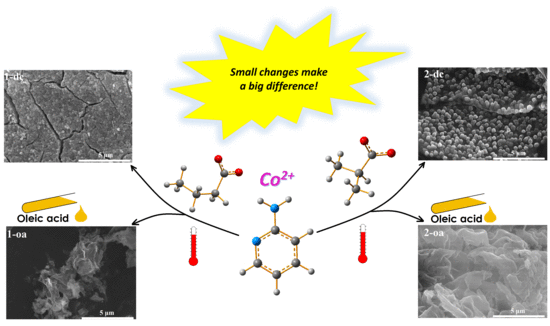
Structural Insights into Influence of Isomerism on Properties of Open Shell Cobalt Coordination System
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.1.1. Synthesis of Cobalt Carboxylates
2.1.2. Synthesis of Coordination Compounds
2.1.3. Synthesis of Cobalt(ii) Oxide Nanoparticles
2.2. Crystal Structure Determination
2.3. Other Physical Measurements
2.4. Quantum-Mechanical Calculations
3. Results and Discussion
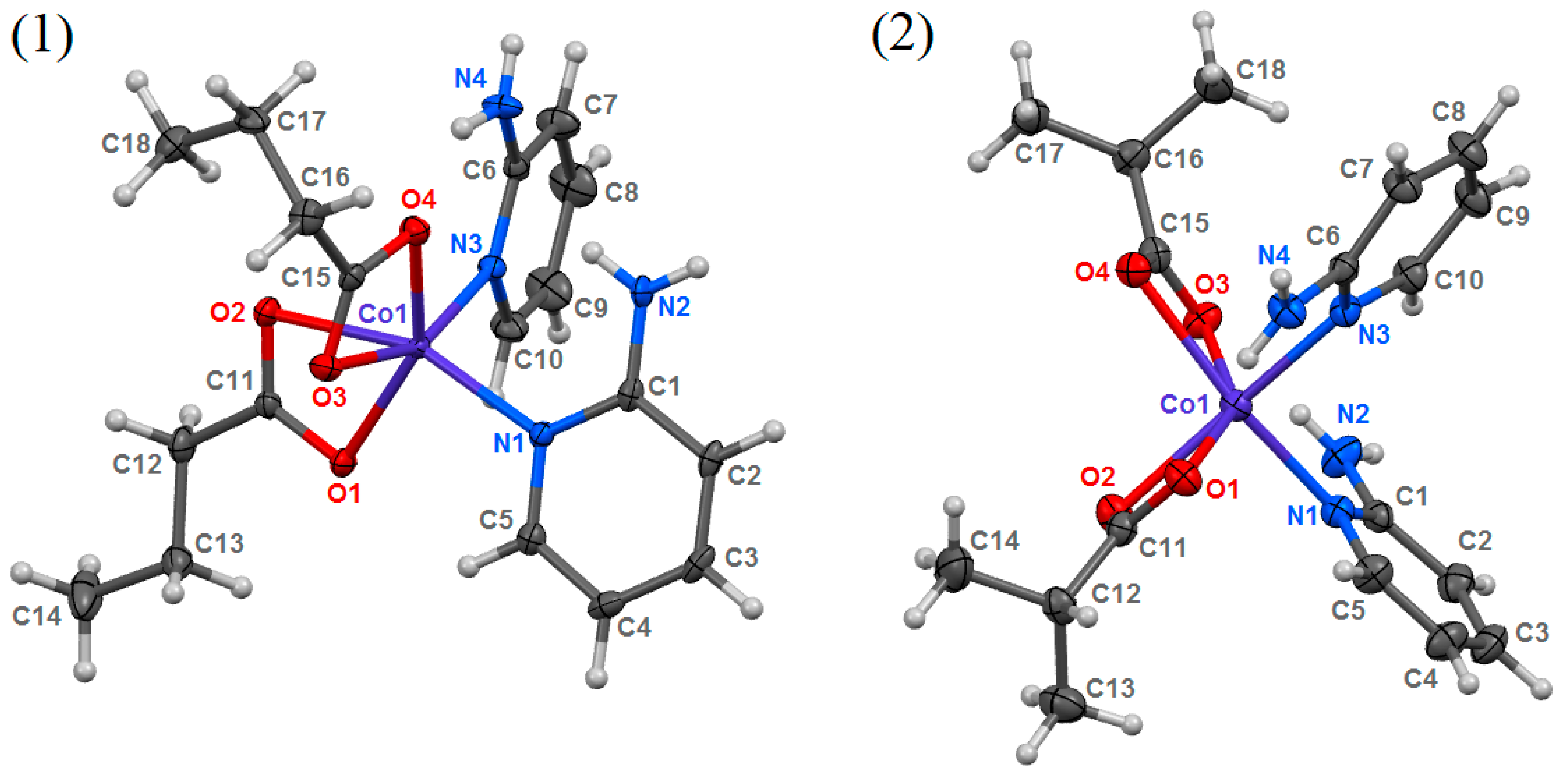
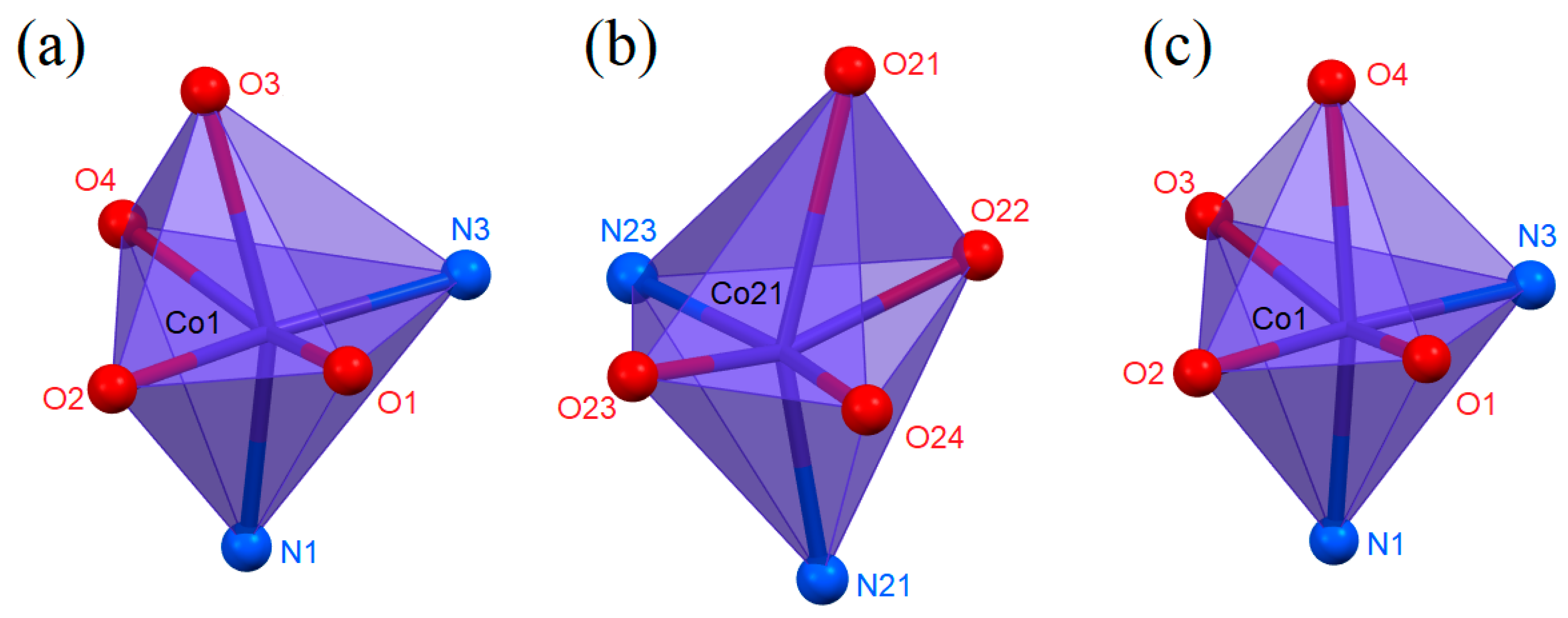
3.1. Structural Analysis
3.1.1. The Bond Valences
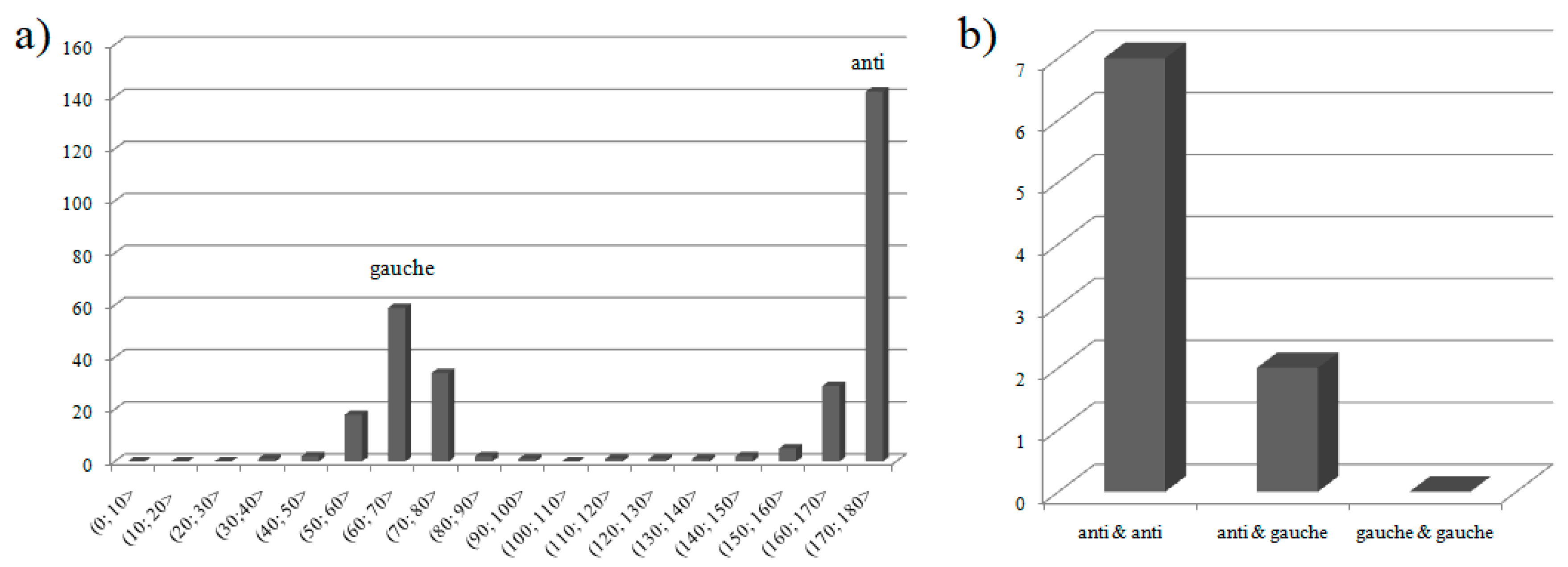
3.1.2. Intermolecular Interactions
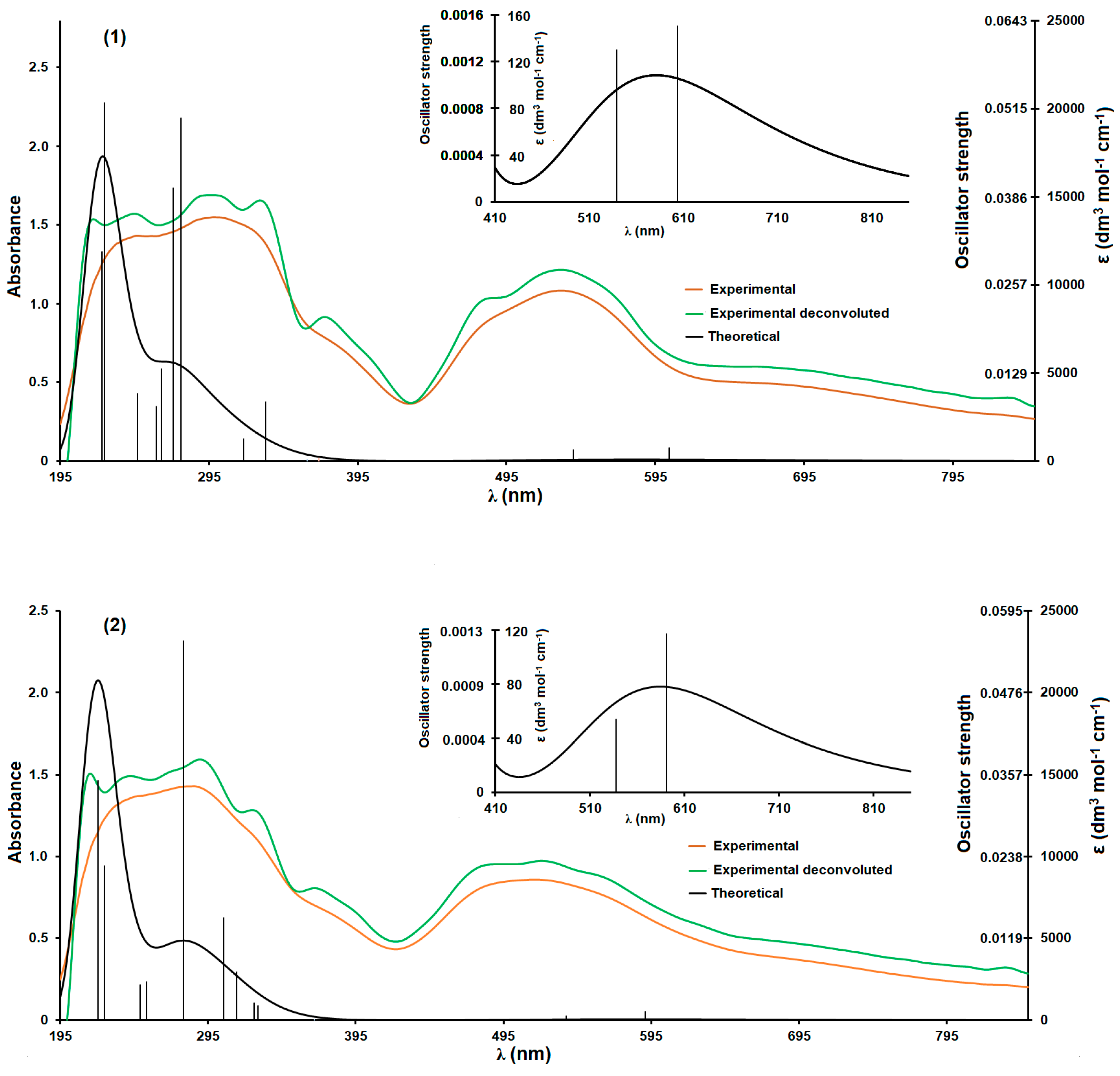
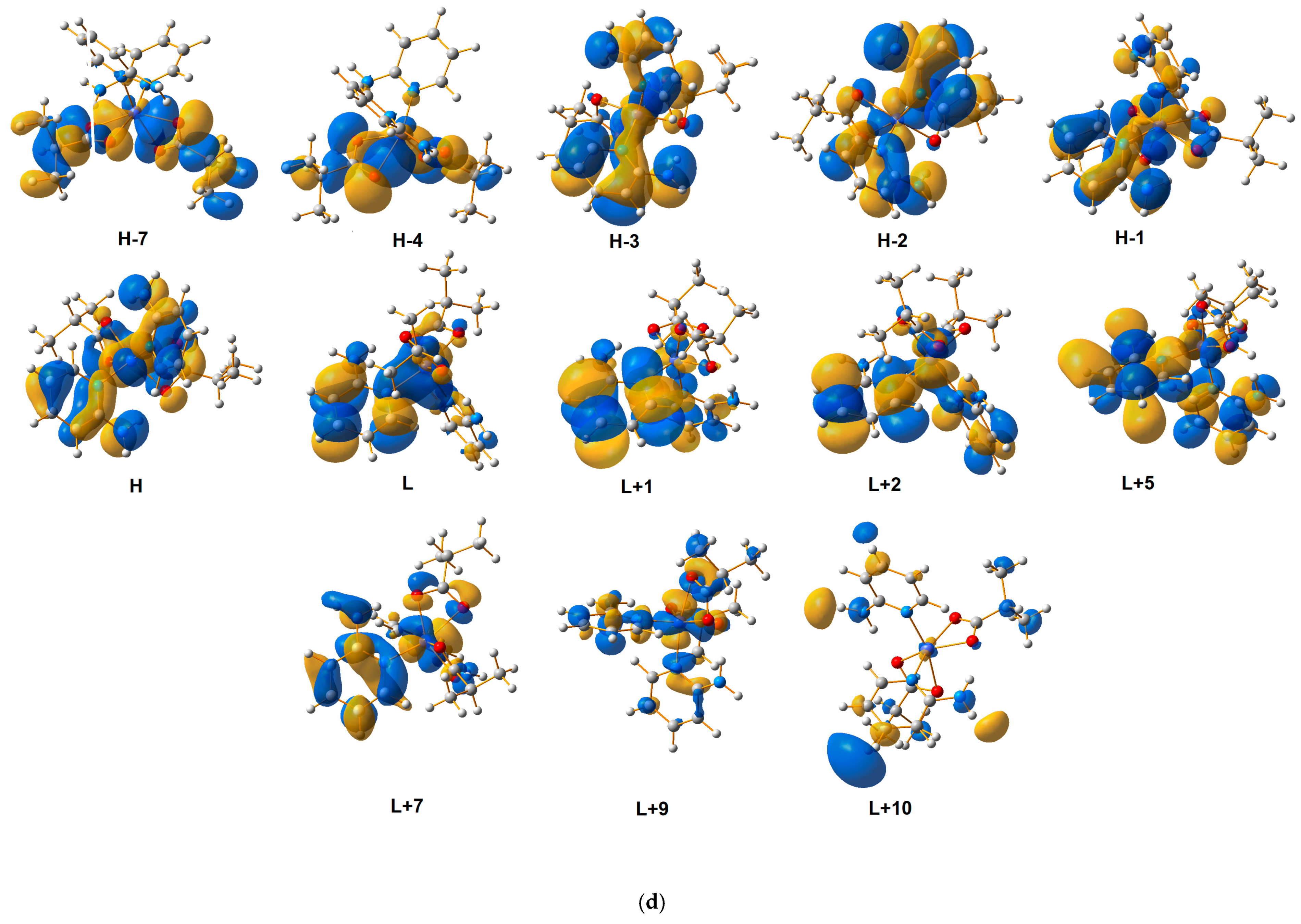
3.2. UV-Vis Spectrometric Analysis
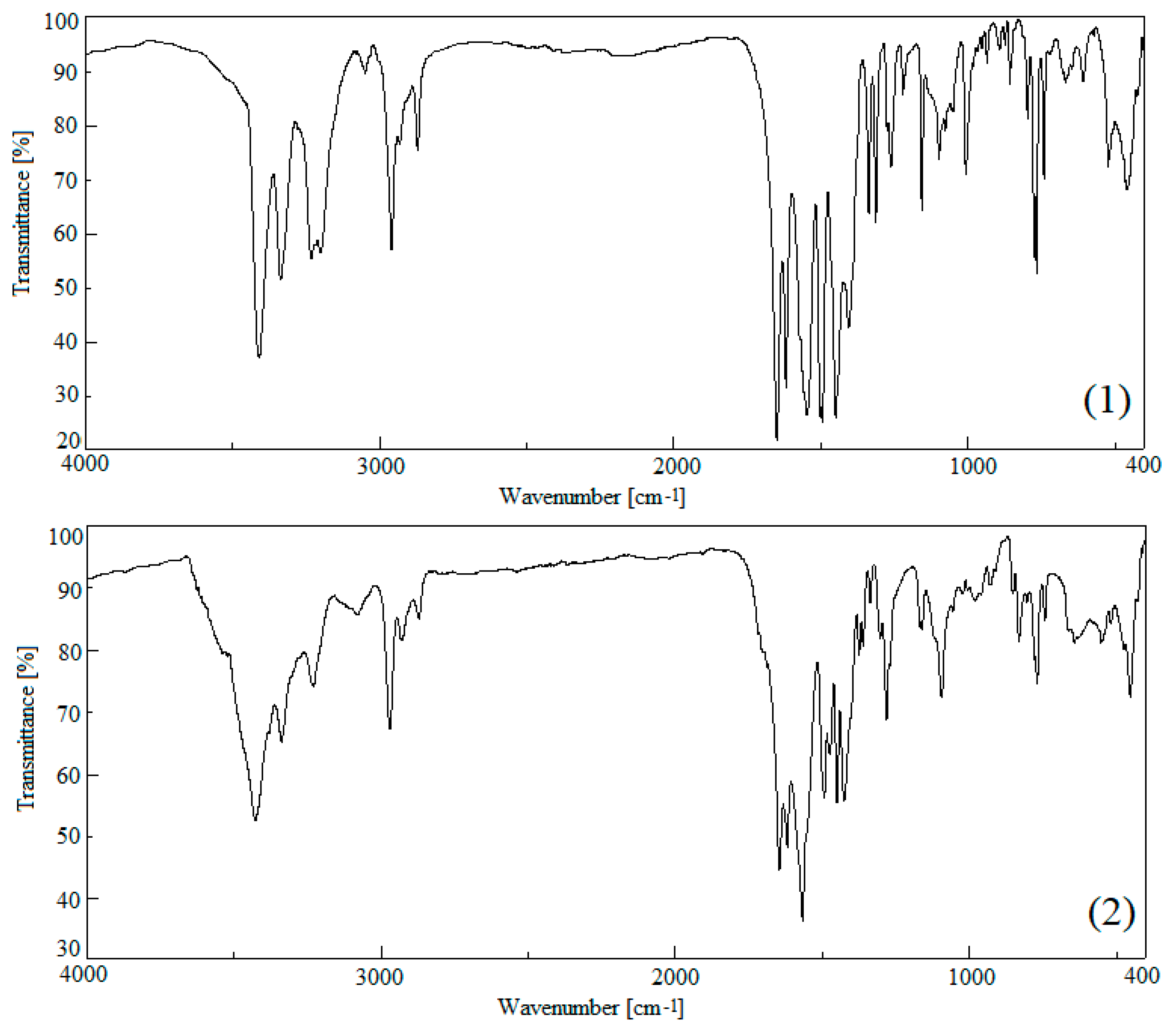
3.3. IR Spectrometric Analysis
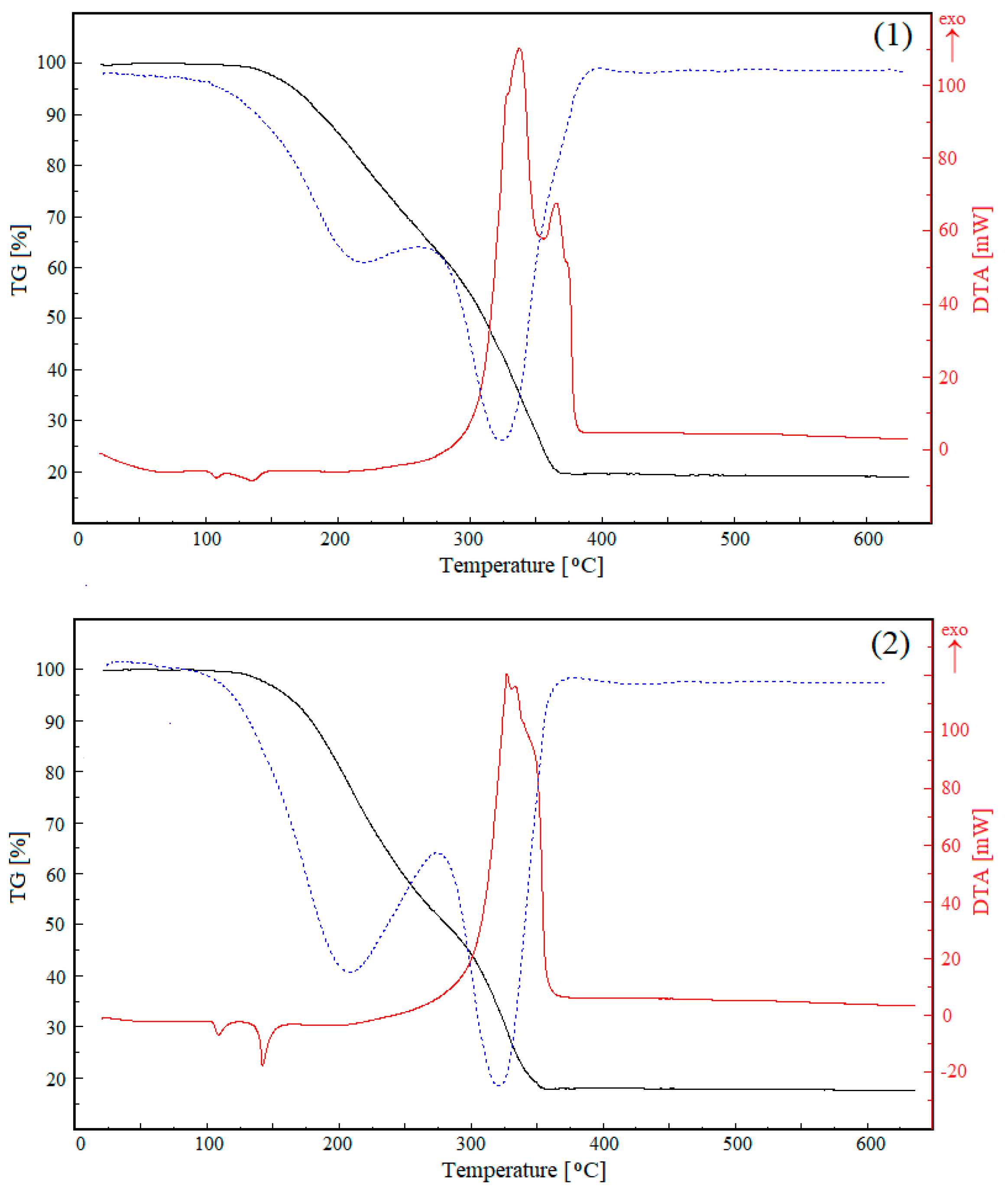
3.4. TG Analysis
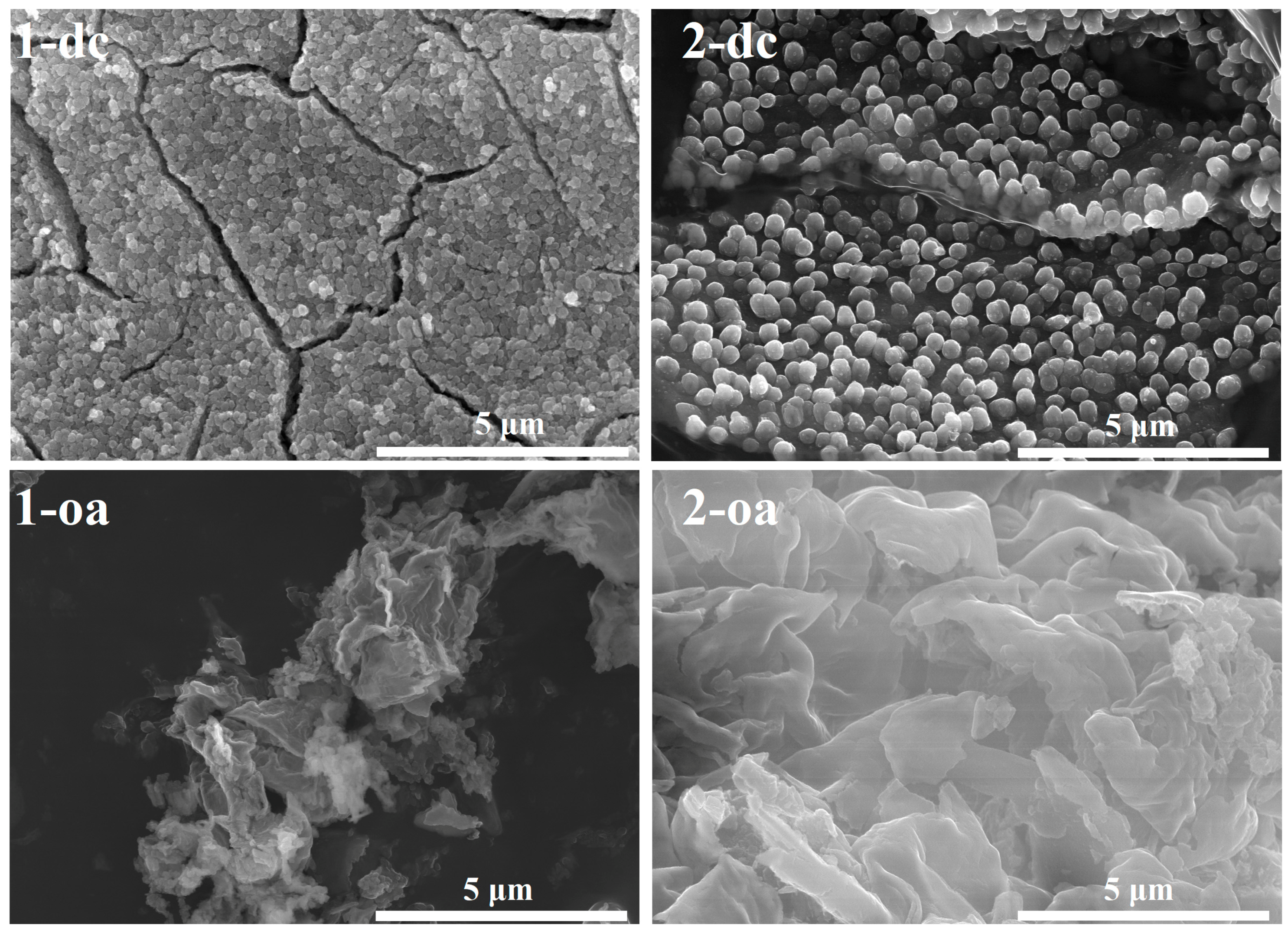
3.5. Nanoparticles of Cobalt(II) Oxide
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dojer, B.; Pevec, A.; Šegedin, P.; Jaglicic, Z.; Stropnik, C.; Kristl, M.; Drofenik, M. Cobalt(II) coordination compounds with acetate and 2-aminopyridine ligands: Synthesis, characterization, structures and magnetic properties of two polymorphic forms. Inorg. Chim. Acta 2010, 363, 1343–1347. [Google Scholar] [CrossRef]
- Wang, C.-C.; Wang, J.-H.; Tang, G.-M.; Wang, J.-T.; Cui, Y.-Z.; Weng Ng, S. Syntheses, crystal structures, and luminescent properties of three metal coordination polymers based on adipic acid and 2-(pyridine-3-yl)-(1H)-benzimidazole. J. Coord. Chem. 2015, 68, 3918–3931. [Google Scholar] [CrossRef]
- Zhao, N.; Li, Y.; Gu, J.; Fernandes, T.A.; Kirillova, M.V.; Kirillov, A.M. New copper(II) coordination compounds assembled from multifunctional pyridine-carboxylate blocks: Synthesis, structures, and catalytic activity in cycloalkane oxidation. Molecules 2019, 24, 6. [Google Scholar] [CrossRef] [PubMed]
- Grabner, S.; Modec, B. Zn(II) Curcuminate Complexes with 2,20-bipyridine and Carboxylates. Molecules 2019, 24, 2540. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.; Sun, X.; Wang, K.; Xu, C.; Li, Z.; Wang, Z. Effect of charge on the structures of Zn(II) coordination polymers with triazole-carboxylate ligands: Syntheses, structures, and luminescent properties. Crystals 2018, 8, 312. [Google Scholar] [CrossRef]
- Majumder, A.; Gramlich, V.; Rosair, G.M.; Batten, S.R.; Masuda, J.D.; El Fallah, M.S.; Ribas, J.; Sutter, J.-P.; Desplanches, C.; Mitra, S. Five New Cobalt(II) and Copper(II)-1,2,4,5-benzenetetracarboxylate Supramolecular Architectures: Syntheses, Structures, and Magnetic Properties. Cryst. Growth Des. 2006, 6, 2355–2368. [Google Scholar] [CrossRef]
- Santana, M.D.; Lozano, A.A.; Garcia, G.; Lopez, G.; Perez, J. Five-coordinate nickel(II) complexes with carboxylate anions and derivatives of 1,5,9-triazacyclododec-1-ene: Structural and 1H NMR spectroscopic studies. Dalton Trans. 2005, 104–109. [Google Scholar] [CrossRef]
- Gina Vasile Scăet, E.; Chifiriuc, M.C.; Bleotu, C.; Kamerzan, C.; Luminiţa Măru, T.; Daniliuc, C.G.; Maxim, C.; Calu, L.; Olar, R.; Badea, M. Synthesis, structural characterization, antimicrobial activity, and in vitro biocompatibility of new unsaturated carboxylate complexes with 2,2’-bipyridine. Molecules 2018, 23, 157. [Google Scholar] [CrossRef]
