Microscopic Analyses of Fruit Cell Plastid Development in Loquat (Eriobotrya japonica) during Fruit Ripening
Abstract
:1. Introduction
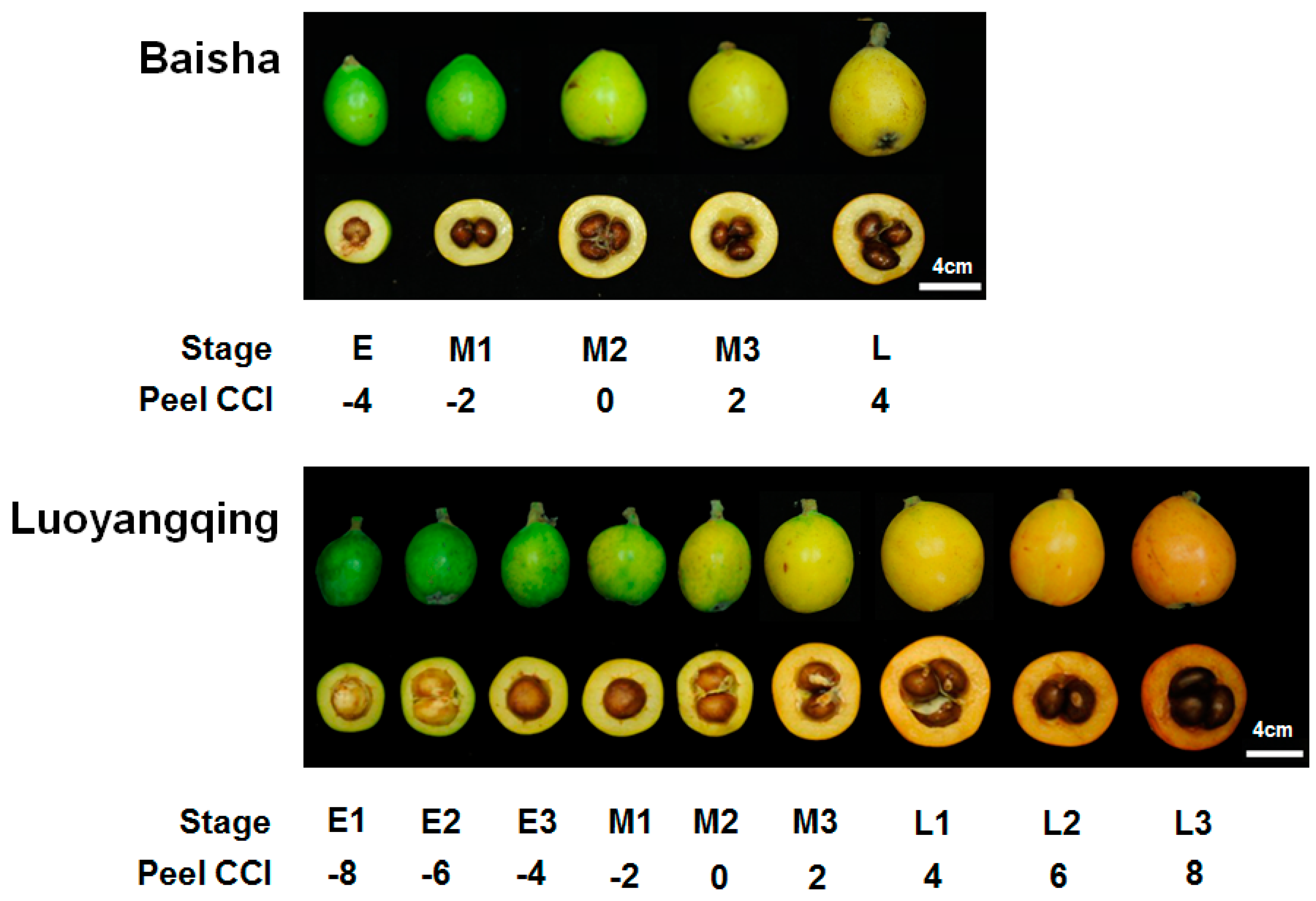
2. Results and Discussion
2.1. Anatomical Changes of Plastids during Loquat Fruit Ripening
2.2. Quantitative Changes in the Abundance and Size of Plastids during Loquat Fruit Ripening
2.3. Plastid Differentiation and the Relationship between Carotenoid Accumulation and Plastid Development during Loquat Fruit Ripening
3. Materials and Methods
3.1. Plant Materials
3.2. Color and Fruit Size Measurement
3.3. Cell Squashing and Differential Interference Contrast Microscopy
3.4. Transmission Electron Microscopy
3.5. Measurement of Quantities and Areas of Cells and Plastids
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Enfissi, E.M.A.; Nogueira, M.; Bramley, P.M.; Fraser, P.D. The regulation of carotenoid formation in tomato fruit. Plant J. 2017, 89, 774–778. [Google Scholar] [CrossRef] [PubMed]
- McQuinn, R.P.; Giovannonni, J.J.; Pogson, B.J. More than meets the eye: From carotenoid biosynthesis, to new insights into apocarotenoid signaling. Curr. Opin. Plant Biol. 2015, 27, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Fraser, P.D.; Bramley, P.M. The biosynthesis and nutritional uses of carotenoids. Prog. Lipid Res. 2004, 43, 228–265. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Yuan, H.; Cao, H.; Yazdani, M.; Tadmor, Y.; Li, L. Carotenoid metabolism in plants: The role of plastids. Mol. Plant 2018, 11, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Egea, I.; Barsan, C.; Bian, W.; Purgatto, E.; Latché, A.; Chervin, C.; Bouzayen, M.; Pech, J.C. Chromoplast differentiation: Current status and perspectives. Plant Cell Physiol. 2010, 51, 1601–1611. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Juez, E.; Pyke, K.A. Plastids unleashed: Their development and their integration in plant development. Int. J. Dev. Biol. 2005, 49, 557–577. [Google Scholar] [CrossRef]
- Cookson, P.J.; Kiano, J.W.; Shipton, C.A.; Fraser, P.D.; Romer, S.; Schuch, W.; Bramley, P.M.; Pyke, K.A. Increases in cell elongation, plastid compartment size and phytoene synthase activity underlie the phenotype of the high pigment-1 mutant of tomato. Planta 2003, 217, 896–903. [Google Scholar] [CrossRef]
- Kolotilin, I.; Koltai, H.; Tadmor, Y.; Bar-Or, C.; Reuveni, M.; Meir, A.; Nahon, S.; Shlomo, H.; Chen, L.; Levin, I. Transcriptional profiling of high pigment-2dg tomato mutant links early fruit plastid biogenesis with its overproduction of phytonutrients. Plant Physiol. 2007, 145, 389–401. [Google Scholar] [CrossRef]
