Antileishmanial Compounds Isolated from Psidium Guajava L. Using a Metabolomic Approach
Abstract
:1. Introduction
2. Results
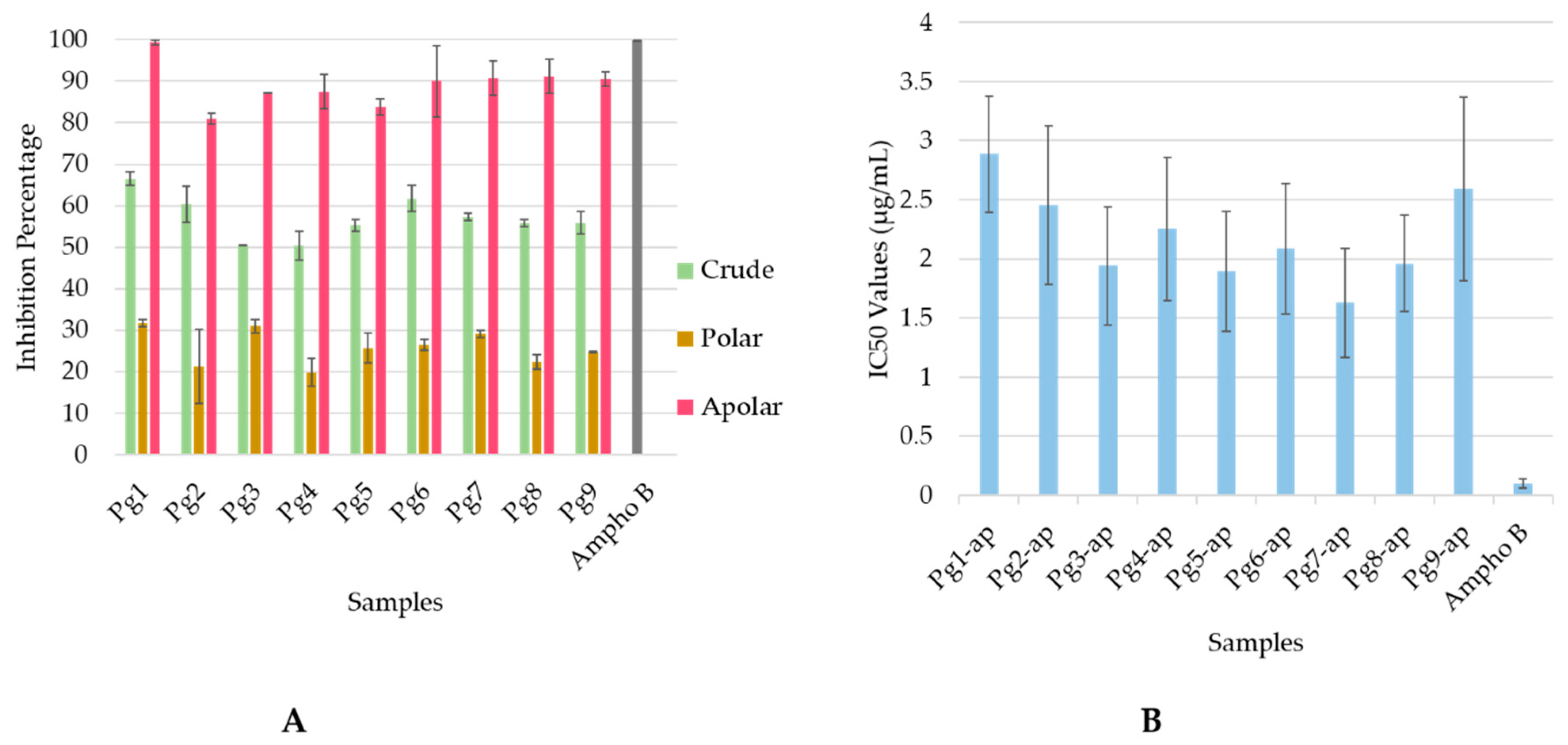
2.1. Antileishmanial Activity
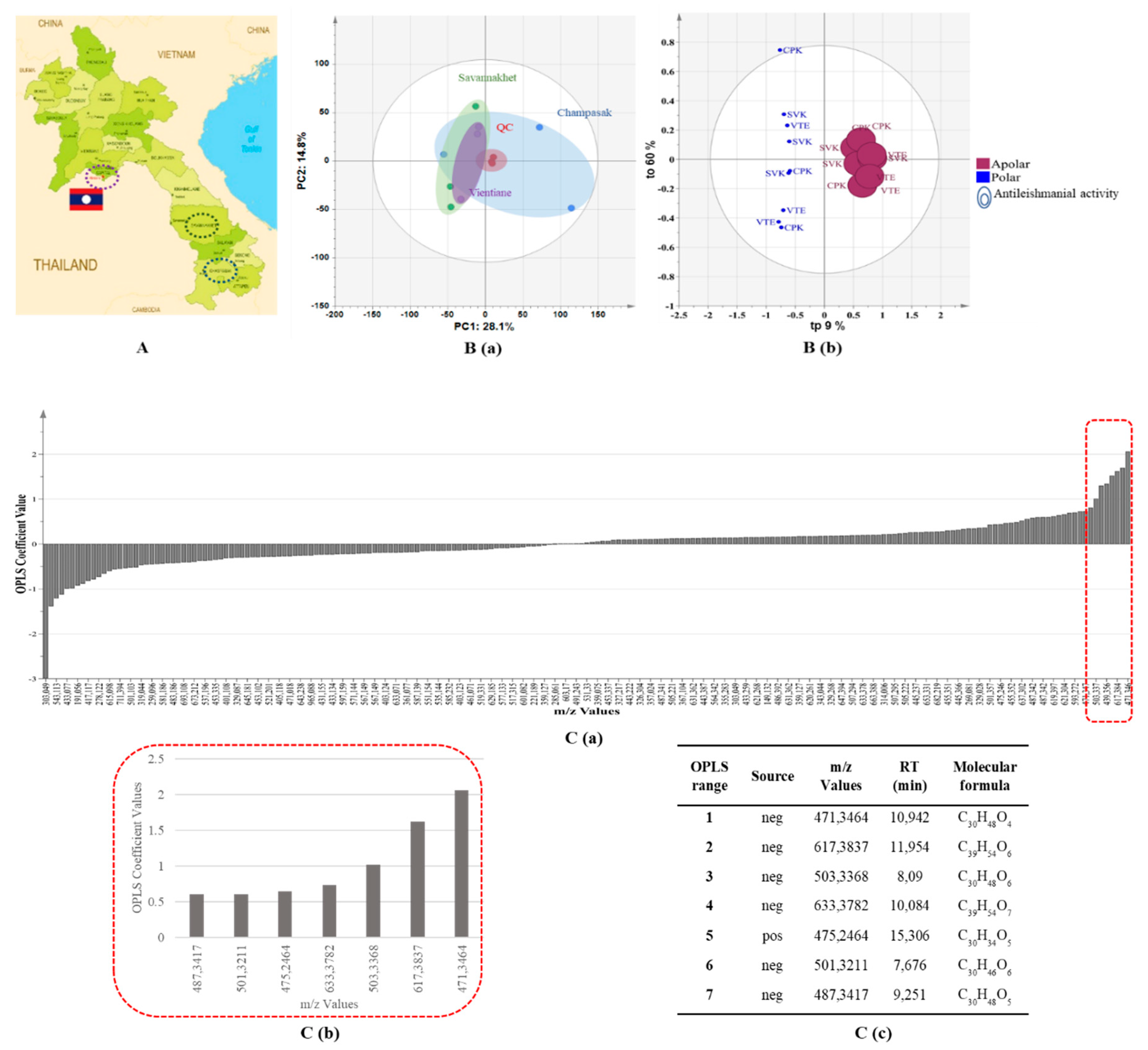
2.2. UHPLC–HRMS-based Metabolomics Approach
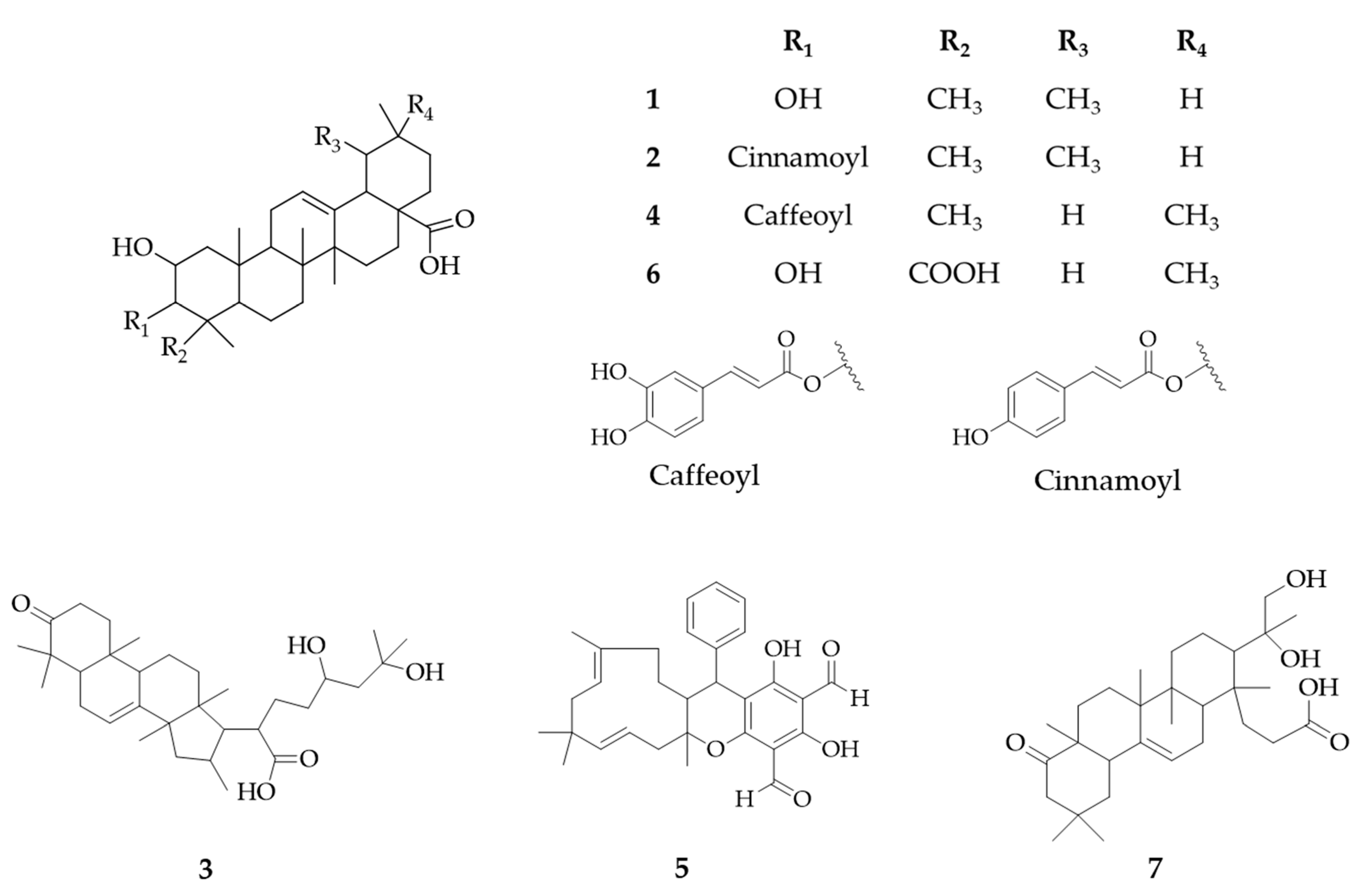
2.3. Identification of Putative Antileishmanial Compounds Based on Liquid–liquid Extraction
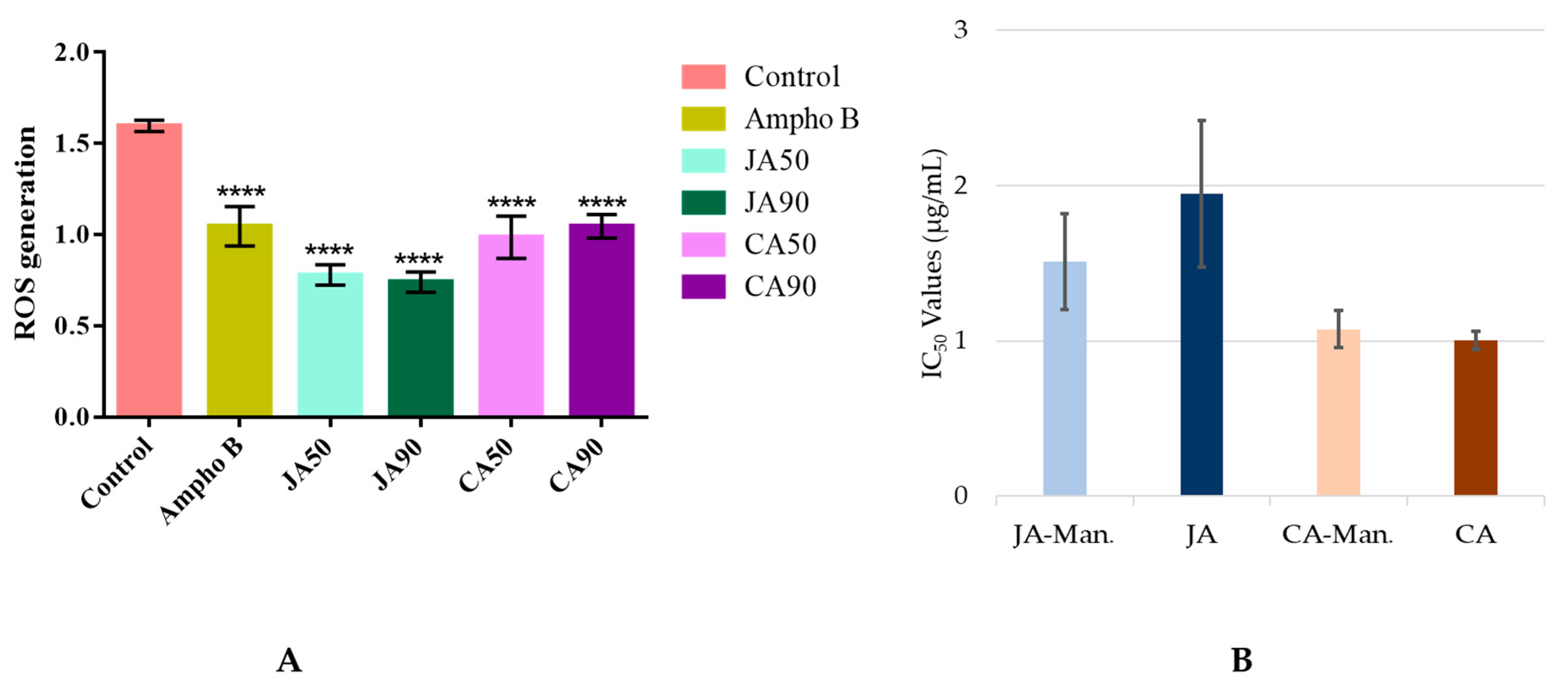
2.4. Putative Mechanism of Action
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Leaf Extraction
4.3. Purification of Compound 1
4.4. UHPLC–HRMS Profiling
4.5. Data Processing
4.6. Statistical Analysis
4.7. Identification of Significant Features
4.8. Antileishmanial Evaluation
4.8.1. Antileishmanial Activity on Promastigotes
4.8.2. Antileishmanial Activity on Axenic Amastigotes
4.9. Cytotoxicity Evaluation
4.10. Determination of Intracellular ROS Generation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef]
- Chappuis, F.; Sundar, S.; Hailu, A.; Ghalib, H.; Rijal, S.; Peeling, R.W.; Alvar, J.; Boelaert, M. Visceral leishmaniasis: What are the needs for diagnosis, treatment and control? Nat. Rev. Microbiol. 2007, 5, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Akbari, M. Worldwide risk factors in leishmaniasis. Asian Pac. J. Trop. Med. 2016, 9, 925–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zulfiqar, B.; Shelper, T.B.; Avery, V.M. Leishmaniasis drug discovery: recent progress and challenges in assay development. Drug Discov. Today 2017, 22, 1516–1531. [Google Scholar] [CrossRef] [PubMed]
- Tiuman, T.S.; Santos, A.O.; Ueda-Nakamura, T.; Filho, B.P.D.; Nakamura, C.V. Recent advances in leishmaniasis treatment. Int. J. Infect. Dis. 2011, 15, 525–532. [Google Scholar] [CrossRef] [Green Version]