- Roy, S.; Paul, B.; Mukherjee, A.; Kundua, B.; Talukdar, A. Copper-catalyzed selective C–N bond formation with 2-amino, 2-hydroxy and 2-bromo-5-halopyridine. RSC Adv. 2017, 7, 44366–44370. [Google Scholar] [CrossRef]
- Pal, S. Pyridine: A Useful Ligand in Transition Metal Complexes. In Pyridine; Pandey, P.P., Ed.; IntechOpen: London, UK, 2018. [Google Scholar]
- Pandey, P.; Kharediya, B.; Elrez, B.; Sutter, J.-P.; Bhargavi, G.; Rajasekharan, M.V.; Sunkari, S.S. Ligand directed structural diversity and magnetism in copper(II)–azido assemblies with isomeric aminopyridines: Synthesis, structure, magnetism and theoretical studies. Dalton Trans. 2017, 46, 15908–15918. [Google Scholar] [CrossRef]
- Shriver, D.F.; Atkins, P.W. Inorganic Chemistry, 5th ed.; Oxford University Press: Oxford, UK, 2010; pp. 473–486. [Google Scholar]
- Kozlevar, B.; Murn, A.; Podlipnik, K.; Lah, N.; Leban, I.; Segedin, P. Two Types of Pyridine Ligands in Mononuclear and Dinuclear Copper(II) Carboxylates. Croat. Chem. Acta 2004, 4, 613–618. [Google Scholar]
- Janiak, C. A critical account on – stacking in metal complexes with aromatic nitrogen-containing ligands. J. Chem. Soc. Dalton Trans. 2000, 3885–3896. [Google Scholar] [CrossRef]
- Yuoh, A.C.B.; Agwara, M.O.; Yufanyi, D.M.; Conde, M.A.; Jagan, R.; Eyong, K.O. Synthesis, Crystal Structure, and Antimicrobial Properties of a Novel 1-D Cobalt Coordination Polymer with Dicyanamide and 2-Aminopyridine. Int. J. Inorg. Chem. 2015, 106838. [Google Scholar] [CrossRef]
- Kuliyev, K.A.; Verdizadeh, N.A.; Suleymanova, G.S. Spectrophotometric Determination of Cobalt (II) with 2,6-Dithiolphenol and Its Derivatives in the Presence of Hydrophobic Amines. Am. J. Chem. 2016, 6, 95–103. [Google Scholar]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. Cambridge Structural Database, CSD v 5.39, August 2018. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.A.; Shamma, A.A.; Kamel, S. New mixed ligand cobalt(II/III) complexes based on the drug sodium valproate and bioactive nitrogen-donor ligands. Synthesis, structure and biological properties. J. Mol. Struct. 2017, 1142, 40–47. [Google Scholar] [CrossRef]
- Ju, W.-Z.; Jiao, R.-H.; Cao, P.; Fang, R.-Q. Bis(2-aminopyridine)dibenzoatocobalt(II). Acta Crystallogr. Sect. E Struct. Rep. Online 2006, 62, m1012–m1013. [Google Scholar] [CrossRef]
- Zhong, D.-C.; Guo, G.-Q.; Zuo, X.-H.; Deng, J.-H.; Yuan, L.; Zhu, R.-H. Bis(2-aminopyridine-κN1)bis(benzoato-κO)cobalt(II). Acta Crystallogr. Sect. E Struct. Rep. Online 2008, 64, m88–m89. [Google Scholar] [CrossRef] [PubMed]
- Bokach, N.A.; Kukushkin, V.Y.; Haukka, M.; Mikhailova, T.B.; Sidorov, A.A.; Eremenko, I.L. Co(II)-Mediated and microwave assisted coupling between 2,6-diaminopyridine and nitriles. A new synthetic route to N-(6-aminopyridin-2-yl)carboximidamides. Russ. Chem. Bull. 2006, 55, 36–43. [Google Scholar] [CrossRef]
- Castillo, O.; Luque, A.; Roman, P.; Lloret, F.; Julve, M. Syntheses, Crystal Structures, and Magnetic Properties of One-Dimensional Oxalato-Bridged Co(II), Ni(II), and Cu(II) Complexes withn-Aminopyridine (n= 2−4) as Terminal Ligand. Inorg. Chem. 2001, 40, 5526–5535. [Google Scholar] [CrossRef]
- Swiatkowski, M.; Kruszynski, R. Structural diversity coordination compounds of zinc as effective precursors of zinc oxide nanoparticles with various morphologies. Appl. Organomet. Chem. 2019, 33, 4812. [Google Scholar] [CrossRef]
