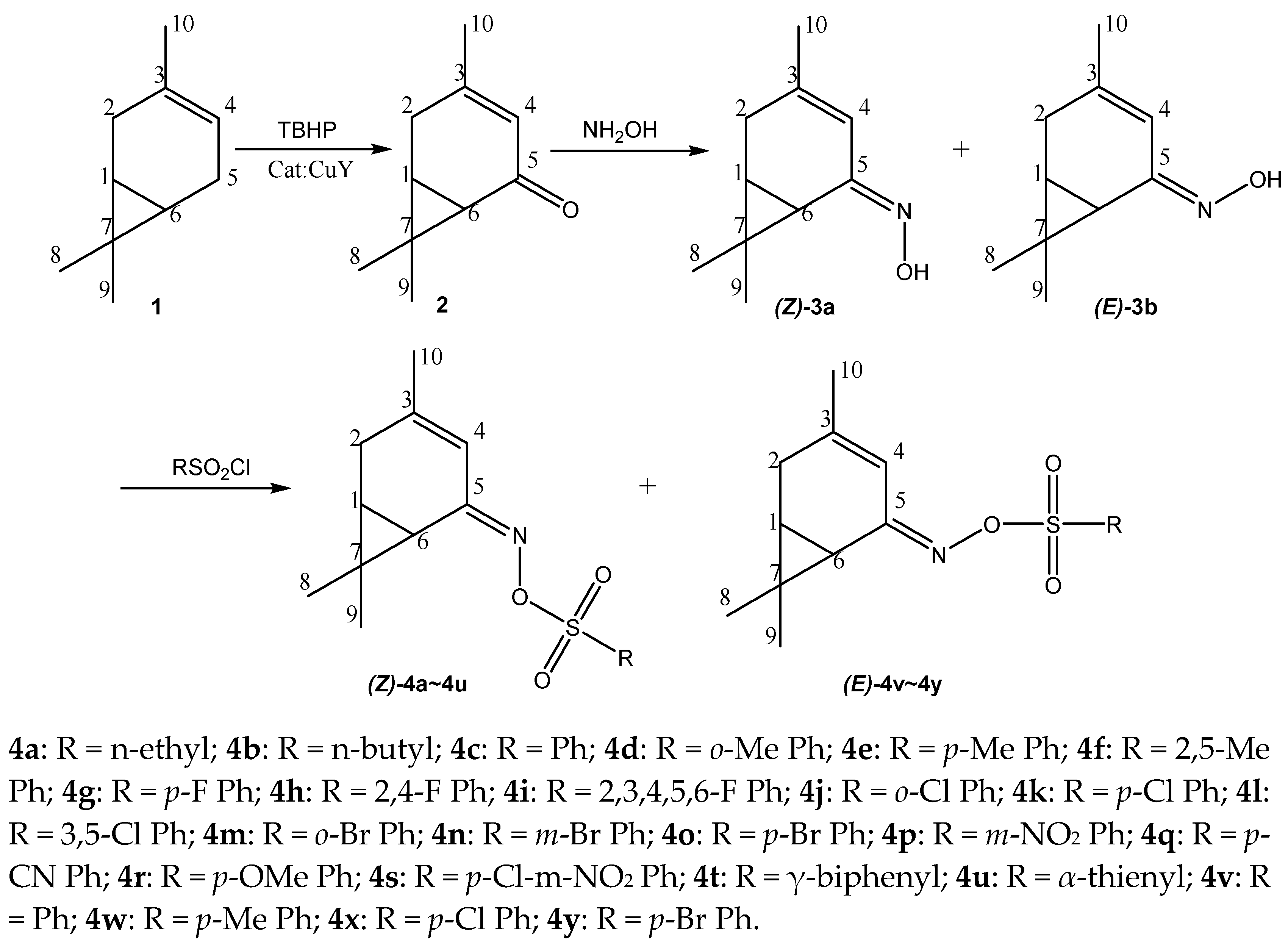
Synthesis of Bioactive Compounds from 3-Carene (II): Synthesis, Antifungal Activity and 3D-QSAR Study of (Z)- and (E)-3-Caren-5-One Oxime Sulfonates
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization
2.2. Antifungal Activity
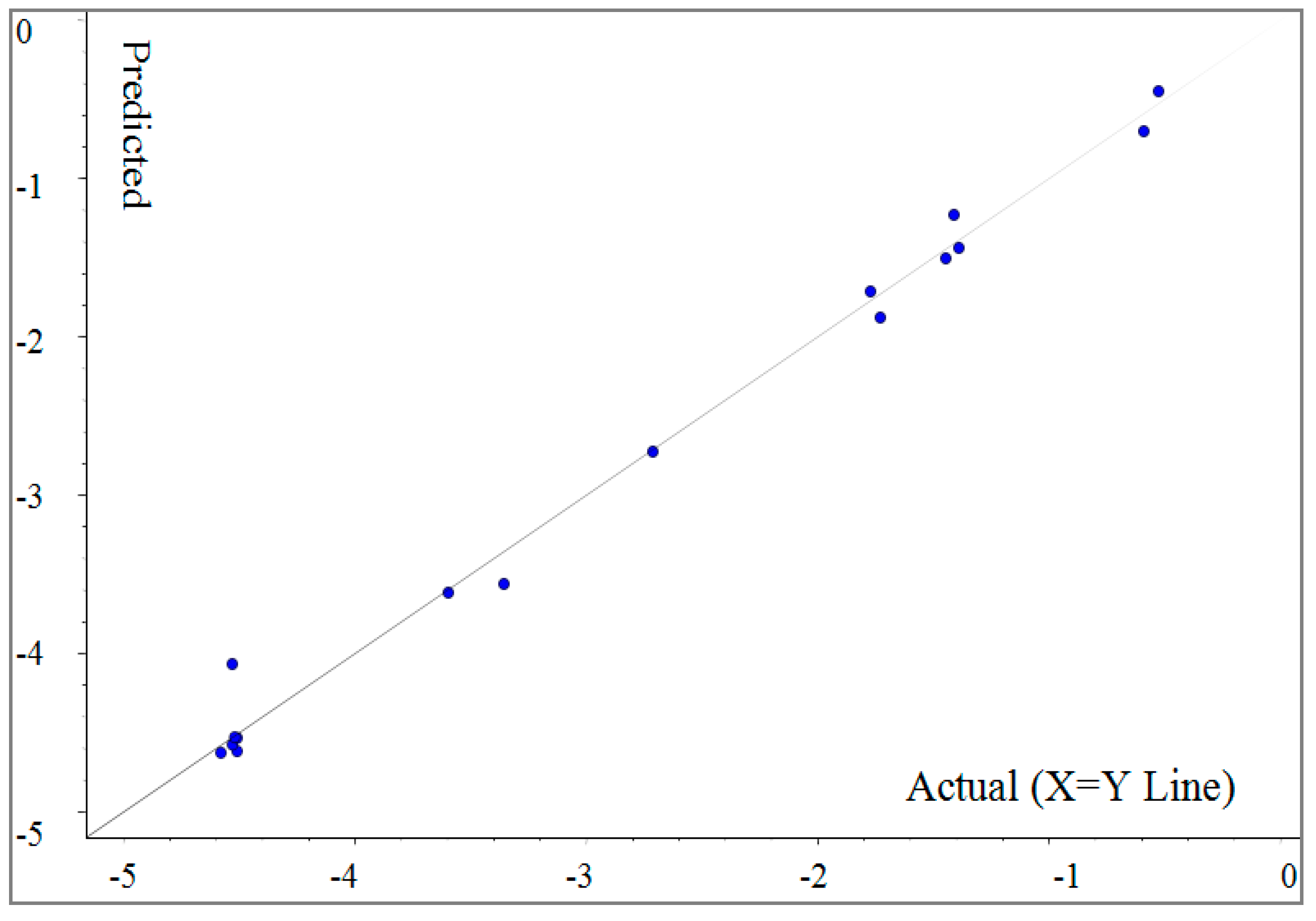
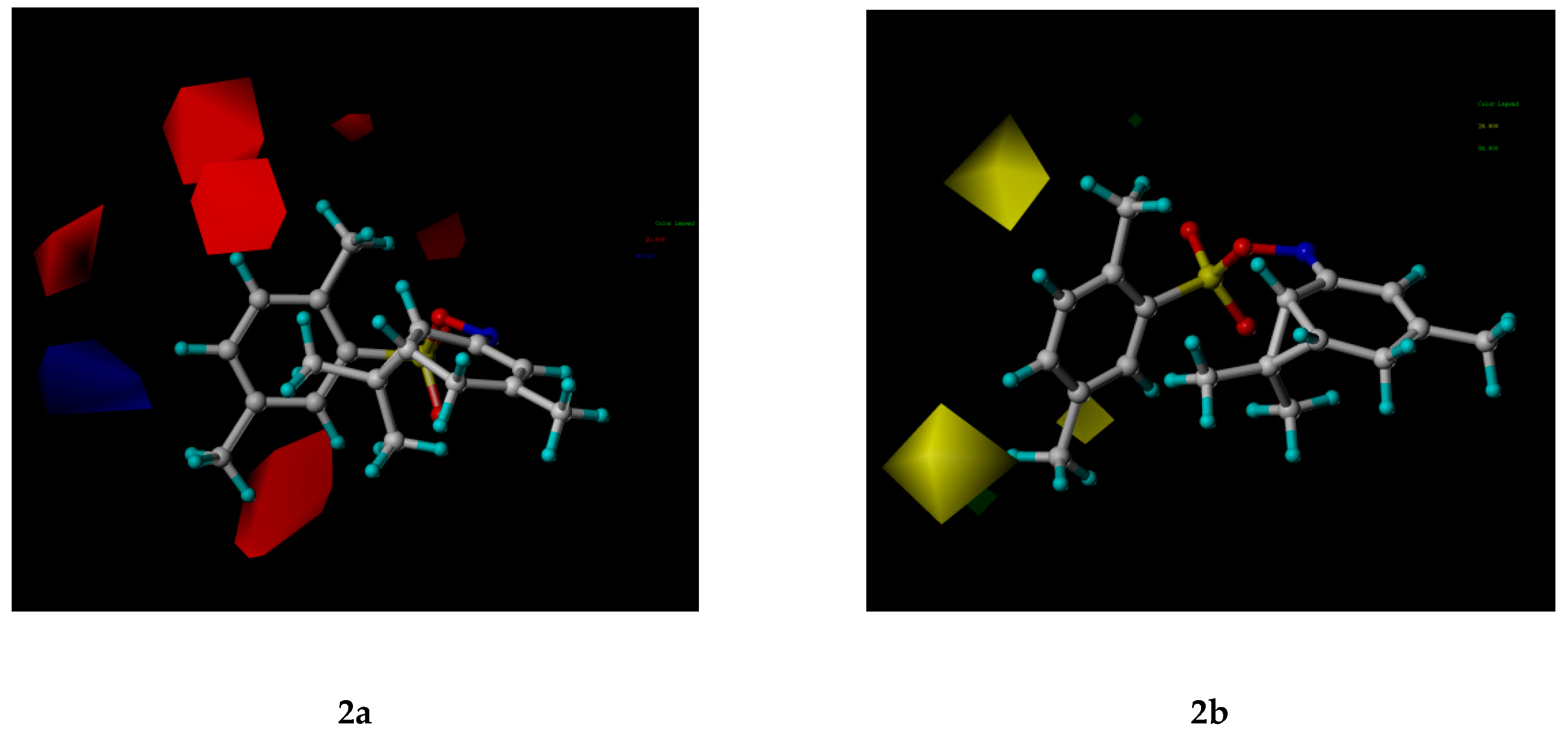
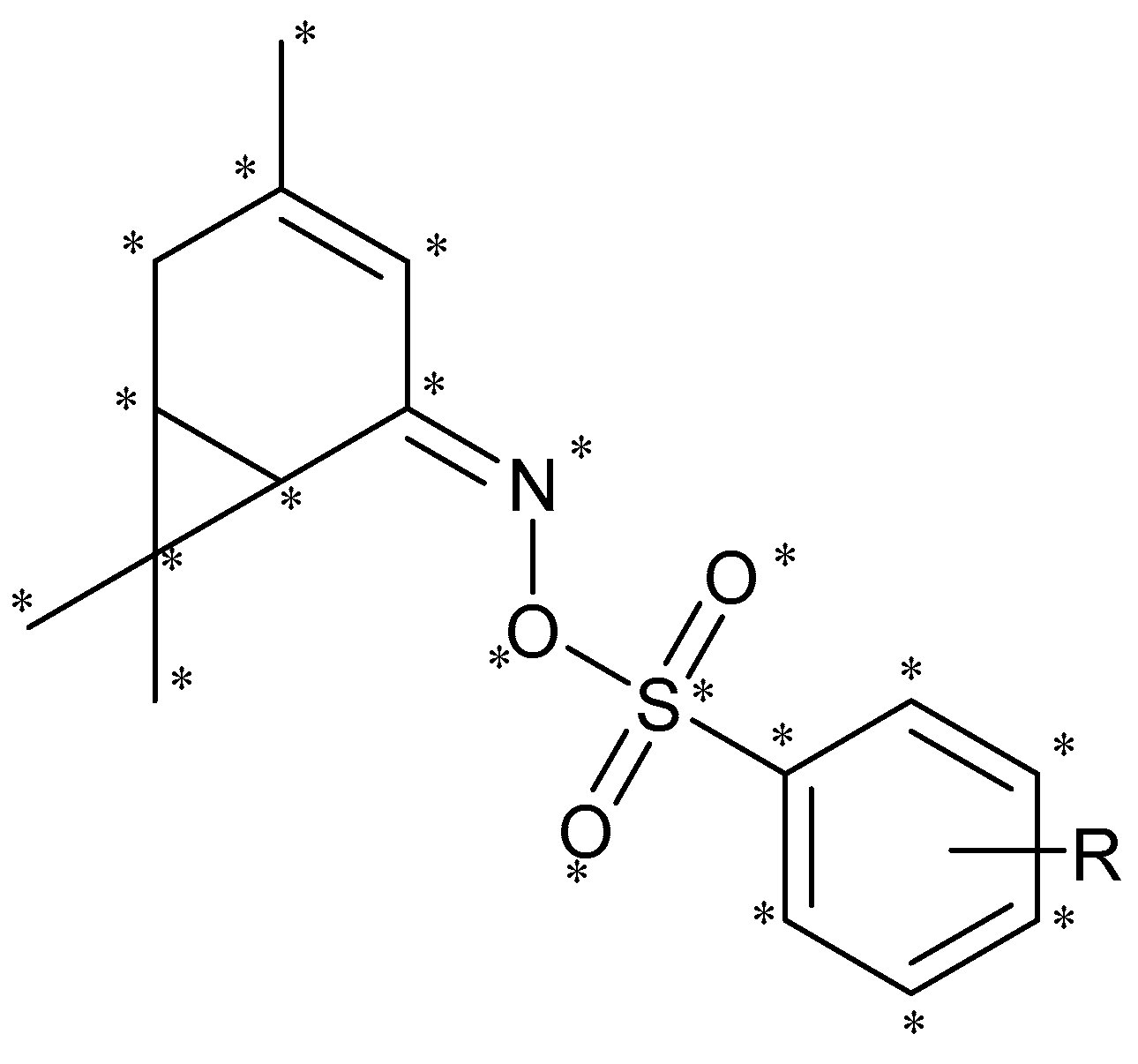
2.3. CoMFA Analysis
3. Experimental Section
3.1. General Information
3.2. Synthesis of 3-caren-5-one (2)
3.3. Synthesis of 3-caren-5-one oxime (3)
3.4. General Procedure for Synthesis of 3-Caren-5-One Oxime Sulfonates (Z)-4a–4u and (E)-4v–4y
3.5. Antifungal Activity Test
3.6. 3D-QSAR Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Qiu, C.L.; Smuts, J.; Schug, K.A. Analysis of terpenes and turpentines using gas chromatography with vacuum ultraviolet detection. J. Sep. Sci. 2017, 40, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Garcia, G.; Tissandie, L.; Filippi, J.J.; Tomi, F. New pinane derivatives found in essential oils of Calocedrus decurrens. Molecules 2017, 22, 921–932. [Google Scholar] [CrossRef]
- Houicher, A.; Hamdi, M.; Hechachna, H.; Ozogul, F. Chemical composition and antifungal activity of Anacyclus valentinus essential oil from Algeria. Food Biosci. 2018, 25, 28–31. [Google Scholar] [CrossRef]
- Uliana, M.P.; Fronza, M.; da Silva, A.G.; Vargas, T.S.; de Andrade, T.U.; Scherer, R. Composition and biological activity of Brazilian rose pepper (Schinus terebinthifolius Raddi) leaves. Ind. Crops Prod. 2016, 83, 235–240. [Google Scholar] [CrossRef]
