Lectin PLL3, a Novel Monomeric Member of the Seven-Bladed β-Propeller Lectin Family
Abstract
:1. Introduction
2. Results
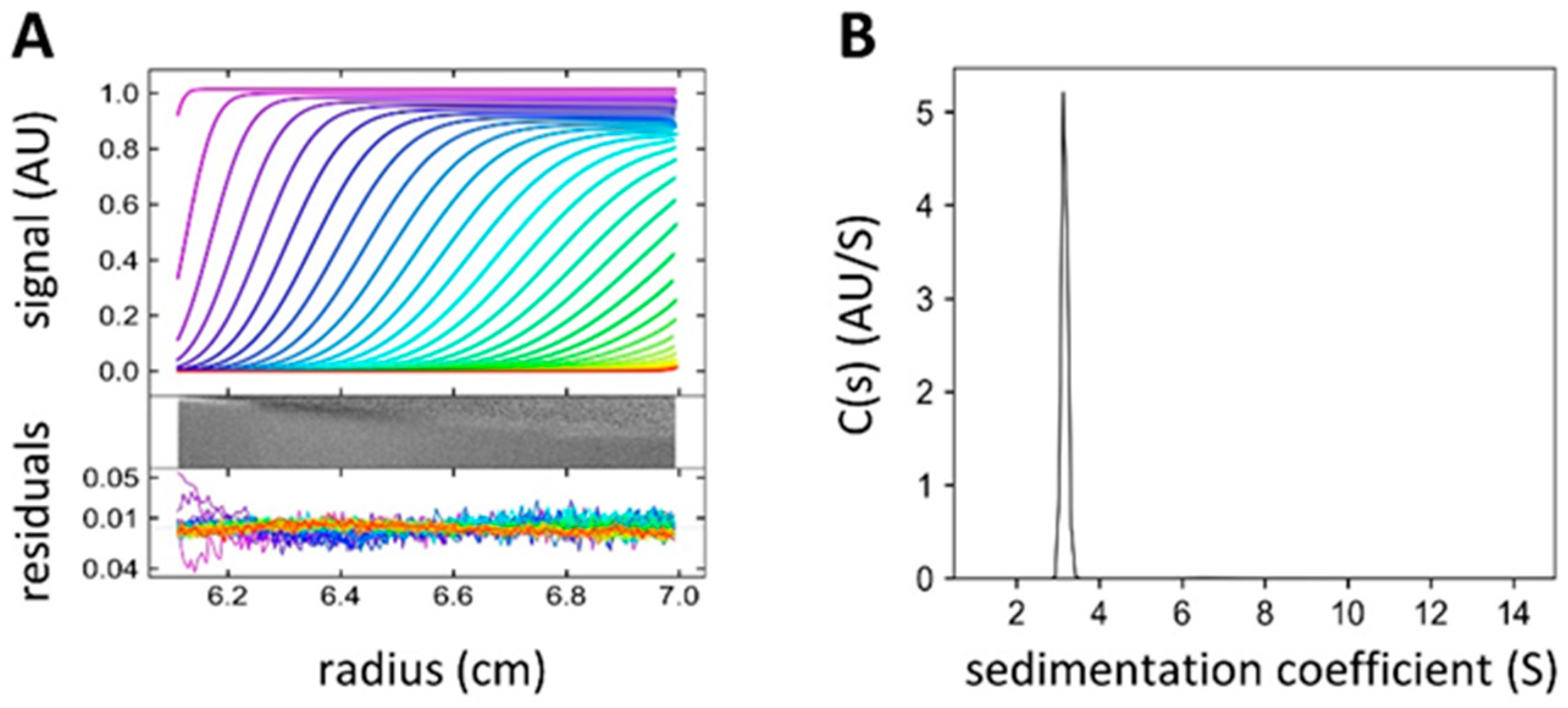
2.1. Production and Basic Characterization of PLL3
2.2. Carbohydrate Specificity
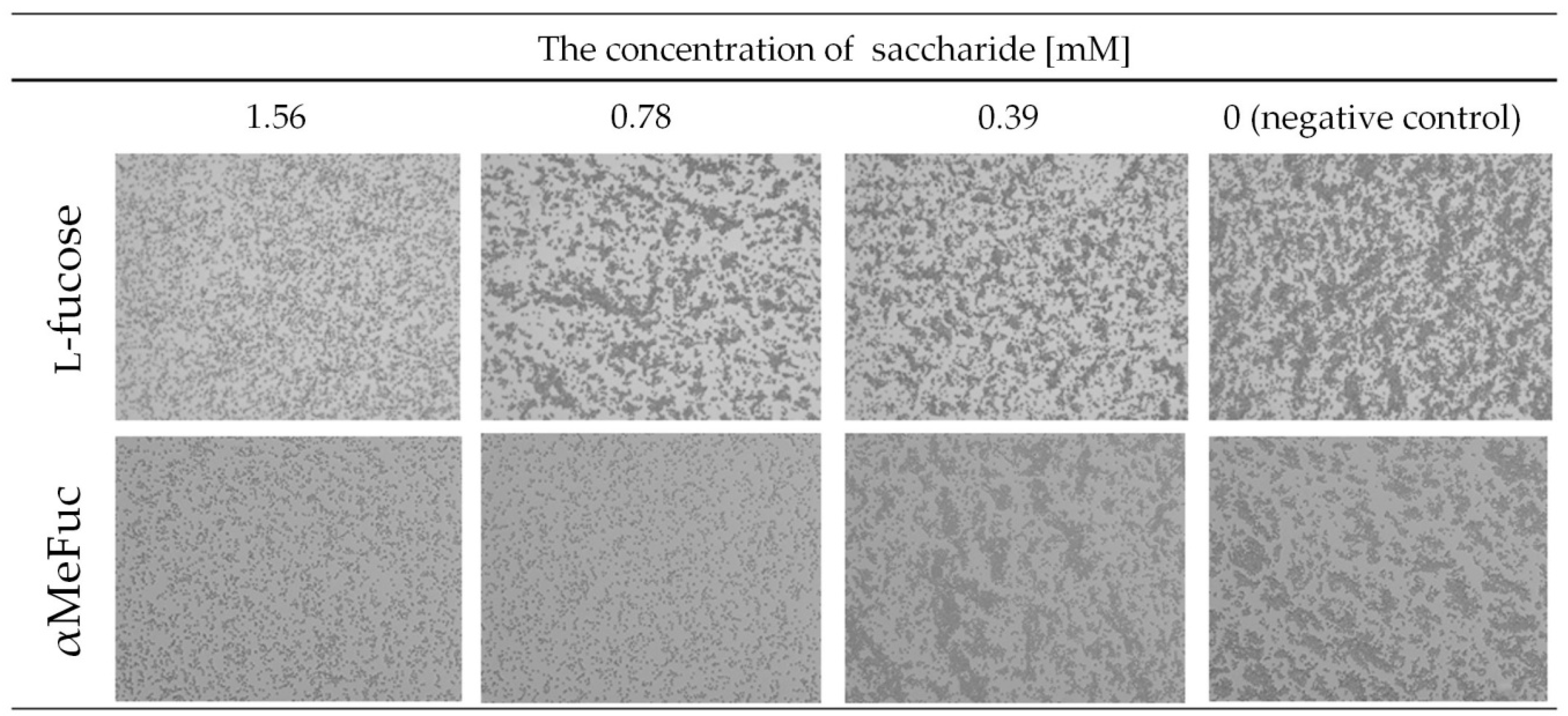
2.2.1. Hemagglutination
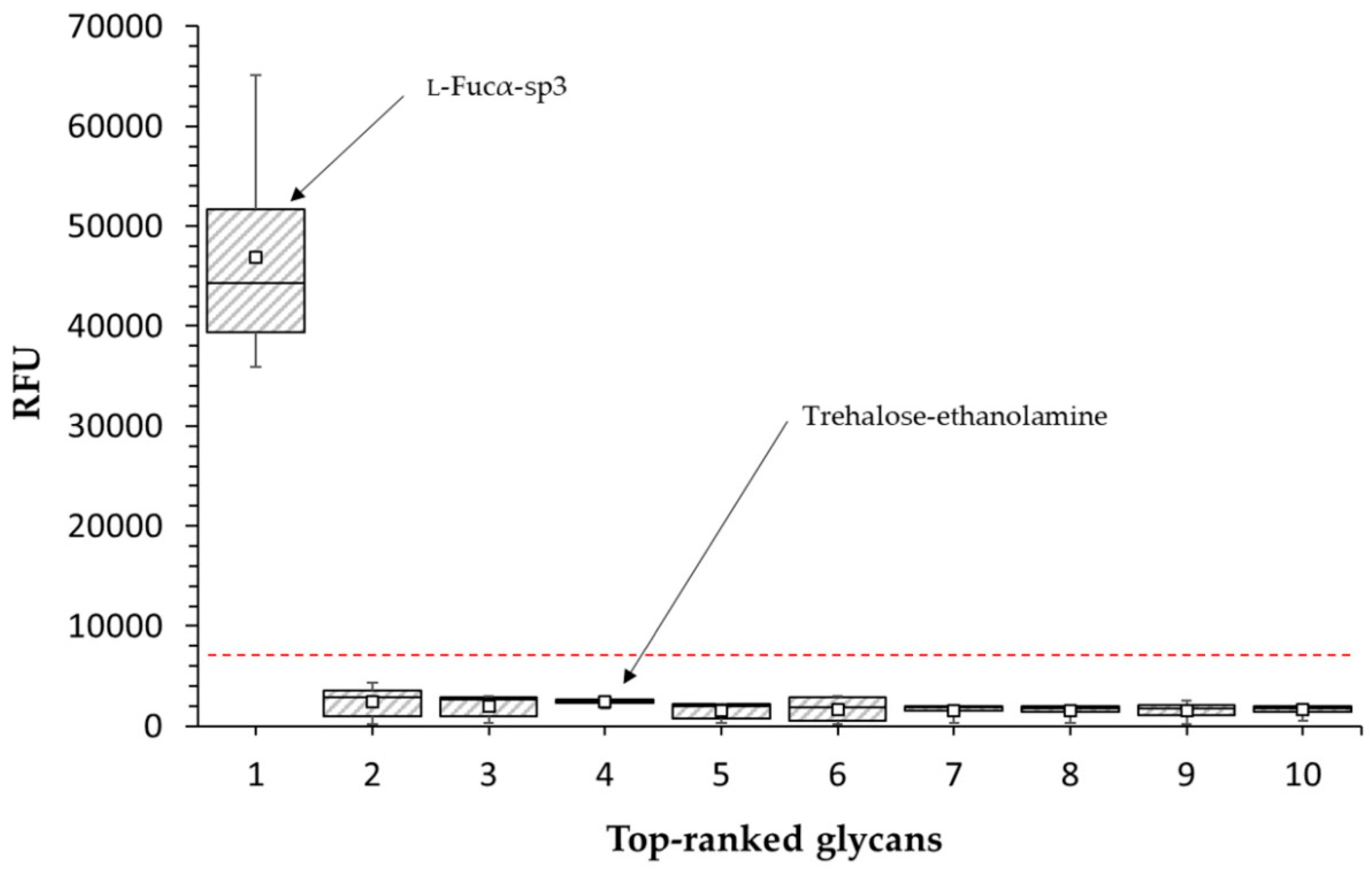
2.2.2. Glycan Array
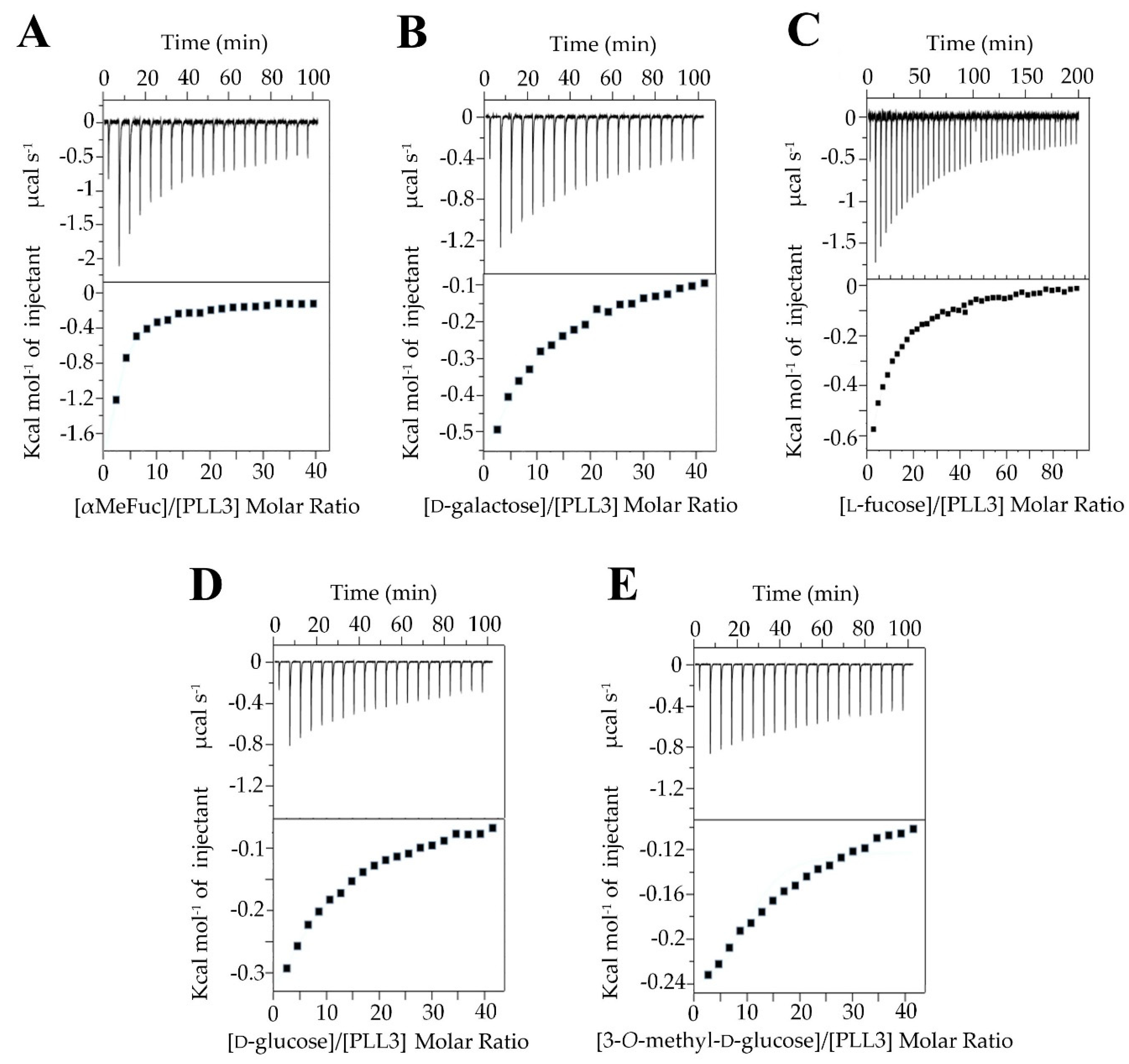
2.2.3. Isothermal Titration Calorimetry (ITC)
2.2.4. Surface Plasmon Resonance (SPR)
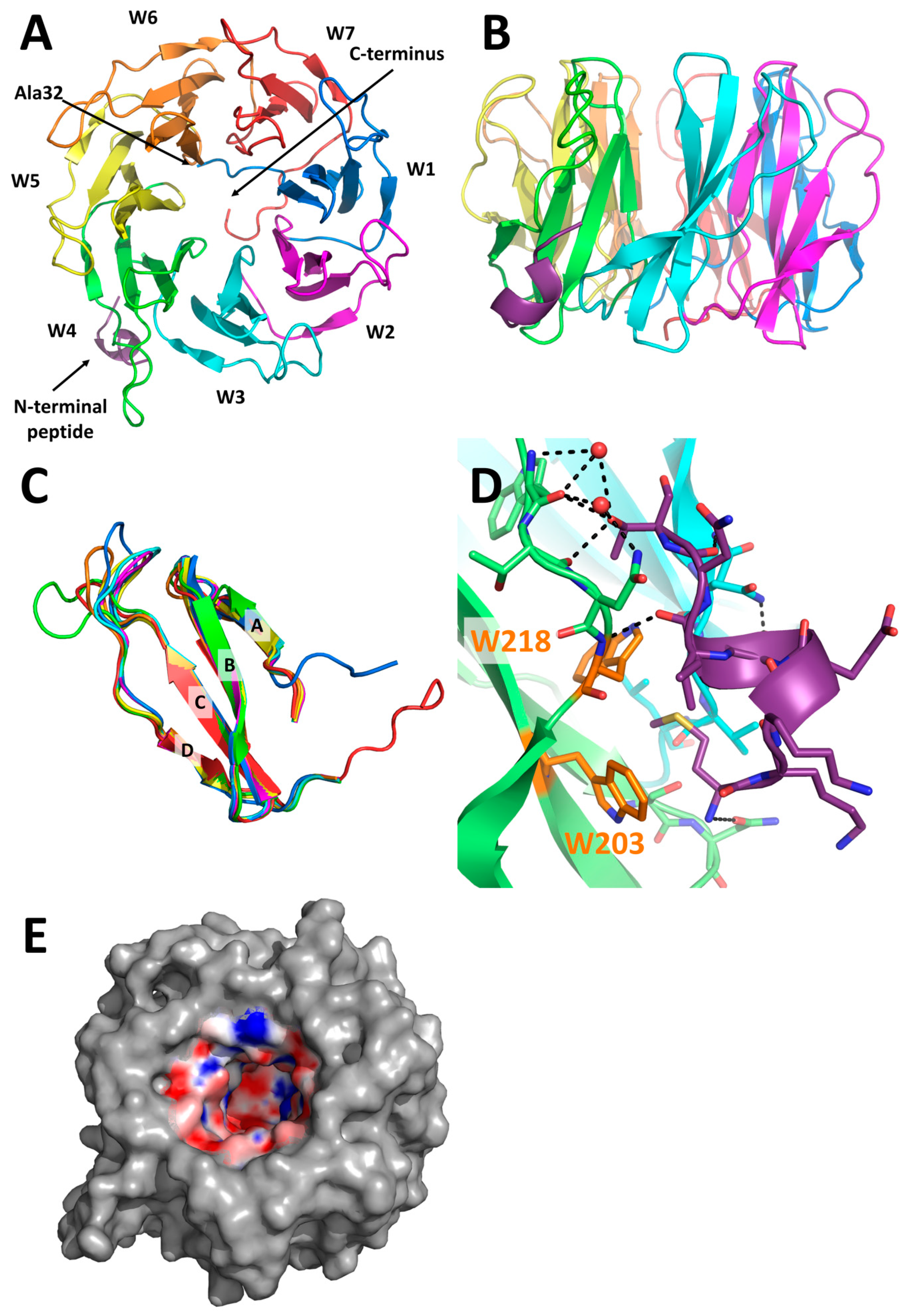
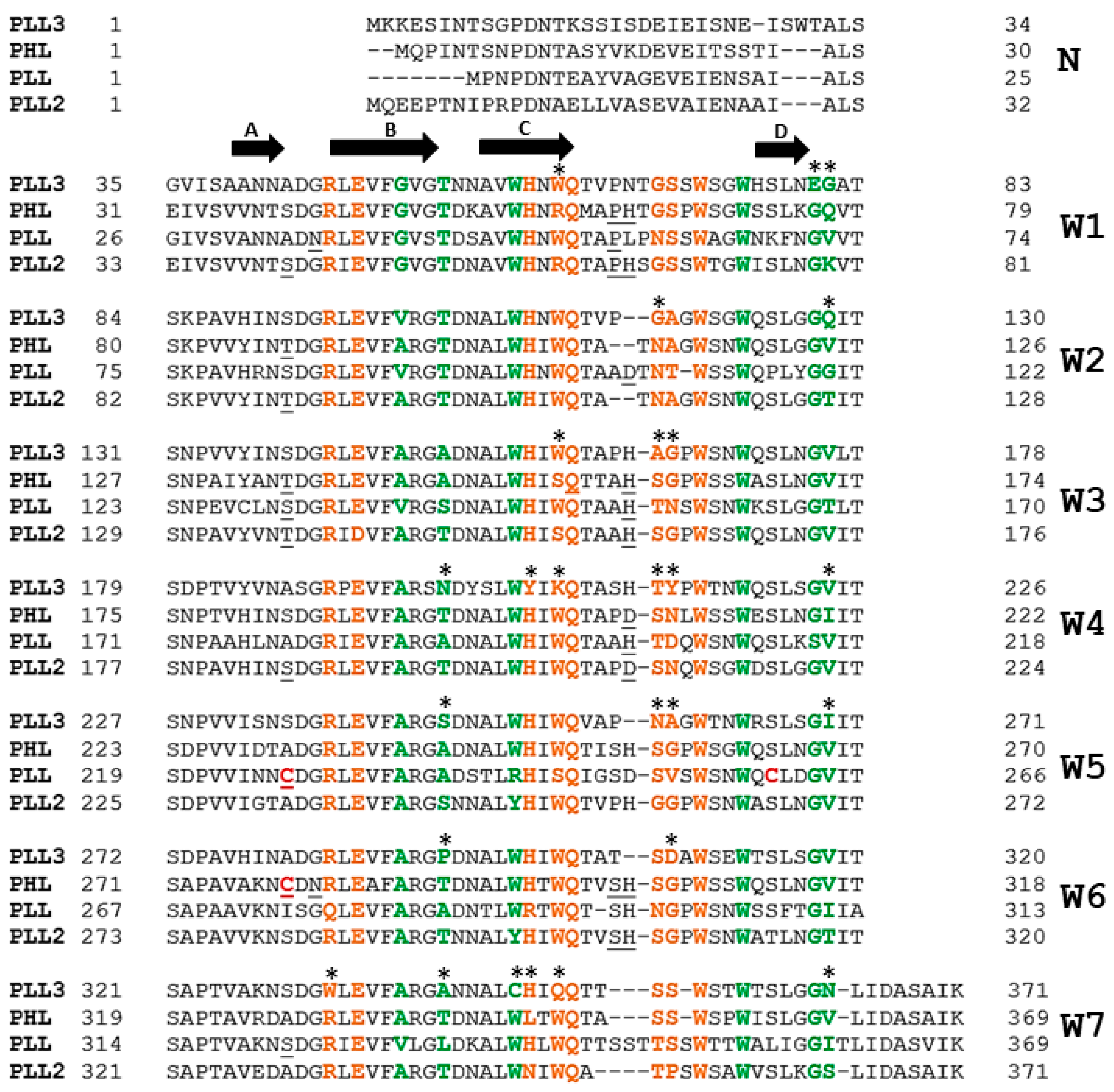
2.3. X-Ray Structure of PLL3
3. Discussion
4. Material and Methods
4.1. Materials
4.2. PLL3 Identification and Cloning
4.3. PLL3 Purification
4.4. Analytical Ultracentrifugation (AUC)
4.5. Hemagglutination
4.6. Glycan Array
4.7. Isothermal Titration Calorimetry
4.8. Surface Plasmon Resonance
4.9. Crystallization
4.10. Data Collection and Structure Determination
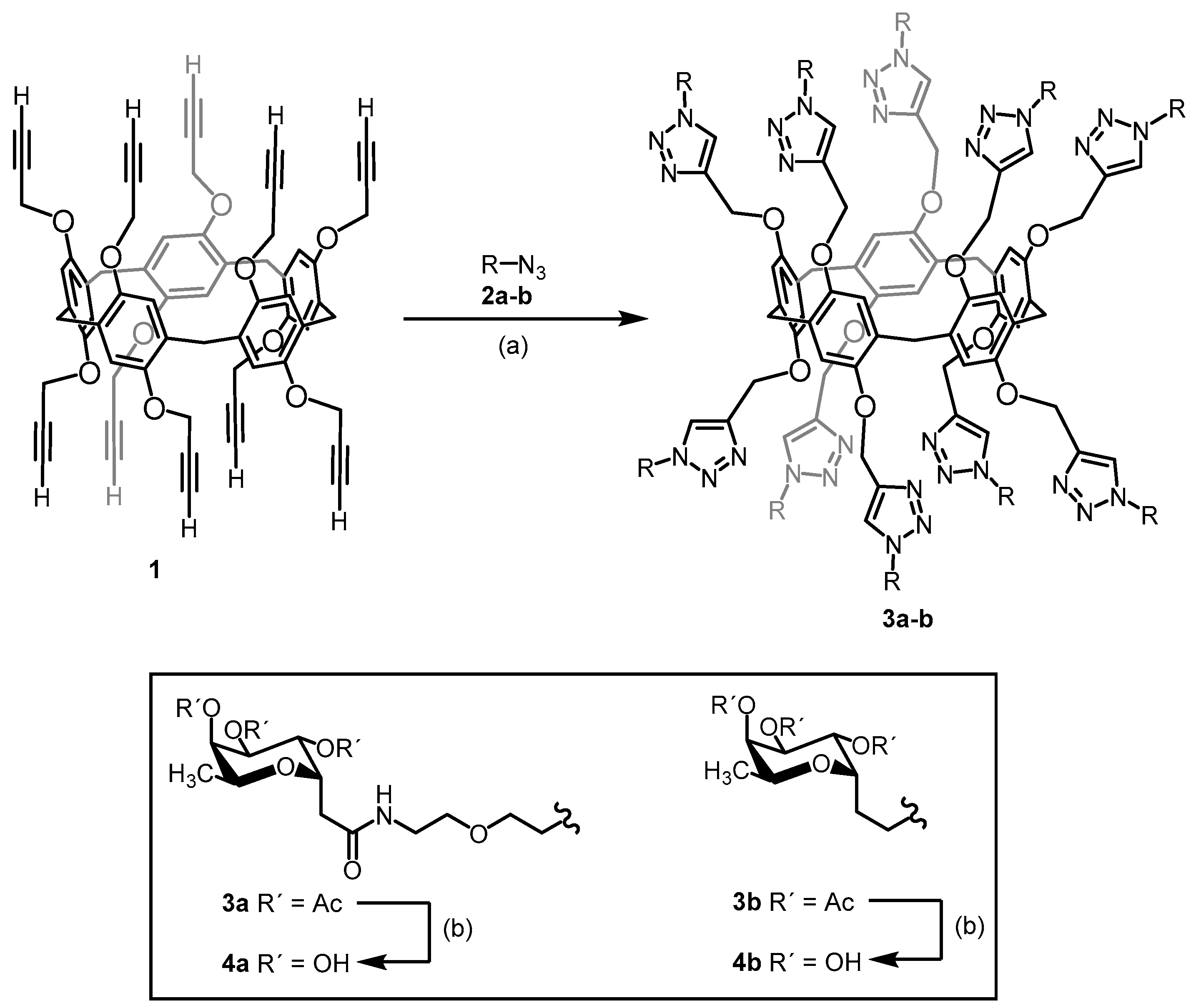
4.11. Synthesis of Decavalent Fucosides
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Ethics Statement
References
- Adeolu, M.; Alnajar, S.; Naushad, S.; Gupta, R.S. Genome-based phylogeny and taxonomy of the ‘Enterobacteriales’: Proposal for Enterobacterales ord. nov. divided into the families Enterobacteriaceae, Erwiniaceae fam. nov., Pectobacteriaceae fam. nov., Yersiniaceae fam. nov., Hafniaceae fam. nov., Morganellaceae fam. nov., and Budviciaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2016, 66, 5575–5599. [Google Scholar] [PubMed]
- Waterfield, N.R.; Ciche, T.; Clarke, D. Photorhabdus and a Host of Hosts. Annu. Rev. Microbiol. 2009, 63, 557–574. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, A.K.; Glaeser, A.; Tobias, N.J.; Heermann, R.; Bode, H.B. Heterogeneous regulation of bacterial natural product biosynthesis via a novel transcription factor. Heliyon 2016, 2, e00197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, D.J. Photorhabdus: A model for the analysis of pathogenicity and mutualism. Cell. Microbiol. 2008, 10, 2159–2167. [Google Scholar] [CrossRef] [PubMed]