- Kruszynski, R.; Swiatkowski, M. The structure of coordination precursors as an effective tool for governing of size and morphology of ZnS and ZnO nanoparticles. J. Saudi Chem. Soc. 2018, 22, 816–825. [Google Scholar] [CrossRef]
- Kang, Q.; Cao, L.; Li, J.; Huang, J.; Xu, Z.; Cheng, Y.; Wang, X.; Bai, J.; Li, Q. Super P enhanced CoO anode for lithium-ion battery with superior electrochemical performance. Ceram. Int. 2016, 42, 15920–15925. [Google Scholar] [CrossRef]
- Chen, C.H.; Hwanga, B.J.; Do, J.S.; Weng, J.H.; Venkateswarlu, M.; Chenga, M.Y.; Santhanam, R.; Ragavendran, K.; Lee, J.F.; Chen, J.M.; et al. An understanding of anomalous capacity of nano-sized CoO anode materials for advanced Li-ion battery. Electrochem. Commun. 2010, 12, 496–498. [Google Scholar] [CrossRef]
- Wang, Q.; Kou, X.; Liu, C.; Zhao, L.; Lin, T.; Liu, F.; Yang, X.; Lin, J.; Lu, G. Hydrothermal synthesis of hierarchical CoO/SnO2 nanostructures for ethanol gas sensor. J. Colloid Interface Sci. 2018, 513, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Koshizaki, N.; Yasumoto, K.; Sasaki, T. A gas-sensing nanocomposite. Nanostruct. Mater. 1999, 12, 971–974. [Google Scholar] [CrossRef]
- Lv, S.; Zhao, X.; Xia, G.; Jin, C.; Wang, L.; Yang, W.; Zhang, Y.; Li, J. Monodisperse and size-tunable CoO nanocrystals synthesized by thermal decomposition and as an active precursor for Fischer-Tropsch synthesis. Chem. Phys. Lett. 2017, 667, 32–37. [Google Scholar] [CrossRef]
- Yuan, X.; Ge, H.; Wang, X.; Dong, C.; Dong, W.; Riaz, M.S.; Xu, Z.; Zhang, J.; Huang, F. Controlled Phase Evolution from Co Nanochains to CoO Nanocubes and Their Application as OER Catalysts. ACS Energy Lett. 2017, 2, 1208–1213. [Google Scholar] [CrossRef]
- Bagheri, M.; Mahjoub, A.R.; Khodadadi, A.A.; Mortazavi, Y. Fast photocatalytic degradation of congo red using CoO-doped β-Ga2O3 nanostructures. RSC Adv. 2014, 63, 33262–33268. [Google Scholar] [CrossRef]
- Rasouli, S.; Dabaee, I. Effect of preparation method on the anti-corrosive properties of nanocrystalline Zn-CoO ceramic pigments. Mater. Corros. 2011, 62, 405–410. [Google Scholar] [CrossRef]
- Ahmed, N.M.; El-Nashar, D.E.; Abd El-Messieh, S.L. Utilization of new micronized and nano-CoO·MgO/kaolin mixed pigments in improving the properties of styrene–butadiene rubber composites. Mater. Des. 2011, 32, 170–182. [Google Scholar] [CrossRef]
- Zouchoune, B.; Mansouri, L. Electronic structure and UV–Vis spectra simulation of square planar Bis(1-(4-methylphenylazo)-2-naphtol)-Transition metal complexes [M(L)2]x (M = Ni, Pd, Pt, Cu, Ag, and x = − 1, 0, + 1): DFT and TD-DFT study. Struct. Chem. 2019, 30, 691–701. [Google Scholar] [CrossRef]
- Małecki, J.G. Half-sandwich ruthenium(II) complexes with N- and N,(N,O)-donor ligands: Molecular, electronic structures, and computational study. Struct. Chem. 2012, 23, 461–472. [Google Scholar] [CrossRef]
- Małecki, J.G.; Bałanda, M.; Groń, T.; Kruszyński, R. Molecular, spectroscopic, and magnetic properties of cobalt(II) complexes with heteroaromatic N(O)-donor ligands. Struct. Chem. 2012, 23, 1219–1232. [Google Scholar] [CrossRef]
- Welcher, F.J. Analityczne zastosowanie kwasu wersenowego (eng. The Analytical Uses of Etylenediamineteraacetic Acid); WNT: Warsaw, Poland, 1963. [Google Scholar]
- Swiatkowski, M.; Kruszynski, R. Structural insights into the usage of carboxylate ions as molecular pins. Polyhedron 2017, 135, 265–277. [Google Scholar] [CrossRef]