- Smeriglio, A.; Denaro, M.; Barreca, D.; Calderaro, A.; Bisignano, C.; Ginestra, G.; Bellocco, E.; Trombetta, D. In vitro evaluation of the antioxidant, cytoprotective, and antimicrobial properties of essential oil from Pistacia vera L. Variety Bronte Hull. Int. J. Mol. Sci. 2017, 18, 1212–1224. [Google Scholar] [CrossRef]
- Kohoude, M.J.; Gbaguidi, F.; Agbani, P.; Ayedoun, M.A.; Cazaux, S.; Bouajila, J. Chemical composition and biological activities of extracts and essential oil of Boswellia dalzielii leaves. Pharm. Biol. 2017, 55, 33–42. [Google Scholar] [CrossRef]
- Harhour, A.; Brada, M.; Fauconnier, M.L.; Lognay, G. Chemical composition and antioxidant activity of algerian juniperus phoenicea essential oil. Nat. Prod. Sci. 2018, 24, 125–131. [Google Scholar] [CrossRef]
- da Silva, B.G.; Fileti, A.M.F.; Foglio, M.A.; Ruiz, A.L.T.G.; Rosa, P.D.V.E. Supercritical carbon dioxide extraction of compounds from Schinus terebinthifolius Raddi fruits: Effects of operating conditions on global yield, volatile compounds, and antiproliferative activity against human tumor cell lines. J. Supercrit. Fluids 2017, 130, 10–16. [Google Scholar] [CrossRef]
- Basholli-Salihu, M.; Schuster, R.; Hajdari, A.; Mulla, D.; Viernstein, H.; Mustafa, B.; Mueller, M. Phytochemical composition, anti-inflammatory activity and cytotoxic effects of essential oils from three Pinus spp. Pharm. Biol. 2017, 55, 1553–1560. [Google Scholar] [CrossRef]
