Design, Synthesis, and Biological Activities of Novel 1,3,5-Trimethylpyrazole-Containing Malonamide Derivatives
Abstract
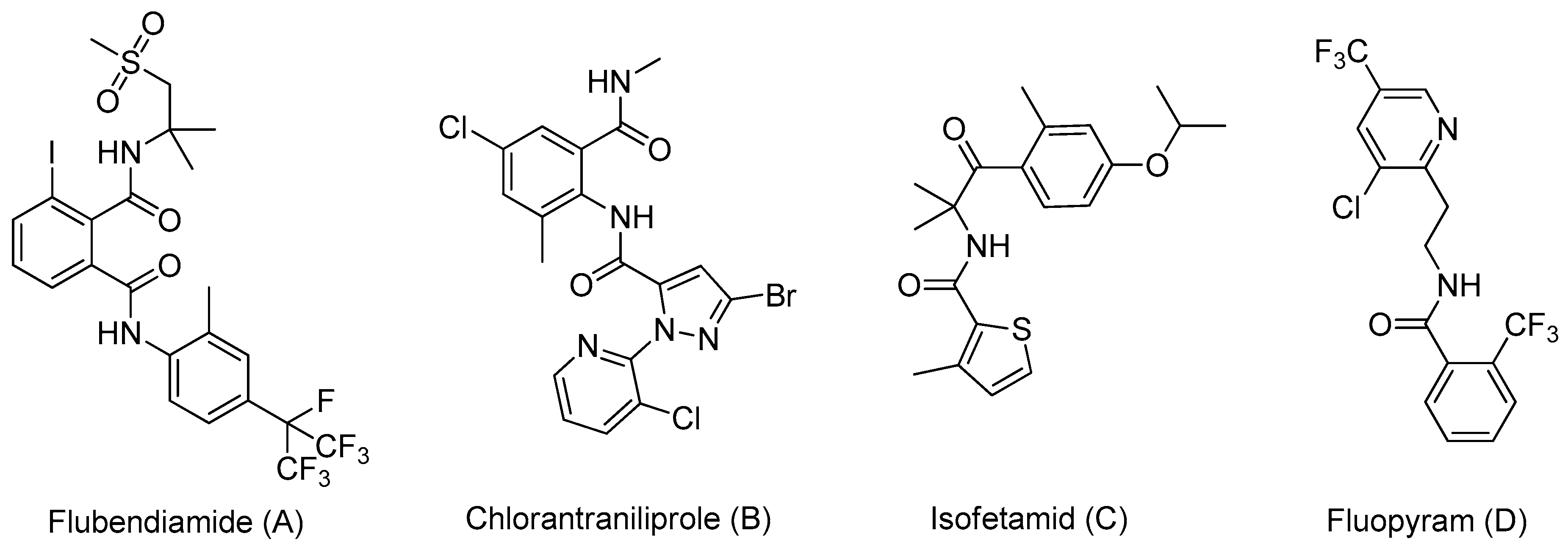
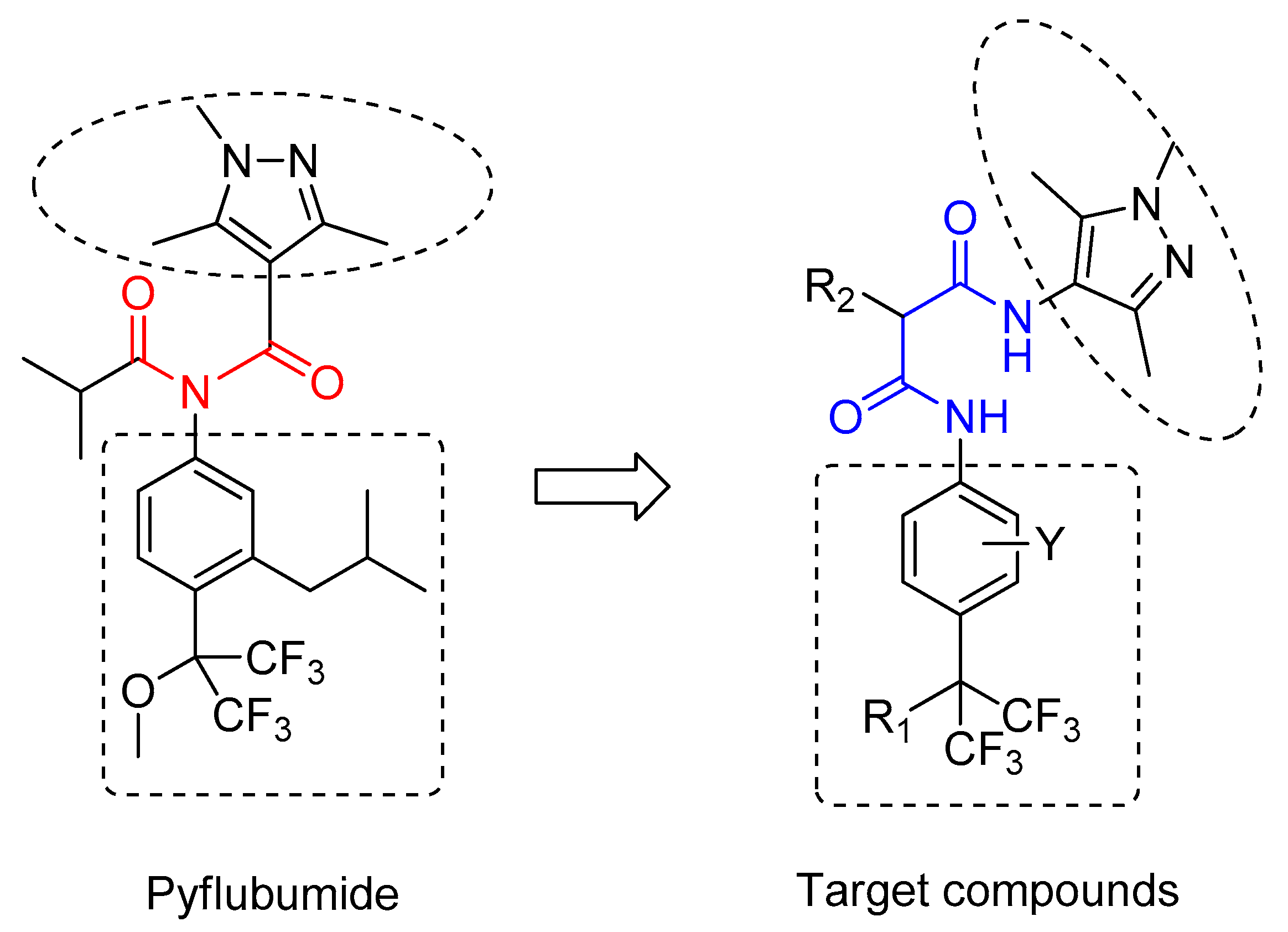
1. Introduction
2. Results and Discussion
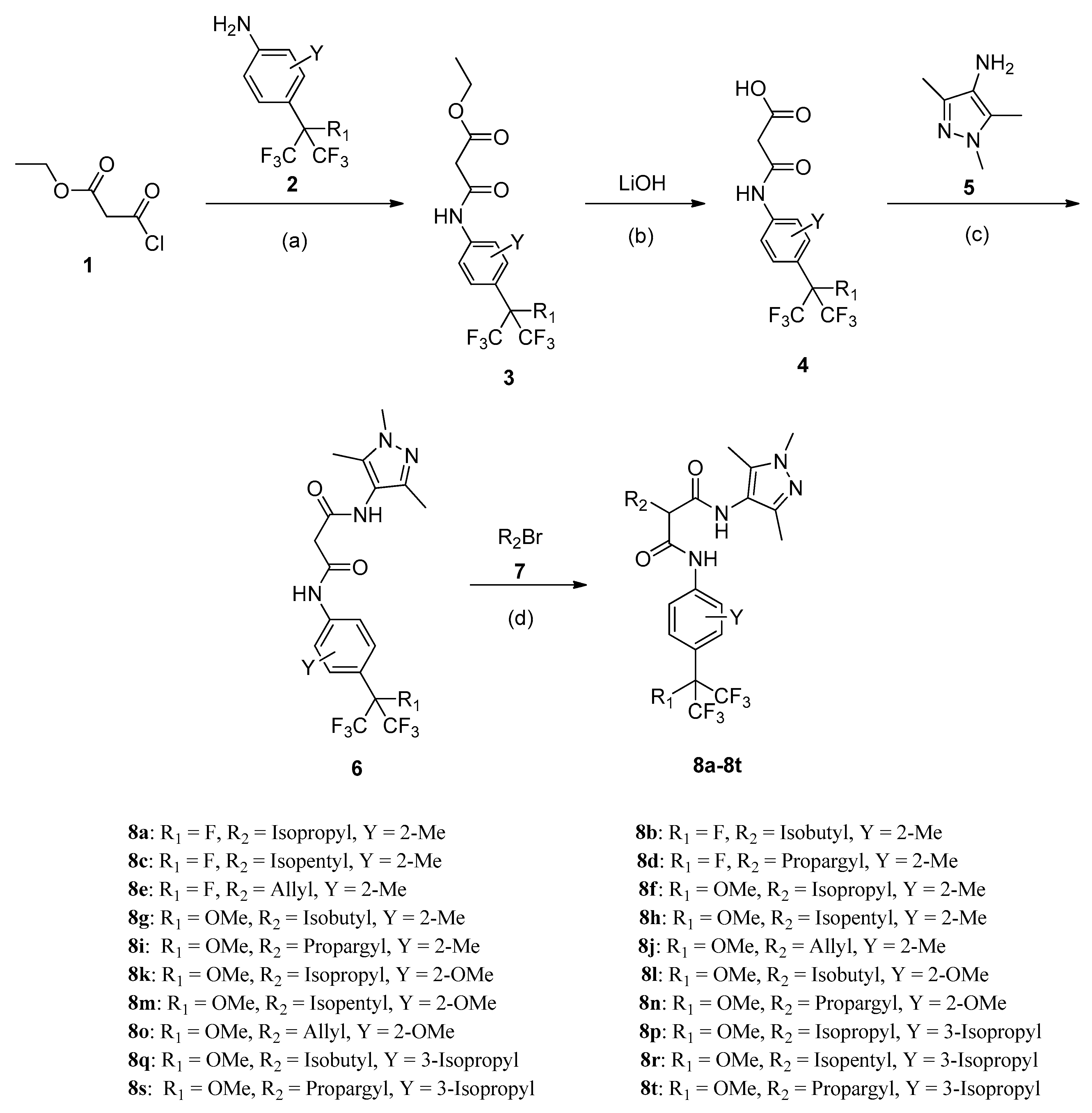
2.1. Synthesis
2.2. Biological Activity
3. Experimental
3.1. Chemicals and Instrumentation
3.2. Synthesis Procedures
3.2.1. General synthesis Procedure for Intermediate 3
3.2.2. General Synthesis Procedure for Intermediate 4
3.2.3. General Synthesis Procedure for Intermediate 6
3.2.4. General Synthesis Procedure for the Target Compounds 8a–8t
3.3. Bioassay Methods
3.3.1. Acaricidal Activity Against Tetranychus Cinnabarinus
3.3.2. Insecticidal Activity Against Plutella xylostella
3.3.3. Insecticidal Activity Against Aphis craccivora
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Xiao, N.W.; Jing, B.; Ge, F.; Liu, X.H. The fate of herbicide acetochlor and its toxicity to Eisenia fetida under laboratory conditions. Chemosphere 2006, 62, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Abigail, M.E.A.; Samuel, S.M.; Ramalingam, C. Addressing the environmental impacts of butachlor and the available remediation strategies: A systematic review. Int. J. Environ. Sci. Technol. 2015, 12, 4025–4036. [Google Scholar] [CrossRef]
- Avenot, H.F.; Michailides, T.J. Resistance to boscalid fungicide in Alternaria alternata isolates from pistachio in California. Plant Dis. 2007, 91, 1345–1350. [Google Scholar] [CrossRef]
- Avenot, H.F.; Michailides, T.J. Progress in understanding molecular mechanisms and evolution of resistance to succinate dehydrogenase inhibiting (SDHI) fungicides in phytopathogenic fungi. Crop Prot. 2010, 29, 643–651. [Google Scholar] [CrossRef]
- Sharma, A.K.; Singh, D.P.; Kumar, J.; Singh, A.; Tewari, A.N.; Singh, K.P.; Karwasra, S.S.; Grewal, A.S. Efficacy of thifluzamide in the control of loose smut of wheat (Triticum aestivum) caused by Ustilago segetum. Indian J. Agric. Sci. 2001, 71, 648–649. [Google Scholar]
