Oxidation of Sodium Deoxycholate Catalyzed by Gold Nanoparticles and Chiral Recognition Performances of Bile Salt Micelles
Abstract
1. Introduction
2. Results and Discussion
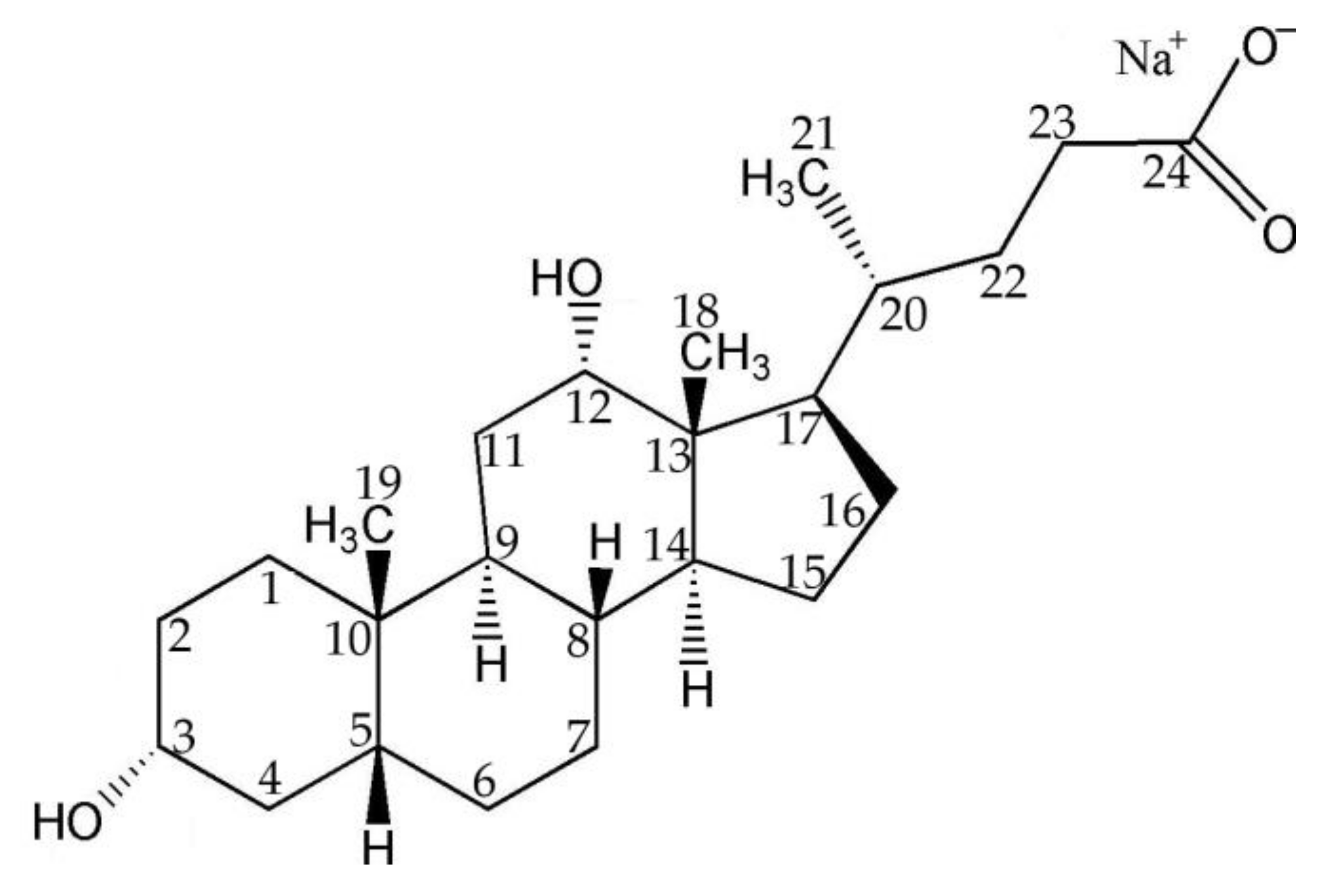
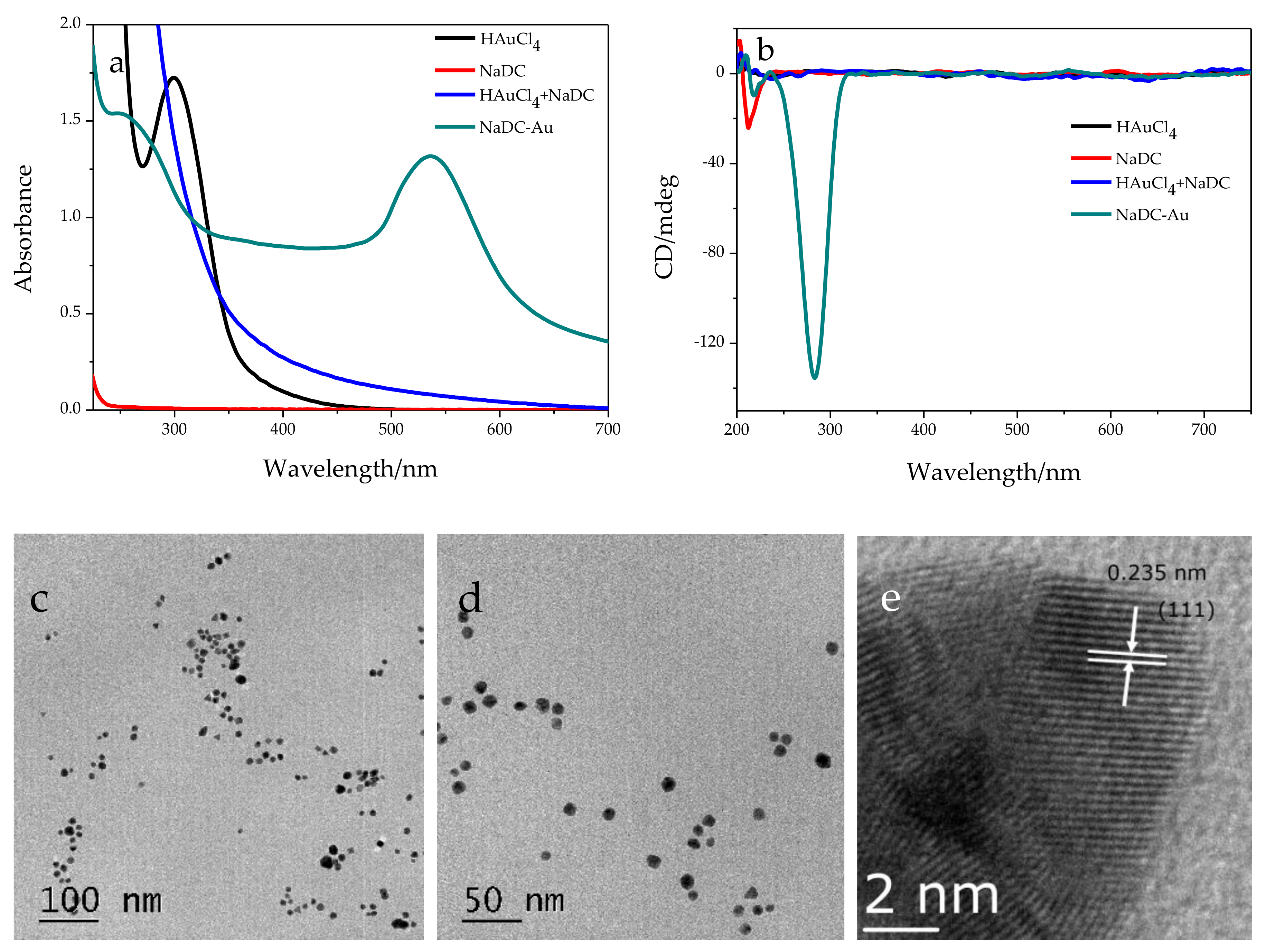
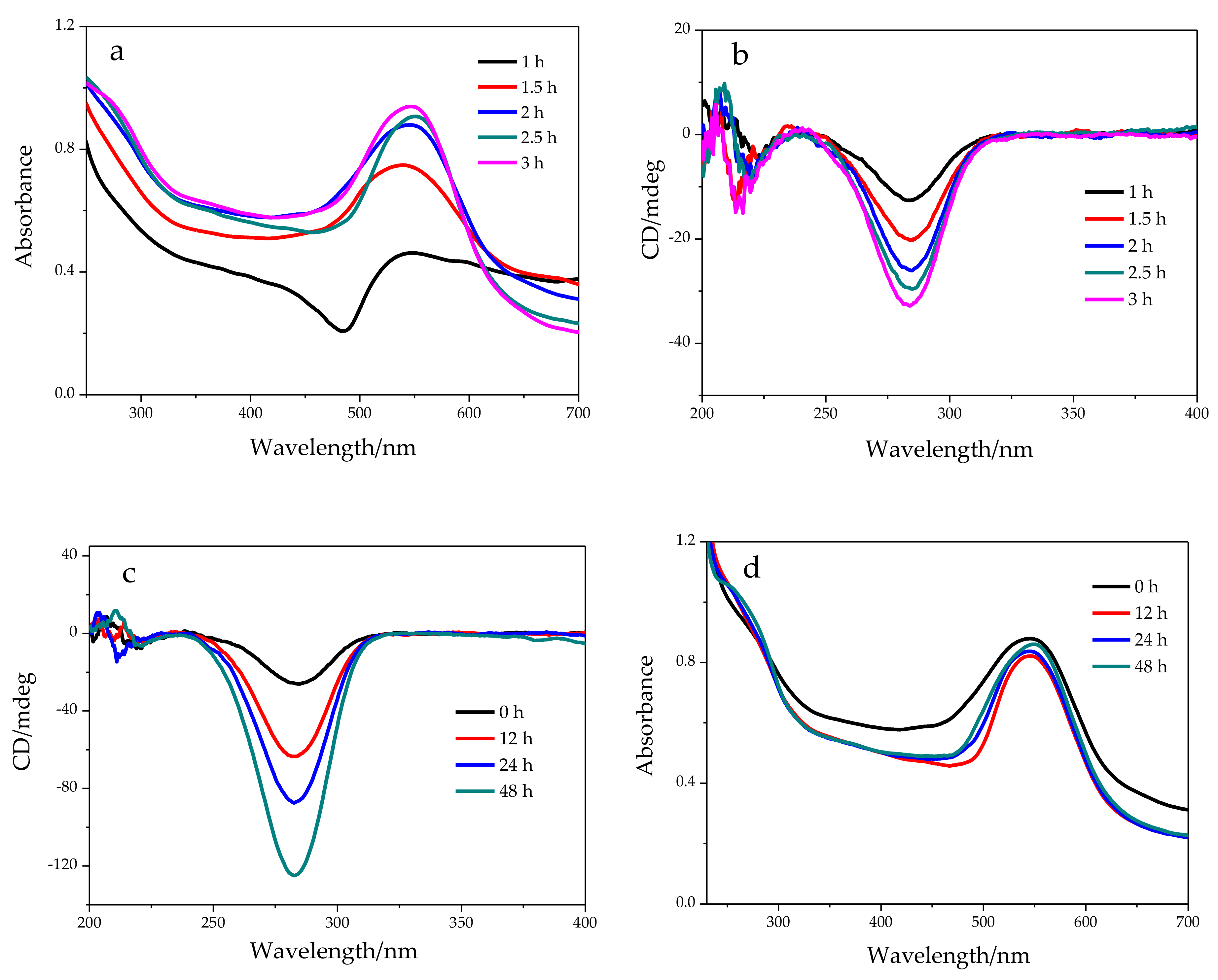
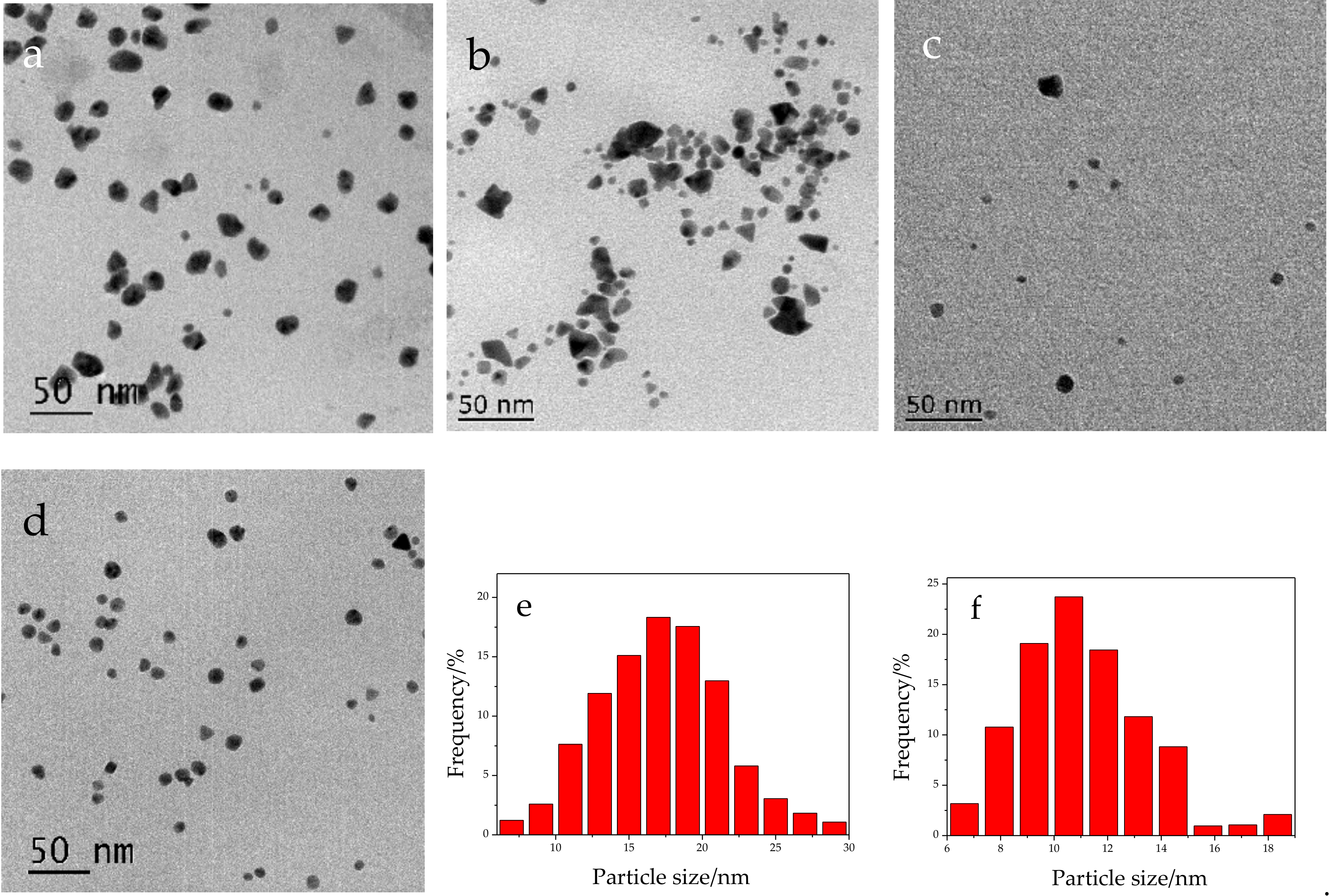
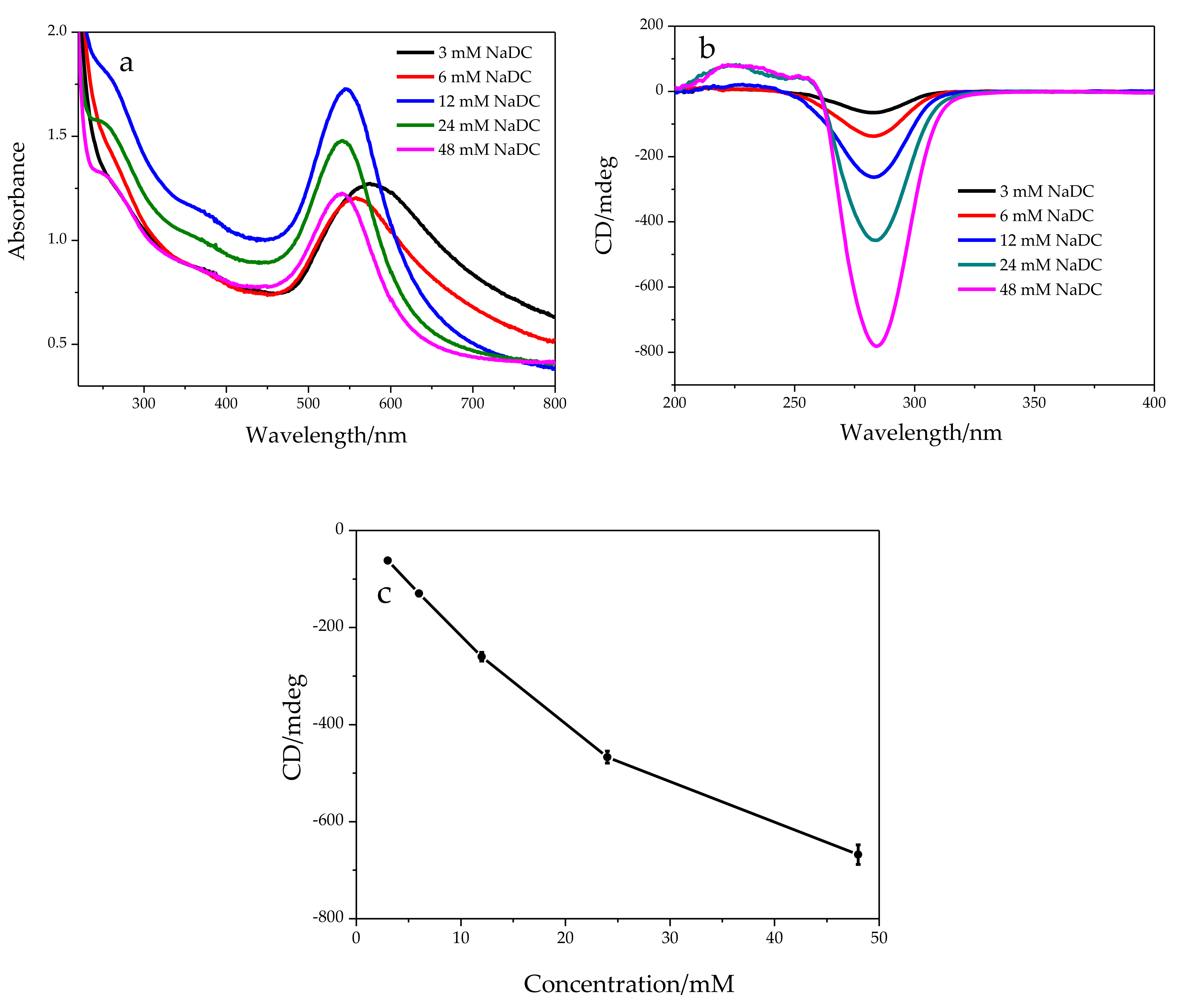
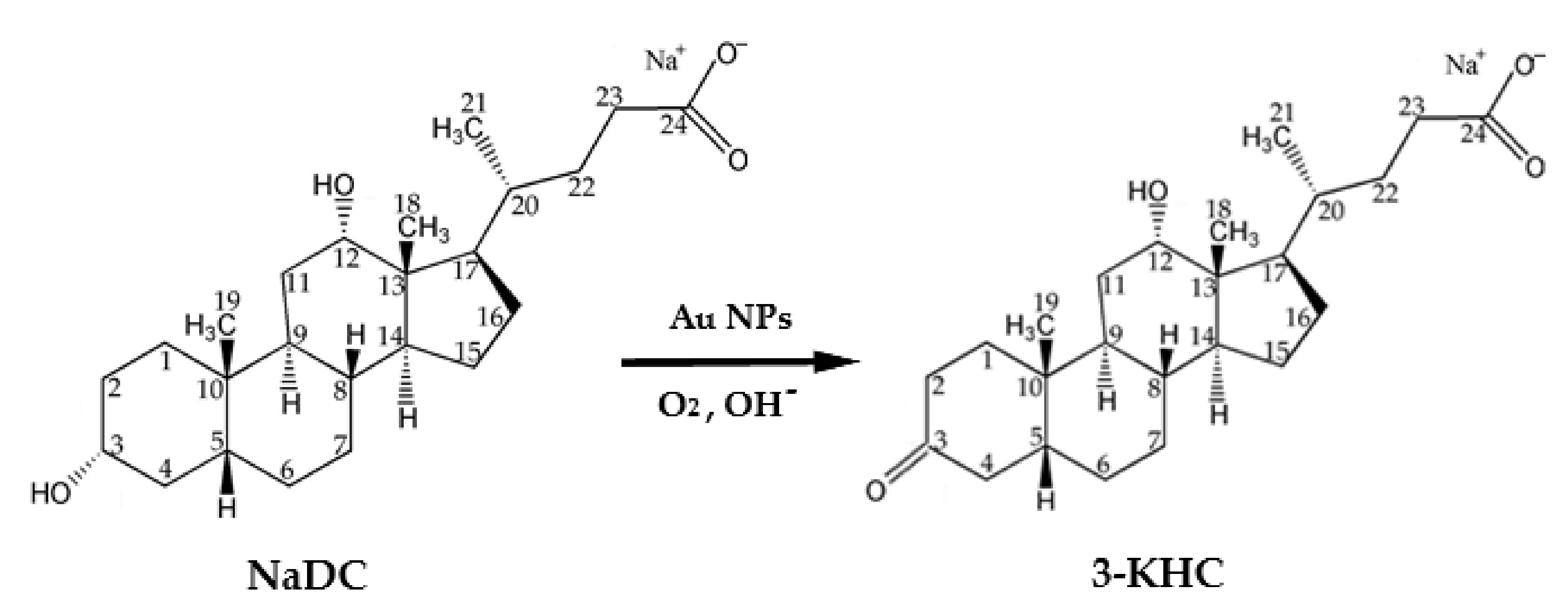
2.1. Chemical Oxidation of NaDC to 3-KHC Catalyzed by Au NPs
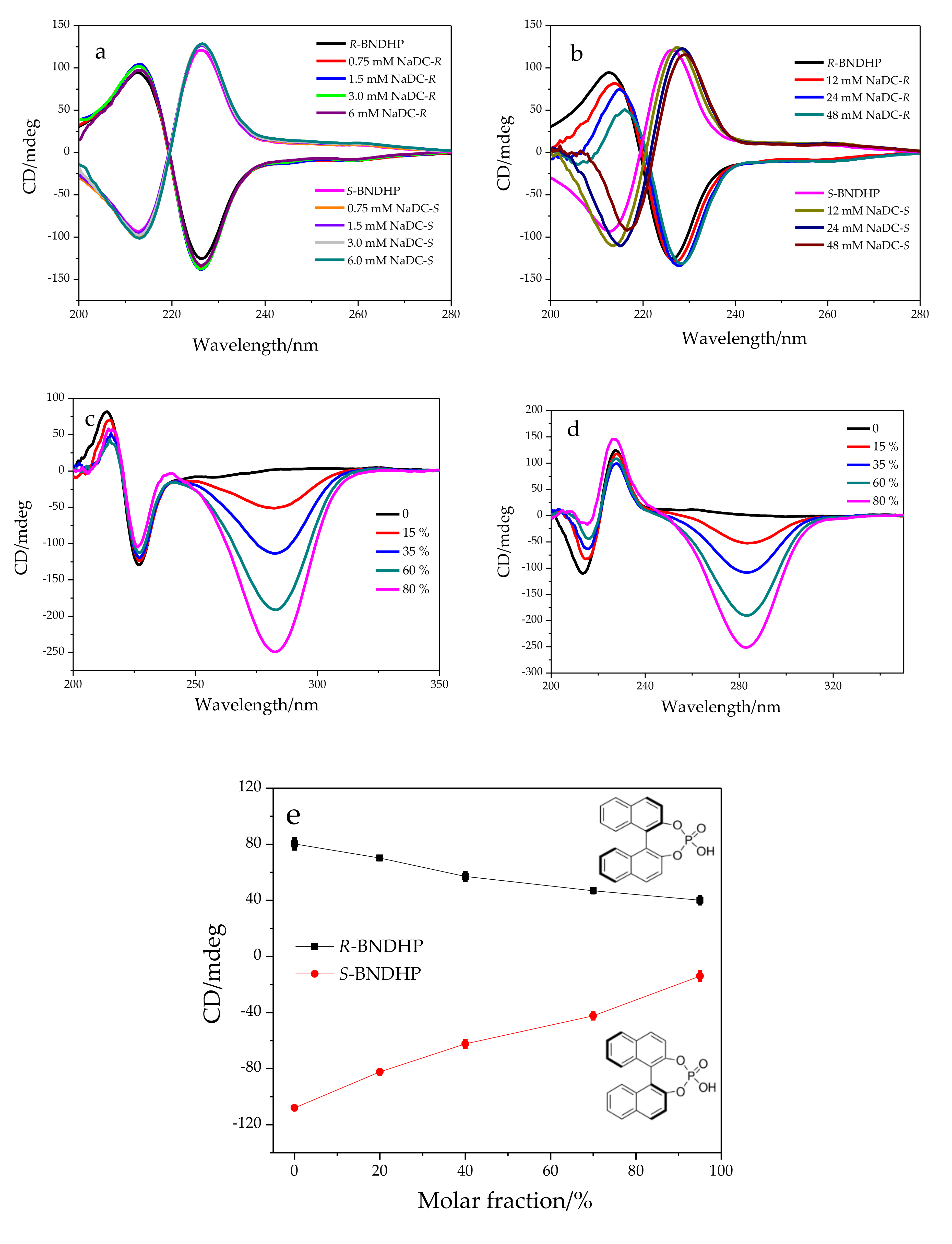
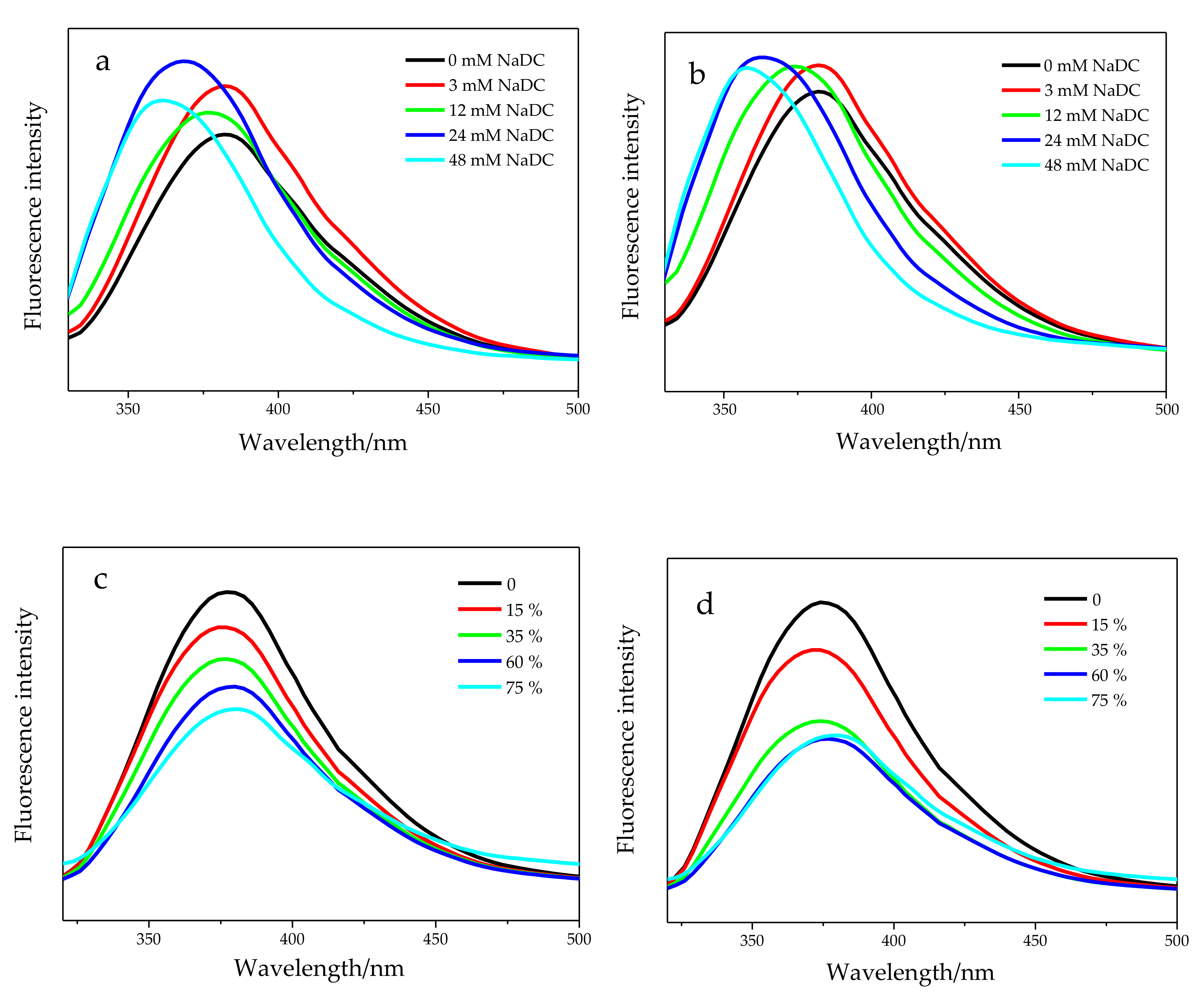
2.2. Chiral Recognition of R,S-BNDHP in Bile Salt Micelles
3. Materials and Methods
3.1. Materials
3.2. Preparation of Au NPs in the Presence of NaDC
3.3. Chiral Recognition of R,S-BNDHP
3.4. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mukhopadhyay, S.; Maitra, U. Chemistry and biology of bile acids. Curr. Sci. 2004, 87, 1666–1683. [Google Scholar]
- Monte, M.J.; Marin, J.J.G.; Antelo, A.; Vazquez-Tato, J. Bile acids: Chemistry, physiology, and pathophysiology. World J. Gastroenterol 2009, 15, 804–816. [Google Scholar] [CrossRef] [PubMed]
- Barnadas-Rodríguez, R.; Cladera, J. Steroidal surfactants: Detection of premicellar aggregation, secondary aggregation changes in micelles, and hosting of a highly charged negative substance. Langmuir 2015, 31, 8980–8988. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Mendoza, M.; Marin, M.L.; Miranda, M.A. Dansyl-labeled cholic acid as a tool to build speciation diagrams for the aggregation of bile acids. J. Phys. Chem. B 2012, 116, 14776–14780. [Google Scholar] [CrossRef] [PubMed]