- Mao, G.F.; Tian, J.X.; Li, T.; Fouad, H.; Ga’al, H.; Mo, J.C. Behavioral responses of anagrus nilaparvatae to common terpenoids, aromatic compounds, and fatty acid derivatives from rice plants. Entomol. Exp. Appl. 2018, 166, 483–490. [Google Scholar] [CrossRef]
- Kelsey, R.G.; Westlind, D.J. Attraction of red turpentine beetle and other scolytinae to ethanol, 3-carene or ethanol + 3-carene in an oregon pine forest. Agr. Forest Entomol. 2018, 20, 272–278. [Google Scholar] [CrossRef]
- Cao, J.Q.; Guo, S.S.; Wang, Y.; Pang, X.; Geng, Z.F.; Du, S.S. Toxicity and repellency of essential oil from Evodia lenticellata Huang fruits and its major monoterpenes against three stored-product insects. Ecotoxicol. Environ. Saf. 2018, 160, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhang, N.; Chen, H.L.; Zhong, B.L.; Yang, A.X.; Kuang, F.; Ouyang, Z.G.; Chun, J. Fumigant activity of sweet orange essential oil fractions against red imported fire ants (Hymenoptera: Formicidae). J. Econ. Entomol. 2017, 4, 1556–1562. [Google Scholar] [CrossRef] [PubMed]
- El-Faham, A.; Elnakdy, Y.A.; El Gazzar, S.A.M.; Abd El-Rahman, M.M.; Khattab, S.N. Synthesis, characterization and anti-proliferation activities of novel cyano oximino sulfonate esters. Chem. Pharm. Bull. 2014, 62, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Surkau, G.; Bohm, K.J.; Muller, K.; Prinz, H. Synthesis, antiproliferative activity and inhibition of tubulin polymerization by anthracenone-based oxime derivatives. Eur. J. Med. Chem. 2010, 45, 3354–3364. [Google Scholar] [CrossRef]