- Illicachi, L.A.; Montalvo-Acosta, J.J.; Insuasty, A.; Quiroga, J.; Abonia, R.; Sortino, M.; Zacchino, S.; Insuasty, B. Synthesis and DFT calculations of novel Vanillin-Chalcones and their 3-aryl-5-(4-(2-(dimethylamino)-ethoxy)-3-methoxyphenyl)-4,5-dihydro-1H-pyrazole-1-carbaldehyde derivatives as antifungal agents. Molecules 2017, 22, 1476. [Google Scholar] [CrossRef] [PubMed]
- Nossier, E.S.; Fahmy, H.H.; Khalifa, N.M.; El-Eraky, W.I.; Baset, M.A. Design and synthesis of novel pyrazole-substituted different nitrogenous heterocyclic ring systems as potential anti-inflammatory agents. Molecules 2017, 22, 512. [Google Scholar] [CrossRef]
- Fahmy, H.H.; Khalifa, N.M.; Ismail, M.M.F.; El-Sahrawy, H.M.; Nossier, E.S. Biological validation of novel polysubstituted pyrazole candidates with in vitro anticancer activities. Molecules 2016, 21, 271. [Google Scholar] [CrossRef]
- Colliot, F.; Kukorowski, K.A.; Hawkins, D.W.; Roberts, D.A. Fipronil: A new soil and foliar broad spectrum insecticide. In Proceedings of the Brighton Crop Protection Conference, Pests and Diseases, Brighton, UK, 23–26 November 1992. [Google Scholar]
- Vicentini, C.B.; Romagnoli, C.; Andreotti, E.; Mares, D. Synthetic pyrazole derivatives as growth inhibitors of some phytopathogenic fungi. J. Agric. Food Chem. 2007, 55, 10331–10338. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Boger, P. Target sites for herbicides: Entering the 21st century. Pest Manag. Sci. 2002, 58, 1149–1154. [Google Scholar] [CrossRef]
- Lein, W.; Bornke, F.; Reindl, A.; Ehrhardt, T.; Stitt, M.; Sonnewald, U. Target-based discovery of novel herbicides. Curr. Opin. Plant Biol. 2004, 7, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.D. Synthesis and QSAR of herbicidal 3-pyrazolyl alpha,alpha,alpha-trifluorotolyl ethers. J. Agric. Food Chem. 1996, 44, 3643–3652. [Google Scholar] [CrossRef]