- Bigdeli, F.; Morsali, A. Synthesis ZnO nanoparticles from a new Zinc(II) coordination polymer precursor. Mater. Lett. 2010, 64, 4–5. [Google Scholar] [CrossRef]
- Ramazani, M.; Morsali, A. Sonochemical syntheses of a new nano-plate cadmium(II) coordination polymer as a precursor for the synthesis of cadmium(II) oxide nanoparticles. Ultrason. Sonochem. 2011, 18, 1160–1164. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT– Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Adv. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Powder Diffraction File; International Center of Diffraction Data: Newton Square, PA, USA, 2003.
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision, D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0 - New Features for the Visualization and Investigation of Crystal Structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Joushaghani, M.; Sotoudehnezhad, M. Potentiometric Study Of Complex Formation Between Some Transition Metal Ions And 2-Aminopyridine. Part, L. A Model For Therapeutic Agent For Wilsons Disease Iran. J. Chem. Chem. Eng. 2003, 22, 17–21. [Google Scholar]
- Kepert, D.L. Aspects of the Stereochemistry of Six-coordination. In Progress in Inorganic Chemistry; Lippard, S.J., Ed.; John Wiley: New York, NY, USA, 1977; Volume 23, pp. 1–65. [Google Scholar]
- Zachariasen, W.H. Bond lengths in oxygen and halogen compounds of d and f elements. J. Less-Common Met. 1978, 62, 1–7. [Google Scholar] [CrossRef]
- Brown, I.D. nfluence of Chemical and Spatial Constraints on the Structures of Inorganic Compounds. Acta Crystallogr. Sect. B Struct. Sci. 1997, 53, 381–393. [Google Scholar] [CrossRef]
- Trzesowska, A.; Kruszynski, R.; Bartczak, T.J. New bond-valence parameters for lanthanides. Acta Crystallogr. Sect. B Struct. Sci. 2004, 60, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Trzesowska, A.; Kruszynski, R.; Bartczak, T.J. New lanthanide–nitrogen bond-valence parameters. Acta Crystallogr. Sect. B Struct. Sci. 2005, 61, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.D. Chemical and steric constraints in inorganic solids. Acta Crystallogr. Sect. B Struct. Sci. 1992, 48, 553–572. [Google Scholar] [CrossRef]
- Trzesowska, A.; Kruszynski, R.; Bartczak, T.J. Bond-valence parameters of lanthanides. Acta Crystallogr. Sect. B Struct. Sci. 2006, 62, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.M.; Palenik, G.J. Bond Valence Sums in Coordination Chemistry. A Simple Method for Calculating the Oxidation State of Cobalt in Complexes Containing Only Co−O Bonds. Inorg. Chem. 1998, 37, 4149–4151. [Google Scholar] [CrossRef] [PubMed]
- Palenik, G.J. Bond Valence Sums in Coordination Chemistry. The Calculation of the Oxidation State of Samarium in Complexes Containing Samarium Bonded Only to Oxygen. Inorg. Chem. 2003, 42, 2725–2728. [Google Scholar] [CrossRef] [PubMed]
- Kruszynski, R.; Sieranski, T. Can Stacking Interactions Exist Beyond the Commonly Accepted Limits? Cryst. Growth Des. 2016, 16, 587–595. [Google Scholar] [CrossRef]
- Van Leeuwen, R. Key Concepts In Time-Dependent Density-Functional Theory. Int. J. Mod. Phys. B 2001, 15, 1969–2023. [Google Scholar] [CrossRef]
- Yenikaya, C.; Poyraz, M.; Sari, M.; Demirci, F.; Ilkimen, H.; Buyukgungor, O. Synthesis, characterization and biological evaluation of a novel Cu(II) complex with the mixed ligands 2,6-pyridinedicarboxylic acid and 2-aminopyridine. Polyhedron 2009, 28, 3526–3532. [Google Scholar] [CrossRef]