- Wang, R.; Zhi, X.Y.; Li, J.; Xu, H. Synthesis of novel oxime sulfonate derivatives of 2′(2′,6′)-(di)chloropicropodophyllotoxins as insecticidal agents. J. Agric. Food Chem. 2015, 63, 6668–6674. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.Q.; Liu, G.C.; Zhang, Y.Y.; Feng, T.; Xu, M.; Xu, H. Design, synthesis and evaluation of novel isoxazolines/oxime sulfonates of 2′(2′,6′)-(di)chloropodophyllotoxins as insecticidal agents. Sci. Rep. 2016, 6, 33062–33073. [Google Scholar] [CrossRef]
- Zhi, X.Y.; Yang, C.; Yu, X.; Xu, H. Synthesis and insecticidal activity of new oxime derivatives of podophyllotoxin-based phenazines against Mythimna separata Walker. Bioorg. Med. Chem. Lett. 2014, 24, 5679–5682. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, A.Q.; Xu, H.T.; Mo, Y.X.; Chen, J.; Shen, L.Q. Design, synthesis, and bioassay of novel compounds of isolongifolenone oxime derivatives. Helv. Chim. Acta. 2016, 99, 696–703. [Google Scholar] [CrossRef]
- Zhang, R.; Duan, W.G.; Lin, G.S.; Chen, Z.C. Synthesis and antifungal activity of verbenone-based oxime sulfonate compounds. Chem. Ind. For. Prod. 2017, 37, 68–78. [Google Scholar]
- Huang, M.; Duan, W.G.; Lin, G.S.; Li, K.; Hu, Q. Synthesis and antifungal activity of novel 3-caren-5-one oxime esters. Molecules 2017, 22, 1538–1552. [Google Scholar] [CrossRef]