- Ciche, T.A.; Kim, K.-S.; Kaufmann-Daszczuk, B.; Nguyen, K.C.Q.; Hall, D.H. Cell invasion and matricide during Photorhabdus luminescens transmission by Heterorhabditis bacteriophora nematodes. Appl. Environ. Microbiol. 2008, 74, 2275–2287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duchaud, E.; Rusniok, C.; Frangeul, L.; Buchrieser, C.; Givaudan, A.; Taourit, S.; Bocs, S.; Boursaux-Eude, C.; Chandler, M.; Charles, J.-F.; et al. The genome sequence of the entomopathogenic bacterium Photorhabdus luminescens. Nat. Biotechnol. 2003, 21, 1307–1313. [Google Scholar] [CrossRef]
- Ciche, T.A.; Bintrim, S.B.; Horswill, A.R.; Ensign, J.C. A phosphopantetheinyl transferase homolog Is essential for Photorhabdus luminescens to support growth and reproduction of the entomopathogenic nematode Heterorhabditis bacteriophora. J. Bacteriol. 2001, 183, 3117–3126. [Google Scholar] [CrossRef] [Green Version]
- Ciche, T.A.; Ensign, J.C. For the insect pathogen Photorhabdus luminescens, which end of a nematode is out? Appl. Environ. Microbiol. 2003, 69, 1890–1897. [Google Scholar] [CrossRef] [Green Version]
- Sharon, N. Lectins: Carbohydrate-specific reagents and biological recognition molecules. J. Biol. Chem. 2007, 282, 2753–2764. [Google Scholar] [CrossRef] [Green Version]
- Lis, H.; Sharon, N. Lectins: Carbohydrate-specific proteins that mediate cellular recognition. Chem. Rev. 1998, 98, 637–674. [Google Scholar] [CrossRef]
- Sharon, N. History of lectins: From hemagglutinins to biological recognition molecules. Glycobiology 2004, 14, 53R–62R. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varki, A. Essentials of Glycobiology, 3rd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2017; ISBN 978-1-62182-132-8. [Google Scholar]
