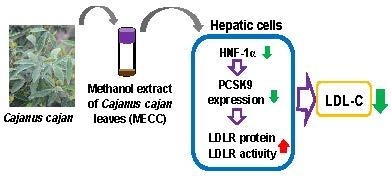
The Cholesterol-Modulating Effect of Methanol Extract of Pigeon Pea (Cajanus cajan (L.) Millsp.) Leaves on Regulating LDLR and PCSK9 Expression in HepG2 Cells
Abstract
1. Introduction
2. Results
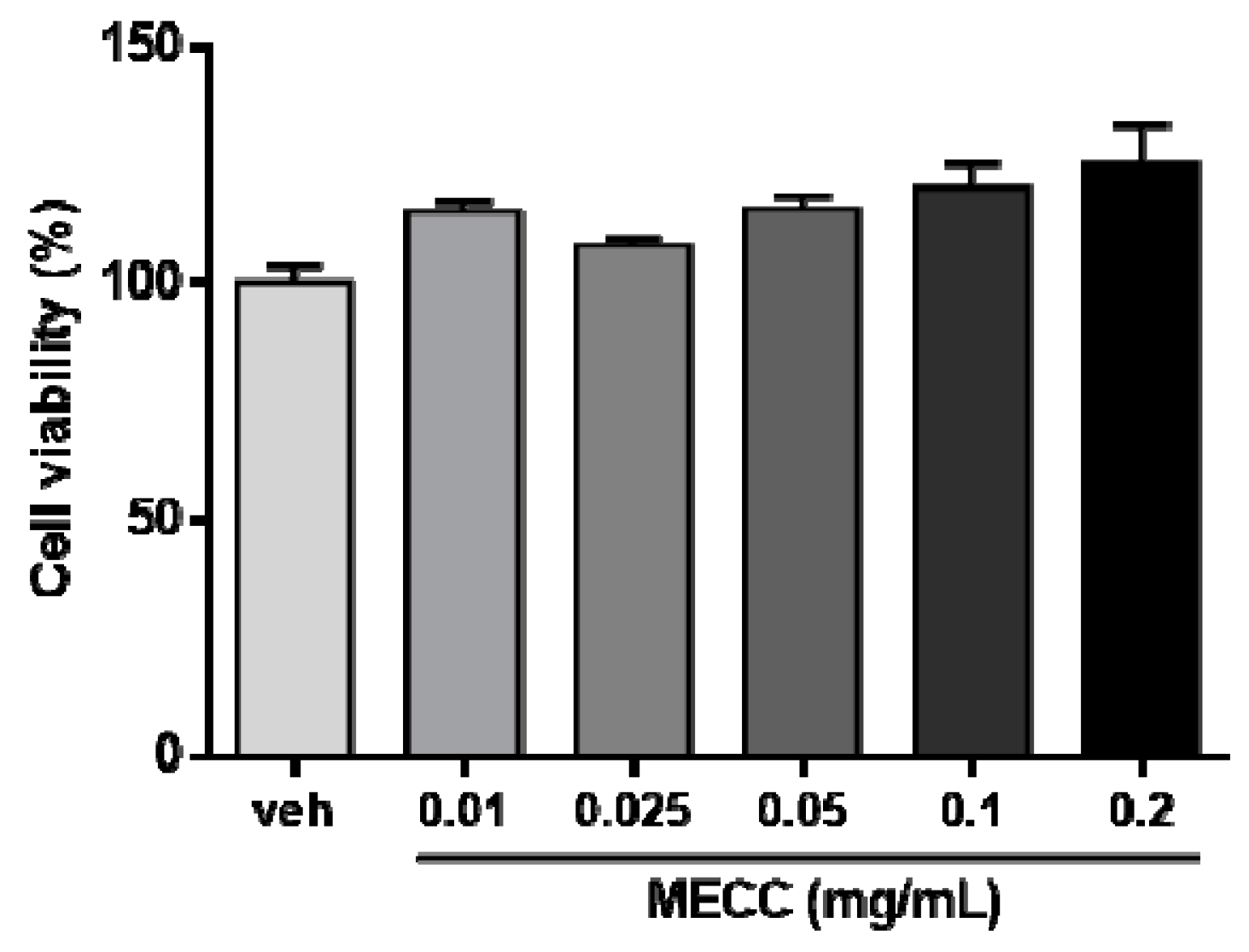
2.1. The Effect of Methanol Extract of Cajanus cajan L. Leaves (MECC) on Cell Viability in HepG2 Cells
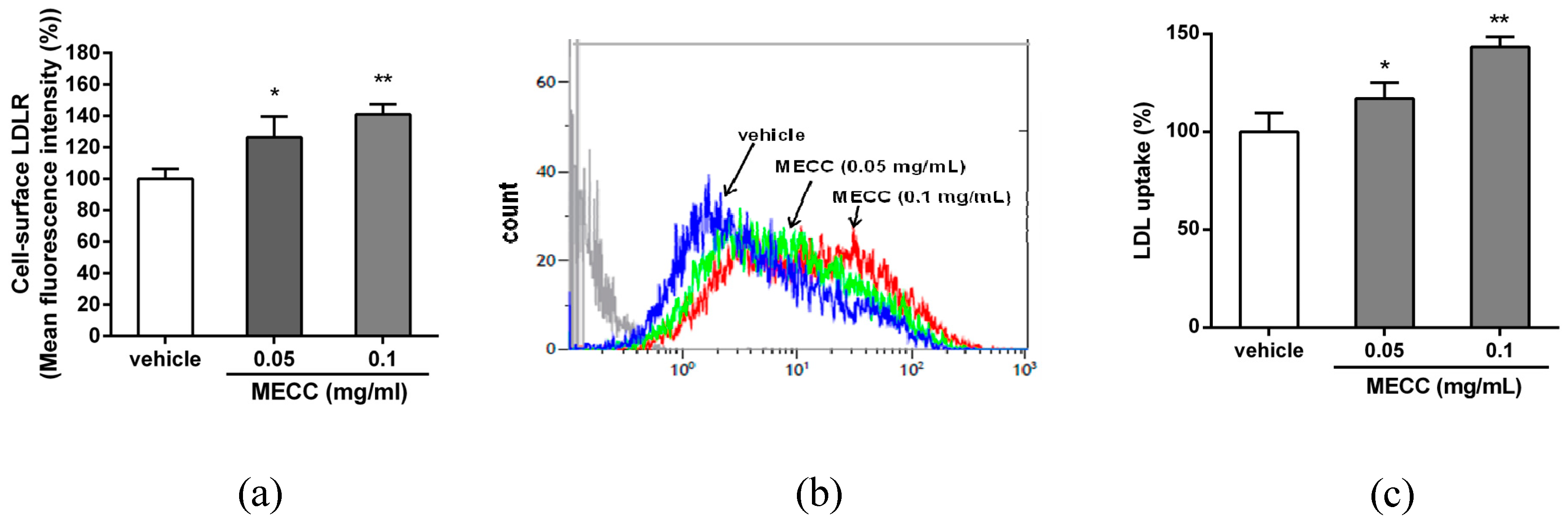
2.2. The Effect of MECC on the LDLR Expression in HepG2 Cells
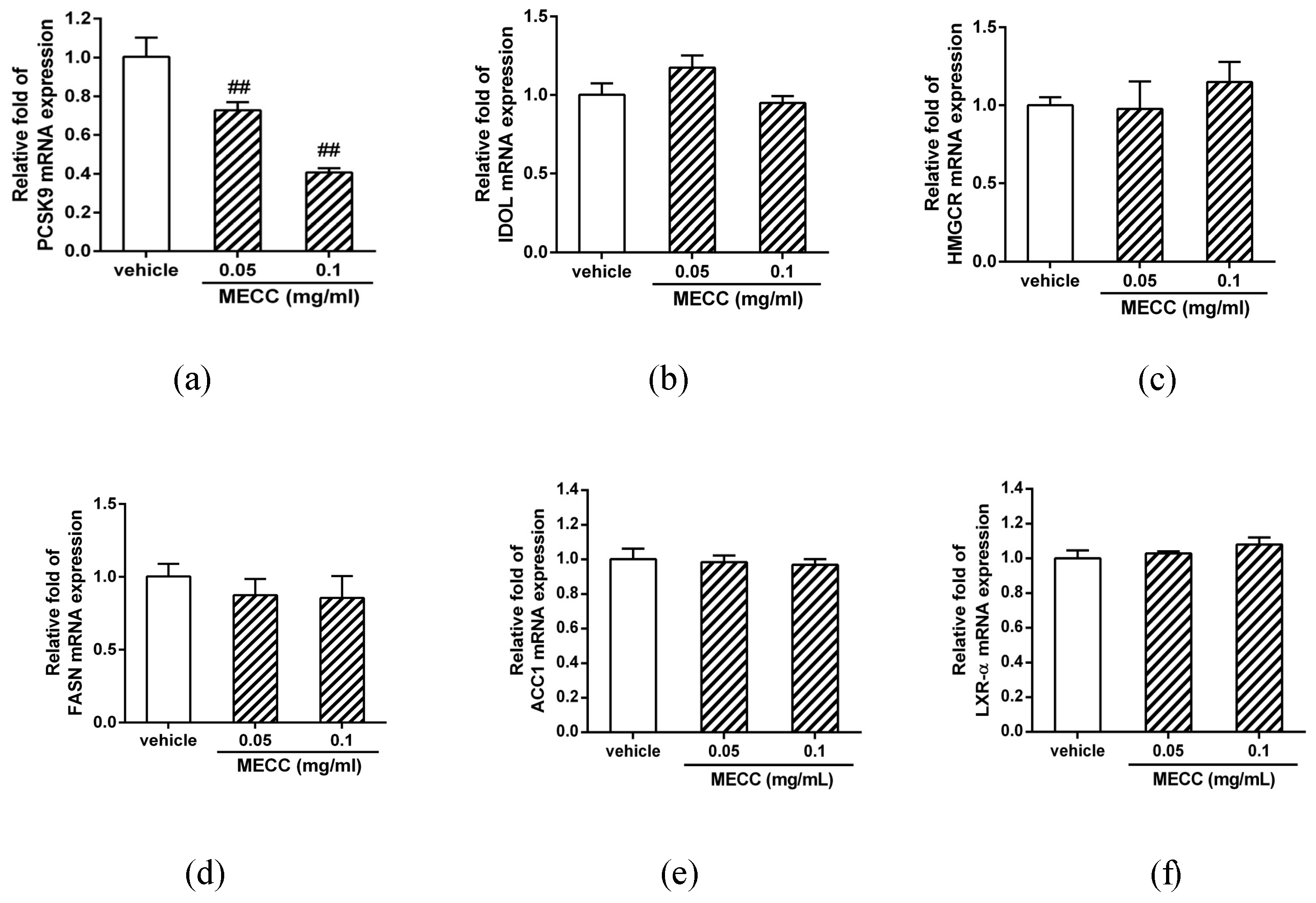
2.3. The Effect of MECC on the mRNA Expression of Selected Genes for Cholesterol Homeostasis and Lipogenesis
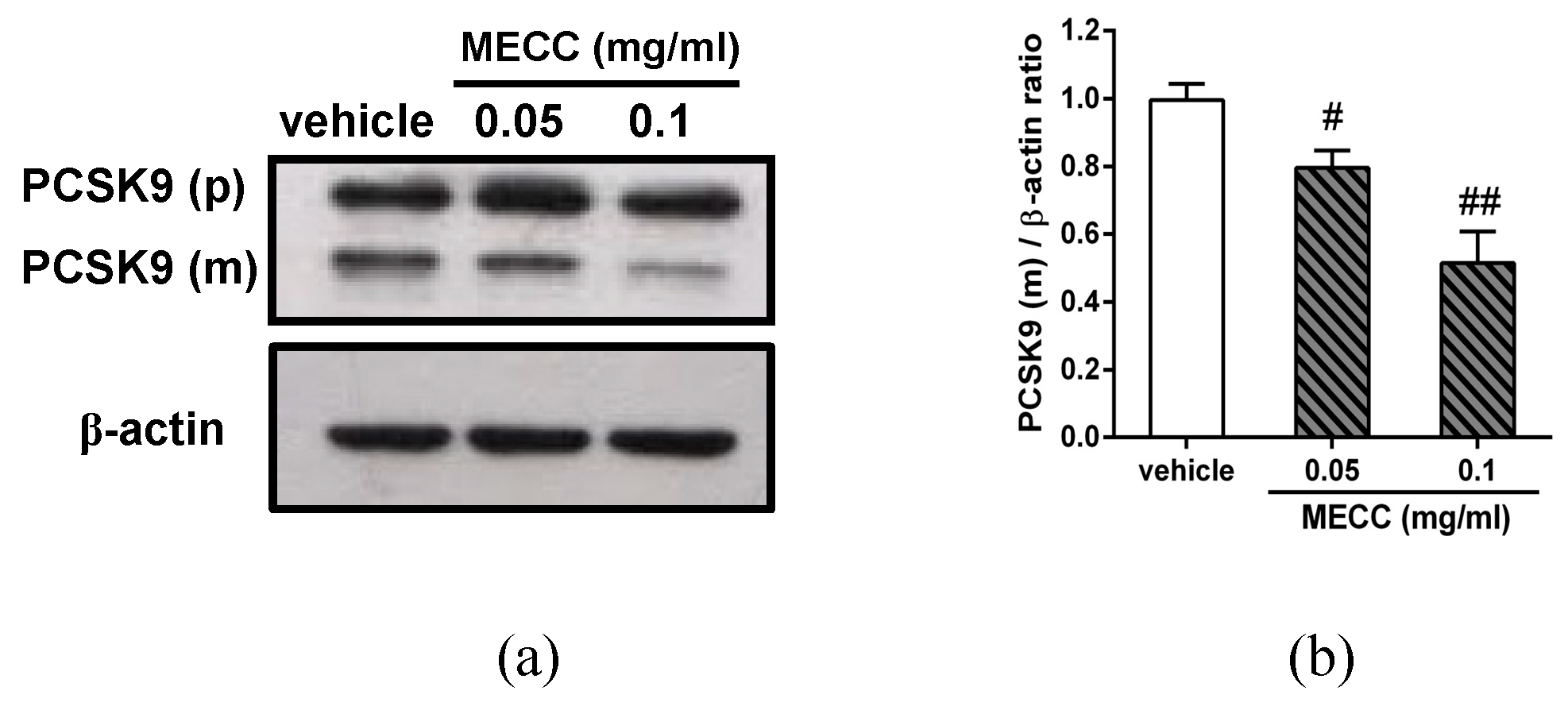
2.4. The Effect of MECC on the PCSK9 Protein Expression in HepG2 Cells
2.5. The Effect of MECC on Regulation of the PCSK9 Promoter and Transcriptional Activators in HepG2 Cells
2.6. Measurement of the Main Bioactive Components in MECC
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Chemicals
4.2. Methanol Extraction of the Leaves of Pigeon Pea (Cajanus cajan (L.) Millsp.)
4.3. Cell Culture and Treatment of Methanol Extract of Cajanus cajan Leaves
4.4. Determination of Cell Viability by MTT Assay
4.5. RNA Extraction and Reverse Transcription Real-Time PCR Analysis
4.6. Western Blot Analysis
4.7. Detection of the Level of Cell-Surface LDLR
4.8. Detection of LDL Uptake
4.9. Transfection, Luciferase Reporter Assay and Estabishment of PCSK9 Promoter/Luciferase Reporter Stable Cell Line
4.10. Identification of Compounds by Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS) Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Grover, J.K.; Yadav, S.; Vats, V. Medicinal plants of India with anti-diabetic potential. J. Ethnopharmacol. 2002, 81, 81–100. [Google Scholar] [CrossRef]