- Chao, M.; Schempp, E.; Rosenstein, R.D. 2-Aminopyridine. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1975, 31, 2922–2924. [Google Scholar] [CrossRef]
- Deacon, G.B.; Phillips, R.J. Relationships between the carbon-oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]
- Sutton, C.C.R.; da Silva, G.; Franks, G.V. Modeling the IR Spectra of Aqueous Metal Carboxylate Complexes: Correlation between Bonding Geometry and Stretching Mode Wavenumber Shifts. Chem. A Eur. J. 2015, 21, 6801–6805. [Google Scholar] [CrossRef] [PubMed]
- Faizan, M.; Alam, M.J.; Afroz, Z.; Bhat, S.A.; Ahmad, S. Anharmonic vibrational spectra and mode-mode couplings analysis of 2-aminopyridine. Spectrochim. Acta Part A 2018, 188, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Akyuz, S. FT-IR spectroscopic investigation of transition metal (II) 2-aminopyridine tetracyanonickelate complexes. Vib. Spectrosc. 2000, 22, 49–54. [Google Scholar] [CrossRef]
- Juibari, N.M.; Abbasi, A.; Ajdari, H.B. Synthesis and Characterization of New Cobalt(II)–Pyrazine Coordination Polymer as Precursor for Preparation of Co(II) Oxide Nanoparticles: Surprising Coordination, DFT Calculation and Spectroscopic Studies. J. Inorg. Organomet. Polym. 2017, 27, S124–S130. [Google Scholar] [CrossRef]
- Hanifehpour, Y.; Soltani, B.; Mirtamizdoust, B.; Khomami, B.; Joo, S.W. Thermolysis Synthesis of Pure Phase Nano-Sized Cobalt(II) Oxide from Novel Cobalt(II)-Pyrazole Discrete Nano Coordination Compound. J. Inorg. Organomet. Polym. 2016, 26, 335–343. [Google Scholar] [CrossRef]
- He, X.; Shi, H. Synthesis and anomalous magnetic properties of hexagonal CoO nanoparticles. Mater. Res. Bull. 2011, 46, 1692–1697. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1 and 2 are available from the authors. |
| Compound | 1 | 2 |
|---|---|---|
| Empirical formula | C18H26CoN4O4 | C18H26CoN4O4 |
| Formula weight | 421.36 | 421.36 |
| Crystal system | Orthorhombic | Orthorhombic |
| Space group | Pna21 (No. 33) | Pbca (No. 61) |
| Temperature (K) | 100.0(1) | 100.0(1) |
| Wavelength (Å) | λ(CuKα) 1.54178 | λ(CuKα) 1.54184 |
| Unit cell dimensions (Å, °) | a = 15.3990(7) | a = 8.43060(10) |
| b = 13.9101(6) | b = 21.4947(4) | |
| c = 19.0482(8) | c = 22.1522(6) | |
| α = 90.00 | α = 90.00 | |
| β = 90.00 | β = 90.00 | |
| γ = 90.00 | γ = 90.00 | |
| Volume (Å3) | 4080.2(3) | 4014.27(14) |
| Z | 8 | 8 |
| Calculated density (Mg/m3) | 1.35 | 1.37 |
| Absorption coefficient (mm−1) | 6.851 | 6.964 |
| F(000) | 1768 | 1768 |
| Crystal size (mm) | 0.095 × 0.107 × 0.119 | 0.019 × 0.072 × 0.099 |
| θ Range for data collection (°) | 2.869 to 72.385 | 3.991 to 80.219 |
| Index ranges | −18 ≤ h ≤ 19, −17 ≤ k ≤ 17, −23 ≤ l ≤ 23 | −10 ≤ h ≤ 10, −27 ≤ k ≤ 25, −28 ≤ l ≤ 28 |
| Reflections collected/unique (Rint) | 45,837/7928 (0.0333) | 72,898/4343 (0.0561) |
| Completeness to θ = 67° (%) | 100.0 | 100.0 |
| Min. and max. transmission | 0.60175 and 1.00000 | 0.51741 and 1.00000 |
| Data/restraints/parameters | 7928/1/516 | 4343/0/260 |
| Goodness-of-fit on F2 | 1.048 | 1.089 |
| Final R indices (I > 2σ(I)) | R1 = 0.0237, wR2 = 0.0602 | R1 = 0.0303, wR2 = 0.0755 |
| R indices (all data) | R1 = 0.0243, wR2 = 0.0606 | R1 = 0.0364, wR2 = 0.0784 |
| Largest diff. peak and hole (e•Å−3) | 0.251 and −0.419 | 0.303 and −0.291 |