- Audfray, A.; Claudinon, J.; Abounit, S.; Ruvoën-Clouet, N.; Larson, G.; Smith, D.F.; Wimmerová, M.; Le Pendu, J.; Römer, W.; Varrot, A.; et al. Fucose-binding Lectin from opportunistic pathogen Burkholderia ambifaria binds to both plant and human oligosaccharidic epitopes. J. Biol. Chem. 2012, 287, 4335–4347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houser, J.; Komarek, J.; Kostlanova, N.; Cioci, G.; Varrot, A.; Kerr, S.C.; Lahmann, M.; Balloy, V.; Fahy, J.V.; Chignard, M.; et al. A soluble fucose-specific lectin from Aspergillus fumigatus conidia—Structure, specificity and possible role in fungal pathogenicity. PLoS ONE 2013, 8, e83077. [Google Scholar] [CrossRef] [PubMed]
- Jančaříková, G.; Houser, J.; Dobeš, P.; Demo, G.; Hyršl, P.; Wimmerová, M. Characterization of novel bangle lectin from Photorhabdus asymbiotica with dual sugar-binding specificity and its effect on host immunity. PLoS Pathog. 2017, 13, e1006564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Sýkorová, P.; Demo, G.; Dobeš, P.; Hyršl, P.; Wimmerová, M. A novel fucose-binding lectin from Photorhabdus luminescens (PLL) with an unusual heptabladed β-propeller tetrameric structure. J. Biol. Chem. 2016, 291, 25032–25049. [Google Scholar] [CrossRef] [Green Version]
- Fujdiarová, E.; Houser, J.; Dobeš, P.; Paulíková, G.; Kondakov, N.; Kononov, L.; Hyršl, P.; Wimmerová, M. Heptabladded β-propeller lectins PLL2 and PHL from Photorhabdus spp. recognize O-methylated sugars and influence the host immune system. submitted.
- Staudacher, E. Methylation--an uncommon modification of glycans. Biol. Chem. 2012, 393, 675–685. [Google Scholar] [CrossRef] [Green Version]