- Lin, G.S.; Duan, W.G.; Yang, L.X.; Huang, M.; Lei, F.H. Synthesis and antifungal activity of novel myrtenal-based 4-methyl-1,2,4-triazole-thioethers. Molecules 2017, 22, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Li, F.Y.; Wang, X.; Duan, W.G.; Lin, G.S. Synthesis and in vitro anticancer activity of novel dehydroabietic acid-based acylhydrazones. Molecules 2017, 22, 1087–1098. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Lin, G.S.; Duan, W.G.; Huang, M.; Lei, F.H. Synthesis and biological activity of novel (Z)- and (E)-verbenone oxime esters. Molecules 2017, 22, 1678–1694. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.L.; Li, F.Y.; Duan, W.G.; Liao, J.N.; Lin, Z.D.; Lin, G.S.; Cen, B.; Lei, F.H. Synthesis and antifungal activity of camphoric acid-based acylhydrazone compounds. Holzforschung 2014, 68, 889–895. [Google Scholar] [CrossRef]
- Lin, G.S.; Ma, C.H.; Duan, W.G.; Cen, B.; Lei, F.H.; Yang, Z.Q. Synthesis and biological activities of α-pinene-based dithiadiazoles. Holzforschung 2014, 68, 75–83. [Google Scholar] [CrossRef]
- Lin, G.S.; Chen, Z.C.; Duan, W.G.; Wang, X.Y.; Lei, F.H. Synthesis and biological activity of novel myrtenal-derived 2-acyl-1,2,4-triazole-3-thione compounds. Chin. J. Org. Chem. 2018, 38, 2085–2092. [Google Scholar] [CrossRef]