- Posa, M.; Pilipovic, A.; Lali, M. The influence of NaCl on hydrophobicity of selected, pharmacologically active bile acids expressed with chromatographic retention index and critical micellar concentration. Colloids Surface. B 2010, 81, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.T.; Li, Q.; Cai, L.R.; Wang, C.; Lei, X.G. Chemoproteomic profiling of bile acid interacting proteins. Acs Cent. Sci. 2017, 3, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, E.; Kolehmainen, E. Use of bile acids in pharmacological and supramolecular applications. Eur. J. Org. Chem. 2004, 16, 3385–3399. [Google Scholar] [CrossRef]
- Maity, B.; Ahmed, S.A.; Seth, D. Interaction of biologically active flavins inside bile salt aggregates: Molecular level investigation. J. Phys. Chem. B 2016, 120, 9854–9866. [Google Scholar] [CrossRef]
- Zhang, M.; Strandman, S.; Waldron, K.C.; Zhu, X.X. Supramolecular hydrogelation with bile acid derivatives: Structures, properties and applications. J. Mater. Chem. B 2016, 4, 7506–7520. [Google Scholar] [CrossRef]
- Dharanivasan, G.; Jesse, D.M.I.; Chandirasekar, S.; Rajendiran, N.; Kathiravan, K. Label free fluorometric characterization of DNA interaction with cholate capped gold nanoparticles using ethidium bromide as a fluorescent probe. J. Fluoresc. 2014, 24, 1397–1406. [Google Scholar] [CrossRef]
- Katona, B.W.; Cummins, C.L.; Ferguson, A.D.; Li, T.T.; Schmidt, D.R.; Mangelsdorf, D.J.; Covey, D.F. Synthesis, characterization, and receptor interaction profiles of enantiomeric bile acids. J. Med. Chem. 2007, 50, 6048–6058. [Google Scholar] [CrossRef] [PubMed]