- Wohlschlager, T.; Butschi, A.; Grassi, P.; Sutov, G.; Gauss, R.; Hauck, D.; Schmieder, S.S.; Knobel, M.; Titz, A.; Dell, A.; et al. Methylated glycans as conserved targets of animal and fungal innate defense. Proc. Natl. Acad. Sci. USA. 2014, 111, E2787–E2796. [Google Scholar] [CrossRef] [Green Version]
- Sweet, L.; Zhang, W.; Torres-Fewell, H.; Serianni, A.; Boggess, W.; Schorey, J. Mycobacterium avium glycopeptidolipids require specific acetylation and methylation patterns for signaling through Toll-like receptor 2. J. Biol. Chem. 2008, 283, 33221–33231. [Google Scholar] [CrossRef] [Green Version]
- Schuck, P. Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and lamm equation modeling. Biophys. J. 2000, 78, 1606–1619. [Google Scholar] [CrossRef] [Green Version]
- Brautigam, C.A. Calculations and publication-quality illustrations for analytical ultracentrifugation data. Meth. Enzym. 2015, 562, 109–133. [Google Scholar] [PubMed]
- Jecklin, M.C.; Schauer, S.; Dumelin, C.E.; Zenobi, R. Label-free determination of protein-ligand binding constants using mass spectrometry and validation using surface plasmon resonance and isothermal titration calorimetry. J. Mol. Recognit. 2009, 22, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Myszka, D.G. Kinetic analysis of macromolecular interactions using surface plasmon resonance biosensors. Curr. Opin. Biotechnol. 1997, 8, 50–57. [Google Scholar] [CrossRef]
- Walski, T.; De Schutter, K.; Van Damme, E.J.M.; Smagghe, G. Diversity and functions of protein glycosylation in insects. Insect Biochem. Mol. Biol. 2017, 83, 21–34. [Google Scholar] [CrossRef]
- Stanton, R.; Hykollari, A.; Eckmair, B.; Malzl, D.; Dragosits, M.; Palmberger, D.; Wang, P.; Wilson, I.B.H.; Paschinger, K. The underestimated N-glycomes of lepidopteran species. Biochim. Et Biophys. Acta (Bba) Gen. Subj. 2017, 1861, 699–714. [Google Scholar] [CrossRef] [Green Version]
