Adsorption and Desorption Performance and Mechanism of Tetracycline Hydrochloride by Activated Carbon-Based Adsorbents Derived from Sugar Cane Bagasse Activated with ZnCl2
Abstract
:1. Introduction
2. Results and Discussions
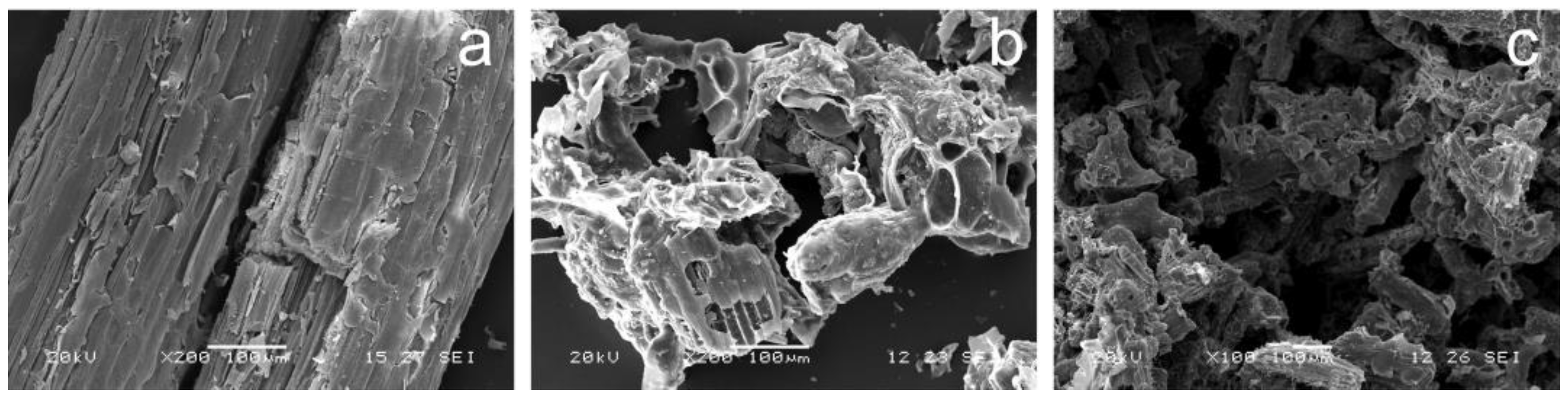
2.1. Materials Structure and Composition
2.2. Batch Adsorption Experiments
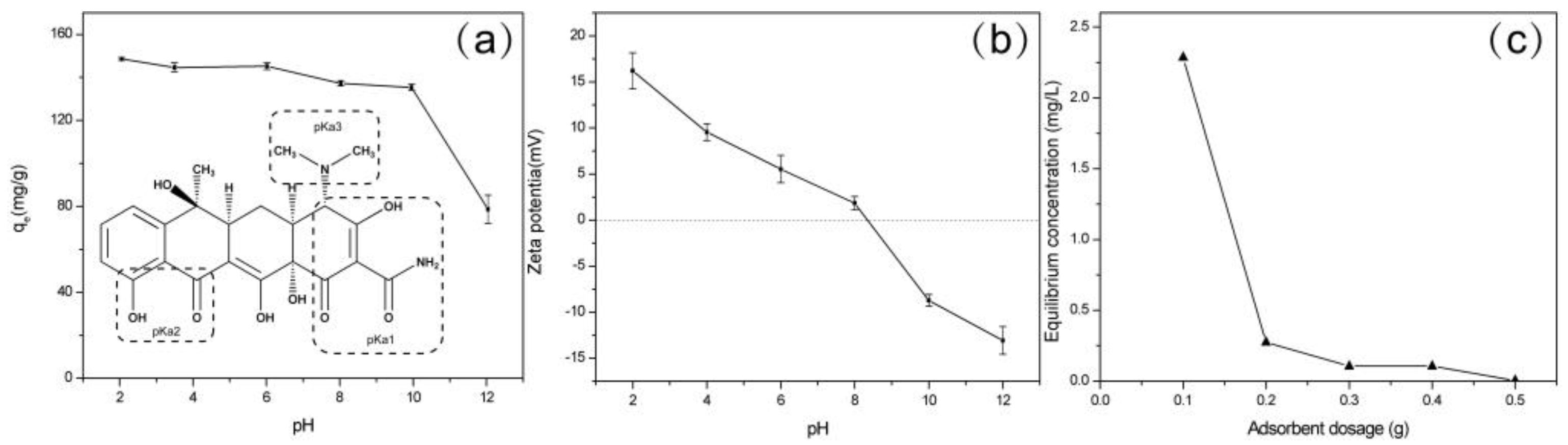
2.2.1. Effect of pH
2.2.2. Adsorbent Dosage Studies
2.2.3. Kinetics Studies
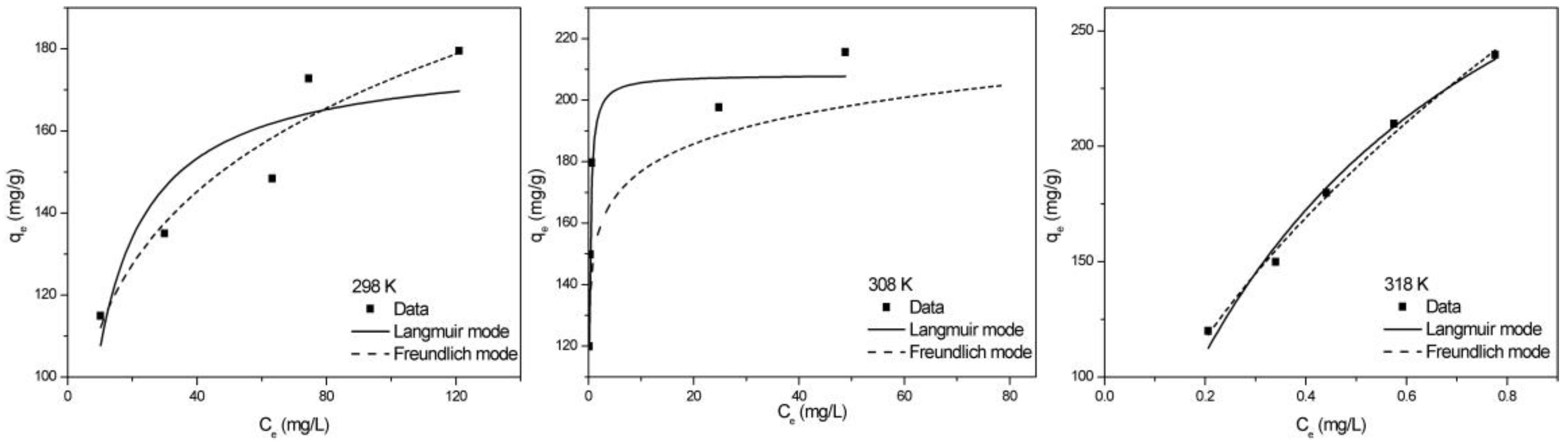
2.2.4. Adsorption Isotherm
2.3. Fixed Bed Adsorption Experiments
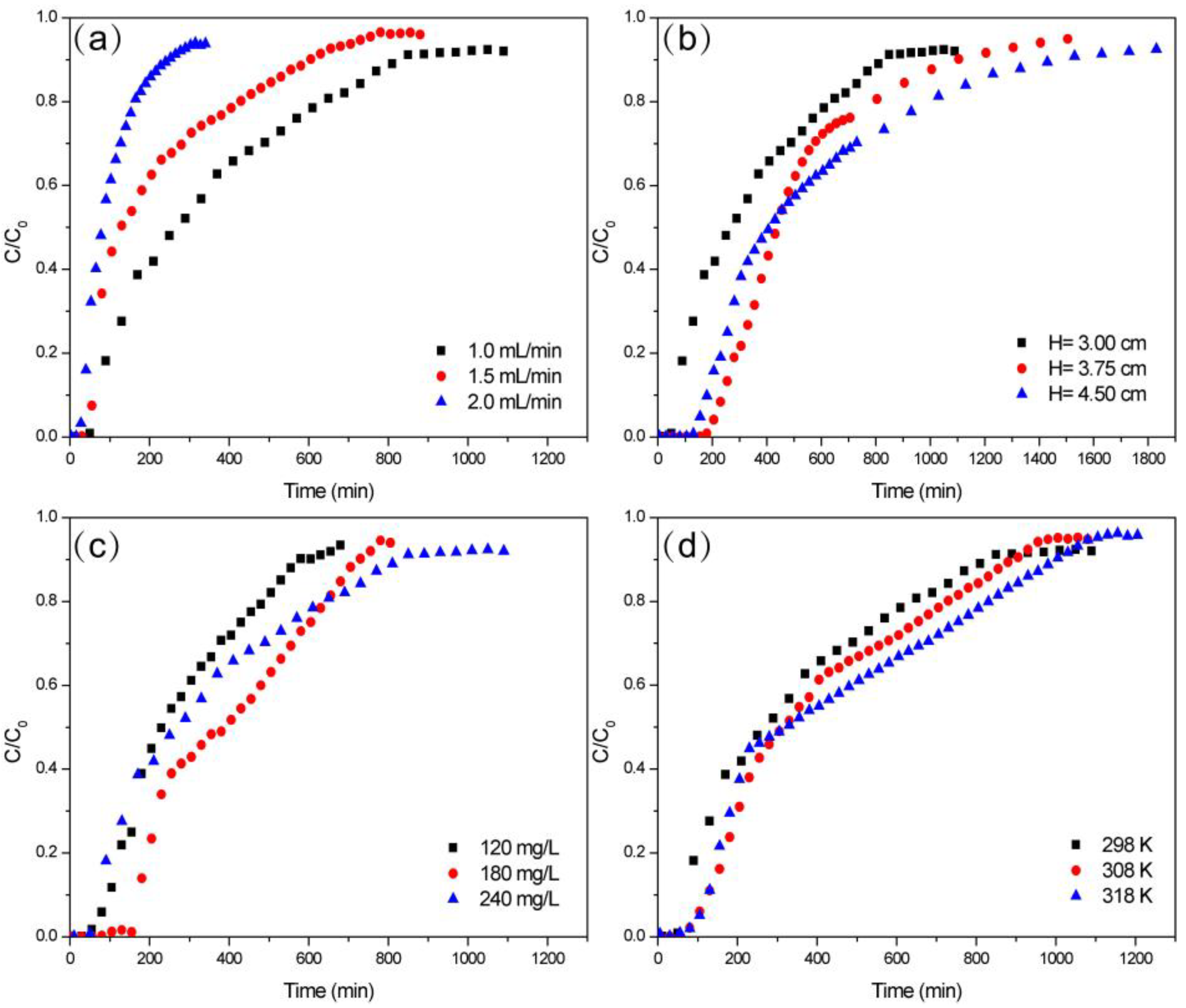
2.3.1. Effect of Flow Rate
2.3.2. Effect of Bed Height
2.3.3. Effect of Initial Concentration
2.3.4. Effect of Temperature
2.3.5. Modelling of Breakthrough Curves
2.4. Fixed Bed Desorption Experiments
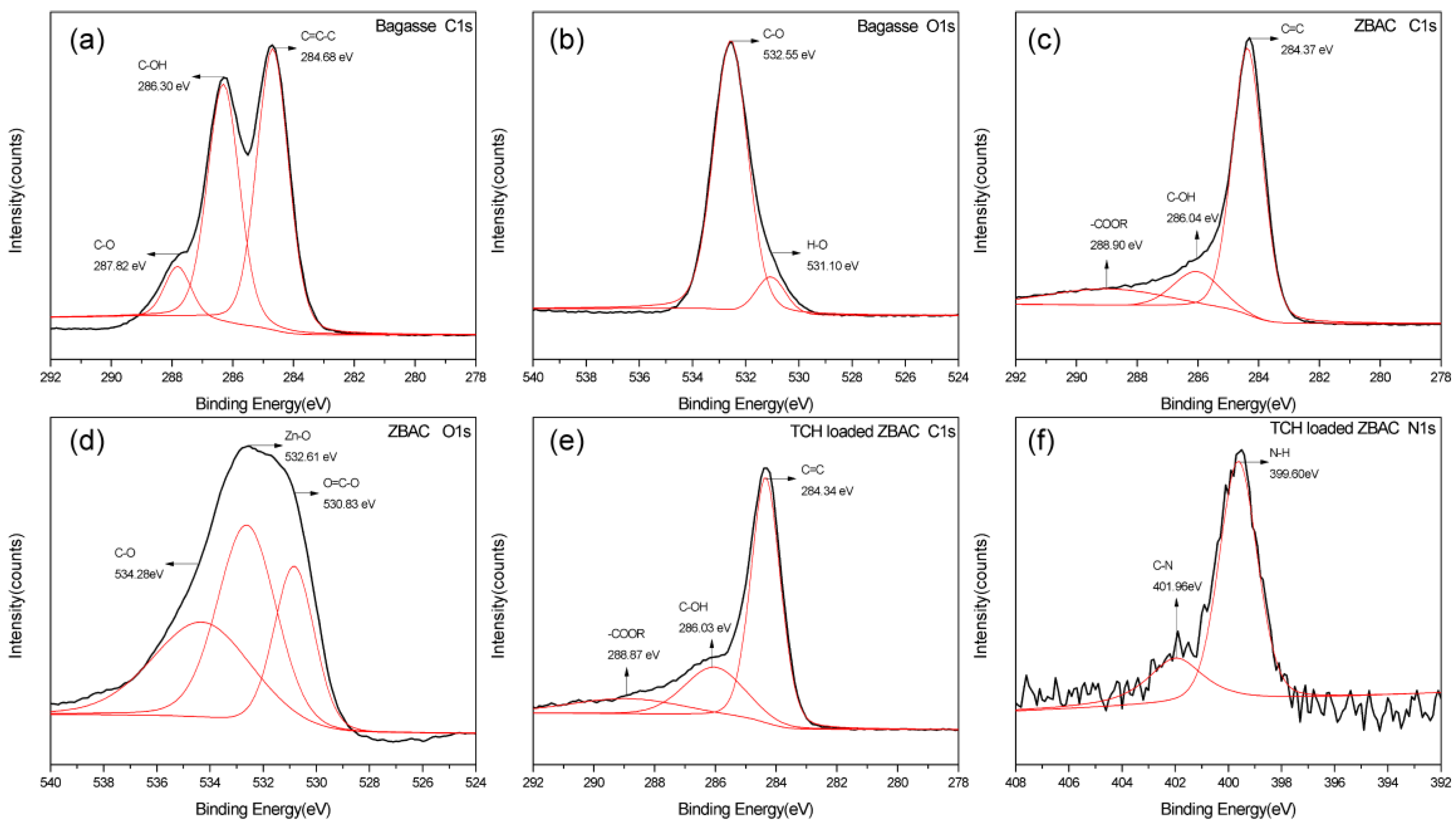
2.5. XPS Analysis
2.6. Adsorption and Desorption Mechanism
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Absorbents
3.3. Characterization
3.4. Adsorption Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| SBET | Brunauer–Emmett–Teller surface area (m2 g−1) |
| Vmic | Micropore volume (cm3 g−1) |
| Dp | Average pore diameter (nm) |
| qm | Maximum adsorption capacity of TCH per unit mass of ZBAC (mg g−1) |
| qt | Amounts of TCH adsorbed at contact time (mg g−1) |
| qe | Amounts of TCH adsorbed at equilibrium time (mg g−1) |
| k1 | Rate constant for first order kinetic (min−1) |
| k2 | Rate constant for second order kinetic (g mg−1 min−1) |
| k0 | Initial adsorption rate (g mg−1 min−1) |
| K | Langmuir adsorption constant (L mg−1) |
| RL | Dimensionless constant separation factor |
| kf | Freundlich constant which indicates the adsorption capacity |
| n | Freundlich constant which related to the adsorption strength of the adsorbent |
| ε | Polanyi potential |
| K’ | Constant of the adsorption energy (mol2 kJ−2) |
| Ki | Rate constant of the intraparticle diffusion (mg g−1 min−1/2) |
| E | Adsorption energy (kJ mol−1) |
| pHpzc | pH at point of zero charge |
| H | Bed height in fixed bed column adsorption (cm) |
| Q | Flow rate (mL min−1) |
| tb | Breakthrough time (min) |
| Vb | Volume of treated solution (mL) |
| qb | Adsorption capacity in Fixed bed adsorption (mg g−1) |
| Rb | Metal removal efficiency of the breakthrough point (%) |
| ts | Saturation time (min) |