- Houten, S.M.; Watanabe, M.; Auwerx, J. Endocrine functions of bile acids. EMBO J. 2006, 25, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Bortolini, O.; Fantin, G.; Fogagnolo, M. Bile acids in asymmetric synthesis and chiral discrimination. Chirality 2010, 22, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Z.; Xie, J.W.; Liu, C.S. Microenvironmental properties and chiral discrimination abilities of bile salt micelles by fluorescence probe technique. Anal. Chem. 2003, 75, 91–97. [Google Scholar] [CrossRef]
- Tyszka, A.; Pikus, G.; Dabrowa, K.; Jurczak, J. Late-stage functionalization of (R)-BINOL-based diazacoronands and their chiral recognition of a-phenylethylamine hydrochlorides. J. Org. Chem. 2019, 84, 6502–6507. [Google Scholar] [CrossRef]
- Meier, A.R.; Yehl, J.B.; Eckenroad, K.W.; Manley, G.A.; Strein, T.G.; Rovnyak, D. Stepwise aggregation of cholate and deoxycholate dictates the formation and loss of surface-available chirally selective binding sites. Langmuir 2018, 34, 6489–6501. [Google Scholar] [CrossRef]
- Aumatell, A.; Wells, R.J. Enantiomeric differentiation of a wide range of pharmacologically active substances by cyclodextrin-modified micellar electrokinetic capillary chromatography using a bile salt. J. Fluoresc. A 1994, 688, 329–337. [Google Scholar] [CrossRef]
- Strei, T.G.; Morris, D.; Palmer, J.; Landers, J.P. Discontinuous electrophoretic stacking system for cholate-based electrokinetic chromatographic separation of 8-hydroxy-2 ‘-deoxyguanosine from unmodified deoxynucleosides Journal of chromatography. B, Biomedical sciences and applications. J. Fluoresc. A 2001, 763, 71–78. [Google Scholar]