- Jannin, J.; Solano, P.; Quick, I.; Debre, P. The francophone network on neglected tropical diseases. PLoS Negl. Trop. Dis. 2017, 11, e0005738. [Google Scholar] [CrossRef] [Green Version]
- Kip, A.E.; Schellens, J.H.M.; Beijnen, J.H.; Dorlo, T.P.C. Clinical pharmacokinetics of systemically administered antileishmanial drugs. Clin. Pharm. 2018, 57, 151–176. [Google Scholar] [CrossRef] [Green Version]
- de Mesquita, M.L.; Desrivot, J.; Bories, C.; Fournet, A.; de Paula, J.E.; Grellier, P.; Espindola, L.S. Antileishmanial and trypanocidal activity of Brazilian Cerrado plants. Mem. Inst. Oswaldo Cruz. Rio De Janerio 2005, 100, 783–787. [Google Scholar] [CrossRef]
- Gutiérrez, R.M.P.; Mitchell, S.; Solis, R.V. Psidium guajava: A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2008, 117, 1–27. [Google Scholar] [CrossRef]
- Dubost, J.M.; Phakeovilay, C.; Her, C.; Bochaton, A.; Elliott, E.; Deharo, E.; Xayvue, M.; Bouamanivong, S.; Bourdy, G. Hmong herbal medicine and herbalists in Lao PDR: pharmacopeia and knowledge transmission. J. Ethnobiol. Ethnomed. 2019, 15, 1–15. [Google Scholar] [CrossRef]
- Sajjekhan, S.; Jha, P.K.; Dakappa, S.S. Pharmacognostic evaluation of Psidium guajava Linn. leaves (Mrytaceae). Med. Aromat. Plant Sci. Biotechnol. 2011, 5, 156–159. [Google Scholar]
- Growther, L.; Sukirtha, K. Phytochemical analysis and antimicrobial properties of leaves and Psidium guajava bark extracts. Asian J. Pharm. Pharm. 2018, 4, 318–323. [Google Scholar] [CrossRef]
- Gawad, S.M.A.; Hetta, M.H.; Ross, S.A.; Badria, F.A.E.R. Antiprotozoal and antimicrobial activity of selected medicinal plants growing in Upper Egypt, Beni-Suef Region. World J. Pharm. Pharm. Sci. 2015, 4, 1720–1740. [Google Scholar]