- Staudacher, E. Mucin-Type O-Glycosylation in Invertebrates. Molecules 2015, 20, 10622–10640. [Google Scholar] [CrossRef]
- Hunter, S.W.; Fujiwara, T.; Brennan, P.J. Structure and antigenicity of the major specific glycolipid antigen of Mycobacterium leprae. J. Biol. Chem. 1982, 257, 15072–15078. [Google Scholar]
- Kondakov, N.N.; Mel’nikova, T.M.; Chekryzhova, T.V.; Mel´nikova, M.V.; Zinin, A.I.; Torgov, V.I.; Chizhov, A.O.; Kononov, L.O. Synthesis of a disaccharide of phenolic glycolipid from Mycobacterium leprae (PGL-I) and its conjugates with bovine serum albumin. Russ. Chem. Bull. 2015, 64, 1142–1148. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Adamová, L.; Malinovská, L.; Wimmerová, M. New sensitive detection method for lectin hemagglutination using microscopy. Microsc. Res. Tech. 2014, 77, 841–849. [Google Scholar] [CrossRef]
- Krug, M.; Weiss, M.S.; Heinemann, U.; Mueller, U. XDSAPP: A graphical user interface for the convenient processing of diffraction data using XDS. J. Appl. Cryst. 2012, 45, 568–572. [Google Scholar] [CrossRef]
- Winn, M.D.; Ballard, C.C.; Cowtan, K.D.; Dodson, E.J.; Emsley, P.; Evans, P.R.; Keegan, R.M.; Krissinel, E.B.; Leslie, A.G.W.; McCoy, A.; et al. Overview of the CCP 4 suite and current developments. Acta Cryst. D Biol. Cryst. 2011, 67, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Vagin, A.; Teplyakov, A. Molecular replacement with MOLREP. Acta Cryst. D Biol. Cryst. 2010, 66, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtan, K. Features and development of Coot. Acta Cryst. D Biol. Cryst. 2010, 66, 486–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murshudov, G.N.; Skubák, P.; Lebedev, A.A.; Pannu, N.S.; Steiner, R.A.; Nicholls, R.A.; Winn, M.D.; Long, F.; Vagin, A.A. REFMAC 5 for the refinement of macromolecular crystal structures. Acta Cryst. D Biol. Cryst. 2011, 67, 355–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nierengarten, I.; Nierengarten, J.-F. The impact of copper-catalyzed alkyne-azide 1,3-dipolar cycloaddition in fullerene chemistry. Chem. Rec. 2015, 15, 31–51. [Google Scholar] [CrossRef] [PubMed]