- Casida, J.E. Golden age of RyR and GABA-R diamide and isoxazoline insecticides: Common genesis, serendipity, surprises, selectivity, and safety. Chem. Res. Toxicol. 2015, 28, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.C.; Xiong, L.X.; Luo, M.; Wang, J.; Hu, C.Y.; Zhang, X.; Yu, S.J.; Li, Y.H.; Sun, D.Q. Synthesis, larvicidal activities and antifungal activities of novel chlorantraniliprole derivatives and their target in the ryanodine receptor. Molecules 2015, 20, 3854–3867. [Google Scholar] [CrossRef] [PubMed]
- Furuya, T.; Machiya, K.; Fujioka, S.; Nakano, M.; Inagaki, K. Development of a novel acaricide, pyflubumide. J. Pestic. Sci. 2017, 42, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Yasokawa, N.; Suwa, A.; Fujioka, S.; Furuya, T.; Sakata, K. Mode of action of novel acaricide pyflubumide: Effects on the mitochondrial respiratory chain. J. Pestic. Sci. 2015, 40, 19–24. [Google Scholar] [CrossRef]
- Tohnishi, M.; Nakao, H.; Furuya, T.; Seo, A.; Kodama, H.; Tsubata, K.; Fujioka, S.; Kodama, H.; Hirooka, T.; Nishimatsu, T. Flubendiamide, a novel insecticide highly active against lepidopterous insect pests. J. Pestic. Sci. 2005, 30, 354–360. [Google Scholar] [CrossRef]
- Masaki, T.; Yasokawa, N.; Tohnishi, M.; Nishimatsu, T.; Tsubata, K.; Inoue, K.; Motoba, K.; Hirooka, T. Flubendiamide, a novel Ca2+ channel modulator, reveals evidence for functional cooperation between Ca2+ pumps and Ca2+ release. Mol. Pharmacol. 2006, 69, 1733–1739. [Google Scholar] [CrossRef] [PubMed]
- Lahm, G.P.; Selby, T.P.; Freudenberger, J.H.; Stevenson, T.M.; Myers, B.J.; Seburyamo, G.; Smith, B.K.; Flexner, L.; Clark, C.E.; Cordova, D. Insecticidal anthranilic diamides: A new class of potent ryanodine receptor activators. Bioorg. Med. Chem. Lett. 2005, 15, 4898–4906. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, T.F.; Panne, U.; Koch, M. New photodegradation products of the fungicide fluopyram: Structural elucidation and mechanism identification. Molecules 2018, 23, 2940. [Google Scholar] [CrossRef] [PubMed]
