Coordination Behavior of 1,4-Disubstituted Cyclen Endowed with Phosphonate, Phosphonate Monoethylester, and H-Phosphinate Pendant Arms
Abstract
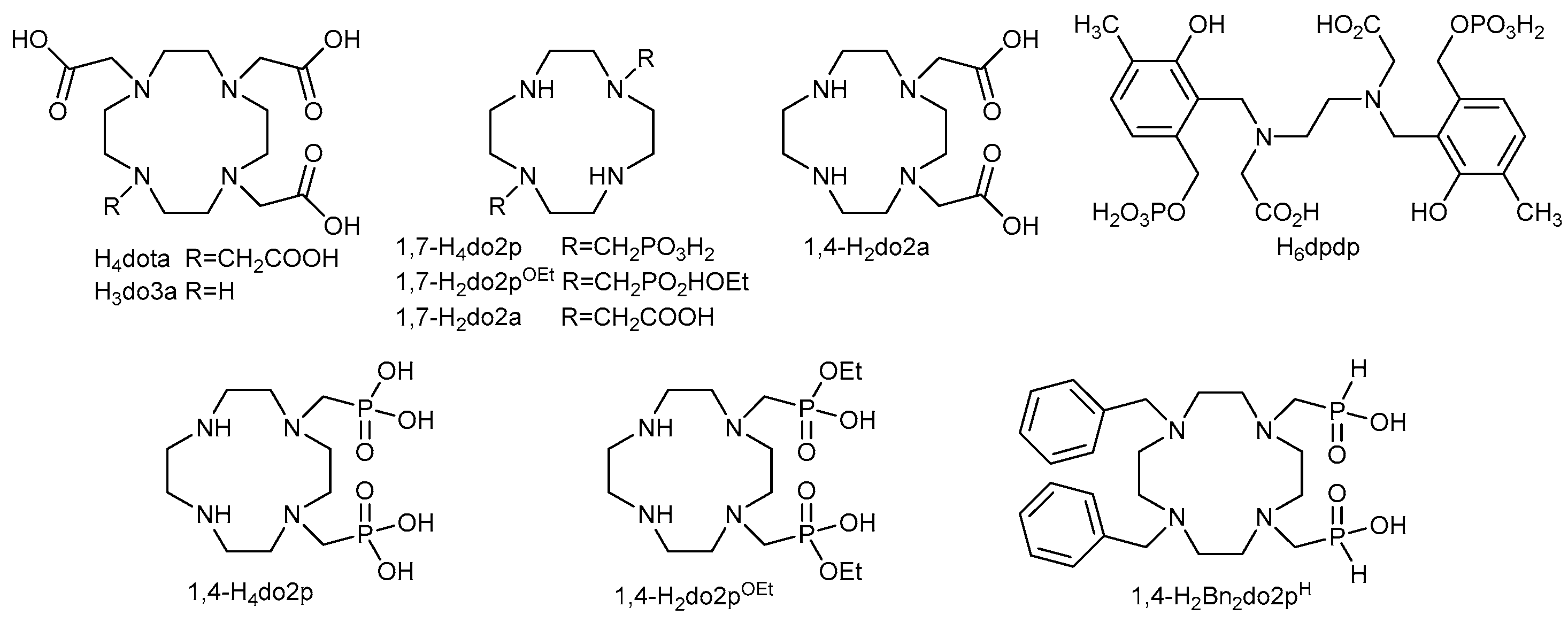
1. Introduction
2. Results and Discussion
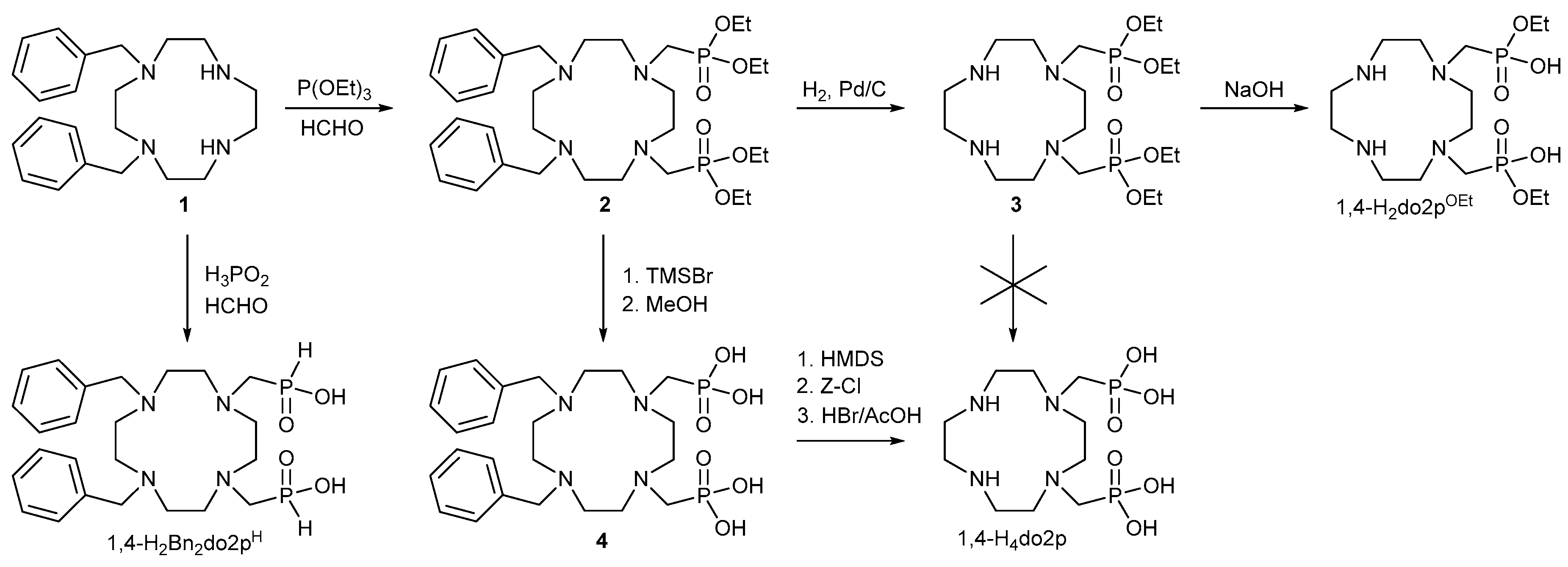
2.1. Synthesis
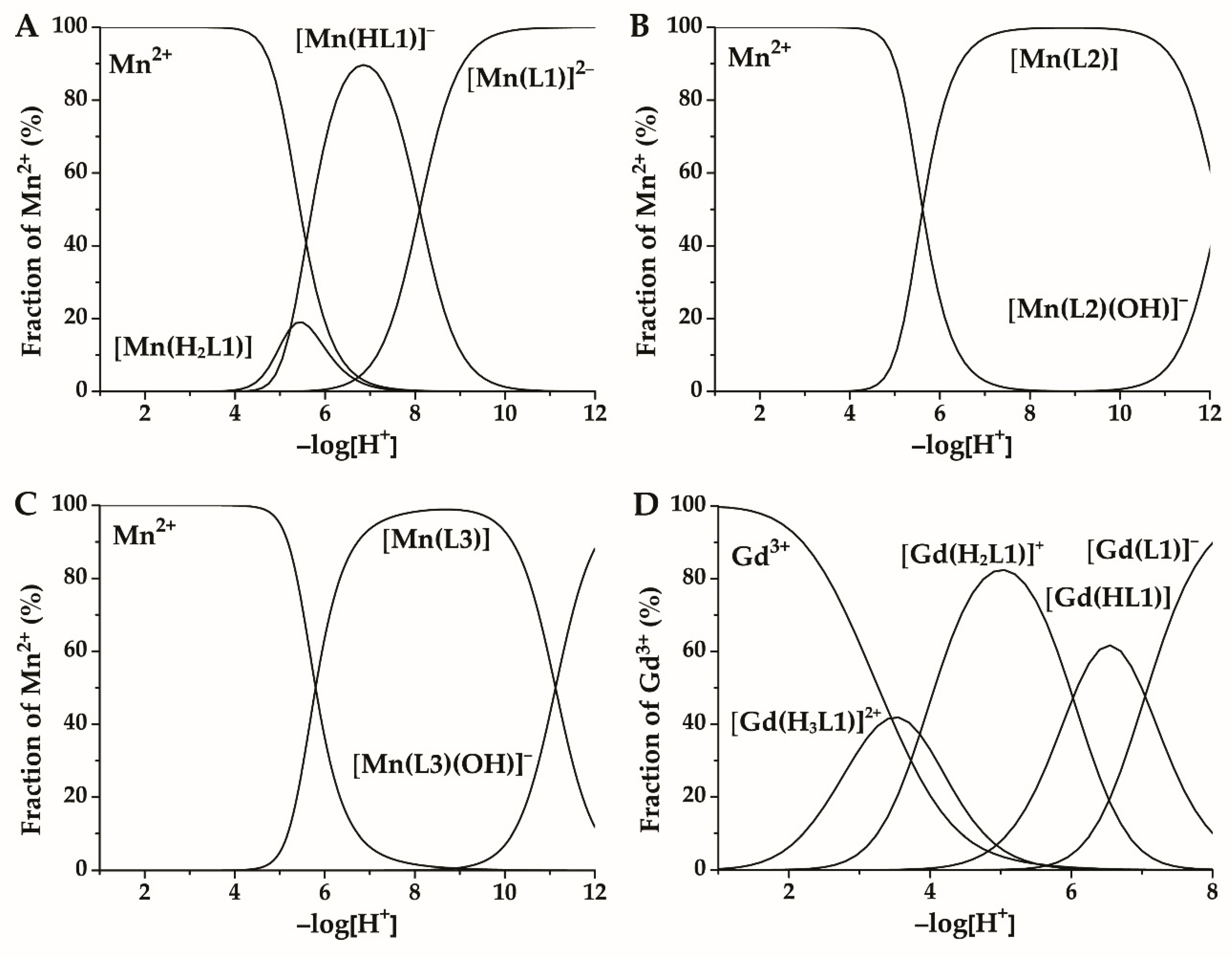
2.2. Protonation of the Ligands and Stability of Their Complexes
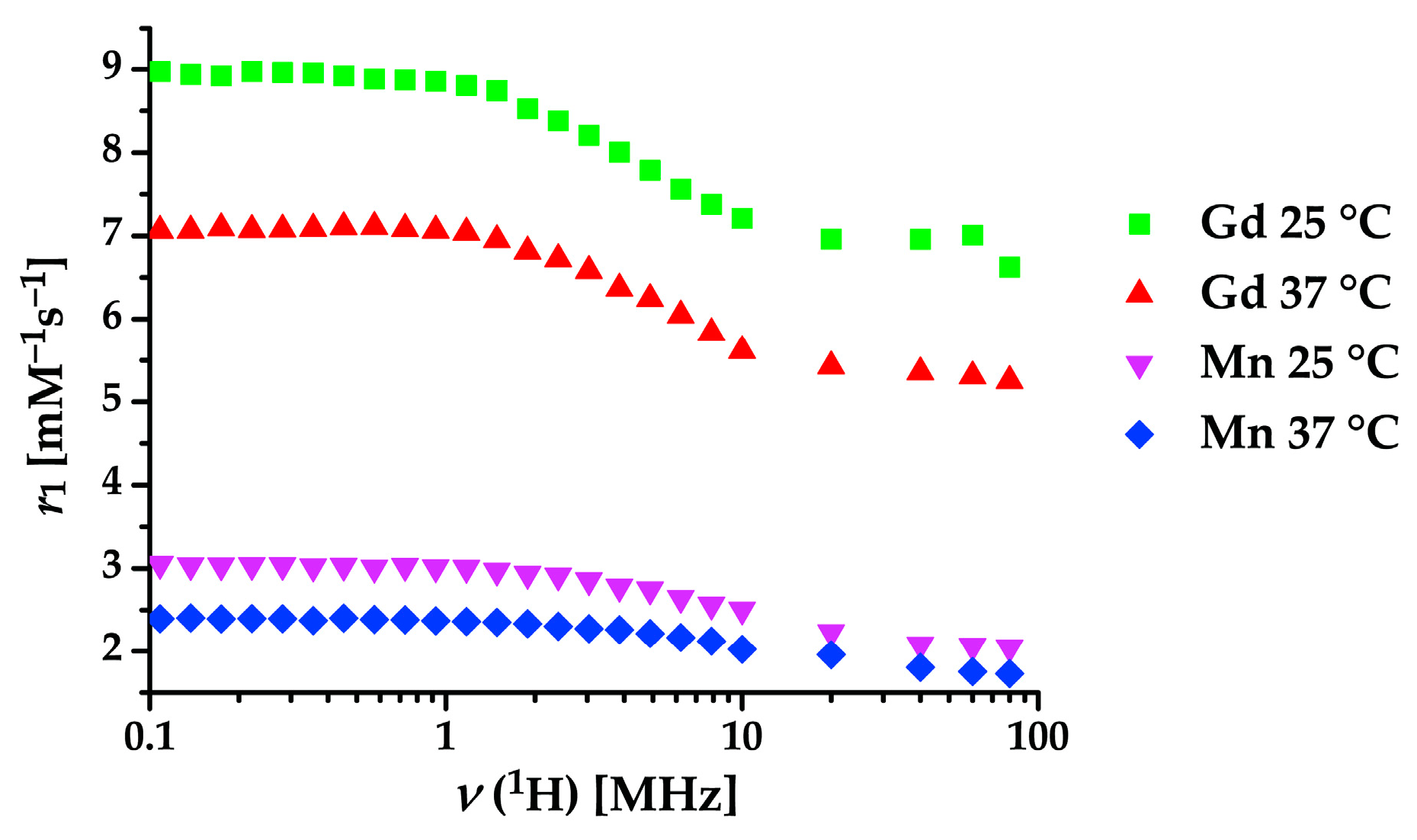
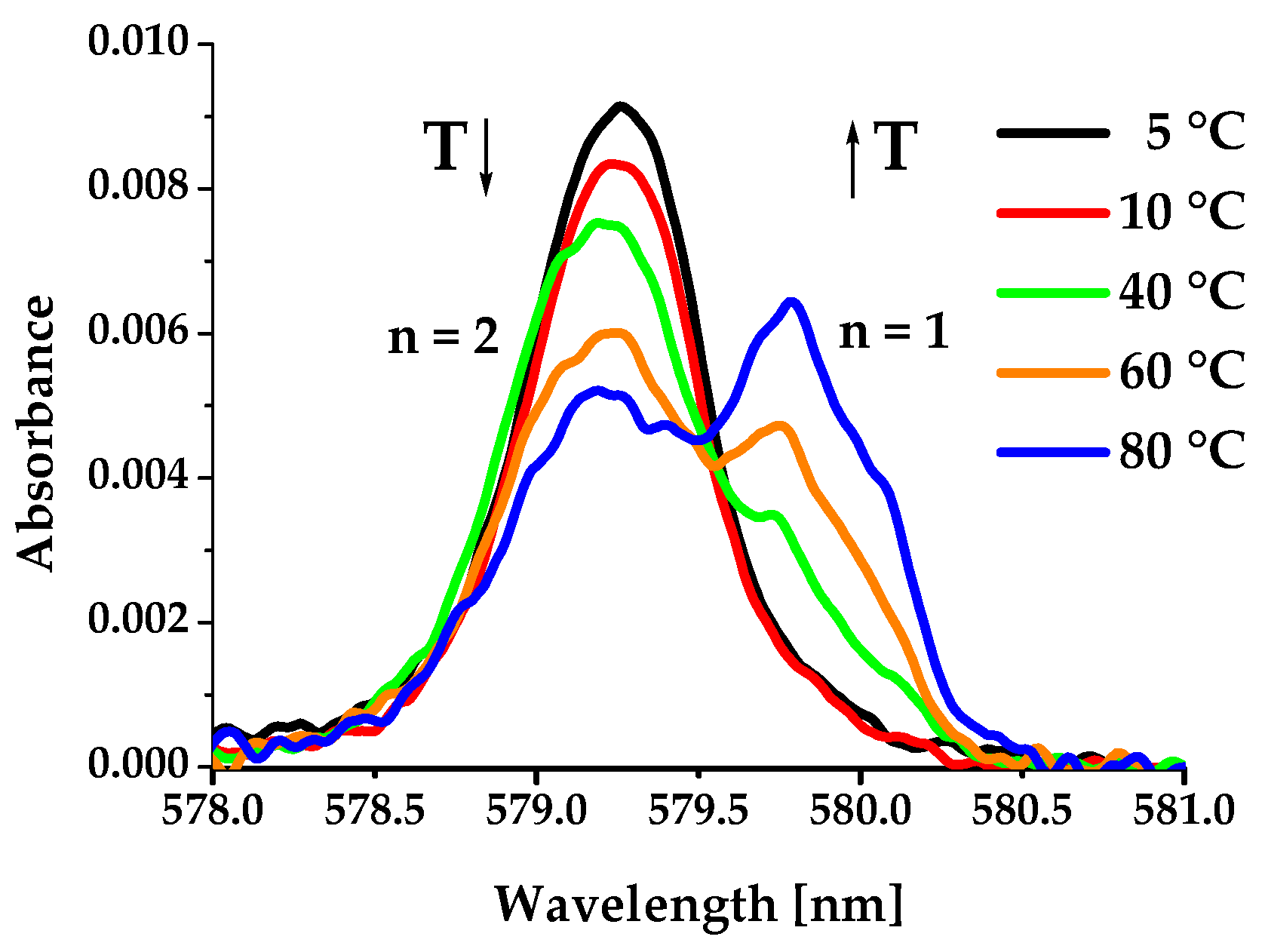
2.3. Hydration Number of Mn(II) and Gd(III) Complexes
3. Materials and Methods
3.1. General
3.2. Synthesis
3.3. Potentiometric Titrations
3.4. Proton Nuclear Magnetic Relaxation Dispersion (1H NMRD) Measurements
3.5. UV-Vis Spectroscopy
3.6. Luminescence Spectroscopy
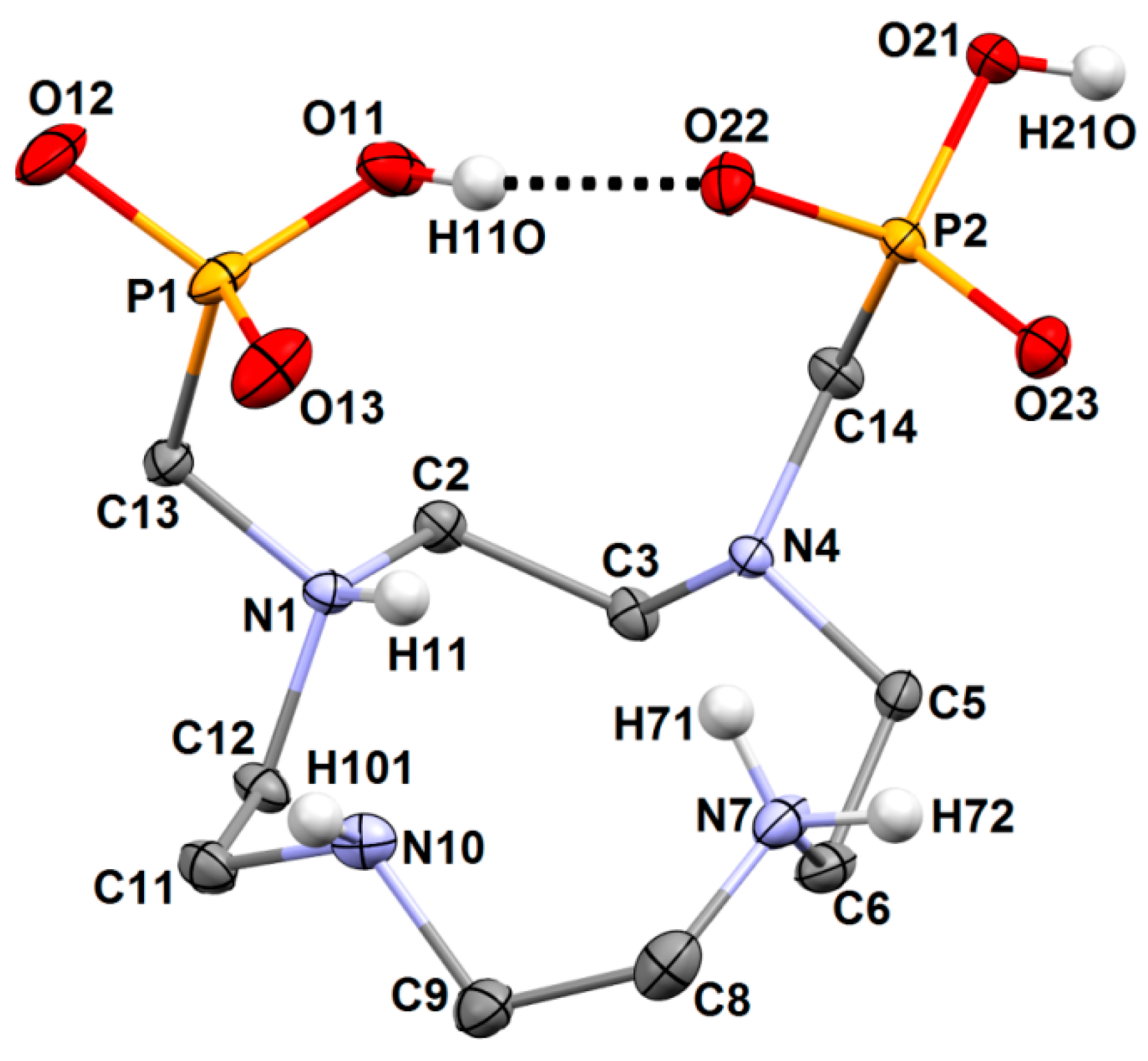
3.7. Single-Crystal X-Ray Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hermann, P.; Kotek, J.; Kubíček, J.; Lukeš, I. Gadolinium(III) Complexes as MRI Contrast Agents: Ligand Design and Properties of the Complexes. Dalton Trans. 2008, 3027–3047. [Google Scholar] [CrossRef] [PubMed]
- Terreno, E.; Castelli, D.D.; Viale, A.; Aime, S. Challenges for Molecular Magnetic Resonance Imaging. Chem. Rev. 2010, 110, 3019–3042. [Google Scholar] [CrossRef] [PubMed]
- Dorazio, S.J.; Olatunde, A.O.; Tsitovich, P.B.; Morrow, J.R. Comparison of divalent transition metal ion paraCEST MRI contrast agents. J. Biol. Inorg. Chem. 2014, 19, 191–205. [Google Scholar] [CrossRef] [PubMed]
- Angelovski, G. Heading toward Macromolecular and Nanosized Bioresponsive MRI Probes for Successful Functional Imaging. Acc. Chem. Res. 2017, 50, 2215–2224. [Google Scholar] [CrossRef] [PubMed]