- Buffet, K.; Nierengarten, I.; Galanos, N.; Gillon, E.; Holler, M.; Imberty, A.; Matthews, S.E.; Vidal, S.; Vincent, S.P.; Nierengarten, J.-F. Pillar[5]arene-based glycoclusters: Synthesis and multivalent binding to pathogenic bacterial lectins. Chemistry 2016, 22, 2955–2963. [Google Scholar] [CrossRef]
- Bertolotti, B.; Sutkeviciute, I.; Ambrosini, M.; Ribeiro-Viana, R.; Rojo, J.; Fieschi, F.; Dvořáková, H.; Kašáková, M.; Parkan, K.; Hlaváčková, M.; et al. Polyvalent C-glycomimetics based on l-fucose or d-mannose as potent DC-SIGN antagonists. Org. Biomol. Chem. 2017, 15, 3995–4004. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Lowe, A.B.; Bowman, C.N. Thiol-click chemistry: A multifaceted toolbox for small molecule and polymer synthesis. Chem. Soc. Rev. 2010, 39, 1355. [Google Scholar] [CrossRef]
- Franc, G.; Kakkar, A.K. “Click” methodologies: Efficient, simple and greener routes to design dendrimers. Chem. Soc. Rev. 2010, 39, 1536. [Google Scholar] [CrossRef]
- Becer, C.R.; Hoogenboom, R.; Schubert, U.S. Click Chemistry beyond metal-catalyzed cycloaddition. Angew. Chem. Int. Ed. 2009, 48, 4900–4908. [Google Scholar] [CrossRef] [PubMed]
- Meldal, M.; Tornøe, C.W. Cu-Catalyzed Azide−Alkyne Cycloaddition. Chem. Rev. 2008, 108, 2952–3015. [Google Scholar] [CrossRef] [PubMed]
- Hein, J.E.; Fokin, V.V. Copper-catalyzed azide–alkyne cycloaddition (CuAAC) and beyond: New reactivity of copper(i) acetylides. Chem. Soc. Rev. 2010, 39, 1302. [Google Scholar] [CrossRef] [PubMed]
- Finn, M.G.; Fokin, V.V. Click chemistry: Function follows form. Chem. Soc. Rev. 2010, 39, 1231. [Google Scholar] [CrossRef]