- Piqueras, C.M.; Latorre, B.A.; Torres, R. Effectiveness of isofetamid, a new succinate dehydrogenase inhibitor fungicide, in the control of grapevine gray mold. Ciencia e Investigación Agraria 2014, 41, 365–374. [Google Scholar] [CrossRef]
- Furuya, T.; Suwa, A.; Nakano, M.; Fujioka, S.; Yasokawa, N.; Machiya, K. Synthesis and biological activity of a novel acaricide, pyflubumide. J. Pestic. Sci. 2015, 40, 38–43. [Google Scholar] [CrossRef]
- Limanto, J.; Krska, S.W.; Dorner, B.T.; Vazquez, E.; Yoshikawa, N.; Tan, L. Dynamic kinetic resolution: Asymmetric transfer hydrogenation of alpha-alkyl-substituted beta-ketoamides. Org. Lett. 2010, 12, 512–515. [Google Scholar] [CrossRef] [PubMed]
- Masanobu, O.; Oona, Y.A.; Eiji, K.; Kenji, T. A Process for Producing Perfluoroalkylaniline Derivatives. EP Patent 1006102 A2, 7 June 2000. [Google Scholar]
- Noboru, A.; Osamu, S. Preparation of Aniline Derivatives. JP Patent 2012067060A, 5 April 2012. [Google Scholar]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Luo, J.X.; Lai, T.; Guo, T.; Chen, F.; Zhang, L.L.; Ding, W.; Zhang, Y.Q. Synthesis and acaricidal activities of scopoletin phenolic ether derivatives: QSAR, molecular docking study and in silico ADME predictions. Molecules 2018, 23, 995. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Kang, S.H.; Yuan, Q.K.; Luo, L.J.; Ma, J.; Shi, Q.C.; Yang, S. N-substituted 5-chloro-6-phenylpyridazin-3(2H)-ones: Synthesis, insecticidal activity against Plutella xylostella (L.) and SAR study. Molecules 2012, 17, 9413–9420. [Google Scholar] [CrossRef]
- Liu, X.Q.; Liu, Y.Q.; Shao, X.S.; Xu, Z.P.; Xu, X.Y.; Li, Z. Synthesis and insecticidal evaluation of tetrahydroimidazo 1,2-a pyridin-5(1H)-one derivatives. Chin. Chem. Lett. 2016, 27, 7–10. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 8a–8t are available from the authors. |