- Contrast Agents for MRI. Experimental Methods; Pierre, V.C., Allen, M.J., Eds.; Royal Society of Chemistry: Croydon, UK, 2018. [Google Scholar]
- Clough, T.J.; Jiang, L.; Wong, K.-L.; Long, N.J. Ligand Design Strategies to Increase Stability of Gadolinium-Based Magnetic Resonance Imaging Contrast Agents. Nat. Commun. 2019, 10, 1420. [Google Scholar] [CrossRef] [PubMed]
- Kueny-Stotz, M.; Garofalo, A.; Felder-Flesch, D. Manganese-Enhanced MRI Contrast Agents: From Small Chelates to Nanosized Hybrids. Eur. J. Inorg. Chem. 2012, 1987–2005. [Google Scholar] [CrossRef]
- Drahoš, B.; Lukeš, I.; Tóth, É. Manganese(II) Complexes as Potential Contrast Agents for MRI. Eur. J. Inorg. Chem. 2012, 1975–1986. [Google Scholar] [CrossRef]
- Caravan, P.; Ellison, J.J.; McMurry, T.J.; Lauffer, R.B. Gadolinium(III) Chelates as MRI Contrast Agents: Structure, Dynamics, and Applications. Chem. Rev. 1999, 99, 2293–2352. [Google Scholar] [CrossRef] [PubMed]
- Wahsner, J.; Gale, E.M.; Rodríguez-Rodríguez, A.; Caravan, P. Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers. Chem. Rev. 2019, 119, 957–1057. [Google Scholar] [CrossRef] [PubMed]
- Merbach, A.E.; Helm, L.; Tóth, É. (Eds.) The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging, 2nd ed.; John Wiley & Sons: Chichester, UK, 2013. [Google Scholar]
- Thomsen, H.S.; Morcos, S.K.; Almén, T.; Bellin, M.-F.; Bertolotto, M.; Bongartz, G.; Clement, O.; Leander, P.; Heinz-Peer, G.; Reimer, P.; et al. Nephrogenic Systemic Fibrosis and Gadolinium-Based Contrast Media: Updated ESUR Contrast Medium Safety Committee Guidelines. Eur. Radiol. 2013, 23, 307–318. [Google Scholar] [CrossRef]
