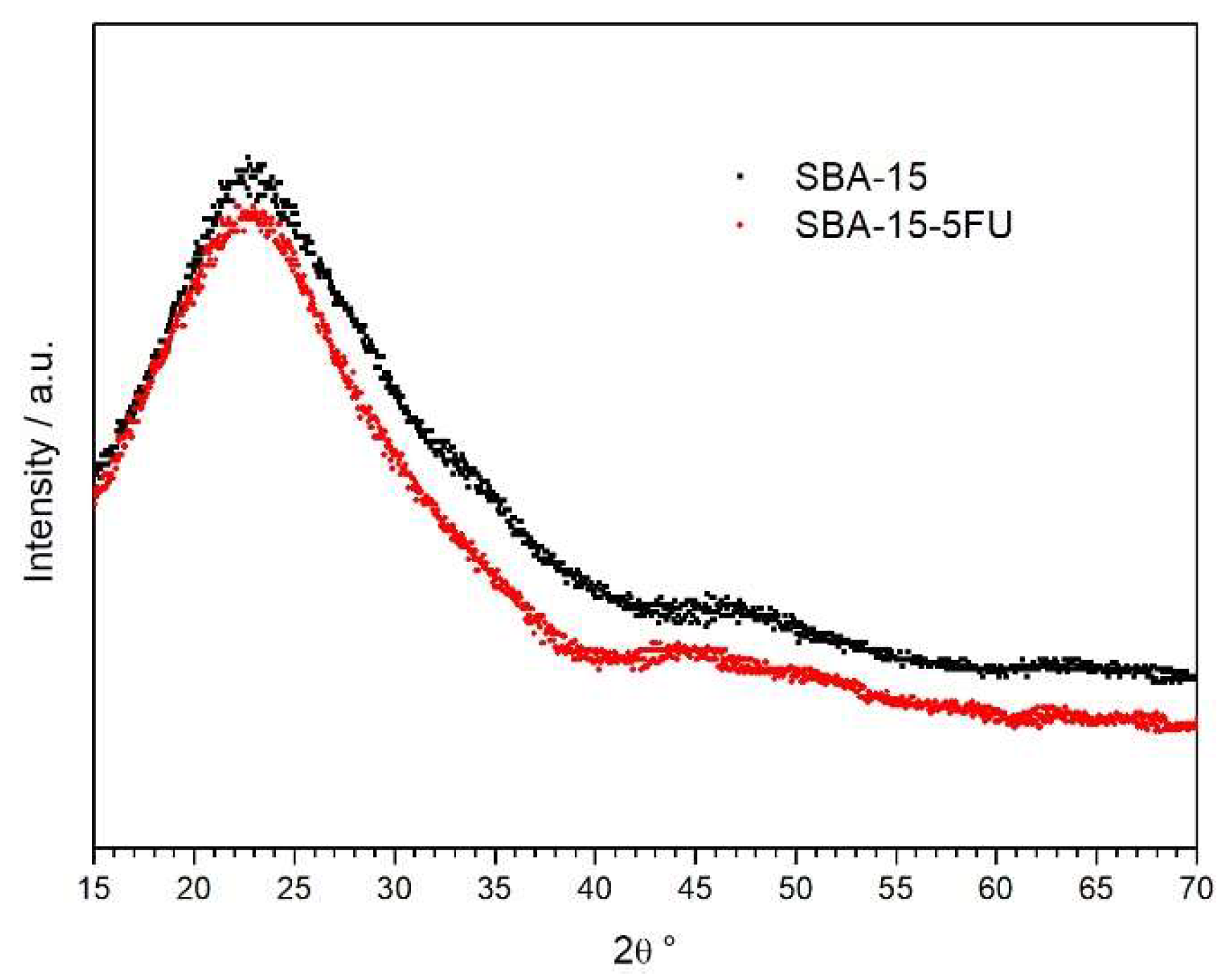
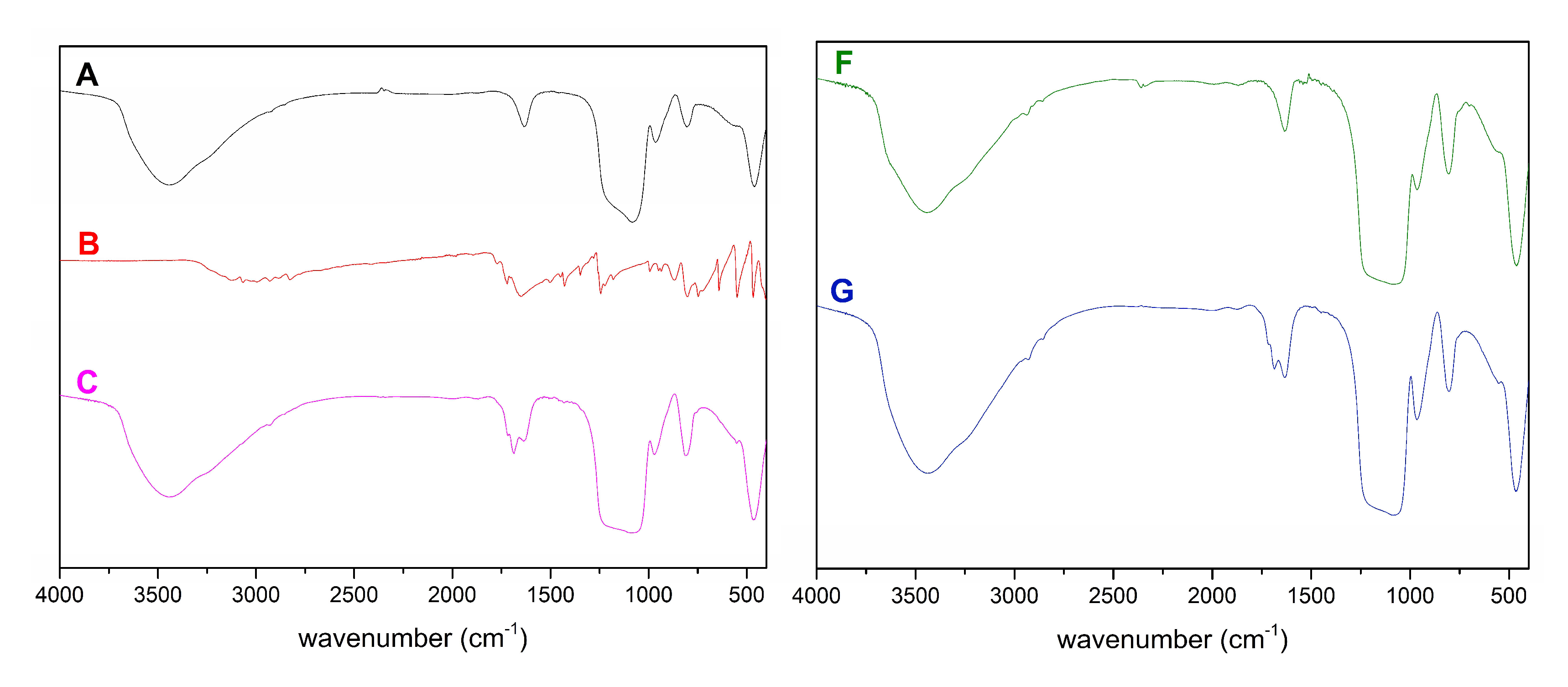
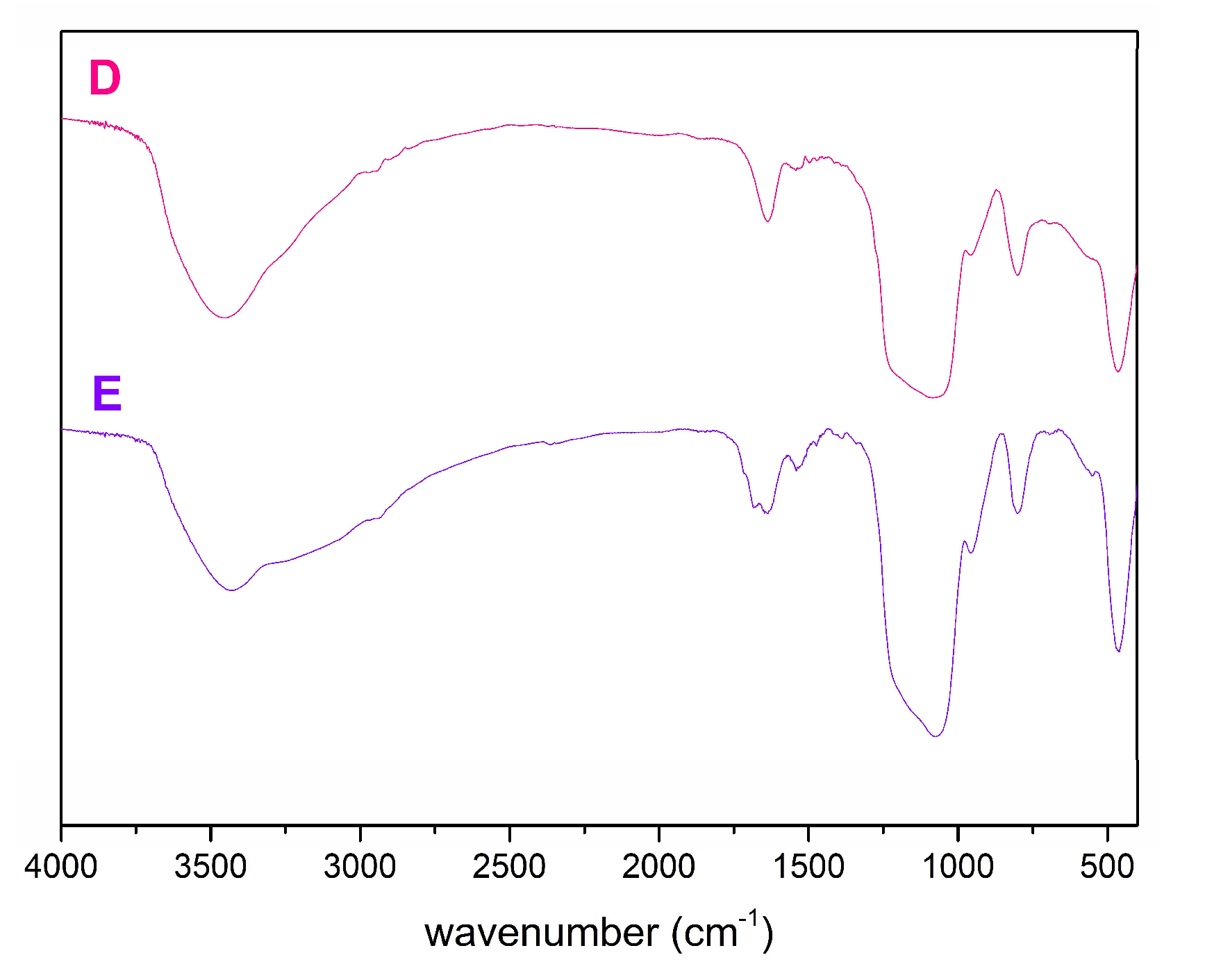
| Material | SBET (m2/g) | DP (nm) | VP (cm3/g) |
|---|---|---|---|
| SBA-15 1 | 528 | 5 | 0.496 |
| nSBA-15 2 | 250 | 4.7 | 0.289 |
| cSBA-15 3 | 477 | 4.9 | 0.435 |
| Parameters | Conditions |
|---|---|
| Mobile phase | methanol: phosphate buffer, pH 6.9 (3:97, v/v) |
| Stationary phase | ODS Hypersil C18 column, (150 × 4.6 mm, 5 µm) |
| Column Temperature | 25 °C |
| Detection Wavelength | 268 nm |
| Flow rate | 1.0 mL/min |
| Injection volume | 20 µL |
| Retention time | 2.9 min |
| Cumulative Release of 5-FU (%) | ||||||
|---|---|---|---|---|---|---|
| Time | pH 2 | pH 7 | ||||