| Compound | Mortality (%) a | LC50 (µg/mL) | 95% CL (µg/mL) | ||||
|---|---|---|---|---|---|---|---|
| 400 µg/mL | 200 µg/mL | 100 µg/mL | 50 µg/mL | 20 µg/mL | |||
| 8a | 90.0 ± 3.3 b | 60.0 ± 5.8 | 24.4 ± 1.9 | 11.1 ± 1.9 | 0.0 ± 0.0 | 157.2 | 127.5–197.0 |
| 8b | 85.6 ± 1.9 | 42.2 ± 1.9 | 20.0 ± 0.0 | 7.8 ± 1.9 | 0.0 ± 0.0 | 197.1 | 158.7–254.9 |
| 8c | 60.0 ± 3.3 | 35.6 ± 1.9 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 308.8 | 254.2–405.3 |
| 8d | 61.1 ± 3.8 | 21.1 ± 1.9 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 333.1 | 278.5–431.0 |
| 8e | 31.1 ± 1.9 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 540.4 | 414.6–1988.6 |
| 8f | 68.9 ± 3.8 | 31.1 ± 1.9 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 290.7 | 244.6–360.1 |
| 8g | 51.1 ± 3.8 | 20.0 ± 3.3 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 376.7 | 305.6–544.9 |
| 8h | 78.9 ± 3.8 | 41.1 ± 1.9 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 250.7 | 212.9–298.7 |
| 8i | 100.0 ± 0.0 | 64.4 ± 1.9 | 30.0 ± 3.3 | 17.8 ± 1.9 | 7.8 ± 1.9 | 119.2 | 52.0–343.6 |
| 8j | 90.0 ± 3.3 | 54.4 ± 1.9 | 20.0 ± 3.3 | 7.8 ± 1.9 | 0.0 ± 0.0 | 172.2 | 140.8–214.8 |
| 8k | 100.0 ± 0.0 | 60.0 ± 3.3 | 20.0 ± 0.0 | 8.9 ± 1.9 | 0.0 ± 0.0 | 149.5 | 124.8–180.9 |
| 8l | 83.3 ± 3.3 | 51.1 ± 1.9 | 20.0 ± 5.8 | 5.6 ± 1.9 | 0.0 ± 0.0 | 190.9 | 154.8–242.8 |
| 8m | 90.0 ± 0.0 | 70.0 ± 3.3 | 35.6 ± 1.9 | 27.8 ± 1.9 | 13.3 ± 3.3 | 107.2 | 79.9–145.5 |
| 8n | 100.0 ± 0.0 | 50.0 ± 3.3 | 20.0 ± 3.3 | 10.0 ± 0.0 | 0.0 ± 0.0 | 158.9 | 84.2–379.2 |
| 8o | 80.0 ± 3.3 | 40.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 250.3 | 212.9–297.1 |
| 8p | 100.0 ± 0.0 | 70.0 ± 3.3 | 37.8 ± 1.9 | 15.6 ± 1.9 | 5.6 ± 1.9 | 112.2 | 90.2–140.4 |
| 8q | 100.0 ± 0.0 | 50.0 ± 3.3 | 20.0 ± 0.0 | 5.6 ± 1.9 | 0.0 ± 0.0 | 164.0 | 103.3–288.0 |