| Vs | Volume of treated solution (mL) |
| qs | Adsorption capacity in Fixed bed adsorption (mg g−1) |
| Rs | Metal removal efficiency of the saturation point (%) |
| M | Adsorbent dosage (g) |
| EBCT | Empty bed contact time (min) |
| MTZ | Mass transfer zone (cm) |
| Cf | Desorbed concentration of TCH |
| qe,d | The amount of TCH desorbed |
| qtotal,d | Amount of TCH desorbed per mass of ZBAC |
| Cp | The maximum concentration in desorption |
| CFp | The overall sorption process concentration factor |
References
- Ocampo-Pérez, R.; Rivera-Utrilla, J.; Gómez-Pacheco, C.; Sánchez-Polo, M.; López-Peñalver, J.J. Kinetic study of tetracycline adsorption on sludge-derived adsorbents in aqueous phase. Chem. Eng. J. 2012, 213, 88–96. [Google Scholar] [CrossRef]
- Parolo, M.E.; Savini, M.C.; Vallés, J.M.; Baschini, M.T.; Avena, M.J. Tetracycline adsorption on montmorillonite: pH and ionic strength effects. Appl. Clay Sci. 2008, 40, 179–186. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y.; Zhang, L.; Huang, H.; Hu, J.; Shah, S.M.; Su, X. Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide. J. Colloid Interface Sci. 2012, 368, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L.; Chen, W.; Duan, L.; Zhu, D.Q. Mechanisms for strong adsorption of tetracycline to carbon nanotubes: A comparative study using activated carbon and graphite as adsorbents. Environ. Sci. Technol. 2009, 43, 2322–2327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lu, Y.; Sun, H.; Zhang, Y.; Zhou, M.; Song, Q.; Gao, Y. Effects of multi−walled carbon nanotubes on pyrene adsorption and desorption in soils: The role of soil constituents. Chemosphere 2019, 221, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Garg, U.K.; Kaur, M.P.; Sud, D.; Garg, V.K. Removal of hexavalent chromium from aqueous solution by adsorption on treated sugarcane bagasse using response surface methodological approach. Desalination 2009, 249, 475–479. [Google Scholar] [CrossRef]
- Yu, Z.; Peldszus, S.; Huck, P.M. Adsorption of selected pharmaceuticals and an endocrine disrupting compound by granular activated carbon. 2. Model prediction. Environ. Sci. Technol. 2009, 43, 1474–1479. [Google Scholar] [CrossRef]
- Lin, Y.C.; Cho, J.; Tompsett, G.A.; Westmoreland, P.R.; Huber, G.W. Kinetics and Mechanism of Cellulose Pyrolysis. J. Phys. Chem. C 2009, 113, 20097–20107. [Google Scholar] [CrossRef] [Green Version]