- Pal, D.; Mishra, P.; Sachan, N.; Ghosh, A.K. Biological activities and medicinal properties of Cajanus cajan (L) Millsp. J. Adv. Pharm. Technol. Res. 2011, 2, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Dai, F.J.; Hsu, W.H.; Huang, J.J.; Wu, S.C. Effect of pigeon pea (Cajanus cajan L.) on high-fat diet-induced hypercholesterolemia in hamsters. Food Chem. Toxicol. 2013, 53, 384–391. [Google Scholar] [CrossRef]
- Wu, N.; Fu, K.; Fu, Y.J.; Zu, Y.G.; Chang, F.R.; Chen, Y.H.; Liu, X.L.; Kong, Y.; Liu, W.; Gu, C.B. Antioxidant activities of extracts and main components of Pigeonpea [Cajanus cajan (L.) Millsp.] leaves. Molecules 2009, 14, 1032–1043. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.S.; Hsu, W.H.; Huang, J.J.; Wu, S.C. Antioxidant and anti-inflammatory effects of pigeon pea (Cajanus cajan L.) extracts on hydrogen peroxide- and lipopolysaccharide-treated RAW264.7 macrophages. Food Funct. 2012, 3, 1294–1301. [Google Scholar] [CrossRef]
- Fu, Y.; Kadioglu, O.; Wiench, B.; Wei, Z.; Gao, C.; Luo, M.; Gu, C.; Zu, Y.; Efferth, T. Cell cycle arrest and induction of apoptosis by cajanin stilbene acid from Cajanus cajan in breast cancer cells. Phytomedicine 2015, 22, 462–468. [Google Scholar] [CrossRef]
- Nix, A.; Paull, C.A.; Colgrave, M. The flavonoid profile of pigeonpea, Cajanus cajan: A review. Springerplus 2015, 4, 125. [Google Scholar] [CrossRef]
- Duker-Eshun, G.; Jaroszewski, J.W.; Asomaning, W.A.; Oppong-Boachie, F.; Brogger Christensen, S. Antiplasmodial constituents of Cajanus cajan. Phytother. Res. 2004, 18, 128–130. [Google Scholar] [CrossRef]
- Liu, W.; Kong, Y.; Zu, Y.; Fu, Y.; Luo, M.; Zhang, L.; Li, J. Determination and quantification of active phenolic compounds in pigeon pea leaves and its medicinal product using liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 4723–4731. [Google Scholar] [CrossRef]