- Shedania, Z.; Kakava, R.; Volonterio, A.; Farkas, T.; Chankvetadze, B. Separation of enantiomers of chiral sulfoxides in high-performance liquid chromatography with cellulose-based chiral selectors using methanol and methanol-water mixtures as mobile phases. J. Chromatogr. A 2018, 1557, 62–74. [Google Scholar] [CrossRef]
- Matarashvili, I.; Ghughunishvili, D.; Chankvetadze, L.; Takaishvili, N.; Khatiashvili, T.; Tsintsadze, M.; Farkas, T.; Chankvetadze, B. Separation of enantiomers of chiral weak acids with polysaccharide-based chiral columns and aqueous-organic mobile phases in high-performance liquid chromatography: Typical reversed-phase behavior? J. Chromatogr. A 2017, 1483, 86–92. [Google Scholar] [CrossRef]
- Zhang, M.; Ye, B.C. Colorimetric chiral recognition of enantiomers using the nucleotide-capped silver nanoparticles. Anal. Chem. 2011, 83, 1504–1509. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.L.; Kong, N.; Conlan, X.A.; Wang, H.B.; Barrow, C.J.; Yan, F.H.; Guo, J.M.; Yang, W.R. Electrochemical evidences of chiral molecule recognition using L/D cysteine modified gold electrodes. Electrochim. Acta 2017, 237, 22–28. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, S.S.; Xu, X.; Fei, K.X.; Peng, Y.X. A surface mediated supramolecular chiral phenomenon for recognition of L- and D-cysteine. Nanomaterials 2018, 8, 1027. [Google Scholar] [CrossRef] [PubMed]
- Ben-Moshe, A.; Teitelboim, A.; Oron, D.; Markovich, G. Probing the interaction of quantum dots with chiral capping molecules using circular dichroism spectroscopy. Nano Lett. 2016, 16, 7467–7473. [Google Scholar] [CrossRef]