| i—j | dij (Å) | νij (v.u.) | i—j—k | αijk (°) | i—j—k | αijk (°) |
|---|---|---|---|---|---|---|
| Compound 1 | ||||||
| Co1—O1 | 2.0749(16) | 0.349 | O1—Co1—O2 | 60.78(6) | O2—Co1—N3 | 88.38(7) |
| Co1—O2 | 2.2447(16) | 0.220 | O1—Co1—O3 | 95.06(7) | O3—Co1—O4 | 59.12(6) |
| Co1—O3 | 2.3271(19) | 0.176 | O1—Co1—O4 | 148.72(7) | O3—Co1—N1 | 93.03(7) |
| Co1—O4 | 2.0840(18) | 0.340 | O1—Co1—N1 | 97.59(7) | O3—Co1—N3 | 157.36(7) |
| Co1—N1 | 2.0758(18) | 0.390 | O1—Co1—N3 | 101.22(8) | O4—Co1—N1 | 100.88(7) |
| Co1—N3 | 2.110(2) | 0.355 | O2—Co1—O3 | 85.93(7) | O4—Co1—N3 | 100.06(7) |
| O2—Co1—O4 | 97.35(7) | N1—Co1—N3 | 100.23(8) | |||
| O2—Co1—N1 | 158.07(7) | |||||
| Co21—O21 | 2.6003(18) | 0.084 | O21—Co21—O22 | 55.36(7) | O22—Co21—N23 | 103.86(7) |
| Co21—O22 | 2.0110(19) | 0.414 | O21—Co21—O23 | 97.79(8) | O23—Co21—O24 | 60.29(6) |
| Co21—O23 | 2.0429(17) | 0.380 | O21—Co21—O24 | 89.79(8) | O23—Co21—N21 | 100.04(8) |
| Co21—O24 | 2.2833(16) | 0.198 | O21—Co21—N21 | 156.67(8) | O23—Co21—N23 | 97.53(7) |
| Co21—N21 | 2.106(2) | 0.359 | O21—Co21—N23 | 87.35(7) | O24—Co21—N21 | 86.11(7) |
| Co21—N23 | 2.0709(18) | 0.395 | O22—Co21—O23 | 143.99(8) | O24—Co21—N23 | 157.02(7) |
| O22—Co21—O24 | 93.11(7) | N21—Co21—N23 | 105.01(8) | |||
| O22—Co21—N21 | 101.90(7) | |||||
| Compound 2 | ||||||
| Co1—O1 | 2.0847(11) | 0.340 | O1—Co1—O2 | 60.68(4) | O2—Co1—N3 | 164.60(5) |
| Co1—O2 | 2.2180(12) | 0.237 | O1—Co1—O3 | 147.00(5) | O3—Co1—O4 | 59.68(4) |
| Co1—O3 | 2.0765(11) | 0.347 | O1—Co1—O4 | 95.78(4) | O3—Co1—N1 | 105.14(5) |
| Co1—O4 | 2.3022(12) | 0.189 | O1—Co1—N1 | 99.57(5) | O3—Co1—N3 | 95.77(5) |
| Co1—N1 | 2.1010(13) | 0.364 | O1—Co1—N3 | 103.96(5) | O4—Co1—N1 | 164.42(5) |
| Co1—N3 | 2.0897(14) | 0.375 | O2—Co1—O3 | 97.49(5) | O4—Co1—N3 | 84.61(5) |
| O2—Co1—O4 | 95.38(4) | N1—Co1—N3 | 94.18(5) | |||
| O2—Co1—N1 | 89.88(5) | |||||
| D–H•••A | d(D—H) | d(H•••A) | d(D•••A) | <(DHA) | Gda(n) |
|---|---|---|---|---|---|
| compound 1 | |||||
| N2—H2N•••O4 | 0.84(3) | 2.35(3) | 3.067(3) | 143(3) | S(6) |
| N2—H2O•••O2 i | 0.81(3) | 2.06(3) | 2.861(3) | 170(3) | C(6) |
| N4—H4N•••O21 | 0.81(4) | 2.17(4) | 2.973(3) | 172(3) | D |
| N4—H4O•••O4 | 0.94(4) | 1.93(4) | 2.800(3) | 153(3) | S(6) |
| N22—H22N•••O3 ii | 0.82(4) | 2.08(4) | 2.902(3) | 175(3) | D |
| N22—H22O•••O22 | 0.73(4) | 2.10(4) | 2.799(3) | 161(4) | S(6) |
| N24—H24N•••O22 | 0.84(4) | 2.30(4) | 3.054(3) | 151(3) | S(6) |
| N24—H24O•••O24 iii | 0.84(3) | 1.99(3) | 2.822(3) | 171(3) | C(6) |
| compound 2 | |||||
| N2—H2N•••O3 | 0.82(2) | 2.10(2) | 2.897(2) | 164(2) | S(6) |
| N2—H2O•••O2 iv | 0.85(2) | 2.04(2) | 2.8733(19) | 166(2) | C(6) |
| N4—H4N•••O4 v | 0.85(2) | 2.12(2) | 2.9546(19) | 166.0(19) | R22(12) |
| N4—H4O•••O1 | 0.86(2) | 2.11(2) | 2.9341(19) | 161.0(19) | S(6) |
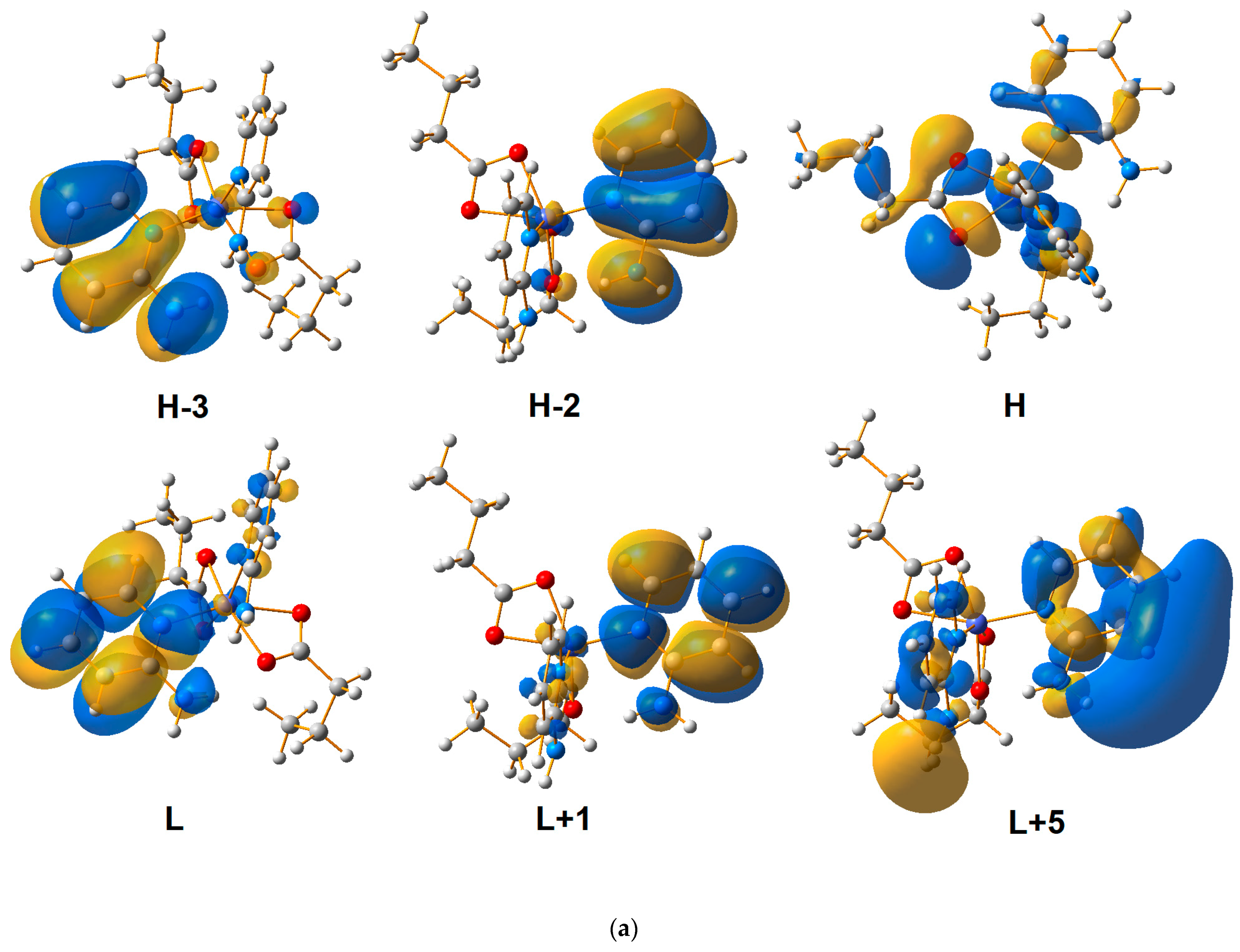
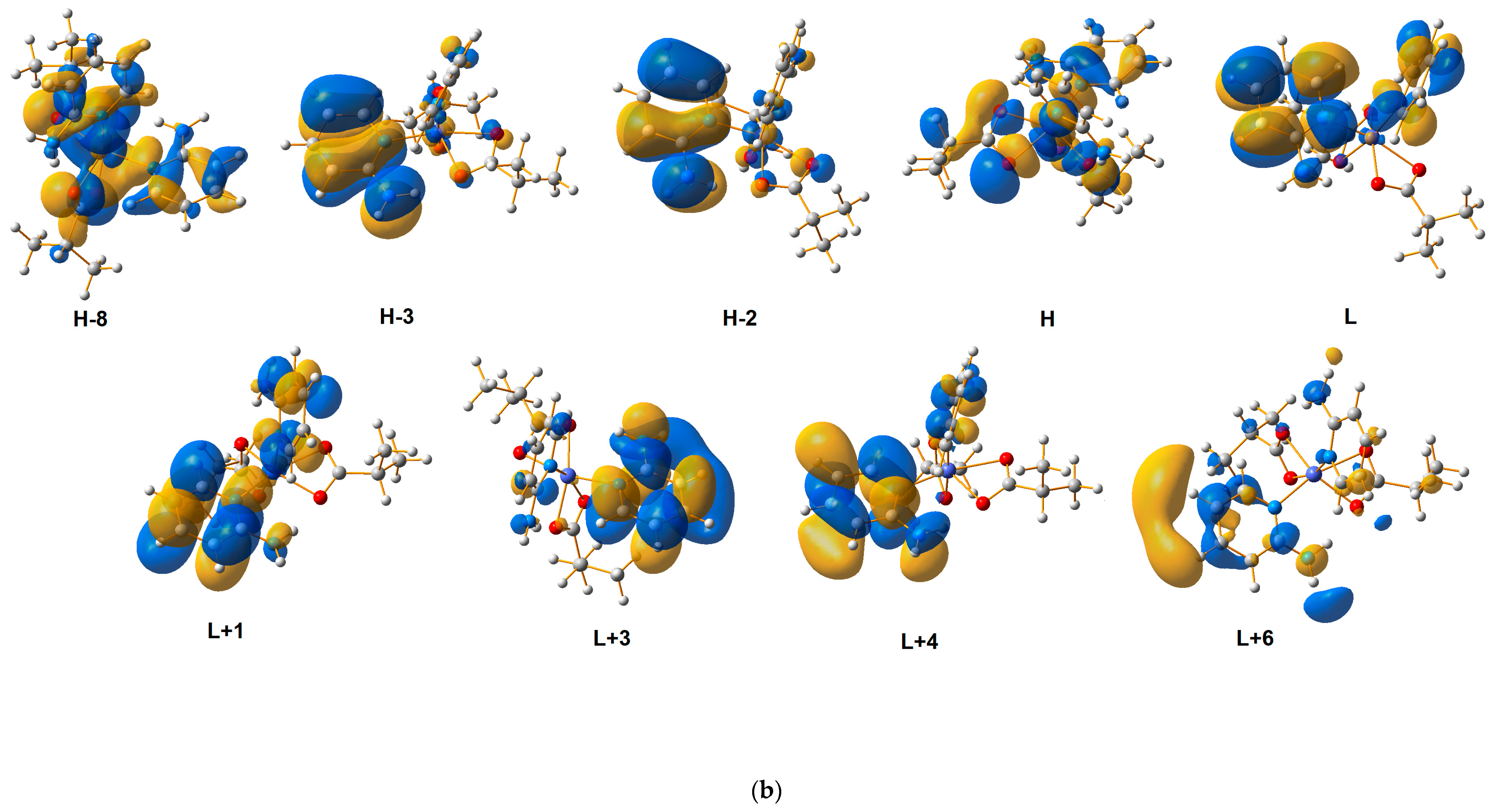
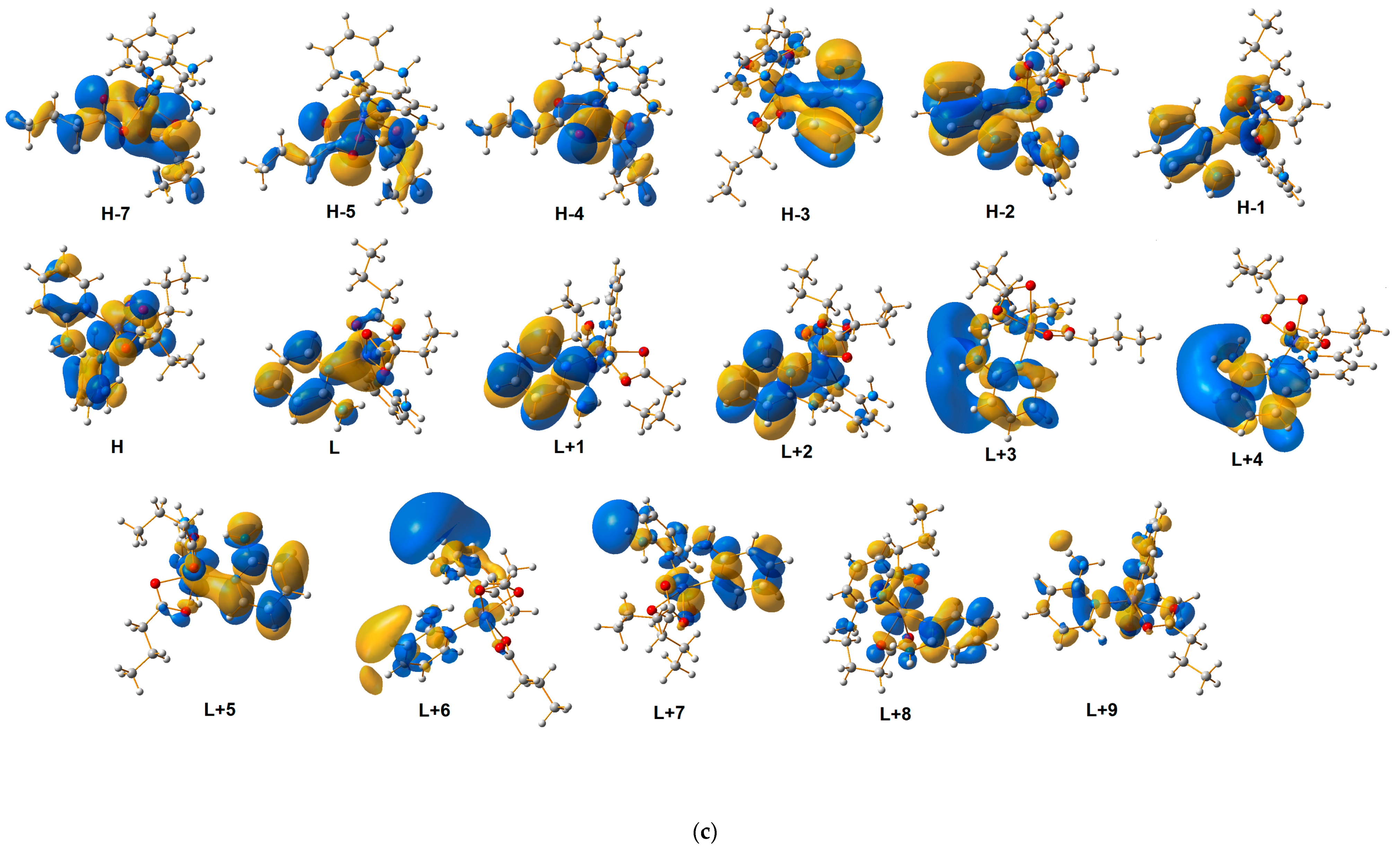
| Theoretical λ (nm) | E (eV) | f | The Most Important Orbitals Involved in Electronic Transitions | Character of Transition | Experimental λ (nm) (Solid State) | |||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 1 | 2 | 2-apy | ||||
| 220.76 | 5.6162 | 0.0349 | αH-8 → αL + 1 βH → βL + 10 | d(Co)/n(2-apy)/π(2-apy) → π*(2-apy) | 217 | 215 | ||
| d(Co)/n(ibut)/n(2-apy) → n(2-apy) | ||||||||