- Wei, Z.F.; Jin, S.; Luo, M.; Pan, Y.Z.; Li, T.T.; Qi, X.L.; Efferth, T.; Fu, Y.J.; Zu, Y.G. Variation in contents of main active components and antioxidant activity in leaves of different pigeon pea cultivars during growth. J. Agric. Food Chem. 2013, 61, 10002–10009. [Google Scholar] [CrossRef]
- Luo, Q.F.; Sun, L.; Si, J.Y.; Chen, D.H. Hypocholesterolemic effect of stilbenes containing extract-fraction from Cajanus cajan L. on diet-induced hypercholesterolemia in mice. Phytomedicine 2008, 15, 932–939. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.J.; Ginsberg, H.N.; Amarenco, P.; Andreotti, F.; Boren, J.; Catapano, A.L.; Descamps, O.S.; Fisher, E.; Kovanen, P.T.; Kuivenhoven, J.A.; et al. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: Evidence and guidance for management. Eur. Heart J. 2011, 32, 1345–1361. [Google Scholar] [CrossRef] [PubMed]
- Turner, T.; Stein, E.A. Non-statin Treatments for Managing LDL Cholesterol and Their Outcomes. Clin. Ther. 2015, 37, 2751–2769. [Google Scholar] [CrossRef] [PubMed]
- Wadhera, R.K.; Steen, D.L.; Khan, I.; Giugliano, R.P.; Foody, J.M. A review of low-density lipoprotein cholesterol, treatment strategies, and its impact on cardiovascular disease morbidity and mortality. J. Clin. Lipidol. 2016, 10, 472–489. [Google Scholar] [CrossRef] [PubMed]
- Go, G.W.; Mani, A. Low-density lipoprotein receptor (LDLR) family orchestrates cholesterol homeostasis. Yale J. Biol. Med. 2012, 85, 19–28. [Google Scholar] [PubMed]
- Beglova, N.; Blacklow, S.C. The LDL receptor: How acid pulls the trigger. Trends Biochem. Sci. 2005, 30, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.S.; Dana, S.E.; Goldstein, J.L. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J. Biol. Chem. 1974, 249, 789–796. [Google Scholar]
- Brown, M.S.; Goldstein, J.L. Familial hypercholesterolemia: Defective binding of lipoproteins to cultured fibroblasts associated with impaired regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity. Proc. Natl. Acad. Sci. USA 1974, 71, 788–792. [Google Scholar] [CrossRef]