- Liu, X.H.; Shi, Y.X.; Ma, Y.; Zhang, C.Y.; Dong, W.L.; Pan, L.; Wang, B.L.; Li, B.J.; Li, Z.M. Synthesis, antifungal activities and 3D-QSAR study of N-(5-substituted-1,3,4-thiadiazol-2-yl)cyclopropane- carboxamides. Eur. J. Med. Chem. 2009, 44, 2782–2786. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 2, (Z)-3a, (E)-3b, (Z)-4a–4u, and (E)-4v–4y are available from the authors. |

| Compounds | Relative Inhibition Rate (%) against the Tested Fungi | |||||
|---|---|---|---|---|---|---|
| C. arachidicola | P. piricola | A. solani | R. solani | B. myadis | C. orbicalare | |
| (Z)-4a (R = n-ethyl) | 74.2 | 35.7 | 34.4 | 29.2 | 5 | 17.5 |
| (Z)-4b (R = n-butyl) | 87.1 | 100 | 1.6 | 46.9 | 36.6 | 34 |
| (Z)-4c (R = Ph) | 9.7 | 7.1 | 27.9 | 23.3 | 20.8 | 13.4 |
| (Z)-4d (R = o-Me Ph) | 61.3 | 85.7 | 27.9 | 58.7 | 28.7 | 25.8 |
| (Z)-4e (R = p-Me Ph) | 48.4 | 92.9 | 27.9 | 42.4 | 36.6 | 17.5 |
| (Z)-4f (R = 2,5-Me Ph) | 0 | 100 | 8.2 | 48.3 | 32.7 | 21.6 |
| (Z)-4g (R = p-F Ph) | 22.6 | 0 | 0 | 35.1 | 24.8 | 29.9 |
| (Z)-4h (R = 2,4-F Ph) | 9.7 | 0 | 0 | 2.6 | 16.8 | 29.9 |
| (Z)-4i (R = 2,3,4,5,6-F Ph) | 74.2 | 0 | 8.2 | 0 | 12.9 | 13.4 |
| (Z)-4j (R = o-Cl Ph) | 9.7 | 92.9 | 21.3 | 43.9 | 16.8 | 21.6 |
| (Z)-4k (R = p-Cl Ph) | 0 | 0 | 1.6 | 23.3 | 20.8 | 25.8 |
| (Z)-4l (R = 3,5-Cl Ph) | 61.3 | 92.9 | 34.4 | 49.8 | 20.8 | 17.5 |
| (Z)-4m (R = o-Br Ph) | 0 | 100 | 27.9 | 23.3 | 20.8 | 25.8 |
| (Z)-4n (R = m-Br Ph) | 0 | 0 | 8.2 | 45.4 | 5 | 21.6 |
| (Z)-4o (R = p-Br Ph) | 48.4 | 14.3 | 8.2 | 20.3 | 12.9 | 29.9 |
