Chemical Diversity of Headspace and Volatile Oil Composition of Two Brown Algae (Taonia atomaria and Padina pavonica) from the Adriatic Sea
Abstract
1. Introduction
2. Results and Discussion
2.1. Headspace and Volatile Oil Composition of T. atomaria
2.2. Headspace and Volatile Oil Composition of P. pavonica
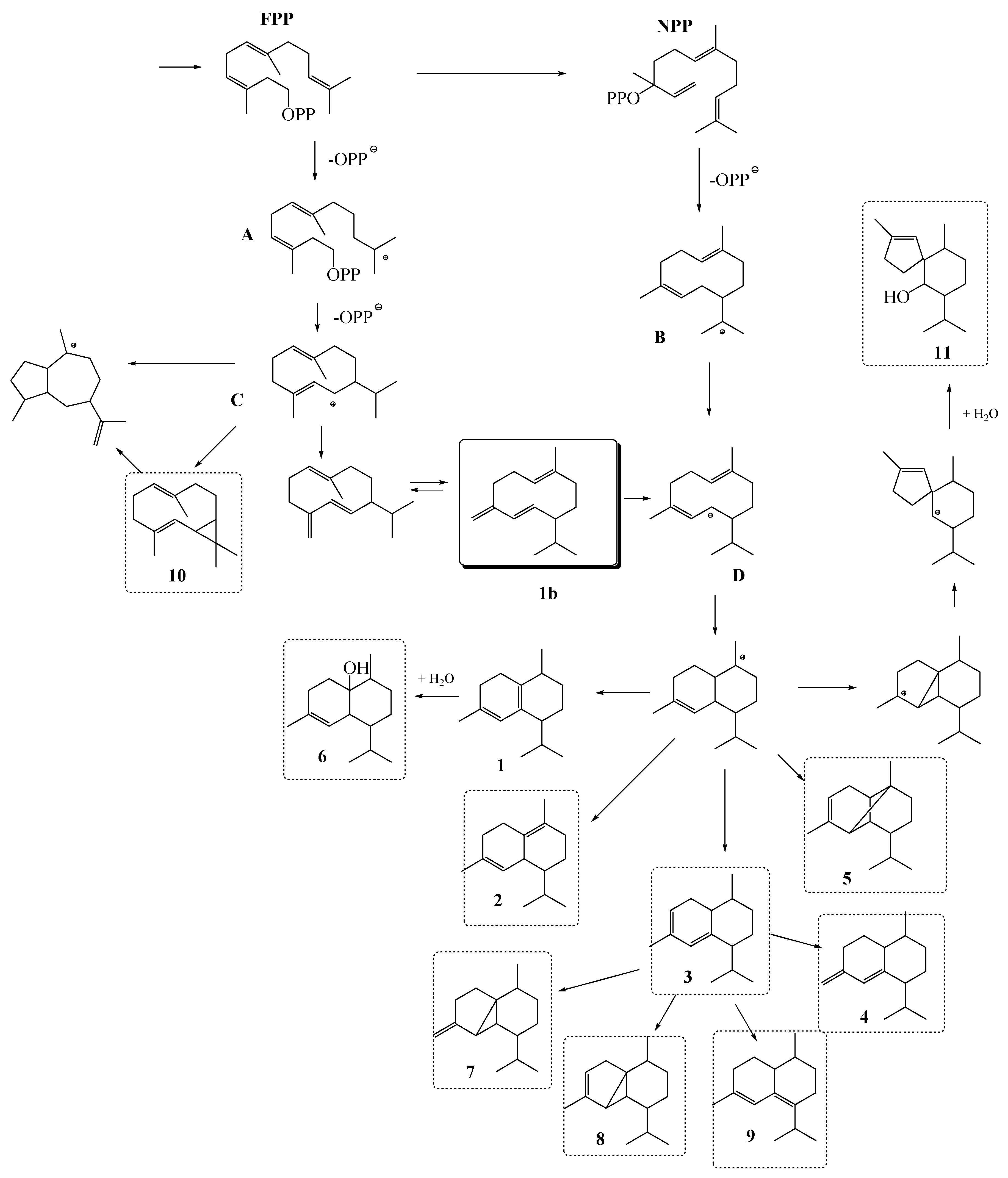
2.3. Possible Biosynthetic Origin of the Major Identified VOCs
3. Materials and Methods
3.1. Headspace Solid-Phase Microextraction (HS-SPME)
3.2. Hydrodistillation (HD)
3.3. Gas Chromatography and Mass Spectrometry (GC-MS) Analyses
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shanura Fernando, L.P.; Nah, J.; Jeon, Y. Potential anti-inflammatory natural products from marine algae. Environ. Toxicol. Pharmacol. 2016, 48, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.G.; Darias, J.; Martin, J.D. Taondiol, a new component from Taonia atomaria. Tetrahedron Lett. 1971, 12, 2729–2732. [Google Scholar] [CrossRef]
- Gonzalez, A.G.; Darias, J.; Martin, J.D.; Norte, M. Atomaric acid, a new component from Taonia atomaria. Tetrahedron Lett 1974, 15, 3951–3954. [Google Scholar] [CrossRef]
- Gonzalez, A.G.; Martin, I.D.; Perez, C.; Rovirosa, J.; Tagle, P.B.; Clardy, J. Isolation and x-ray structural determination of three new diterpenoids from the marine alga Taonia atomaria. Chem. Lett. 1984, 13, 1649–1652. [Google Scholar] [CrossRef]
- De Rosa, S.; De Giulio, A.; Iodice, C.; Zavodink, N. Sesquiterpenes from the brown alga Taonia atomaria. Phytochemistry 1994, 37, 1327–1330. [Google Scholar] [CrossRef]
- Abatis, D.; Vagias, C.; Galanakis, D.; Norris, J.N.; Moreau, D.; Roussakis, C.; Roussis, V. Atomarianones A and B: Two cytotoxic meroditerpenes from the brown alga Taonia atomaria. Tetrahedron Lett. 2005, 46, 8525–8529. [Google Scholar] [CrossRef]
- Tziveleka, L.-A.; Abatis, D.; Paulus, K.; Bauer, R.; Vagiasa, C.; Roussis, V. Marine polyprenylated hydroquinones, quinones, and chromenols with inhibitory effects on leukotriene formation. Chem. Biodivers. 2005, 2, 901–909. [Google Scholar] [CrossRef]
- El Baz, F.K.; El Baroty, G.S.; Abd El Baky, H.H.; Abd El-Salam, O.I.; Ibrahim, E.A. Structural characterization and biological activity of sulfolipids from selected marine algae. Grasas Aceites 2013, 64, 561–571. [Google Scholar]