- Nierengarten, I.; Nothisen, M.; Sigwalt, D.; Biellmann, T.; Holler, M.; Remy, J.-S.; Nierengarten, J.-F. Polycationic pillar[5]arene derivatives: Interaction with DNA and biological applications. Chem. Eur. J. 2013, 19, 17552–17558. [Google Scholar] [CrossRef]
- Kašáková, M.; Bertolotti, B.; Dong, L.; Rousset, A.; Kánya, N.; Moravcová, J.; Vidal, S. 3-(2,3,4-Tri-O-acetyl-α-l-fucopyranosyl)-prop-1-ene. In Carbohydrate Chemistry: Proven Synthetic Methods; Kosma, P., Ed.; CRC Press: Boca Raton, FL, USA, 2020; Volume 5, in press. [Google Scholar]
- Kolomiets, E.; Johansson, E.M.V.; Renaudet, O.; Darbre, T.; Reymond, J.-L. Neoglycopeptide dendrimer libraries as a source of lectin binding ligands. Org. Lett. 2007, 9, 1465–1468. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
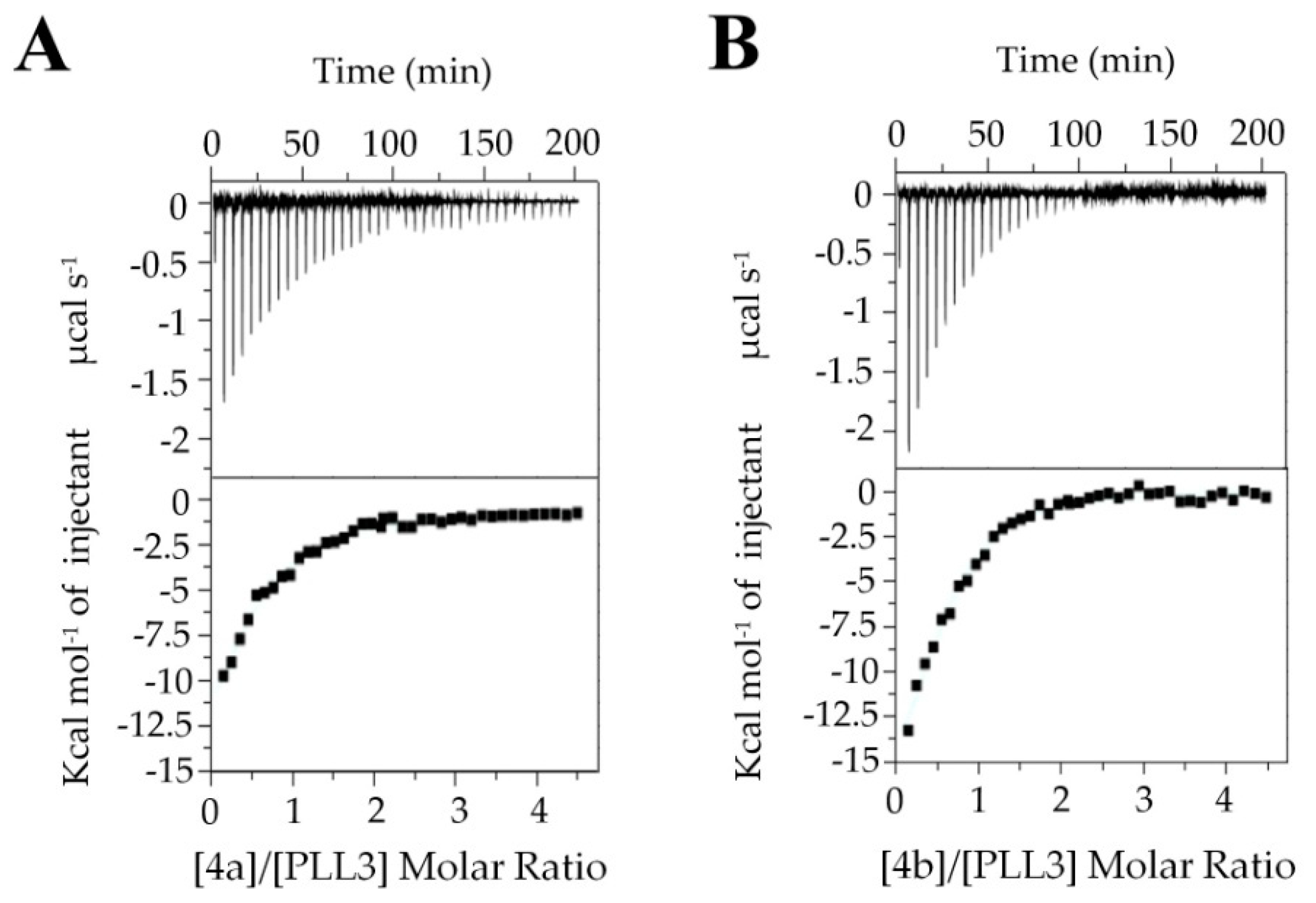
| Ligand | Stoichiometry | ||||
|---|---|---|---|---|---|
| 3 | 4 | 5 | 6 | 7 | |
| methyl α-l-fucopyranoside | 0.7 ± 0.02 | 0.7 ± 0.03 | 0.6 ± 0.03 | 0.6 ± 0.03 | 0.6 ± 0.03 |
| d-galactose | 2.8 ± 0.14 | 2.7 ± 0.14 | 2.6 ± 0.13 | 2.5 ± 0.13 | 2.3 ± 0.13 |
| l-fucose | 3.4 ± 0.12 | 3.3 ± 0.12 | 3.2 ± 0.11 | 3.1 ± 0.10 | 3.0 ± 0.09 |
| d-glucose | 3.7 ± 0.08 | 3.6 ± 0.08 | 3.4 ± 0.08 | 3.3 ± 0.07 | 3.1 ± 0.07 |
| 3-O-methyl-d-glucose | 11.3 ± 1.55 | 11.1 ± 1.50 | 10.9 ± 1.50 | 10.7 ± 1.50 | 10.4 ± 1.45 |
| Ligand | IC50 [mM] |
|---|---|
| methyl α-l-fucopyranoside | 1.04 ± 0.05 |
| l-fucose | 2.51 ± 0.09 |
| 3-O-methyl-d-glucose | 26.36 ± 2.84 |
| d-galactose | 156.46 ± 53.87 |
| d-glucose | >400 |
| Data Collection | |
| Protein Data Bank Code | 6T96 |
| Space group | P212121 |
| Wavelength (Å) | 0.91842 |
| Unit cell parameters (Å) | |
| a/b/c | 56.58/69.42/76.71 |
| α/β/γ | 90/90/90 |
| Resolution range (Å) | 51.46–1.65 (1.74–1.65) |
| Total number of observations | 271406 |
| Unique reflections | 37089 |
| Completeness (%) | 99.9 (99.2) |
| Rmerge | 0.143 (0.989) |