- Galpaz, N.; Wang, Q.; Menda, N.; Zamir, D.; Hirschberg, J. Abscisic acid deficiency in the tomato mutant high-pigment 3 leading to increased plastid number and higher fruit lycopene content. Plant J. 2008, 53, 717–730. [Google Scholar] [CrossRef]
- Enfissi, E.M.; Barneche, F.; Ahmed, I.; Lichtlé, C.; Gerrish, C.; McQuinn, R.P.; Giovannoni, J.J.; Lopez-Juez, E.; Bowler, C.; Bramley, P.M.; Fraser, P.D. Integrative transcript and metabolite analysis of nutritionally enhanced DE-ETIOLATED1 downregulated tomato fruit. Plant Cell 2010, 22, 1190–1215. [Google Scholar] [CrossRef]
- Peng, G.; Wang, C.Y.; Song, S.; Fu, X.M.; Azam, M.; Grierson, D.; Xu, C.J. The role of 1-deoxy-d-xylulose-5-phosphate synthase and phytoene synthase gene family in citrus carotenoid accumulation. Plant Physiol. Biochem. 2013, 71, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.J.; Wang, C.Y.; Yin, T.T.; Zhong, S.L.; Grierson, D.; Chen, K.S.; Xu, C.J. Cytological and molecular characterization of carotenoid accumulation in normal and high-lycopene mutant oranges. Sci. Rep. 2017, 7, 761. [Google Scholar] [CrossRef] [PubMed]
- Harris, W.M.; Spurr, A.R. Chromoplasts of tomato fruits. II. The red tomato. Am. J. Bot. 1969, 56, 380–389. [Google Scholar] [CrossRef]
- Egea, I.; Bian, W.; Barsan, C.; Jauneau, A.; Pech, J.C.; Latché, A.; Li, Z.G.; Chervin, C. Chloroplast to chromoplast transition in tomato fruit: Spectral confocal microscopy analyses of carotenoids and chlorophylls in isolated plastids and time-lapse recording on intact live tissue. Ann. Bot. 2011, 108, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Schweiggert, R.M.; Steingass, C.B.; Heller, A.; Esquivel, P.; Carle, R. Characterization of chromoplasts and carotenoids of red-and yellow-fleshed papaya (Carica papaya L.). Planta 2011, 234, 1031–1044. [Google Scholar] [CrossRef] [PubMed]
- Grilli Caiola, M.; Canini, A. Ultrastructure of chromoplasts and other plastids in Crocus sativus L. (Iridaceae). Plant Biosyst. 2004, 138, 43–52. [Google Scholar] [CrossRef]
- Paolillo, D.J.; Garvin, D.F.; Parthasarathy, M.V. The chromoplasts of mutants of cauliflower (Brassica oleracea L. var. botrytis). Protoplasma 2004, 224, 245–253. [Google Scholar] [CrossRef]
- Zhou, C.H.; Xu, C.J.; Sun, C.D.; Li, X.; Chen, K.S. Carotenoids in white- and red-fleshed loquat fruits. J. Agric. Food Chem. 2007, 55, 7822–7830. [Google Scholar] [CrossRef]