- Mayer, A.M.S.; Lehmann, V.K.B. Marine Pharmacology in 1998: Marine compounds with antibacterial, anticoagulant, anti-inflammatory, anthelmintic, antiplatelet, antiprotozoan, and antiviral activities; with actions on the cardiovascular, endocrine, immune, and nervous systems; and other miscellaneous mechanisms of action. Pharmacologist 2000, 42, 62–69. [Google Scholar]
- Jacobs, R.S.; Culver, P.; Langdon, R.; O’Brien, E.T.; White, S. Some pharmacological observations on marine natural products. Tetrahedron 1985, 41, 981–984. [Google Scholar] [CrossRef]
- Wessels, M.; Konig, G.M.; Wright, A.D. A new tyrosine kinase inhibitor from the marine brown alga Stypopodium zonale. J. Nat. Prod. 1999, 62, 927–930. [Google Scholar] [CrossRef] [PubMed]
- Gerwick, W.H.; Fenical, W. Ichthyotoxic and cytotoxic metabolites of the tropical brown alga Stypopodium zonale (Lamouroux) Papenfuss. J. Org. Chem. 1981, 46, 22–27. [Google Scholar] [CrossRef]
- Nahas, R.; Abatis, D.; Anagnostopoulou, M.A.; Kefalas, P.; Vagias, C.; Roussis, V. Radical-scavenging activity of Aegean Sea marine algae. Food Chem. 2007, 102, 577–581. [Google Scholar] [CrossRef]
- Othmani, A.; Briand, J.-F.; Ayé, M.; Molmeret, M.; Culioli, G. Surface metabolites of the brown alga Taonia atomaria have the ability to regulate epibiosis. Biofouling 2016, 32, 801–813. [Google Scholar] [CrossRef] [PubMed]
- Khafaji, A.K. Alginate and laminarin of some brown algae from Red Sea near Jeddah, Saudi Arabia. Pak. J. Bot. 1986, 18, 351–353. [Google Scholar]
- Hegazi, M.M.; Perez-Ruzafa, A.; Almela, L.; Candela, M.-E. Separation and identification of chlorophylls and carotenoids from Caulerpa prolifera, Jania rubens and Padina pavonica by reversed-phase high-performance liquid chromatography. J. Chromatogr. A 1998, 829, 153–159. [Google Scholar] [CrossRef]
- Kamenarska, Z.; Gasic, M.J.; Zlatovic, M.; Rasovic, A.; Sladic, D.; Kljajic, Z.; Stefanov, K.; Seizova, K.; Najdenski, H.; Kujumgiev, A.; et al. Chemical Composition of the Brown Alga Padina pavonia (L.) Gaill. from the Adriatic Sea. Bot. Mar. 2002, 45, 339–345. [Google Scholar] [CrossRef]
- Ibtissam, C.; Hassane, R.; José, M.-L.; Francisco, D.S.J.; Antonio, G.V.J.; Hassan, B.; Mohamed, K. Screening of antibacterial activity in marine green and brown macroalgae from the coast of Morocco. Afr. J. Biotechnol. 2009, 8, 1258–1262. [Google Scholar]
- Sultana, V.; Ehteshamul-Haque, S.; Ara, J.; Athar, M. Comparative efficacy of brown, green and red seaweeds in the control of root infecting fungi and okra. Int. J. Environ. Sci. Technol. 2005, 2, 129–132. [Google Scholar] [CrossRef]