- Runge, V.M.; Dechelation (Transmetalation). Consequences and Safety Concerns With the Linear Gadolinium-Based Contrast Agents, In View of Recent Health Care Rulings by the EMA (Europe), FDA (United States), and PMDA (Japan). Invest. Radiol. 2018, 53, 571–578. [Google Scholar] [PubMed]
- Le Fur, M.; Caravan, P. The biological fate of gadolinium-based MRI contrast agents: A call to action for bioinorganic chemists. Metallomics 2019, 11, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Christianson, D.W. Structural Chemistry and Biology of Manganese Metalloenzymes. Prog. Biophys. Mol. Biol. 1997, 67, 217–252. [Google Scholar] [CrossRef]
- Koretsky, A.P.; Silva, A.C. Manganese Enhanced Magnetic Resonance Imaging (MEMRI). NMR Biomed. 2004, 17, 527–631. [Google Scholar] [CrossRef] [PubMed]
- Wolf, G.L.; Burnett, K.R.; Goldstein, E.J.; Joseph, P.M. Magnetic Resonance Annual 1985; Kressel, H., Ed.; Raven: New York, NY, USA, 1985; pp. 231–266. [Google Scholar]
- Rocklage, S.M.; Cacheris, W.P.; Quay, S.C.; Hahn, F.E.; Raymond, K.N. Manganese(II) N,N′-dipyridoxylethylenediamine-N,N′-diacetate 5,5′-bis(phosphate). Synthesis and Characterization of a Paramagnetic Chelate for Magnetic Resonance Imaging Enhancement. Inorg. Chem. 1989, 28, 477–485. [Google Scholar] [CrossRef]
- Kettritz, U.; Schlund, J.F.; Wilbur, K.; Eisenberg, L.B.; Semelka, R.C. Comparison of Gadolinium Chelates with Manganese-DPDP for Liver Lesion Detection and Characterization: Preliminary Results. Magn. Reson. Imaging 1996, 14, 1185–1190. [Google Scholar] [CrossRef]
- Murakami, T.; Baron, R.L.; Peterson, M.S.; Oliver, J.H., 3rd; Davis, P.L.; Confer, S.R.; Federle, M.P. Hepatocellular Carcinoma: MR Imaging with Mangafodipir Trisodium (Mn-DPDP). Radiology 1996, 200, 69–77. [Google Scholar] [CrossRef]