- Lagace, T.A. PCSK9 and LDLR degradation: Regulatory mechanisms in circulation and in cells. Curr. Opin. Lipidol. 2014, 25, 387–393. [Google Scholar] [CrossRef]
- Costet, P.; Krempf, M.; Cariou, B. PCSK9 and LDL cholesterol: Unravelling the target to design the bullet. Trends Biochem. Sci. 2008, 33, 426–434. [Google Scholar] [CrossRef]
- Stein, E.A.; Swergold, G.D. Potential of proprotein convertase subtilisin/kexin type 9 based therapeutics. Curr. Atheroscler. Rep. 2013, 15, 310. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, M.P. How Do PCSK9 Inhibitors Stack Up to Statins for Low-Density Lipoprotein Cholesterol Control? Am. Health Drug Benefits 2015, 8, 436–442. [Google Scholar] [PubMed]
- Peng, W.; Qiang, F.; Qian, Z.; Ke, Z.; Yi, L.; Jian, Z.; Chongrong, Q. Therapeutic efficacy of PCSK9 monoclonal antibodies in statin-nonresponsive patients with hypercholesterolemia and dyslipidemia: A systematic review and meta-analysis. Int. J. Cardiol. 2016, 222, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Cameron, J.; Ranheim, T.; Kulseth, M.A.; Leren, T.P.; Berge, K.E. Berberine decreases PCSK9 expression in HepG2 cells. Atherosclerosis 2008, 201, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Dong, B.; Park, S.W.; Lee, H.S.; Chen, W.; Liu, J. Hepatocyte nuclear factor 1alpha plays a critical role in PCSK9 gene transcription and regulation by the natural hypocholesterolemic compound berberine. J. Biol. Chem. 2009, 284, 28885–28895. [Google Scholar] [CrossRef]
- Tai, M.H.; Chen, P.K.; Chen, P.Y.; Wu, M.J.; Ho, C.T.; Yen, J.H. Curcumin enhances cell-surface LDLR level and promotes LDL uptake through downregulation of PCSK9 gene expression in HepG2 cells. Mol. Nutr. Food Res. 2014, 58, 2133–2145. [Google Scholar] [CrossRef]