- Khaled, N.; Hiba, M.; Astma, C. Antioxidant and Antifungal activities of Padina pavonica and Sargassum vulgare from the Lebanese Mediterranean Coast. Adv. Environ. Biol. 2012, 6, 42–48. [Google Scholar]
- Ktari, L.; Guyot, M. A cytotoxic oxysterol from the marine alga Padina pavonica (L.) Thivy. J. Appl. Phycol. 1999, 11, 511–513. [Google Scholar] [CrossRef]
- Awad, N.E.; Selim, M.A.; Metawe, H.M.; Matloub, A.A. Cytotoxic xenicane diterpenes from the brown alga Padina pavonia (L.) Gaill. Phytother. Res. 2008, 22, 1610–1613. [Google Scholar] [CrossRef] [PubMed]
- Tringali, C.; Piatelli, M.; Spatafora, C. Sesquiterpenes and geranylgeranylglycerol from the brown algae Taonia lacheana and Taonia atomaria f. ciliata: Their chemotaxonomic significance. Phytochemistry 1995, 40, 827–831. [Google Scholar] [CrossRef]
- El Shoubaky, G.A.; Essam, A.; Salem, E.A. Terpenes and sterols composition of marine brown algae Padina pavonica (Dictyotales) and Hormophysa triquetra (Fucales). Int. J. Pharmacogn. Phytochem. Res. 2014, 6, 894–900. [Google Scholar]
- Stranden, M.; Borg-Karlson, A.-K.; Mustaparta, H. Receptor neuron discrimination of the germacrene D enantiomers in the moth Helicoverpa armigera. Chem. Senses 2002, 27, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Kiran, S.R.; Devi, P.S. Evaluation of mosquitocidal activity of essential oil and sesquiterpenes from leaves of Chloroxylon swietenia DC. Parasitol. Res. 2007, 101, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Bruce, T.J.A.; Birkett, M.A.; Blande, J.; Hooper, A.M.; Martin, J.L.; Khambay, B.; Prosser, I.; Smart, L.E.; Wadhams, L.J. Response of economically important aphids to components of Hemizygia petiolata essential oil. Pest. Manag. Sci. 2005, 61, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Casiglia, S.; Bruno, M.; Bramucci, M.; Quassinti, L.; Lupidi, G.; Fiorini, D.; Maggi, F. Kundmannia sicula (L.) DC: A rich source of germacrene D. J. Essent. Oil Res. 2017, 29, 437–442. [Google Scholar] [CrossRef]
- Setzer, W.N.; Schmidt, J.M.; Noletto, J.A.; Vogler, B. Leaf oil compositions and bioactivities of abaco bush medicines. Pharmacologyonline 2006, 3, 794–802. [Google Scholar]
- Kuiate, J.-R.; Bessìere, J.M.; Amvam Zollo, P.H.; Philibert Kuate, S. Chemical composition and antidermatophytic properties of volatile fractions of hexanic extract from leaves of Cupressus lusitanica Mill. from Cameroon. J. Ethnopharmacol. 2006, 103, 160–165. [Google Scholar] [CrossRef]