- Gallez, B.; Baudelet, C.; Geurts, M. Regional Distribution of Manganese Found in the Brain after Injection of a Single Dose of Manganese-Based Contrast Agents. Magn. Reson. Imaging 1998, 16, 1211–1215. [Google Scholar] [CrossRef]
- Cersosimo, M.G.; Koller, W.C. The Diagnosis of Manganese-Induced Parkinsonism. Neurotoxicology 2006, 27, 340–346. [Google Scholar] [CrossRef]
- Price, E.W.; Orvig, C. Matching Chelators to Radiometals for Radiopharmaceuticals. Chem. Soc. Rev. 2014, 43, 260–290. [Google Scholar] [CrossRef]
- Boros, E.; Packard, A.B. Radioactive Transition Metals for Imaging and Therapy. Chem. Rev. 2019, 119, 870–901. [Google Scholar]
- Kostelnik, T.I.; Orvig, C. Radioactive Main Group and Rare Earth Metals for Imaging and Therapy. Chem. Rev. 2019, 119, 902–956. [Google Scholar] [CrossRef] [PubMed]
- McInnes, L.E.; Rudd, S.E.; Donnelly, P.S. Copper, Gallium and Zirconium Positron Emission Tomography Imaging Agents: The Importance of Metal Ion Speciation. Coord. Chem. Rev. 2017, 352, 499–516. [Google Scholar] [CrossRef]
- Amoroso, A.J.; Fallis, I.A.; Pope, S.J.A. Chelating Agents for Radiolanthanides: Applications to Imaging and Therapy. Coord. Chem. Rev. 2017, 340, 198–219. [Google Scholar] [CrossRef]
- Huskens, J.; Torres, D.A.; Kovacs, Z.; André, J.P.; Geraldes, C.F.G.C.; Sherry, A.D. Alkaline Earth Metal and Lanthanide(III) Complexes of Ligands Based upon 1,4,7,10-Tetraazacyclododecane-1,7-bis(acetic acid). Inorg. Chem. 1997, 36, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, A.; Calabi, L.; Giorgi, C.; Losi, P.; Mariani, P.; Palano, D.; Paoli, P.; Rossi, P.; Valtancoli, B. Thermodynamic