- Souza, C.E.S.; Silva, A.R.P.; Gomez, M.C.V.; Rolóm, M.; Coronel, C.; Costa, J.G.M.; Sousa, A.K.; Rolim, L.A.; Souza, F.H.S.; Coutinho, H.D.M. Anti-trypanosoma, anti-leishmania and cytotoxic activities of natural products from Psidium brownianum Mart. ex DC. and Psidium guajava var. Pomifera analysed by LC–MS. Acta Trop. 2017, 176, 380–384. [Google Scholar] [CrossRef]
- Chervin, J.; Perio, P.; Martins-Froment, N.; Pharkeovilay, C.; Reybier, K.; Nepveu, F.; Fabre, N.; Talou, T.; Bonzon-Ponnet, V.; Marti, G. Dereplication of natural products from complex extracts by regression analysis and molecular networking: Case study of redox-active compounds from Viola alba subsp. dehnhardtii. Metabolomics 2017, 13, 1–12. [Google Scholar] [CrossRef]
- Chassagne, F.; Haddad, M.; Amiel, A.; Phakeovilay, C.; Manithip, C.; Bourdy, G.; Deharo, E.; Marti, G. A metabolomic approach to identify anti-hepatocarcinogenic compounds from plants used traditionally in the treatment of liver diseases. Fitoterapia 2018, 127, 226–236. [Google Scholar] [CrossRef]
- Ayouni, K.; Berboucha-Rahmani, M.; Kim, H.K.; Atmani, D.; Verpoorte, R.; Choi, Y.H. Metabolomic tool to identify antioxidant compounds of Fraxinus angustifolia leaf and stem bark extracts. Ind. Crops Prod. 2016, 88, 65–77. [Google Scholar] [CrossRef]
- Tsugawa, H.; Kind, T.; Nakabayashi, R.; Yukihira, D.; Tanaka, W.; Cajka, T.; Saito, K.; Fiehn, O.; Arita, M. Hydrogen rearrangement rules: Computational MS/MS fragmentation and structure elucidation using ms-finder software. Anal. Chem. 2016, 88, 7946–7958. [Google Scholar] [CrossRef]
- Fukushima, M.; Matsuyama, F.; Ueda, N.; Egawa, K.; Takemoto, J.; Kajimoto, Y.; Yonaha, N.; Miura, T.; Kaneko, T.; Nishi, Y.; et al. Effect of corosolic acid on postchallenge plasma glucose levels. Diabetes Res. Clin. Pract. 2006, 73, 174–177. [Google Scholar] [CrossRef]