| SBA-15 | nSBA-15 | cSBA-15 | SBA-15 | nSBA-15 | cSBA-15 | |
| 5 min | 3.06 | 2.26 | 1.92 | 31.40 | 62.82 | 7.27 |
| 15 min | 3.20 | 2.27 | 1.94 | 38.77 | 63.09 | 10.53 |
| 30 min | 3.23 | 2.29 | 1.94 | 43.83 | 64.34 | 13.94 |
| 1 h | 3.23 | 2.32 | 1.98 | 50.08 | 65.43 | 14.82 |
| 2 h | 3.24 | 2.33 | 1.99 | 57.76 | 67.68 | 15.35 |
| 4 h | 3.24 | 2.34 | 2.00 | 73.51 | 71.74 | 15.57 |
| 6 h | 3.25 | 2.36 | 2.00 | 87.42 | 72.99 | 15.94 |
| 24 h | 3.26 | 2.44 | 2.01 | 98.48 | 74.93 | 16.13 |
| 48 h | 3.26 | 2.49 | 2.03 | 98.69 | 75.16 | 16.81 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šuleková, M.; Váhovská, L.; Hudák, A.; Žid, L.; Zeleňák, V. A Study of 5-Fluorouracil Desorption from Mesoporous Silica by RP-UHPLC. Molecules 2019, 24, 1317. https://doi.org/10.3390/molecules24071317
Šuleková M, Váhovská L, Hudák A, Žid L, Zeleňák V. A Study of 5-Fluorouracil Desorption from Mesoporous Silica by RP-UHPLC. Molecules. 2019; 24(7):1317. https://doi.org/10.3390/molecules24071317
Chicago/Turabian StyleŠuleková, Monika, Lucia Váhovská, Alexander Hudák, Lukáš Žid, and Vladimír Zeleňák. 2019. "A Study of 5-Fluorouracil Desorption from Mesoporous Silica by RP-UHPLC" Molecules 24, no. 7: 1317. https://doi.org/10.3390/molecules24071317
APA StyleŠuleková, M., Váhovská, L., Hudák, A., Žid, L., & Zeleňák, V. (2019). A Study of 5-Fluorouracil Desorption from Mesoporous Silica by RP-UHPLC. Molecules, 24(7), 1317. https://doi.org/10.3390/molecules24071317