- Ahmadpour, A.; Do, D.D. The preparation of activated carbon from macadamia nutshell by chemical activation. Carbon 1997, 35, 1723–1732. [Google Scholar] [CrossRef]
- Yubing, S.; Shubin, Y.; Yue, C.; Congcong, D.; Wencai, C.; Xiangke, W. Adsorption and desorption of U(VI) on functionalized graphene oxides: a combined experimental and theoretical study. Environ. Sci. Technol. 2015, 49, 4255–4262. [Google Scholar] [CrossRef]
- Kumar, A.; Mohan Jena, H. High surface area microporous activated carbons prepared from Fox nut (Euryale ferox) shell by zinc chloride activation. Appl. Surf. Sci. 2015, 356, 753–761. [Google Scholar] [CrossRef]
- Ozdemir, I.; Şahin, M.; Orhan, R.; Erdem, M. Preparation and characterization of activated carbon from grape stalk by zinc chloride activation. Fuel Process. Technol. 2014, 125, 200–206. [Google Scholar] [CrossRef]
- Li, H.; Hu, J.; Meng, Y.; Su, J.; Wang, X. An investigation into the rapid removal of tetracycline using multilayered graphene-phase biochar derived from waste chicken feather. Sci. Total Environ. 2017, 603–604, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Shang, C.; Shao, J.; Lin, Y.; Luo, S.; Zhang, J.; Huang, H.; Lei, M.; Zeng, Q. Carbon disulfide-modified magnetic ion-imprinted chitosan-Fe(III): A novel adsorbent for simultaneous removal of tetracycline and cadmium. Carbohydr. Polym. 2017, 155, 19–27. [Google Scholar] [CrossRef]
- Shan, D.; Deng, S.; Zhao, T.; Yu, G.; Winglee, J.; Wiesner, M.R. Preparation of regenerable granular carbon nanotubes by a simple heating-filtration method for efficient removal of typical pharmaceuticals. Chem. Eng. J. 2016, 294, 353–361. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.J.; Islam, M.A.; Asif, M.; Hameed, B.H. Human hair-derived high surface area porous carbon material for the adsorption isotherm and kinetics of tetracycline antibiotics. Bioresour. Technol. 2017, 243. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, F.; Duan, L.; Yang, H.; Gao, J. Tetracycline adsorption onto rice husk ash, an agricultural waste: Its kinetic and thermodynamic studies. J. Mol. Liq. 2016, 222, 487–494. [Google Scholar] [CrossRef]