| 223.29 | 5.5525 | 0.0238 | βH-2 → βL + 7 αH-3 → αL + 5 | d(Co)/n(2-apy)/π(2-apy) → d(Co)/n(2-apy)/π*(2-apy) | ||||
| π(2-apy) → π*(2-apy) | ||||||||
| 225.00 | 5.5104 | 0.0407 | βH-2 → βL + 6 | d(Co)/n(2-apy)/π(2-apy) → d(Co)/n(2-apy) | ||||
| 225.30 | 5.5030 | 0.0224 | βH-7 → βL + 2 βH-7 → βL + 1 αH-3 → αL + 6 | n(ibut) → d(co)/π*(2-apy) | ||||
| n(ibut) → d(co)/π*(2-apy) | ||||||||
| π(2-apy) → n(2-apy) | ||||||||
| 246.94 | 5.0209 | 0.0077 | βH-2 → βL + 3 βH-1→ βL + 5 | d(Co)/n(2-apy)/π(2-apy) → d(Co)/n(2-apy)/π*(2-apy) | 245 | 243 | 234 | |
| d(Co)/n(2-apy)/n(but)/π(2-apy) → d(Co)/π*(2-apy) | ||||||||
| 249.10 | 4.9772 | 0.0051 | βH → βL + 5 | d(Co)/n(ibut)/n(2-apy) → d(Co)/π*(2-apy) | ||||
| 253.45 | 4.8918 | 0.0056 | βH-4 → βL + 1 βH-4 → βL + 2 | n(ibut) → d(co)/π*(2-apy) | ||||
| n(ibut) → d(co)/π*(2-apy) | ||||||||
| 259.67 | 4.7747 | 0.0062 | βH-5 → βL + 1 βH → βL + 3 | n(but) → π*(2-apy) | ||||
| d(Co)/n(2-apy)/n(but) → d(Co)/n(2-apy)/π*(2-apy) | ||||||||
| 263.31 | 4.7088 | 0.0105 | βH → βL + 4 βH → βL + 3 | d(Co)/n(2-apy)/n(but) → n(2-apy)/π*(2-apy) | ||||
| d(Co)/n(2-apy)/n(but) → d(Co)/n(2-apy)/π*(2-apy) | ||||||||
| 270.86 | 4.5775 | 0.0310 | βH-2 → βL + 2 αH-2 → αL + 1 βH-7 → βL | d(Co)/n(2-apy)/π(2-apy) → d(Co)/π*(2-apy) | 297 | 290 | 298 | |
| π(2-apy)/n(2-apy) → π*(2-apy) | ||||||||
| n(but) → d(Co)/π*(2-apy) | ||||||||
| 276.20 | 4.4889 | 0.0389 | βH-3 → βL + 1 αH-3 → αL | d(Co)/n(2-apy)/π(2-apy) → π*(2-apy) | ||||
| π(2-apy) → π*(2-apy) | ||||||||
| 278.62 | 4.4500 | 0.0551 | βH-2 → βL + 1 αH-2 → αL | d(Co)/π(2-apy) → d(Co)/π*(2-apy) | ||||
| π(2-apy) → π*(2-apy) | ||||||||
| 305.73 | 4.0554 | 0.0149 | βH-2 → βL | d(Co)/π(2-apy) → d(Co)/π*(2-apy) | ||||
| 314.31 | 3.9447 | 0.0070 | βH → βL | d(Co)/n(ibut)/n(2-apy) → d(Co)/π*(2-apy) | 329 | 325 | ||
| 318.29 | 3.7702 | 0.0025 | βH-4 → βL | n(but) → d(Co)/π*(2-apy) | ||||
| 316.39 | 3.9187 | 0.0065 | αH → αL | d(Co)/n(ibut)/n(2-apy) → π*(2-apy) | ||||
| 326.13 | 3.8017 | 0.0025 | βH → βL + 1 αH-3 → αL + 4 | d(Co)/n(ibut)/n(2-apy) → d(Co)/π*(2-apy) | ||||
| π(2-apy) → π*(2-apy) | ||||||||
| 329.02 | 3.7683 | 0.0021 | αH-2 → αL + 3 | π(2-apy) → π*(2-apy) | ||||
| 333.03 | 3.7229 | 0.0067 | βH → βL + 1 | d(Co)/n(2-apy)/n(but) → π*(2-apy) | ||||
| 361.20 | 3.4325 | 0.0002 | αH-3 → αL βH-3 → βL + 1 | π(2-apy) → π*(2-apy) | 373 | 367 | ||
| d(Co)/n(2-apy)/π(2-apy) → d(Co)/π*(2-apy) | ||||||||
| 367.12 | 3.3772 | 0.0001 | αH-3 → αL + 1 βH-3 → βL + 1 | π(2-apy) → π*(2-apy) | ||||
| d(Co)π(2-apy) → d(Co)/π*(2-apy) | ||||||||
| 368.90 | 3.3609 | 0.0003 | αH-2 → αL + 1 βH-2 → βL | π(2-apy)/n(2-apy) → π*(2-apy) | ||||
| d(Co)/n(2-apy)/π(2-apy) → d(Co)/π*(2-apy) | ||||||||
| 378.50 | 3.2757 | 00030 | αH-2 → αL | π(2-apy) → π*(2-apy) | ||||
| βH-2 → βL | d(Co)/n(2-apy)/π(2-apy) → d(Co)/π*(2-apy) | |||||||
| 537.58 | 2.3063 | 0.0006 | βH-1 → βL + 9 βH-1 → βL + 7 | d(Co)/n(ibut)π(2-apy) → d(Co)/n(ibut)/n(2-apy) | 485 | 492 | ||
| d(Co)/n(ibut)π(2-apy) → d(Co)/n(ibut)/n(2-apy)/π*(2-apy) | ||||||||
| 539.69 | 2.2973 | 0.0013 | βH-1 → βL + 9 | d(Co)/n(2-apy)/n(but)/π(2-apy) → d(Co)/n(2-apy) | ||||
| 591.11 | 2.0975 | 0.0013 | βH → βL + 7 | d(Co)//n(ibut)n(2-apy) → d(Co)/n(ibut)/n(2-apy)/π*(2-apy) | 532 | 521 | ||
| 604.34 | 2.0516 | 0.0015 | βH-1 → βL + 9 βH → βL + 8 | d(Co)/n(2-apy)/n(but)/π(2-apy) → d(Co)/n(2-apy) | ||||
| d(Co)/n(2-apy)/n(but) → d(Co)/π*(2-apy) | ||||||||
| 1 | Cobalt Butyrate [23,38] | 2-apy [63,64] | Assignment | 2 | Cobalt Isobutyrate [23,38] | 2-apy [63,64] | Assignment |