and Structural Aspects of Manganese(II) Complexes with Polyaminopolycarboxylic Ligands Based upon 1,4,7,10-Tetraazacyclododecane (Cyclen). Crystal Structure of Dimeric [MnL]2·2CH3OH Containing the New Ligand 1,4,7,10-Tetraazacyclododecane-1,4-diacetate. J. Chem. Soc. Dalton Trans. 2001, 917–922. [Google Scholar]
- Rolla, G.A.; Platas-Iglesias, C.; Botta, M.; Tei, L.; Helm, L. 1H and 17O NMR Relaxometric and Computational Study on Macrocyclic Mn(II) Complexes. Inorg. Chem. 2013, 52, 3268–3279. [Google Scholar]
- Rolla, G.; De Biasio, V.; Giovenzana, G.B.; Botta, M.; Tei, L. Supramolecular Assemblies Based on Amphiphilic Mn2+-Complexes as High Relaxivity MRI Probes. Dalton Trans. 2018, 47, 10660–10670. [Google Scholar] [CrossRef] [PubMed]
- Garda, Z.; Forgács, A.; Do, Q.N.; Kálmán, F.K.; Timári, S.; Baranyai, Z.; Tei, L.; Tóth, I.; Kovács, Z.; Tircsó, G. Physico-Chemical Properties of MnII Complexes Formed with Cis- and Trans-DO2A: Thermodynamic, Electrochemical and Kinetic Studies. J. Inorg. Biochem. 2016, 163, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Forgács, A.; Tei, L.; Baranyai, Z.; Tóth, I.; Zékány, L.; Botta, M. A Bisamide Derivative of [Mn(1,4-DO2A)]—Solution Thermodynamic, Kinetic, and NMR Relaxometric Studies. Eur. J. Inorg. Chem. 2016, 1165–1174. [Google Scholar] [CrossRef]
- Burai, L.; Ren, J.; Kovacs, Z.; Brücher, E.; Sherry, A.D. Synthesis, Potentiometry, and NMR Studies of Two New 1,7-Disubstituted Tetraazacyclododecanes and Their Complexes Formed with Lanthanide, Alkaline Earth Metal, Mn2+, and Zn2+ Ions. Inorg. Chem. 1998, 37, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Bellouard, F.; Chuburu, F.; Kervarec, N.; Toupet, L.; Triki, S.; Le Mest, Y.; Handel, H. Cis-Diprotected Cyclams and Cyclens: A New Route to Symmetrically or Asymmetrically 1,4-Disubstituted Tetraazamacrocycles and to Asymmetrically Tetrasubstituted Derivatives. J. Chem. Soc. Perkin Trans. 1 1999, 3499–3505. [Google Scholar] [CrossRef]
- Sun, X.; Wuest, M.; Kovacs, Z.; Sherry, D.A.; Motekaitis, R.; Wang, Z.; Martell, A.E.; Welch, M.J.; Anderson, C.J. In Vivo Behavior of Copper-64-Labeled Methanephosphonate Tetraaza Macrocyclic Ligands. J. Biol. Inorg. Chem. 2003, 8, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Lebdušková, P.; Hermann, P.; Helm, L.; Tóth, É.; Kotek, J.; Binnemans, K.; Rudovský, J.; Lukeš, I.; Merbach, A.E. Gadolinium(III) Complexes of Mono- and Diethyl Esters of Monophosphonic Acid Analogue of DOTA as Potential MRI Contrast Agents: Solution Structures and Relaxometric Studies. Dalton Trans. 2007, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Försterová, M.; Jandurová, Z.; Marques, F.; Gano, L.; Lubal, P.; Vaněk, J.; Hermann, P.; Santos, I. Chemical and Biological Evaluation of 153Sm and 166Ho Complexes of 1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetrakis(methylphosphonic acid monoethylester) (H4dotpOEt). J. Inorg. Biochem. 2008, 102, 1531–1540. [Google Scholar] [CrossRef] [PubMed]
- Kotek, J.; Vojtíšek, P.; Císařová, I.; Hermann, P.; Jurečka, P.; Rohovec, J.; Lukeš, I. Bis(methylphosphonic Acid) Derivatives of 1,4,8,11-Tetraazacyclotetradecane (Cyclam). Synthesis, Crystal and Molecular Structures, and Solution Properties. Collect. Czech. Chem. Commun. 2000, 65, 1289–1316. [Google Scholar] [CrossRef]