- Liao, P.; Zhan, Z.; Dai, J.; Wu, X.; Zhang, W.; Wang, K.; Yuan, S. Adsorption of tetracycline and chloramphenicol in aqueous solutions by bamboo charcoal: A batch and fixed-bed column study. Chem. Eng. J. 2013, 228, 496–505. [Google Scholar] [CrossRef]
- Liu, P.; Liu, W.J.; Jiang, H.; Chen, J.J.; Li, W.W.; Yu, H.Q. Modification of bio-char derived from fast pyrolysis of biomass and its application in removal of tetracycline from aqueous solution. Bioresour. Technol. 2012, 121, 235–240. [Google Scholar] [CrossRef]
- Luo, X.; Lei, X.; Cai, N.; Xie, X.; Xue, Y.; Yu, F. Removal of Heavy Metal Ions from Water by Magnetic Cellulose-Based Beads with Embedded Chemically Modified Magnetite Nanoparticles and Activated Carbon. ACS Sustain. Chem. Eng. 2016, 4, 3960–3969. [Google Scholar] [CrossRef]
- Ahmad, Z.U.; Yao, L.G.; Wang, J.; Gang, D.D.; Islam, F.; Lian, Q.Y.; Zappi, M.E. Neodymium embedded ordered mesoporous carbon (OMC) for enhanced adsorption of sunset yellow: Characterizations, adsorption study and adsorption mechanism. Chem. Eng. J. 2019, 359, 814–826. [Google Scholar] [CrossRef]
- Luo, X.; Lei, X.; Xie, X.; Yu, B.; Cai, N.; Yu, F. Adsorptive removal of Lead from water by the effective and reusable magnetic cellulose nanocomposite beads entrapping activated bentonite. Carbohydr. Polym. 2016, 151, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Dai, X.; Long, S.; Cai, N.; Ma, Z.; Luo, X. Facile Design of Green Engineered Cellulose/Metal Hybrid Macrogels for Efficient Trace Phosphate Removal. Ind. Eng. Chem. Res. 2017, 56, 7525–7533. [Google Scholar] [CrossRef]
- Naeem, A.; Westerhoff, P.; Mustafa, S. Vanadium removal by metal (hydr)oxide adsorbents. Water Res. 2007, 41, 1596–1602. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zeng, J.; Liu, S.; Zhang, L. An effective and recyclable adsorbent for the removal of heavy metal ions from aqueous system: Magnetic chitosan/cellulose microspheres. Bioresour. Technol. 2015, 194, 403–406. [Google Scholar] [CrossRef]
- Luo, X.; Liu, C.; Yuan, J.; Zhu, X.; Liu, S. Interfacial Solid-Phase Chemical Modification with Mannich Reaction and Fe(III) Chelation for Designing Lignin-Based Spherical Nanoparticle Adsorbents for Highly Efficient Removal of Low Concentration Phosphate from Water. ACS Sustain. Chem. Eng. 2017, 5, 6539–6547. [Google Scholar] [CrossRef]