- Herrera, B.T.; Moor, S.R.; McVeigh, M.; Roesner, E.K.; Marini, F.; Anslyn, E.V. Rapid optical determination of enantiomeric excess, diastereomeric excess, and total concentration using dynamic-covalent assemblies: A demonstration using 2-aminocyclohexanol and chemometrics. J. Am. Chem. Soc. 2019, 141, 11151–11160. [Google Scholar] [CrossRef]
- Zhu, Y.Y.; Wu, X.D.; Gu, S.X.; Pu, L. Free amino acid recognition: A Bisbinaphthyl-based fluorescent probe with high enantioselectivity. J. Am. Chem. Soc. 2019, 141, 175–181. [Google Scholar] [CrossRef]
- Wang, J.; Fei, K.-X.; Zhang, S.-S.; Peng, Y.-X. Synthesis and plasmonic chiroptical studies of sodium deoxycholate modified silver nanoparticles. Materials 2018, 11, 1291. [Google Scholar] [CrossRef]
- Wang, J.; Xu, X.; Qiu, X.L.; Zhang, S.S.; Peng, Y.X. Yolk–shell structured Au@Ag@mSiO2 as a probe for sensing cysteine enantiomers and Cu2+ based on circular dichroism. Analyst 2019, 144, 7489–7497. [Google Scholar] [CrossRef]
- Song, H.W.; Li, Z.B.; Peng, Y.X.; Li, X.; Xu, X.C.; Pan, J.M.; Niu, X.H. enzyme-triggered in situ formation of ag nanoparticles with oxidase-mimicking activity for amplified detection of alkaline phosphatase activity. Analyst 2019, 144, 2416–2422. [Google Scholar] [CrossRef]
- Qiao, Y.; Chen, H.F.; Lin, Y.Y.; Huang, J.B. Controllable synthesis of water-soluble gold nanoparticles and their applications in electrocatalysis and Surface-Enhanced Raman Scattering. Langmuir 2011, 27, 11090–11097. [Google Scholar] [CrossRef]
- Chandirasekar, S.; Chandrasekaran, C.; Muthukumarasamyvel, T.; Sudhandiran, G.; Rajendiran, N. Sodium cholate-templated blue light-emitting Ag subnanoclusters: In vivo toxicity and imaging in Zebrafish Embryos. Acs Appl. Mater. Interfaces 2015, 7, 1422–1430. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.S.; Chen, Y.L.; Huang, J.L.; Chen, J.D.; Zhao, C.; Zheng, Y.Q.; Yu, T.; Yang, Y.; Zhang, H.W. Supramolecular hydrogels for creating gold and silver nanoparticles in situ. Soft Matter 2013, 9, 2017–2023. [Google Scholar] [CrossRef]
- Gu, Q.Y.; Sautet, P.; Michel, C. Unraveling the role of base and catalyst polarization in alcohol oxidation on Au and Pt in water. ACS Catal. 2018, 8, 11716–11721. [Google Scholar] [CrossRef]
- Lei, L.J.; Liu, H.; Wu, Z.W.; Qin, Z.F.; Wang, G.F.; Ma, J.Y.; Luo, L.; Fan, W.B.; Wang, J.G. Aerobic oxidation of alcohols over isolated single Au atoms supported on CeO2 nanorods: Catalysis of interfacial [O–Ov–Ce–O–Au] sites. Acs Appl. Nano Mater. 2019, 2, 5214–5223. [Google Scholar] [CrossRef]
- Li, H.Y.; Shinde, P.B.; Lee, H.J.; Yoo, E.S.; Lee, C.; Hong, J.K.; Choi, S.H.; Jung, J.H. Bile acid derivatives from a sponge-associated bacterium psychrobacter sp. Arch. Pharm. Res. 2009, 32, 857–862. [Google Scholar] [CrossRef]
- Bettarello, L.; Bortolini, O.; Fantin, G.; Guerrini, A. Mixed oxo-hydroxy bile acids as actual or potential impurities in ursodeoxycholic acid preparation: A 1H and 13C NMR study. Il Farm. 2000, 55, 51–55. [Google Scholar] [CrossRef]
- Bortolini, O.; Fantin, G.; Fogagnolob, M.; Mari, L. Two-way enantioselective control in the epoxidation of alkenes with the keto bile acid–Oxone® system. Tetrahedron 2006, 62, 4482–4490. [Google Scholar] [CrossRef]
- Bertolasi, V.; Bortolini, O.; Fantin, G.; Fogagnolo, M.; Perrone, D. Preparation and characterization of some keto-bile acid azines. Steroids 2007, 72, 756–764. [Google Scholar] [CrossRef]