| 8r | 100.0 ± 0.0 | 60.0 ± 0.0 | 25.6 ± 1.9 | 12.2 ± 1.9 | 7.8 ± 1.9 | 132.3 | 39.7–1172.9 |
| 8s | 85.6 ± 1.9 | 43.3 ± 3.3 | 20.0 ± 3.3 | 8.9 ± 1.9 | 0.0 ± 0.0 | 194.2 | 156.0–252.1 |
| 8t | 38.9 ± 1.9 | 18.9 ± 3.8 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 460.3 | 348.5–879.2 |
| FEN | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | — | — |
| Compound | Mortality (%) a | LC50 (µg/mL) | 95% CL (µg/mL) | ||||
|---|---|---|---|---|---|---|---|
| 400 µg/mL | 200 µg/mL | 100 µg/mL | 50 µg/mL | 20 µg/mL | |||
| 8a | 80.0 ± 10.0 b | 60.0 ± 0.0 | 30.0 ± 10.0 | 16.7 ± 5.8 | 10.0 ± 0.0 | 153.2 | 89.8–333.2 |
| 8b | 53.3 ± 5.8 | 30.0 ± 0.0 | 16.7 ± 5.8 | 6.7 ± 5.8 | 0.0 ± 0.0 | 359.0 | 204.7–2371.2 |
| 8c | 63.3 ± 5.8 | 36.7 ± 5.8 | 10.0 ± 0.0 | 6.7 ± 5.8 | 0.0 ± 0.0 | 289.3 | 183.8–808.9 |
| 8d | 90.0 ± 0.0 | 73.3 ± 5.8 | 30.0 ± 0.0 | 23.3 ± 5.8 | 16.7 ± 5.8 | 109.5 | 63.0–204.2 |
| 8e | 100.0 ± 0.0 | 76.7 ± 5.8 | 33.3 ± 5.8 | 26.7 ± 5.8 | 13.3 ± 5.8 | 94.5 | 58.7–153.6 |
| 8f | 20.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | — | — |
| 8g | 100.0 ± 0.0 | 100.0 ± 0.0 | 43.3 ± 5.8 | 20.0 ± 0.0 | 6.7 ± 5.8 | 82.6 | 57.2–119.3 |
| 8h | 86.7 ± 5.8 | 60.0 ± 0.0 | 30.0 ± 10.0 | 13.3 ± 5.8 | 0.0 ± 0.0 | 154.0 | 102.2–250.5 |
| 8i | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 83.3 ± 5.8 | 46.7 ± 5.8 | 22.1 | 6.6–33.3 |
| 8j | 70.0 ± 0.0 | 33.3 ± 5.8 | 20.0 ± 0.0 | 6.7 ± 5.8 | 0.0 ± 0.0 | 257.3 | 164.4–636.1 |
| 8k | 60.0 ± 0.0 | 50.0 ± 0.0 | 30.0 ± 0.0 | 16.7 ± 5.8 | 6.7 ± 5.8 | 235.4 | 124.0–1322.9 |
| 8l | 70.0 ± 0.0 | 46.7 ± 5.8 | 26.7 ± 5.8 | 10.0 ± 0.0 | 0.0 ± 0.0 | 93.3 | 57.9–149.3 |
| 8m | 63.3 ± 5.8 | 30.0 ± 10.0 | 20.0 ± 0.0 | 6.7 ± 5.8 | 0.0 ± 0.0 | 294.2 | 179.9–1000.9 |
| 8n | 100.0 ± 0.0 | 66.7 ± 5.8 | 40.0 ± 0.0 | 23.3 ± 5.8 | 13.3 ± 5.8 | 99.9 | 61.2–166.9 |
| 8o | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 76.7 ± 5.8 | 43.3 ± 5.8 | 24.2 | 8.9–36.5 |