- Bozan, B.; Ozek, T.; Kurkcuoglu, M.; Kirimer, N.; Baser, K.; Husnu, C. Analysis of essential oil and headspace volatiles of the flowers of Pelargonium endlicherianum used as an anthelmintic in folk medicine. Planta Med. 1999, 65, 781–782. [Google Scholar] [CrossRef]
- Jerković, I.; Marijanović, Z.; Roje, M.; Kuś, P.M.; Jokić, S.; Čoz-Rakovac, R. Phytochemical study of the headspace volatile organic compounds of fresh algae and seagrass from the Adriatic Sea (single point collection). PLoS ONE 2018, 13, e0196462. [Google Scholar]
- Moore, R.E. Volatile compounds from marine algae. Acc. Chem. Res. 1977, 10, 40–47. [Google Scholar] [CrossRef]
- Jüttner, F. Biologically active compounds released during algal blooms. Int. Ver. Theor. Angew. 1981, 21, 227–230. [Google Scholar] [CrossRef]
- Seymour, J.R.; Simo, R.; Ahmed, T.; Stocker, R. Chemoattraction to dimethylsulfoniopropionate throughout the marine microbial food web. Science 2010, 329, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K. Handbook of Marine Macroalgae: Biotechnology and Applied Phycology; John Wiley & Sons Ltd.: Chichester, UK, 2012. [Google Scholar]
- Le Bideau, F.L.; Kousara, L.; Chen, M.; Lai Wei, L.; Dumas, F. Tricyclic sesquiterpenes from marine origin. Chem. Rev. 2017, 117, 6110–6159. [Google Scholar] [CrossRef] [PubMed]
- Arinoni, D. Stereochemical aspects of sesquiterpene biosynthesis. Pure Appl. Chem. 1975, 4, 219–245. [Google Scholar] [CrossRef]
- Alcolombri, U.; Ben-Dor, S.; Feldmesser, E.; Levin, Y.; Tawfik, D.S.; Vardi, A. Identification of the algal dimethyl sulfide–releasing enzyme: A missing link in the marine sulfur cycle. Science 2015, 348, 1466–1469. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, A. The biogeneration of green odor by green leaves. Phytochemistry 1993, 34, 1201–1218. [Google Scholar] [CrossRef]
- Pohnert, G.; Boland, W. The oxylipin chemistry of attraction and defense in brown algae and diatoms. Nat. Prod. Rep. 2002, 19, 108–122. [Google Scholar]
- Hombeck, M.; Boland, W. Biosynthesis of the algal pheromone fucoserratene by the fresh- water diatom Asterionella formosa (Bacillariophyceae). Tetrahedron 1998, 54, 11033–11042. [Google Scholar] [CrossRef]
- Tanchotikul, U.; Hsieh, T.C.-Y. Volatile flavor components in crayfish waste. J. Food Sci. 1989, 54, 1515–1520. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology (NIST) Chemistry WebBook. NIST Standard Reference Database Number 69. Available online: http://webbook.nist.gov/chemistry/ (accessed on 12 December 2018).