|---|---|---|---|---|---|---|---|
| 3410 s | 3446 s | νas NH2 | 3430 s | 3446 s | νas NH2 | ||
| 3338 m | νas NH2 | 3343 m | νas NH2 | ||||
| 3233 m | νs NH2 | 3230 m | 3188 m | νs NH2 | |||
| 3201 m | 3188 m | νs NH2 | |||||
| 3052 w | 3026 w | ν CH(ar) | 3078 w | 3026 w | ν CH(ar) | ||
| 2960 m | 2962 s | νas CH3 | 2970 m | 2967 m | νas CH3 | ||
| 2934 w | 2936 w | νs CH3 | 2928 w | 2929 w | νs CH3 | ||
| 2870 m | 2876 m | νs CH2 | 2870 w | 2872 w | ν CH | ||
| 1648 s | 1628 s | σ NH2 | 1646 s | 1628 s | σ NH2 | ||
| 1617 s | 1605 m | σ NH2, ν CC(ar) | 1617 s | 1605 m | σ NH2, ν CC(ar) | ||
| 1547 s | 1557 s | νas COO | 1552 s | 1557 s | νas COO | ||
| 1494 s | 1488 s | δ-α CHar | 1492 m | 1488 s | δ-α CH(ar) | ||
| 1476 m | 1472 m | δas CH3 | |||||
| 1449 s | 1448 m | 1440 s | δas CH3, δ-α CH(ar) | 1450 m | 1440 s | δ-α CH(ar) | |
| 1416 m | 1408 s | νs COO | 1425 m | 1416 s | νs COO | ||
| 1375 w | 1376 w | δs CH3 | |||||
| 1361 w | 1361 m | δs CH3 | |||||
| 1336 m | 1332 m | 1340 m | δs CH3, δ-α CH(ar) | 1337 w | 1340 m | δ-α CH(ar) | |
| 1312 m | 1314 m | 1324 m | ω CH2, ν CN(NH2) | ||||
| 1303 w | 1307 m | δ CαH | |||||
| 1260 m | 1245 m | 1275 m | τ CH2, ν CN(ar) | 1281 m | 1284 m | 1275 m | δ CαH, ν CN(ar) |
| 1220 w | 1212 w | τ CH2 | |||||
| 1167 w | 1168 m | ρas CH3 | |||||
| 1155 m | 1156 m | δ-α CH(ar) | 1159 w | 1156 m | δ-α CH(ar) | ||
| 1098 w | 1100 w | ν CβCα | 1094 m | 1098 m | ρs CH3 | ||
| 1076 w | 1079 w | δ-γ CH3 | |||||
| 1049 w | 1047 w | 1044 w | δ-γ CH3, ν CN(ar), ν CC(ar) | 1054 w | 1044 w | ν CN(ar), ν CC(ar) | |
| 1006 m | 1015 w | 987 m | δ-γ CH3, δ-γ CH(ar) | 980 w | 987 m | δ-γ CH(ar) | |
| 934 w | 934 m | ν CγCβ | 930 w | 925 w | ρs CH3 | ||
| 890 vw | 893 m | ν CαC | |||||
| 872 vw | 879 w | ν CαC | |||||
| 857 w | 860 w | δ-γ CH(ar) | 850 w | 860 w | δ-γ CH(ar) | ||
| 831 m | 845 w | νs (Cβ)2CαC, σ COO | |||||
| 796 m | 800 m | δ-γ CH2 | |||||
| 766 s | 753 m | 772 s | δ-γ CH2, T ar | 768 m | 762 w | 772 s | ω COO, T ar |
| 741 m | 720 m | 736 w | δ-γ CH2, T ar | 742 w | 736 w | T ar | |
| 664 vw | 645 w | 665 m | σ COO, δ-α ar | 641 w | 664 w | 665 m | σ COO, δ-α ar |
| 607 w | 585 w | ω COO | |||||
| 552 w | 560 w | δCαC | |||||
| 522 w | 522 s | T ar | 518 w | 522 s | T ar | ||
| 454 m | 435 m | ω NH2 | 451 m | 435 m | ω NH2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swiatkowski, M.; Sieranski, T.; Bogdan, M.; Kruszynski, R. Structural Insights into Influence of Isomerism on Properties of Open Shell Cobalt Coordination System. Molecules 2019, 24, 3357. https://doi.org/10.3390/molecules24183357
Swiatkowski M, Sieranski T, Bogdan M, Kruszynski R. Structural Insights into Influence of Isomerism on Properties of Open Shell Cobalt Coordination System. Molecules. 2019; 24(18):3357. https://doi.org/10.3390/molecules24183357
Chicago/Turabian StyleSwiatkowski, Marcin, Tomasz Sieranski, Marta Bogdan, and Rafal Kruszynski. 2019. "Structural Insights into Influence of Isomerism on Properties of Open Shell Cobalt Coordination System" Molecules 24, no. 18: 3357. https://doi.org/10.3390/molecules24183357
APA StyleSwiatkowski, M., Sieranski, T., Bogdan, M., & Kruszynski, R. (2019). Structural Insights into Influence of Isomerism on Properties of Open Shell Cobalt Coordination System. Molecules, 24(18), 3357. https://doi.org/10.3390/molecules24183357