- Fu, X.M.; Kong, W.B.; Peng, G.; Zhou, J.Y.; Azam, M.; Xu, C.J.; Grierson, D.; Chen, K.S. Plastid structure and carotenogenic gene expression in red- and white-fleshed loquat (Eriobotrya japonica) fruits. J. Exp. Bot. 2012, 63, 341–354. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Sun, C.D.; Zhang, L.L.; Dai, X.; Xu, C.J.; Chen, K.S. Preferential accumulation of orange-colored carotenoids in Ponkan (Citrus reticulata) fruit peel following postharvest application of ethylene or ethephon. Sci. Hort. 2010, 126, 229–235. [Google Scholar] [CrossRef]
- Olmo, M.; García, J.M. Nondestructive methods to evaluate maturity level of oranges. J. Food Sci. 2000, 65, 365–369. [Google Scholar] [CrossRef]
- Forth, D.; Pyke, K.A. The suffulta mutation in tomato reveals a novel method of plastid replication during fruit ripening. J. Exp. Bot. 2006, 57, 1971–1979. [Google Scholar] [CrossRef] [PubMed]
- Barsan, C.; Zouine, M.; Maza, E.; Bian, W.; Egea, I.; Rossignol, M.; Bouyssie, D.; Pichereaux, C.; Purgatto, E.; Bouzayen, M.; Latché, A.; Pech, J.C. Proteomic analysis of chloroplast-to-chromoplast transition in tomato reveals metabolic shifts coupled with disrupted thylakoid biogenesis machinery and elevated energy-production components. Plant Physiol. 2012, 160, 708–725. [Google Scholar] [CrossRef] [PubMed]
- Maass, D.; Arango, J.; Wüst, F.; Beyer, P.; Welsch, R. Carotenoid crystal formation in Arabidopsis and carrot roots caused by increased phytoene synthase protein levels. PLoS ONE 2009, 4, e6373. [Google Scholar] [CrossRef] [PubMed]
- McGuire, R.G. Reporting of objective color measurements. HortScience 1992, 27, 1254–1255. [Google Scholar]
Sample Availability: Not applicable. |



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, P.; Wang, R.; Zhu, C.; Fu, X.; Wang, S.; Grierson, D.; Xu, C. Microscopic Analyses of Fruit Cell Plastid Development in Loquat (Eriobotrya japonica) during Fruit Ripening. Molecules 2019, 24, 448. https://doi.org/10.3390/molecules24030448
Lu P, Wang R, Zhu C, Fu X, Wang S, Grierson D, Xu C. Microscopic Analyses of Fruit Cell Plastid Development in Loquat (Eriobotrya japonica) during Fruit Ripening. Molecules. 2019; 24(3):448. https://doi.org/10.3390/molecules24030448
Chicago/Turabian StyleLu, Pengjun, Ruqian Wang, Changqing Zhu, Xiumin Fu, Shasha Wang, Don Grierson, and Changjie Xu. 2019. "Microscopic Analyses of Fruit Cell Plastid Development in Loquat (Eriobotrya japonica) during Fruit Ripening" Molecules 24, no. 3: 448. https://doi.org/10.3390/molecules24030448
APA StyleLu, P., Wang, R., Zhu, C., Fu, X., Wang, S., Grierson, D., & Xu, C. (2019). Microscopic Analyses of Fruit Cell Plastid Development in Loquat (Eriobotrya japonica) during Fruit Ripening. Molecules, 24(3), 448. https://doi.org/10.3390/molecules24030448