Sample Availability: The samples of the marine algae are available from the authors for limited time. |
| No. | Compound | RI | Area Percentages (%) | ||
|---|---|---|---|---|---|
| HS-SPME (PDMS/DVB) ± SD | HS-SPME (DVB/CAR/PDMS) ± SD | HD ± SD | |||
| 1 | Dimethyl sulfide S | 523 | 0.06 ± 0.01 | 0.02 ± 0.01 | - |
| 2 | p-Xylene S | 870 | - | - | 0.01 ± 0.01 |
| 3 | 2-Ethyl-1-hexyl acetate | 1151 | 0.03 ± 0.01 | 0.03 ± 0.00 | - |
| 4 | Albene | 1160 | - | - | 0.01 ± 0.00 |
| 5 | Bornyl acetate S | 1286 | - | 0.01 ± 0.00 | - |
| 6 | γ-Elemene | 1341 | 0.06 ± 0.01 | 0.09 ± 0.01 | - |
| 7 | α-Cubebene S | 1353 | 2.53 ± 0.06 | 2.48 ± 0.05 | 0.16 ± 0.01 |
| 8 | Cyclosativene S | 1368 | 0.01 ± 0.00 | 0.01 ± 0.00 | - |
| 9 | α-Ylangene | 1374 | 0.02 ± 0.00 | 0.06 ± 0.01 | - |
| 10 | α-Copaene S | 1379 | 0.04 ± 0.01 | 0.12 ± 0.02 | 0.02 ± 0.00 |
| 11 | β-Patchoulene | 1383 | 0.02 ± 0.00 | 0.02 ± 0.01 | - |
| 12 | β-Bourbonene | 1387 | 0.71 ± 0.04 | 0.63 ± 0.02 | 0.49 ± 0.01 |
| 13 | β-Cubebene | 1393 | 12.80 ± 0.53 | 10.74 ± 0.01 | 6.10 ± 0.25 |
| 14 | β-Ylangene | 1421 | 0.50 ± 0.03 | 0.61 ± 0.04 | 0.17 ± 0.01 |
| 15 | β-Copaene | 1432 | 0.31 ± 0.02 | 0.45 ± 0.03 | 0.12 ± 0.01 |
| 16 | trans-α-Bergamotene | 1437 | - | - | 0.01 ± 0.00 |
| 17 | Aromandendrene S | 1442 | 0.01 ± 0.00 | 0.02 ± 0.01 | - |
| 18 | Isogermacrene D | 1447 | 0.10 ± 0.02 | 0.13 ± 0.02 | 0.03 ± 0.01 |
| 19 | Cadina-3,5-diene | 1454 | 2.45 ± 0.07 | 3.60 ± 0.02 | 0.39 ± 0.02 |
| 20 | (E)-β-Farnesene S | 1461 | 0.04 ± 0.00 | - | 0.02 ± 0.00 |
| 21 | cis-Muurola-4(15),5-diene | 1467 | - | - | 0.07 ± 0.01 |
| 22 | α-Muurolene | 1470 | - | - | 0.01 ± 0.00 |
| 23 | Alloaromadendrene S | 1464 | 0.13 ± 0.02 | 0.26 ± 0.03 | 0.19 ± 0.02 |
| 24 | γ-Gurjunene S | 1473 | - | - | 0.11 ± 0.01 |
| 25 | trans-Cadina-1(6),4-diene | 1477 | 1.21 ± 0.02 | 2.39 ± 0.04 | - |
| 26 | α-Amorphene | 1479 | - | - | 0.17 ± 0.02 |
| 27 | Germacrene D S | 1486 | 32.06 ± 2.16 | 27.89 ± 2.00 | 22.24 ± 1.92 |
| 28 | γ-Muurolene | 1487 | - | - | 0.11 ± 0.02 |
| 29 | epi-Bicyclosesquiphellandrene | 1496 | 27.49 ± 2.03 | 25.13 ± 2.20 | 20.83 ± 1.90 |
| 30 | γ-Amorphene | 1495 | - | - | 2.10 ± 0.05 |
| 31 | Bicyclogermacrene | 1498 | 0.87 ± 0.05 | 0.63 ± 0.03 | - |
| 32 | Epizonarene | 1500 | - | 0.66 ± 0.08 | - |
| 33 | α-Muurolene | 1502 | 0.27 ± 0.02 | 0.57 ± 0.06 | 0.33 ± 0.04 |
| 34 | β-Cadinene | 1509 | 0.14 ± 0.01 | 0.22 ± 0.03 | - |
| 35 | Tridecanal S | 1511 | 0.05 ± 0.01 | 0.01 ± 0.00 | 0.08 ± 0.01 |
| 36 | γ-Cadinene | 1517 | 0.40 ± 0.02 | 0.97 ± 0.05 | 2.14 ± 0.09 |
| 37 | Cubebol | 1519 | 1.08 ± 0.02 | 1.10 ± 0.01 | - |
| 38 | δ-Cadinene S | 1527 | 1.13 ± 0.03 | 2.14 ± 0.05 | 1.09 ± 0.02 |