- Xiao, D.B.; Yang, W.S.; Yao, J.N.; Xi, L.; Yang, X.; Shuai, Z.G. Size-dependent exciton chirality in (R)-(+)-1,1′-bi-2-naphthol dimethyl ether nanoparticles. J. Am. Chem. Soc. 2004, 126, 15439–15444. [Google Scholar] [CrossRef]
- Yanli, Z.; Aidong, P.; Jing, W.; Wensheng, Y.; Jiannian, Y. Size-tunable exciton chirality and fluorescence emission in (R)-(−)-2,2-bis-(p-toluenesulfonyloxy)-1,1-binaphthalene nanoparticles. J. Photoch. Photobio. A 2006, 181, 94–98. [Google Scholar]
- Eckenroad, K.W.; Manley, G.A.; Yehl, J.B.; Pirnie, R.T.; Strein, T.G.; Rovnyak, D. An edge selection mechanism for chirally selective solubilization of binaphthyl atropisomeric guests by cholate and deoxycholate micelles. Chirality 2016, 28, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.L.; Rovnyak, D.; Strein, T.G. Direct measurement of the thermodynamics of chiral recognition in bile salt micelles. Chirality 2016, 28, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; He, G.; Wang, H.Y.; Sun, X.H.; Liu, T.H.; Ding, L.P.; Fang, Y. Fabrication of a novel cholic acid modified OPE-based fluorescent film and its sensing performances to inorganic acids in acetone. Acs Appl. Mater. Interfaces 2012, 4, 6935–6941. [Google Scholar] [CrossRef] [PubMed]
- Boudiombo, J.S.B.; Su, H.; Ravenscroft, N.; Bourne, S.A.; Nassimbeni, L.R. Separation and resolution of methylcyclohexanones by enclathration with deoxycholic acid. Cryst. Growth Des. 2019, 19, 3962–3968. [Google Scholar] [CrossRef]