| CC1/2 (%) | 99.7 (70.8) |
| Multiplicity | 7.3 (7.4) |
| <I/σ(I)> | 8.2 (1.7) |
| Refinement Statistics | |
| Refine resolution (Å) | 45.53–1.65 |
| Reflection used | 35215 |
| Reflection used for Rfree | 1804 |
| Rwork factor (%) | 16.93 |
| Rfree factor (%) | 19.60 |
| Root mean square deviation (RMSD) | |
| Bond lengths (Å) | 0.009 |
| Bond angles (degree) | 1.48 |
| Chiral volumes (Å3) | 0.073 |
| Number of non-hydrogen atoms (total) | 3114 |
| Number of water molecules | 325 |
| Ramachandran plot (%) | |
| Residues in most favorable regions | 96.6 |
| Residues in allowed regions | 3.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faltinek, L.; Fujdiarová, E.; Melicher, F.; Houser, J.; Kašáková, M.; Kondakov, N.; Kononov, L.; Parkan, K.; Vidal, S.; Wimmerová, M. Lectin PLL3, a Novel Monomeric Member of the Seven-Bladed β-Propeller Lectin Family. Molecules 2019, 24, 4540. https://doi.org/10.3390/molecules24244540
Faltinek L, Fujdiarová E, Melicher F, Houser J, Kašáková M, Kondakov N, Kononov L, Parkan K, Vidal S, Wimmerová M. Lectin PLL3, a Novel Monomeric Member of the Seven-Bladed β-Propeller Lectin Family. Molecules. 2019; 24(24):4540. https://doi.org/10.3390/molecules24244540
Chicago/Turabian StyleFaltinek, Lukáš, Eva Fujdiarová, Filip Melicher, Josef Houser, Martina Kašáková, Nikolay Kondakov, Leonid Kononov, Kamil Parkan, Sébastien Vidal, and Michaela Wimmerová. 2019. "Lectin PLL3, a Novel Monomeric Member of the Seven-Bladed β-Propeller Lectin Family" Molecules 24, no. 24: 4540. https://doi.org/10.3390/molecules24244540
APA StyleFaltinek, L., Fujdiarová, E., Melicher, F., Houser, J., Kašáková, M., Kondakov, N., Kononov, L., Parkan, K., Vidal, S., & Wimmerová, M. (2019). Lectin PLL3, a Novel Monomeric Member of the Seven-Bladed β-Propeller Lectin Family. Molecules, 24(24), 4540. https://doi.org/10.3390/molecules24244540