- Ogura, M.; Cordell, G.A.; Farnsworth, N.R. Jacoumaric acid, a new triterpene ester from Jacaranda caucana. Phytochemistry 1977, 16, 286–287. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, G.Q.; Wei, K.; Hai, P.; Wang, F.; Liu, J.-K. Isolation and Biomimetic Synthesis of (±)-Guajadial B, a Novel Meroterpenoid from Psidium guajava. Org. Lett. 2012, 14, 5936–5939. [Google Scholar] [CrossRef]
- Maestrini, M.; Tava, A.; Mancini, S.; Salari, F.; Perrucci, S. In vitro anthelmintic activity of saponins derived from medicago spp. plants against donkey gastrointestinal nematodes. Vet. Sci. 2019, 6, 35. [Google Scholar] [CrossRef] [Green Version]
- Martins, R.C.; Dorneles, G.P.; Teixeira, V.O.N.; Antonello, A.M.; Couto, J.L.; Rodrigues Júnior, L.C.; Monteiro, M.C.; Peres, A.; Schrekker, H.S.; Romão, P.R.T. Imidazolium salts as innovative agents against Leishmania amazonensis. Int. Immunopharmacol. 2018, 63, 101–109. [Google Scholar] [CrossRef]
- Pramanik, P.K.; Paik, D.; Pramanik, A.; Chakraborti, T. White jute (Corchorus capsularis L.) leaf extract has potent leishmanicidal activity against Leishmania donovani. Parasitol. Int. 2019, 71, 41–45. [Google Scholar] [CrossRef]
- Paula, R.C.; Silva, S.M.; Faria, K.F.; Frézard, F.; Moreira, C.P.S.; Foubert, K.; Lopes, J.C.D.; Campana, P.R.V.; Rocha, M.P.; Silva, A.F.; et al. In vitro antileishmanial activity of leaf and stem extracts of seven Brazilian plant species. J. Ethnopharmacol. 2019, 232, 155–164. [Google Scholar] [CrossRef]
- Bouyahya, A.; Et-Touys, A.; Dakka, N.; Fellah, H.; Abrini, J.; Bakri, Y. Antileishmanial potential of medicinal plant extracts from the North-West of Morocco. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 50–54. [Google Scholar] [CrossRef]
- Mehwish, S.; Islam, A.; Ullah, I.; Wakeel, A.; Qasim, M.; Khan, M.A.; Ahmad, A.; Ullah, N. In vitro antileishmanial and antioxidant potential, cytotoxicity evaluation and phytochemical analysis of extracts from selected medicinally important plants. Biocatal. Agric. Biotechnol. 2019, 19, 1–9. [Google Scholar] [CrossRef]