| 39 | Zonarene | 1529 | 2.25 ± 0.05 | 2.53 ± 0.08 | 1.45 ± 0.05 |
| 40 | trans-Cadina-1,4-diene | 1536 | 0.46 ± 0.02 | 0.83 ± 0.04 | 0.24 ± 0.01 |
| 41 | α-Cadinene | 1541 | 0.18 ± 0.01 | 0.49 ± 0.03 | 0.03 ± 0.01 |
| 42 | α-Calacorene | 1546 | 0.01 ± 0.00 | 0.08 ± 0.01 | - |
| 43 | Gleenol | 1590 | 9.68 ± 0.80 | 11.02 ± 0.70 | 15.35 ± 0.95 |
| 44 | Junenol | 1518 | - | - | 0.17 ± 0.02 |
| 45 | Di-epi-1,10-cubenol | 1629 | - | - | 0.54 ± 0.01 |
| 46 | T-Cadinol | 1646 | 0.31 ± 0.05 | 0.39 ± 0.04 | 1.59 ± 0.05 |
| 47 | α-Muurolol (Torreyol) | 1648 | - | - | 0.49 ± 0.05 |
| 48 | δ-Cadinol | 1654 | - | - | 0.25 ± 0.02 |
| 49 | α-Cadinol | 1659 | 0.03 ± 0.00 | 0.01 ± 0.00 | 1.45 ± 0.09 |
| 50 | Pentadecanal | 1714 | 0.03 ± 0.00 | 0.01 ± 0.00 | 0.16 ± 0.01 |
| 51 | 4,10(14)-Cadinadien-8-β-ol * | 1784 | 1.03 ± 0.05 | 0.84 ± 0.08 | 1.94 ± 0.09 |
| 52 | Diisobutyl phthalate S | 1870 | 0.01 ± 0.00 | 0.09 ± 0.01 | 4.23 ± 0.09 |
| 53 | Isocembrol (Thunbergol) | 2040 | - | - | 0.73 ± 0.02 |
| 54 | Pachydictyol A | 2123 | - | - | 3.26 ± 0.06 |
| 55 | Cembra-4,7,11,15-tetraen-3-ol * | 2226 | - | - | 2.46 ± 0.05 |
| No. | Compound | RI | Area Percentages (%) | ||
|---|---|---|---|---|---|
| HS-SPME (PDMS/DVB) ± SD | HS-SPME (DVB/CAR/PDMS) ± SD | HD ± SD | |||
| 1 | Dimethyl sulfide S | 523 | 18.27 ± 1.46 | 26.37 ± 2.10 | - |
| 2 | p-Xylene S | 869 | - | - | 0.06 ± 0.01 |
| 3 | Benzaldehyde S | 970 | 2.48 ± 0.07 | 0.33 ± 0.02 | - |
| 4 | 6-Methylhept-5-en-2-one | 990 | 0.87 ± 0.01 | - | - |
| 5 | Octanal S | 1005 | 9.63 ± 0.91 | 7.98 ± 0.20 | - |
| 6 | 1,8-Cineole S | 1038 | 2.43 ± 0.05 | 0.87 ± 0.04 | - |
| 7 | 2-Ethylhexan-1-ol | 1039 | - | 0.87 ± 0.03 | - |
| 8 | 2,2,6-Trimethylcyclohexanone S | 1041 | 0.68 ± 0.02 | 0.55 ± 0.01 | - |
| 9 | Octan-1-ol S | 1079 | 37.73 ± 2.80 | 38.60 ± 2.71 | - |
| 10 | α-Cumyl alcohol | 1092 | - | 1.71 ± 0.05 | - |
| 11 | Dictyopterene A | 1119 | 1.27 ± 0.02 | 0.87 ± 0.03 | - |
| 12 | Dictyopterene D′ | 1157 | 1.15 ± 0.05 | - | - |
| 13 | Berkheyaradulene | 1387 | - | - | 0.18 ± 0.01 |
| 14 | β-Ylangene | 1421 | - | - | 0.10 ± 0.00 |
| 15 | trans-α-Bergamotene | 1439 | - | - | 0.05 ± 0.01 |
| 16 | epi-β-Santalene | 1449 | - | - | 0.16 ± 0.03 |
| 17 | α-Humulene | 1455 | - | - | 0.05 ± 0.01 |
| 18 | (E)-β-Farnesene S | 1460 | 7.92 ± 0.85 | 6.28 ± 0.99 | 0.03 ± 0.01 |
| 19 | β-Santalene | 1462 | - | - | 0.04 ± 0.01 |
| 20 | (E)-β-Guaiene | 1487 | - | - | 0.24 ± 0.02 |
| 21 | epi-Bicyclosesquiphellandrene | 1496 | - | - | 0.04 ± 0.00 |
| 22 | Pentadecane S | 1500 | 4.30 ± 0.10 | 3.56 ± 0.09 | 1.11 ± 0.20 |
| 23 | β-Bisabolene | 1509 | - | - | 0.08 ± 0.00 |
| 24 | α-Farnesene | 1510 | 4.43 ± 0.11 | - | - |