- Chen, H.C.; Chen, P.Y.; Wu, M.J.; Tai, M.H.; Yen, J.H. Tanshinone IIA Modulates Low Density Lipoprotein Uptake via Down-Regulation of PCSK9 Gene Expression in HepG2 Cells. PLoS ONE 2016, 11, e0162414. [Google Scholar] [CrossRef]
- Gao, W.Y.; Chen, P.Y.; Chen, S.F.; Wu, M.J.; Chang, H.Y.; Yen, J.H. Pinostrobin Inhibits Proprotein Convertase Subtilisin/Kexin-type 9 (PCSK9) Gene Expression through the Modulation of FoxO3a Protein in HepG2 Cells. J. Agri. Food Chem. 2018, 66, 6083–6093. [Google Scholar] [CrossRef]
- Maxwell, K.N.; Soccio, R.E.; Duncan, E.M.; Sehayek, E.; Breslow, J.L. Novel putative SREBP and LXR target genes identified by microarray analysis in liver of cholesterol-fed mice. J. Lipid Res. 2003, 44, 2109–2119. [Google Scholar] [CrossRef]
- Lambert, G.; Charlton, F.; Rye, K.A.; Piper, D.E. Molecular basis of PCSK9 function. Atherosclerosis 2009, 203, 1–7. [Google Scholar] [CrossRef]
- Zelcer, N.; Hong, C.; Boyadjian, R.; Tontonoz, P. LXR regulates cholesterol uptake through Idol-dependent ubiquitination of the LDL receptor. Science 2009, 325, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Poirier, S.; Mayer, G.; Poupon, V.; McPherson, P.S.; Desjardins, R.; Ly, K.; Asselin, M.C.; Day, R.; Duclos, F.J.; Witmer, M.; et al. Dissection of the endogenous cellular pathways of PCSK9-induced low density lipoprotein receptor degradation: Evidence for an intracellular route. J. Biol. Chem. 2009, 284, 28856–28864. [Google Scholar] [CrossRef] [PubMed]
- Grassi, D.; Desideri, G.; Ferri, C. Flavonoids: Antioxidants against atherosclerosis. Nutrients 2010, 2, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Coban, D.; Milenkovic, D.; Chanet, A.; Khallou-Laschet, J.; Sabbe, L.; Palagani, A.; Vanden Berghe, W.; Mazur, A.; Morand, C. Dietary curcumin inhibits atherosclerosis by affecting the expression of genes involved in leukocyte adhesion and transendothelial migration. Mol. Nutr. Food Res. 2012, 56, 1270–1281. [Google Scholar] [CrossRef]