- Qin, X.J.; Yu, Q.; Yan, H.; Khan, A.; Feng, M.-Y.; Li, P.-P.; Hao, X.J.; An, L.K.; Liu, H.Y. Meroterpenoids with antitumor activities from Guava (Psidium guajava). J. Agric. Food Chem. 2017, 65, 4993–4999. [Google Scholar] [CrossRef]
- Abbruscato, P.; Tosi, S.; Crispino, L.; Biazzi, E.; Menin, B.; Picco, A.M.; Pecetti, L.; Avato, P.; Tava, A. Triterpenoid glycosides from Medicago sativa as antifungal agents against Pyricularia oryzae. J. Agric. Food Chem. 2014, 62, 11030–11036. [Google Scholar] [CrossRef]
- Torres-Santos, E.C.; Lopes, D.; Rodrigues Oliveira, R.; Carauta, J.P.P.; Bandeira Falcao, C.A.; Kaplan, M.A.C.; Rossi-Bergmann, B. Antileishmanial activity of isolated triterpenoids from Pourouma guianensis. Phytomedicine 2004, 11, 114–120. [Google Scholar] [CrossRef]
- Moreira, R.R.D.; Santos, A.G.D.; Carvalho, F.A.; Perego, C.H.; Crevelin, E.J.; Crotti, A.E.M.; Cogo, J.; Cardoso, M.L.C.; Nakamura, C.V. Antileishmanial activity of Melampodium divaricatum and Casearia sylvestris essential oils on Leishmania amazonensis. Rev. Inst. Med. Trop. Sao Paulo 2019, 61, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Mukherjee, N.; Gajbhiye, R.L.; Jaisankar, P.; Datta, S.; Das Saha, K. Intracellular anti-leishmanial effect of Spergulin-A, a triterpenoid saponin of Glinus oppositifolius. Infect. Drug Resistance 2019, 12, 2933–2942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngbolua, K.N.; Lufuluabo, L.G.; Moke, L.E.; Bango, G.N.; Liyongo, C.I.; Ashande, C.M.; Sapo, B.S.; Zoawe, B.G.; Mpiana, P.T. A review on the phytochemistry and pharmacology of psidium guajava L. (Myrtaceae) and future direction. Discov. Phytomed. 2018, 5, 7–13. [Google Scholar] [CrossRef]