- Miyata, M.; Tohnai, N.; Hisaki, I. Crystalline host-guest assemblies of steroidal and related molecules: Diversity, hierarchy, and supramolecular chirality. Acc. Chem. Res. 2007, 40, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Nonappa; Maitra, U. Unlocking the potential of bile acids in synthesis, supramolecular/materials chemistry and nanoscience. Org. Biomol. Chem. 2008, 6, 657–669. [Google Scholar] [CrossRef]
- Adhikari, A.; Dey, S.; Mandal, U.; Das, D.K.; Ghosh, S.; Bhattacharyya, K. Femtosecond solvation dynamics in different regions of a bile salt aggregate: Excitation wavelength dependence. Phys. Chem. B 2008, 112, 3575–3580. [Google Scholar] [CrossRef]
Sample Availability: Sample of the compound 3-KHC is available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Xu, X.; Chen, H.; Zhang, S.-S.; Peng, Y.-X. Oxidation of Sodium Deoxycholate Catalyzed by Gold Nanoparticles and Chiral Recognition Performances of Bile Salt Micelles. Molecules 2019, 24, 4508. https://doi.org/10.3390/molecules24244508
Wang J, Xu X, Chen H, Zhang S-S, Peng Y-X. Oxidation of Sodium Deoxycholate Catalyzed by Gold Nanoparticles and Chiral Recognition Performances of Bile Salt Micelles. Molecules. 2019; 24(24):4508. https://doi.org/10.3390/molecules24244508
Chicago/Turabian StyleWang, Jing, Xu Xu, Hao Chen, Shuai-Shuai Zhang, and Yin-Xian Peng. 2019. "Oxidation of Sodium Deoxycholate Catalyzed by Gold Nanoparticles and Chiral Recognition Performances of Bile Salt Micelles" Molecules 24, no. 24: 4508. https://doi.org/10.3390/molecules24244508
APA StyleWang, J., Xu, X., Chen, H., Zhang, S.-S., & Peng, Y.-X. (2019). Oxidation of Sodium Deoxycholate Catalyzed by Gold Nanoparticles and Chiral Recognition Performances of Bile Salt Micelles. Molecules, 24(24), 4508. https://doi.org/10.3390/molecules24244508