- Chanet, A.; Milenkovic, D.; Manach, C.; Mazur, A.; Morand, C. Citrus flavanones: What is their role in cardiovascular protection? J. Agri. Food Chem. 2012, 60, 8809–8822. [Google Scholar] [CrossRef]
- Ochiai, A.; Miyata, S.; Iwase, M.; Shimizu, M.; Inoue, J.; Sato, R. Kaempferol stimulates gene expression of low-density lipoprotein receptor through activation of Sp1 in cultured hepatocytes. Sci. Rep. 2016, 6, 24940. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.C.; Boerwinkle, E.; Mosley, T.H. Jr.; Hobbs, H.H. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl. J. Med. 2006, 354, 1264–1272. [Google Scholar] [CrossRef]
- Horton, J.D.; Cohen, J.C.; Hobbs, H.H. Molecular biology of PCSK9: Its role in LDL metabolism. Trends Biochem. Sci. 2007, 32, 71–77. [Google Scholar] [CrossRef]
- Maxwell, K.N.; Fisher, E.A.; Breslow, J.L. Overexpression of PCSK9 accelerates the degradation of the LDLR in a post-endoplasmic reticulum compartment. Proc. Natl. Acad. Sci. USA 2005, 102, 2069–2074. [Google Scholar] [CrossRef]
- Page, M.M.; Watts, G.F. PCSK9 inhibitors-mechanisms of action. Aust Prescr 2016, 39, 164–167. [Google Scholar] [CrossRef]
- Della Badia, L.A.; Elshourbagy, N.A.; Mousa, S.A. Targeting PCSK9 as a promising new mechanism for lowering low-density lipoprotein cholesterol. Pharmacol. Ther. 2016, 164, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, V.; Zelcer, N. Post-transcriptional regulation of lipoprotein receptors by the E3-ubiquitin ligase inducible degrader of the low-density lipoprotein receptor. Curr. Opin. Lipidol. 2012, 23, 213–219. [Google Scholar] [CrossRef]