- Garcia, A.R.; Oliveira, D.M.P.; Amaral, A.C.F.; Jesus, J.B.; Sodero, A.C.R.; Souza, A.M.T.; Supuran, C.T.; Vermelho, A.B.; Rodrigues, I.A.; Pinheiro, A.S. Leishmania infantum arginase: biochemical characterization and inhibition by naturally occurring phenolic substances. J. Enzym. Inhib. Med. Chem. 2019, 34, 1100–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, Y.; Yamada, K.; Yoshikawa, N.; Nakamura, K.; Haginaka, J.; Kunitomo, M. Corosolic acid prevents oxidative stress, inflammation and hypertension in SHR/NDmcr-cp rats, a model of metabolic syndrome. Life Sci. 2006, 79, 2474–2479. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, S.; Shin, Y.K.; Kang, H.; Kim, K.Y. In vitro antibacterial activity of macelignan and corosolic acid against the bacterial bee pathogens Paenibacillus larvae and Melissococcus plutonius. Acta Vet. Brno 2018, 87, 277–284. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Q.W.; Li, S.L.; Yi, Y.; Zhao, J.; Wang, Y.; Ye, W.C. Psidium guajava, a potential resource rich in corosolic acid revealed by high performance liquid chromatography. J. Med. Plants Res. 2011, 5, 4261–4266. [Google Scholar]
- Hsiao, Y.H.; Lin, C.W.; Wang, P.H.; Hsin, M.C.; Yang, S.F. The potential of chinese herbal medicines in the treatment of cervical cancer. Integr. Cancer Ther. 2019, 18, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Numata, A.; Yang, P.; Takahashi, C.; Fujiki, R.; Nabae, M.; Fujita, E. Cytotoxic triterpenes from a chinese medicine, Goreishi. Chem. Pharm. Bull. 1989, 37, 648–651. [Google Scholar] [CrossRef]
- Hou, W.; Li, Y.; Zhang, Q.; Wei, X.; Peng, A.; Chen, L.; Wei, Y. Triterpene acids isolated from Lagerstroemia speciosa leaves as α-glucosidase inhibitors. Phytother. Res. 2009, 23, 614–618. [Google Scholar] [CrossRef]