| 8p | 30.0 ± 0.0 | 10.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 569.8 | 405.6–2013.3 |
| 8q | 76.7 ± 5.8 | 40.0 ± 0.0 | 20.0 ± 0.0 | 10.0 ± 0.0 | 0.0 ± 0.0 | 220.0 | 142.9–454.3 |
| 8r | 56.7 ± 5.8 | 26.7 ± 5.8 | 10.0 ± 0.0 | 6.7 ± 5.8 | 0.0 ± 0.0 | 359.0 | 213.2–1857.2 |
| 8s | 90.0 ± 0.0 | 56.7 ± 5.8 | 26.7 ± 5.8 | 13.3 ± 5.8 | 6.7 ± 5.8 | 149.9 | 95.6–268.6 |
| 8t | 30.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | — | — |
| FLU | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ±0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | — | — |
| Compound | Mortality (%) a | LC50 (µg/mL) | 95% CL (µg/mL) | ||||
|---|---|---|---|---|---|---|---|
| 200 µg/mL | 100 µg/mL | 50 µg/mL | 20 µg/mL | 10 µg/mL | |||
| 8a | 100.0 ± 0.0 b | 100.0 ± 0.0 | 80.0 ± 0.0 | 56.7 ± 2.9 | 21.7 ± 2.9 | 19.1 | 13.5–25.4 |
| 8b | 100.0 ± 0.0 | 100.0 ± 0.0 | 70.0 ± 0.0 | 43.3 ± 2.9 | 20.0 ± 5.0 | 23.3 | 16.8–31.1 |
| 8c | 100.0 ± 0.0 | 90.0 ± 0.0 | 65.0 ± 0.0 | 36.7 ± 2.9 | 15.0 ± 0.0 | 29.0 | 20.8–39.1 |
| 8d | 71.7 ± 2.9 | 45.0 ± 0.0 | 20.0 ± 0.0 | 13.3 ± 2.9 | 8.3 ± 2.9 | 111.7 | 73.0–224.0 |
| 8e | 40.0 ± 0.0 | 20.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 225.3 | 162.9–612.0 |
| 8f | 100.0 ± 0.0 | 81.7 ± 2.9 | 58.3 ± 2.9 | 31.7 ± 2.9 | 18.3 ± 2.9 | 32.6 | 22.7–45.1 |
| 8g | 90.0 ± 0.0 | 70.0 ± 0.0 | 50.0 ± 5.0 | 26.7 ± 2.9 | 11.7 ± 2.9 | 46.6 | 32.0–68.0 |
| 8h | 80.0 ± 5.0 | 50.0 ± 5.0 | 30.0 ± 0.0 | 13.3 ± 2.9 | 8.3 ± 2.9 | 85.0 | 58.3–141.1 |
| 8i | 100.0 ± 0.0 | 75.0 ± 5.0 | 50.0 ± 0.0 | 28.3 ± 5.8 | 16.7 ± 2.9 | 38.3 | 26.8–53.7 |
| 8j | 100.0 ± 0.0 | 91.7 ± 2.9 | 60.0 ± 5.0 | 36.7 ± 2.9 | 21.7 ± 2.9 | 27.6 | 19.2–37.9 |
| 8k | 100.0 ± 0.0 | 100.0 ± 0.0 | 76.7 ± 2.9 | 41.7 ± 2.9 | 25.0 ± 0.0 | 21.4 | 15.2–28.5 |
| 8l | 71.7 ± 2.9 | 50.0 ± 5.0 | 40.0 ± 0.0 | 26.7 ± 2.9 | 13.3 ± 2.9 | 79.2 | 47.6–173.5 |
| 8m | 100.0 ± 0.0 | 80.0 ± 0.0 | 50.0 ± 5.0 | 30.0 ± 5.0 | 18.3 ± 2.9 | 35.7 | 24.9–49.8 |