- Ibrahim, S.; Somanathan, S.; Billheimer, J.; Wilson, J.M.; Rader, D.J. Stable liver-specific expression of human IDOL in humanized mice raises plasma cholesterol. Cardiovasc. Res. 2016, 110, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Naureckiene, S.; Ma, L.; Sreekumar, K.; Purandare, U.; Lo, C.F.; Huang, Y.; Chiang, L.W.; Grenier, J.M.; Ozenberger, B.A.; Jacobsen, J.S.; et al. Functional characterization of Narc 1, a novel proteinase related to proteinase K. Arch. Biochem. Biophy. 2003, 420, 55–67. [Google Scholar] [CrossRef]
- Kourimate, S.; Chetiveaux, M.; Jarnoux, A.L.; Lalanne, F.; Costet, P. Cellular and secreted pro-protein convertase subtilisin/kexin type 9 catalytic activity in hepatocytes. Atherosclerosis 2009, 206, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.Y.; Lin, J.; Lu, K.; Xu, H.G.; Geng, Z.Z.; Sun, P.H.; Chen, W.M. Anti-Inflammatory Effects of Cajaninstilbene Acid and Its Derivatives. J. Agri. Food Chem. 2016, 64, 2893–2900. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Kadioglu, O.; Wiench, B.; Wei, Z.; Wang, W.; Luo, M.; Yang, X.; Gu, C.; Zu, Y.; Efferth, T. Activity of the antiestrogenic cajanin stilbene acid towards breast cancer. J. Nutr. Biochem. 2015, 26, 1273–1282. [Google Scholar] [CrossRef]
- Jiang, B.P.; Liu, Y.M.; Le, L.; Li, Z.Y.; Si, J.Y.; Liu, X.M.; Chang, Q.; Pan, R.L. Cajaninstilbene acid prevents corticosterone-induced apoptosis in PC12 cells by inhibiting the mitochondrial apoptotic pathway. Cell Physiol. Biochem. 2014, 34, 1015–1026. [Google Scholar] [CrossRef]
- Carmichael, J.; DeGraff, W.G.; Gazdar, A.F.; Minna, J.D.; Mitchell, J.B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: Assessment of chemosensitivity testing. Cancer Res. 1987, 47, 936–942. [Google Scholar]
Sample Availability: Not available. |



| Cajaninstilbene Acid (mg/g Extract) | Pinostrobin (mg/g Extract) | Vitexin (mg/g Extract) | Orientin (mg/g Extract) | |
|---|---|---|---|---|
| MECC | 0.23 ± 0.04 | 0.01 ± 0.001 | 0.04 ± 0.005 | 0.13 ± 0.01 |
| Genes | Primers |
|---|---|
| LDLR | 5′-AGTTGGCTGCGTTAATGTGA-3′ |
| 5′-TGATGGGTTCATCTGACCAGT-3′ | |