- Meyer, M.; Dahaoui-Gindrey, V.; Lecomte, C.; Guilard, R. Conformations and Coordination Schemes of Carboxylate and Carbamoyl Derivatives of the Tetraazamacrocycles Cyclen and Cyclam, and the Relation to their Protonation States. Coord. Chem. Rev. 1998, 178–180, 1313–1405. [Google Scholar] [CrossRef]
- Pniok, M.; Kubíček, V.; Havlíčková, J.; Kotek, J.; Sabatie-Gogová, A.; Plutnar, J.; Huclier-Markai, S.; Hermann, P. Thermodynamic and Kinetic Study of Scandium(III) Complexes of DTPA and DOTA: A Step Toward Scandium Radiopharmaceuticals. Chem. Eur. J. 2014, 20, 7944–7955. [Google Scholar] [CrossRef]
- Lukeš, I.; Kotek, J.; Vojtíšek, P.; Hermann, P. Complexes of Tetraazacycles Bearing Methylphosphinic/phosphonic Acid Pendant Arms with Copper(II), Zinc(II) and Lanthanides(III). A Comparison with their Acetic Acid Analogues. Coord. Chem. Rev. 2001, 216–217, 287–312. [Google Scholar] [CrossRef]
- Lima, L.M.P.; Delgado, R.; Hermann, P.; Ševčík, R.; Lubal, P.; Carvalho, H.F.; Martins, A.F.; Tóth, É.; Geraldes, C.F.G.C. Tris(phosphonomethyl)cyclen Derivatives: Thermodynamic Stability, Kinetics, Solution Structure, and Relaxivity of Ln3+ Complexes. Eur. J. Inorg. Chem. 2012, 2548–2559. [Google Scholar] [CrossRef]
- Lima, L.M.P.; Esteves, C.V.; Delgado, R.; Hermann, P.; Kotek, J.; Ševčíková, R.; Lubal, P. Tris(phosphonomethyl) Cyclen Derivatives: Synthesis, Acid–Base Properties and Complexation Studies with Cu2+ and Zn2+ Ions. Eur. J. Inorg. Chem. 2012, 15, 2533–2547. [Google Scholar] [CrossRef]