| 8n | 90.0 ± 0.0 | 68.3 ± 2.9 | 50.0 ± 5.0 | 30.0 ± 0.0 | 18.3 ± 2.9 | 42.9 | 27.8–65.3 |
| 8o | 75.0 ± 0.0 | 50.0 ± 5.0 | 30.0 ± 0.0 | 18.3 ± 2.9 | 10.0 ± 0.0 | 88.4 | 57.7–166.1 |
| 8p | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 88.3 ± 2.9 | 63.3 ± 2.9 | 8.1 | 1.8–11.3 |
| 8q | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 83.3 ± 2.9 | 63.3 ± 2.9 | 8.1 | 2.1–11.6 |
| 8r | 100.0 ± 0.0 | 70.0 ± 5.0 | 60.0 ± 0.0 | 43.3 ± 2.9 | 20.0 ± 5.0 | 30.9 | 19.7–44.9 |
| 8s | 90.0 ± 5.0 | 65.0 ± 5.0 | 45.0 ± 0.0 | 25.0 ± 0.0 | 13.3 ± 2.9 | 50.7 | 34.5–76.0 |
| 8t | 50.0 ± 5.0 | 30.0 ± 5.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 181.2 | 137.8–320.3 |
| IMI | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | 100.0 ± 0.0 | — | — |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.-B.; Liao, M.; Liu, Q.; Feng, T.; Xu, Z.-Y.; Rui, C.-H.; Liu, S.-Z. Design, Synthesis, and Biological Activities of Novel 1,3,5-Trimethylpyrazole-Containing Malonamide Derivatives. Molecules 2019, 24, 562. https://doi.org/10.3390/molecules24030562
Li Q-B, Liao M, Liu Q, Feng T, Xu Z-Y, Rui C-H, Liu S-Z. Design, Synthesis, and Biological Activities of Novel 1,3,5-Trimethylpyrazole-Containing Malonamide Derivatives. Molecules. 2019; 24(3):562. https://doi.org/10.3390/molecules24030562
Chicago/Turabian StyleLi, Qi-Bo, Min Liao, Qing Liu, Tong Feng, Zhi-Yuan Xu, Chang-Hui Rui, and Shang-Zhong Liu. 2019. "Design, Synthesis, and Biological Activities of Novel 1,3,5-Trimethylpyrazole-Containing Malonamide Derivatives" Molecules 24, no. 3: 562. https://doi.org/10.3390/molecules24030562
APA StyleLi, Q.-B., Liao, M., Liu, Q., Feng, T., Xu, Z.-Y., Rui, C.-H., & Liu, S.-Z. (2019). Design, Synthesis, and Biological Activities of Novel 1,3,5-Trimethylpyrazole-Containing Malonamide Derivatives. Molecules, 24(3), 562. https://doi.org/10.3390/molecules24030562