| (Z)-4p (R = m-NO2 Ph) | 48.4 | 85.7 | 8.2 | 52.8 | 5 | 29.9 |
| (Z)-4q (R = p-CN Ph) | 22.6 | 0 | 14.8 | 17.3 | 12.9 | 13.4 |
| (Z)-4r (R = p-MeO Ph) | 9.7 | 0 | 27.9 | 29.2 | 5 | 17.5 |
| (Z)-4s (R = p-Cl-m-NO2 Ph) | 16.1 | 42.9 | 1.6 | 52.8 | 5 | 17.5 |
| (Z)-4t (R = γ-biphenyl) | 22.6 | 28.6 | 14.8 | 18.8 | 20.8 | 9.3 |
| (Z)-4u (R = α-thienyl) | 9.7 | 64.3 | 21.3 | 24.7 | 5 | 17.5 |
| (E)-4v (R = Ph) | 0 | 14.3 | 0 | 23.3 | 8.9 | 0 |
| (E)-4w (R = p-Me Ph) | 0 | 42.9 | 21.3 | 29.2 | 20.8 | 9.3 |
| (E)-4x (R = p-Cl Ph) | 9.7 | 57.1 | 8.2 | 89.7 | 100 | 1 |
| (E)-4y (R = p-Br Ph) | 0 | 92.9 | 27.9 | 100 | 100 | 9.3 |
| Chlorothanil | 73.3 | 75 | 73.9 | 96.1 | 90.4 | 91.3 |
| Compounds | ED | ED’’ | Residue |
|---|---|---|---|
| (Z)-4c (R = Ph) | −3.60 | −3.61 | 0.01 |
| (Z)-4d (R = o-Me Ph) | −1.73 | −1.88 | 0.15 |
| (Z)-4e (R = p-Me Ph) | −1.39 | −1.44 | 0.05 |
| (Z)-4f (R = 2,5-Me Ph) | −0.53 | −0.45 | −0.08 |
| (Z)-4g (R = p-F Ph) | −4.51 | −4.61 | 0.10 |
| (Z)-4n (R = 2,4-F Ph) | −4.53 | −4.57 | 0.04 |
| (Z)-4j (R = o-Cl Ph) | −1.41 | −1.23 | −0.18 |
| (Z)-4k (R = p-Cl Ph) | −4.53 | −4.06 | −0.47 |
| (Z)-4l (R = 3,5-Cl Ph) | −1.45 | −1.50 | 0.05 |
| (Z)-4m (R = o-Br Ph) | −0.59 | −0.70 | 0.11 |
| (Z)-4n (R = m-Br Ph) | −4.58 | −4.62 | 0.04 |
| (Z)-4o (R = p-Br Ph) | −3.36 | −3.56 | 0.20 |
| (Z)-4p (R = m-NO2 Ph) | −1.77 | −1.71 | −0.06 |
| (Z)-4q (R = p-CN Ph) | −4.51 | −4.53 | 0.02 |
| (Z)-4r (R = p-MeO Ph) | −4.52 | −4.53 | 0.01 |