| PCSK9 | 5′-GCTGAGCTGCTCCAGTTTCT-3′ |
| 5′-AATGGCGTAGACACCCTCAC-3′ | |
| IDOL | 5′-AAGTTCTTCGTGGAGCCTCA-3′ |
| 5′-ACTGAGTTCCACTGCCTGCT-3′ | |
| HMGCR | 5′-TGATTGACCTTTCCAGAGCAAG-3′ |
| 5′-CTAAAATTGCCATTCCACGAGC-3′ | |
| ACC1 | 5′-GAGGGAAGGGAATTAGAA-3′ |
| 5′-ATCACCCCAGGGAGATAC-3′ | |
| FASN | 5′-TATGCTTCTTCGTGCAGCAGTT-3′ |
| 5′-GCTGCCACACGCTCCTCTAG-3′ | |
| LXR-α | 5′-GCCGAGTTTGCCTTGCTCA-3′ |
| 5′-TCCGGAGGCTCACCAGTTTC-3′ | |
| GAPDH | 5′-ATGAGAAGTATGACAACAGCCT-3′ |
| 5′-AGTCCTTCCACGATACCAAAGT-3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, H.-Y.; Wu, J.-R.; Gao, W.-Y.; Lin, H.-R.; Chen, P.-Y.; Chen, C.-I.; Wu, M.-J.; Yen, J.-H. The Cholesterol-Modulating Effect of Methanol Extract of Pigeon Pea (Cajanus cajan (L.) Millsp.) Leaves on Regulating LDLR and PCSK9 Expression in HepG2 Cells. Molecules 2019, 24, 493. https://doi.org/10.3390/molecules24030493
Chang H-Y, Wu J-R, Gao W-Y, Lin H-R, Chen P-Y, Chen C-I, Wu M-J, Yen J-H. The Cholesterol-Modulating Effect of Methanol Extract of Pigeon Pea (Cajanus cajan (L.) Millsp.) Leaves on Regulating LDLR and PCSK9 Expression in HepG2 Cells. Molecules. 2019; 24(3):493. https://doi.org/10.3390/molecules24030493
Chicago/Turabian StyleChang, Heng-Yuan, Jia-Ru Wu, Wan-Yun Gao, Huei-Ru Lin, Pei-Yi Chen, Chen-I Chen, Ming-Jiuan Wu, and Jui-Hung Yen. 2019. "The Cholesterol-Modulating Effect of Methanol Extract of Pigeon Pea (Cajanus cajan (L.) Millsp.) Leaves on Regulating LDLR and PCSK9 Expression in HepG2 Cells" Molecules 24, no. 3: 493. https://doi.org/10.3390/molecules24030493
APA StyleChang, H.-Y., Wu, J.-R., Gao, W.-Y., Lin, H.-R., Chen, P.-Y., Chen, C.-I., Wu, M.-J., & Yen, J.-H. (2019). The Cholesterol-Modulating Effect of Methanol Extract of Pigeon Pea (Cajanus cajan (L.) Millsp.) Leaves on Regulating LDLR and PCSK9 Expression in HepG2 Cells. Molecules, 24(3), 493. https://doi.org/10.3390/molecules24030493