- Kul, A.; Koyuncu, H. Adsorption of Pb(II) Ions From Aqueous Solution by Native and Activated Bentonite: Kinetic, Equilibrium and Thermodynamic Study. J. Hazard. Mater. 2010, 179, 332–339. [Google Scholar] [CrossRef]
- Luo, X.; Cai, Y.; Liu, L.; Zeng, J. Cr(VI) adsorption performance and mechanism of an effective activated carbon prepared from bagasse with a one-step pyrolysis and ZnCl2 activation method. Cellulose 2019, 26, 4921–4934. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, C.; Lu, Y.; Luo, X.; Zeng, J. Bagasse-based adsorbent with nitric acid esterification and Fe(iii) chelation for the highly efficient removal of low concentration phosphate from water. New J. Chem. 2019, 43, 2966–2973. [Google Scholar] [CrossRef]
- Luo, X.; Yuan, J.; Liu, Y.; Liu, C.; Zhu, X.; Dai, X.; Ma, Z.; Wang, F. Improved Solid-Phase Synthesis of Phosphorylated Cellulose Microsphere Adsorbents for Highly Effective Pb2+ Removal from Water: Batch and Fixed-Bed Column Performance and Adsorption Mechanism. ACS Sustain. Chem. Eng. 2017, 5, 5108–5117. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Teong, L.C.; Toh, R.H.; Hanafiah, M.A.K.M. Utilization of chitosan–zeolite composite in the removal of Cu(II) from aqueous solution: Adsorption, desorption and fixed bed column studies. Chem. Eng. J. 2012, 209, 46–53. [Google Scholar] [CrossRef]
- Bulgariu, D.; Bulgariu, L. Sorption of Pb(II) onto a mixture of algae waste biomass and anion exchanger resin in a packed-bed column. Bioresour. Technol. 2013, 129, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Calero, M.; Hernáinz, F.; Blázquez, G.; Tenorio, G.; Martínlara, M.A. Study of Cr (III) biosorption in a fixed-bed column. J. Hazard. Mater. 2009, 171, 886–893. [Google Scholar] [CrossRef] [PubMed]
- Aschermann, G.; Zietzschmann, F.; Jekel, M. Influence of dissolved organic matter and activated carbon pore characteristics on organic micropollutant desorption. Water Res. 2018, 133, 123–131. [Google Scholar] [CrossRef]
- Martín-Lara, M.A.; Blázquez, G.; Ronda, A.; Rodríguez, I.L.; Calero, M. Multiple biosorption–desorption cycles in a fixed-bed column for Pb(II) removal by acid-treated olive stone. J. Ind. Eng. Chem. 2012, 18, 1006–1012. [Google Scholar] [CrossRef]
- Lodeiro, P.; Herrero, R.; Sastre de Vicente, M.E. Batch desorption studies and multiple sorption-regeneration cycles in a fixed-bed column for Cd(II) elimination by protonated Sargassum muticum. J. Hazard. Mater. 2006, 137, 1649–1655. [Google Scholar] [CrossRef] [Green Version]