- Sifaoui, I.; López-Arencibia, A.; Martín-Navarro, C.M.; Reyes-Batlle, M.; Mejri, M.; Valladares, B.; Lorenzo-Morales, J.; Abderabba, M.; Enrique, J. Selective activity of oleanolic and maslinic acids on the amastigote form of Leishmania spp. Iran J. Pharm. Res. 2017, 16, 1190–1193. [Google Scholar] [PubMed]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Silva, F.; Inacio, J.D.F.; Canto-Cavalheiro, M.M.; Almeida-Amaral, E.E. Reactive oxygen species production and mitochondrial dysfunction contribute to quercetin induced death in Leishmania amazonensis. PLoS ONE 2011, 6, e0014666. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds corosolic acid are available from the authors. |
| OPLS Rank * | Source | m/z Values | RT (min) | Adduct Type | MF | Δm/z (mDa) | Main MS/MS Fragments | UV (nm) | Putative Annotation | Chemical Class | Biology Source |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | neg | 4,713,464 | 10.942 | [M − H]− | C30H48O4 | 1.5836 | 451.2516 393.3562 289.2787 | ND | Corosolic acid a,c (2α-hydroxyursolic acid) | Ursane triterpenoids | Lagerstroemia speciosa [19] |
| 2 | neg | 6,173,837 | 11.954 | [M − H]− | C39H54O6 | 1.063 | 597.0866 537.0319 497.4617 453.3008 | 310 | Jacoumaric acid a,b | Ursane triterpenoids | Jacaranda caucana [20] |
| 3 | neg | 5,033,368 | 8.090 | [M − H]− | C30H48O6 | 1.0128 | 485.3478 437.4027 389.4317 183.3482 | ND | 16,24,25-Trihydroxy-3-oxoeuph-7-en-21-oic acid a | Dammarane triterpenoids | Psidium guajava |
| 4 | neg | 6,333,782 | 10.084 | [M − H]− | C39H54O7 | 1.4776 | 563.7110 513.4430 469.5226 392.2423 | 300 | 2,3-Dihydroxy-12-oleanen-28-oic acid; 3-O-(3,4-Dihydroxy-E-cinnamoyl) a | Oleanane triterpenoids | Psidium guajava |
| 5 | pos | 4,752,464 | 15.306 | [M + H]+ | C30H34O5 | 1.5006 | 323.2012 293.3159 271.0567 205.2643 | 278 | Guajadial B a,b | Meroterpenoids | Psidium guajava [21] |