| 25 | cis-Calamenene | 1526 | - | 0.62 ± 0.09 | - |
| 26 | trans-Calamenene | 1528 | - | 0.19 ± 0.02 | - |
| 27 | (E)-α-Bisabolene | 1544 | - | - | 0.01 ± 0.00 |
| 28 | Tetradecanal S | 1613 | - | - | 0.10 ± 0.02 |
| 29 | 3-Oxo-α-ionol | 1655 | - | - | 0.76 ± 0.09 |
| 30 | Tetradecan-1-ol S | 1680 | - | - | 0.26 ± 0.03 |
| 31 | Pentadecanal | 1714 | - | - | 0.13 ± 0.01 |
| 32 | Hexadecan-2-one S | 1772 | - | - | 0.23 ± 0.03 |
| 33 | Pentadecan-1-ol S | 1782 | - | - | 0.10 ± 0.01 |
| 34 | 6,10,14-Trimethylpentadecan-2-one S | 1846 | - | - | 0.10 ± 0.00 |
| 35 | (Z)-Hexadec-11-en-1-ol | 1862 | - | - | 1.15 ± 0.08 |
| 36 | Diisobutyl phthalate S | 1870 | - | 0.24 ± 0.02 | 2.15 ± 0.03 |
| 37 | (E)-Hexadec-11-en-1-ol | 1870 | - | - | 2.58 ± 0.05 |
| 38 | Hexadecan-1-ol S | 1882 | - | - | 17.29 ± 2.50 |
| 39 | Isopachydictol A | 1889 | - | - | 0.15 ± 0.01 |
| 40 | Octadecanal S | 2019 | - | - | 0.09 ± 0.00 |
| 41 | Methyl 5,8,11,14-eicosatetraenoate (Methyl arachidonate) * | 2035 | - | - | 0.66 ± 0.02 |
| 42 | Methyl 5,8,11,14,17-eicosapentaenoate * | 2039 | - | - | 3.98 ± 0.09 |
| 43 | Methyl 8,11,14,17-eicosatetraenoate * | 2045 | - | - | 1.84 ± 0.08 |
| 44 | (Z,Z)-Octadeca-9,12-dien-1-ol | 2051 | - | - | 3.60 ± 0.03 |
| 45 | (Z)-Octadec-9-en-1-ol S | 2058 | - | - | 25.68 ± 2.26 |
| 46. | (Z,Z)-Octadeca-3,13-dien-1-ol * | 2070 | - | - | 7.55 ± 0.09 |
| 47 | trans-Phytol S | 2112 | - | - | 6.45 ± 0.08 |
| 48 | Pachydictol A | 2123 | - | - | 6.03± 0.09 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jerković, I.; Kranjac, M.; Marijanović, Z.; Roje, M.; Jokić, S. Chemical Diversity of Headspace and Volatile Oil Composition of Two Brown Algae (Taonia atomaria and Padina pavonica) from the Adriatic Sea. Molecules 2019, 24, 495. https://doi.org/10.3390/molecules24030495
Jerković I, Kranjac M, Marijanović Z, Roje M, Jokić S. Chemical Diversity of Headspace and Volatile Oil Composition of Two Brown Algae (Taonia atomaria and Padina pavonica) from the Adriatic Sea. Molecules. 2019; 24(3):495. https://doi.org/10.3390/molecules24030495
Chicago/Turabian StyleJerković, Igor, Marina Kranjac, Zvonimir Marijanović, Marin Roje, and Stela Jokić. 2019. "Chemical Diversity of Headspace and Volatile Oil Composition of Two Brown Algae (Taonia atomaria and Padina pavonica) from the Adriatic Sea" Molecules 24, no. 3: 495. https://doi.org/10.3390/molecules24030495
APA StyleJerković, I., Kranjac, M., Marijanović, Z., Roje, M., & Jokić, S. (2019). Chemical Diversity of Headspace and Volatile Oil Composition of Two Brown Algae (Taonia atomaria and Padina pavonica) from the Adriatic Sea. Molecules, 24(3), 495. https://doi.org/10.3390/molecules24030495