- Qu, R.; Feng, M.; Wang, X.; Qin, L.; Wang, C.; Wang, Z.; Wang, L. Metal accumulation and oxidative stress biomarkers in liver of freshwater fish Carassius auratus following in vivo exposure to waterborne zinc under different pH values. Aquat. Toxicol. 2014, 150, 9–16. [Google Scholar] [CrossRef]
- Barroso-Bogeat, A.; Alexandre-Franco, M.; Fernández-González, C.; Gómez-Serrano, V. Activated carbon surface chemistry: Changes upon impregnation with Al(III), Fe(III) and Zn(II)-metal oxide catalyst precursors from NO3− aqueous solutions. Arab. J. Chem. 2016. [Google Scholar] [CrossRef] [Green Version]
- Acharya, J.; Sahu, J.N.; Sahoo, B.K.; Mohanty, C.R.; Meikap, B.C. Removal of chromium(VI) from wastewater by activated carbon developed from Tamarind wood activated with zinc chloride. Chem. Eng. J. 2009, 150, 25–39. [Google Scholar] [CrossRef]
- Angin, D. Production and characterization of activated carbon from sour cherry stones by zinc chloride. Fuel 2014, 115, 804–811. [Google Scholar] [CrossRef]
- Antón-Herrero, R.; García-Delgado, C.; Alonso-Izquierdo, M.; García-Rodríguez, G.; Cuevas, J.; Eymar, E. Comparative adsorption of tetracyclines on biochars and stevensite: Looking for the most effective adsorbent. Appl. Clay Sci. 2018, 160, 162–172. [Google Scholar] [CrossRef]
- Song, Q.; Fang, Y.; Liu, Z.; Li, L.; Wang, Y.; Liang, J.; Huang, Y.; Lin, J.; Hu, L.; Zhang, J.; et al. The performance of porous hexagonal BN in high adsorption capacity towards antibiotics pollutants from aqueous solution. Chem. Eng. J. 2017, 325, 71–79. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |




| ACs | qm (mg g−1) | References |
|---|---|---|
| Human hair | 128.5 | Ahmed, et al. [16] |
| Chicken feather | 388.3 | Li, Hu, Meng, Su and Wang [13] |
| Rice-husk | 58.8 | Chen, et al. [17] |
| Bamboo charcoal | 27.7 | Liao, et al. [18] |
| Rice husk ash | 8.37 | Liu, et al. [19] |
| Sugar cane bagasse | 239.6 | This study |
| Model ID | qe,exp mg g−1 | ki mg−1 g min−1/2 | C mg g−1 | R2 |
|---|---|---|---|---|
| Sector #1 | ||||
| 298 K | 19.938 | 1.073 | 17.938 | 0.9013 |
| 308 K | 21.885 | 1.250 | 13.310 | 0.9629 |
| 318 K | 22.335 | 2.079 | 13.330 | 0.9696 |
| Sector #2 | ||||
| 298 K | 23.818 | 0.3948 | 17.938 | 0.9743 |