| (Z)-4s (R = p-Cl-m-NO2 Ph) | −2.71 | −2.73 | 0.02 |
| Contribution (%) | ||||||
|---|---|---|---|---|---|---|
| q2 | r2 | S | F | Steric | Electrostatic | |
| CoMFA | 0.569 | 0.990 | 0.243 | 68.914 | 95.0 | 5.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, G.-Q.; Duan, W.-G.; Lin, G.-S.; Yu, Y.-P.; Wang, X.-Y.; Lu, S.-Z. Synthesis of Bioactive Compounds from 3-Carene (II): Synthesis, Antifungal Activity and 3D-QSAR Study of (Z)- and (E)-3-Caren-5-One Oxime Sulfonates. Molecules 2019, 24, 477. https://doi.org/10.3390/molecules24030477
Kang G-Q, Duan W-G, Lin G-S, Yu Y-P, Wang X-Y, Lu S-Z. Synthesis of Bioactive Compounds from 3-Carene (II): Synthesis, Antifungal Activity and 3D-QSAR Study of (Z)- and (E)-3-Caren-5-One Oxime Sulfonates. Molecules. 2019; 24(3):477. https://doi.org/10.3390/molecules24030477
Chicago/Turabian StyleKang, Guo-Qiang, Wen-Gui Duan, Gui-Shan Lin, You-Pei Yu, Xiao-Yu Wang, and Sun-Zhong Lu. 2019. "Synthesis of Bioactive Compounds from 3-Carene (II): Synthesis, Antifungal Activity and 3D-QSAR Study of (Z)- and (E)-3-Caren-5-One Oxime Sulfonates" Molecules 24, no. 3: 477. https://doi.org/10.3390/molecules24030477
APA StyleKang, G.-Q., Duan, W.-G., Lin, G.-S., Yu, Y.-P., Wang, X.-Y., & Lu, S.-Z. (2019). Synthesis of Bioactive Compounds from 3-Carene (II): Synthesis, Antifungal Activity and 3D-QSAR Study of (Z)- and (E)-3-Caren-5-One Oxime Sulfonates. Molecules, 24(3), 477. https://doi.org/10.3390/molecules24030477