| 6 | neg | 5,013,211 | 7.676 | [M − H]− | C30H46O6 | 1.0627 | 483.6488 389.3652 321.4315 189.4521 | ND | Medicagenic acid a,b | Ursane triterpenoids | Medicago sativa [22] |
| 7 | neg | 4,873,417 | 9.251 | [M − H]− | C30H48O5 | 1.1982 | 469.5041 424.4036 268.196 | ND | 4,23-Dihydroxy-22-oxo-3,4-seco-12-oleanen-3-oic acid a | Oleanane triterpenoids | Streptomyces sp. |
| Compounds | Promastigotes | Axenic Amastigotes | J774A1 | SI | |
|---|---|---|---|---|---|
| IC50 (µg/mL) | IC50 (µg/mL) | CC50 (µg/mL) | Promastigotes | Amastigotes | |
| Pooled apolar fraction | >50 | 1.96 ± 0.47 | 51.19 ± 9.21 | ND | 26.12 |
| Corosolic acid (1) | 18.43 ± 1.20 | 1.01 ± 0.06 | 5.77 ± 0.50 | 0.31 | 5.71 |
| Jacoumaric acid (2) | >50 | 1.32 ± 0.59 | 12.88 ± 2.50 | ND | 9.76 |
| Guajadial B (5) | >50 | >50 | NT | ND | ND |
| Medicagenic acid (6) | >50 | >50 | NT | ND | ND |
| Amphotericin B | 0.09 ± 0.01 | 0.09 ± 0.04 | 5.79 ± 0.65 | 64.33 | 64.33 |
| Doxorubicin | NT | NT | 0.04 ± 0.004 | ND | ND |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phakeovilay, C.; Bourgeade-Delmas, S.; Perio, P.; Valentin, A.; Chassagne, F.; Deharo, E.; Reybier, K.; Marti, G. Antileishmanial Compounds Isolated from Psidium Guajava L. Using a Metabolomic Approach. Molecules 2019, 24, 4536. https://doi.org/10.3390/molecules24244536
Phakeovilay C, Bourgeade-Delmas S, Perio P, Valentin A, Chassagne F, Deharo E, Reybier K, Marti G. Antileishmanial Compounds Isolated from Psidium Guajava L. Using a Metabolomic Approach. Molecules. 2019; 24(24):4536. https://doi.org/10.3390/molecules24244536
Chicago/Turabian StylePhakeovilay, Chiobouaphong, Sandra Bourgeade-Delmas, Pierre Perio, Alexis Valentin, François Chassagne, Eric Deharo, Karine Reybier, and Guillaume Marti. 2019. "Antileishmanial Compounds Isolated from Psidium Guajava L. Using a Metabolomic Approach" Molecules 24, no. 24: 4536. https://doi.org/10.3390/molecules24244536