| 308 K | 23.999 | 0.5875 | 17.593 | 0.9891 |
| 318 K | 23.999 | 0.7908 | 18.812 | 0.9571 |
| No. | mL/min | cm | K | mg/L | pH | min | mL | mg/g | % | min | mL | mg/g | % | g | mg/L | min | cm |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q | H | T | C0 | tb | Vb | qb | Rb | ts | Vs | qs | Rs | M | Ce | EBTC | MTZ | ||
| 1 | 1 | 3 | 298 | 120 | 3.5 | 105 | 105 | 15.24 | 96.77 | 580 | 580 | 42.07 | 49.21 | 0.8 | 112.8 | 2.85 | 2.46 |
| 2 | 1 | 3 | 298 | 180 | 3.5 | 180 | 180 | 39.87 | 98.45 | 730 | 730 | 91.73 | 55.84 | 0.8 | 169.2 | 2.85 | 2.26 |
| 3 | 1 | 3 | 298 | 240 | 3.5 | 70 | 70 | 14.94 | 99.63 | 850 | 850 | 105.3 | 41.3 | 0.8 | 227.5 | 2.85 | 2.75 |
| 4 | 1 | 3.75 | 298 | 240 | 3.5 | 230 | 230 | 54.62 | 98.95 | 1105 | 1105 | 126.4 | 47.67 | 1 | 236.5 | 3.56 | 2.97 |
| 5 | 1 | 4.5 | 298 | 240 | 3.5 | 180 | 180 | 35.46 | 98.51 | 1530 | 1530 | 118.9 | 38.88 | 1.2 | 237.8 | 4.27 | 3.97 |
| 6 | 1.5 | 3 | 298 | 240 | 3.5 | 55 | 82.5 | 24.03 | 98.23 | 605 | 907.5 | 100.1 | 46.77 | 0.8 | 230.4 | 1.9 | 2.73 |
| 7 | 2 | 3 | 298 | 240 | 3.5 | 40 | 80 | 23.15 | 96.45 | 240 | 480 | 61.55 | 42.75 | 0.8 | 225.2 | 1.43 | 2.5 |
| 8 | 1 | 3 | 308 | 240 | 3.5 | 130 | 130 | 37.95 | 97.31 | 905 | 905 | 123.5 | 45.5 | 0.8 | 232.8 | 2.85 | 2.57 |
| 9 | 1 | 3 | 318 | 240 | 3.5 | 130 | 130 | 37.94 | 97.34 | 1005 | 1005 | 134.9 | 44.5 | 0.8 | 238.8 | 2.85 | 2.61 |
| Model | Parameters | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|---|
| Adams–Bohart | KAB (L mg−1 min−1) × 10−5 | 6.951 | 3.684 | 2.126 | 1.406 | 3.137 | 3.008 | 9.307 | 2.143 | 1.710 |
| N0 (mg L−1) × 104 | 0.932 | 2.041 | 2.367 | 2.293 | 2.469 | 2.154 | 1.300 | 2.692 | 2.984 | |
| R2 | 0.958 | 0.955 | 0.9459 | 0.966 | 0.9659 | 0.937 | 0.945 | 0.945 | 0.928 | |
| Thomas | Kth (L mg−1 min−1) × 10−5 | 6.967 | 3.685 | 2.071 | 1.969 | 3.137 | 3.002 | 9.309 | 2.164 | 1.694 |
| q0 (mg g−1) | 41.13 | 91.69 | 97.15 | 97.43 | 112.6 | 84.77 | 56.72 | 115.2 | 126.4 | |
| R2 | 0.958 | 0.955 | 0.9412 | 0.923 | 0.9659 | 0.938 | 0.946 | 0.945 | 0.928 | |
| Yoon–Nelson | KYN (min−1) × 10−2 | 0.836 | 0.675 | 0.502 | 0.472 | 0.753 | 0.720 | 2.234 | 0.520 | 0.411 |
| τcal (min) | 274.1 | 403.5 | 320.6 | 487.1 | 469.4 | 188.4 | 94.53 | 384.2 | 417.0 | |
| R2 | 0.958 | 0.955 | 0.9412 | 0.964 | 0.9659 | 0.938 | 0.945 | 0.945 | 0.928 | |
| BDST | KBDST (L mg−1 min−1) × 10−5 | 6.967 | 3.727 | 2.072 | 1.969 | 3.137 | 3.002 | 9.309 | 2.164 | 1.711 |
| N0 (mg L−1) × 104 | 0.894 | 1.974 | 2.112 | 2.118 | 2.449 | 1.843 | 0.616 | 25.06 | 2.720 | |
| R2 | 0.958 | 0.955 | 0.9412 | 0.923 | 0.9659 | 0.937 | 0.945 | 0.945 | 0.928 | |
| Dose Response | q0 (mg g−1) | 36.68 | 78.25 | 82.15 | 89.09 | 108.9 | 66.66 | 49.28 | 98.66 | 103.4 |
| α | 2.166 | 2.421 | 1.618 | 1.892 | 3.025 | 1.519 | 2.037 | 1.947 | 1.668 | |
| R2 | 0.9940 | 0.974 | 0.9906 | 0.99 | 0.9934 | 0.98 | 0.997 | 0.988 | 0.968 | |
| Clark | A*103 | 3.922 | 14.13 | 0.617 | 7.736 | 2.076 | 0.281 | 3.323 | 1.581 | 2.087 |
| r (min−1) × 10−2 | 1.764 | 1.492 | 0.981 | 1.152 | 1.869 | 1.302 | 4.995 | 1.036 | 0.956 | |
| R2 | 0.902 | 0.918 | 0.8841 | 0.868 | 0.9246 | 0.837 | 0.89 | 0.884 | 0.882 |
| Q | qtotal,d | qe,d | tp | Cp | CFp |
|---|---|---|---|---|---|
| mL/min | mg/g | mg/g | min | mg/L | |
| 1.0 | 10.54 | 13.18 | 20 | 117.5 | 0.489 |
| 1.5 | 5.276 | 6.595 | 15 | 58.50 | 0.244 |
| 2.0 | 4.479 | 5.599 | 10 | 16.31 | 0.068 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, Y.; Liu, L.; Tian, H.; Yang, Z.; Luo, X. Adsorption and Desorption Performance and Mechanism of Tetracycline Hydrochloride by Activated Carbon-Based Adsorbents Derived from Sugar Cane Bagasse Activated with ZnCl2. Molecules 2019, 24, 4534. https://doi.org/10.3390/molecules24244534
Cai Y, Liu L, Tian H, Yang Z, Luo X. Adsorption and Desorption Performance and Mechanism of Tetracycline Hydrochloride by Activated Carbon-Based Adsorbents Derived from Sugar Cane Bagasse Activated with ZnCl2. Molecules. 2019; 24(24):4534. https://doi.org/10.3390/molecules24244534
Chicago/Turabian StyleCai, Yixin, Liming Liu, Huafeng Tian, Zhennai Yang, and Xiaogang Luo. 2019. "Adsorption and Desorption Performance and Mechanism of Tetracycline Hydrochloride by Activated Carbon-Based Adsorbents Derived from Sugar Cane Bagasse Activated with ZnCl2" Molecules 24, no. 24: 4534. https://doi.org/10.3390/molecules24244534
APA StyleCai, Y., Liu, L., Tian, H., Yang, Z., & Luo, X. (2019). Adsorption and Desorption Performance and Mechanism of Tetracycline Hydrochloride by Activated Carbon-Based Adsorbents Derived from Sugar Cane Bagasse Activated with ZnCl2. Molecules, 24(24), 4534. https://doi.org/10.3390/molecules24244534