- Marques, F.; Gano, L.; Paula Campello, M.; Lacerda, S.; Santos, I.; Lima, L.M.P.; Costa, J.; Antunes, P.; Delgado, R. 13- and 14-Membered Macrocyclic Ligands Containing Methylcarboxylate or Methylphosphonate Pendant Arms: Chemical and Biological Evaluation of their 153Sm and 166Ho Complexes as Potential Agents for Therapy or Bone Pain Palliation. J. Inorg. Biochem. 2006, 100, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Chang, C.A.; Francesconi, L.C.; Dischino, D.D.; Malley, M.F.; Gougoutas, J.Z.; Tweedle, M.F. Synthesis, Stability, and Structure of Gadolinium(III) and Yttrium(III) Macrocyclic Poly(amino carboxylates). Inorg. Chem. 1994, 33, 3567–3575. [Google Scholar] [CrossRef]
- Takács, A.; Napolitano, R.; Purgel, M.; Bényei, A.C.; Zékány, L.; Brücher, E.; Tóth, I.; Baranyai, Z.; Aime, S. Solution Structures, Stabilities, Kinetics, and Dynamics of DO3A and DO3A–Sulphonamide Complexes. Inorg. Chem. 2014, 53, 2858–2872. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, A.; Calabi, L.; Ferrini, L.; Losi, P.; Uggeri, F.; Valtancoli, B. Thermodynamics of Gd(III) Complexation by Macrocyclic and Acyclic Polyamino Carboxylates. Inorg. Chim. Acta 1996, 249, 13–15. [Google Scholar] [CrossRef]
- Bianchi, A.; Calabi, L.; Giorgi, C.; Losi, P.; Mariani, P.; Paoli, P.; Rossi, P.; Valtancoli, B.; Virtuani, M. Thermodynamic and Structural Properties of Gd3+ Complexes with Functionalized Macrocyclic Ligands Based upon 1,4,7,10-Tetraazacyclododecane. J. Chem. Soc. Dalton Trans. 2000, 697–705. [Google Scholar] [CrossRef]
- Kumar, K.; Chang, C.A.; Tweedle, M.F. Equilibrium and Kinetic Studies of Lanthanide Complexes of Macrocyclic Polyamino Carboxylates. Inorg. Chem. 1993, 32, 587–593. [Google Scholar] [CrossRef]
- Polášek, M.; Caravan, P. Is Macrocycle a Synonym for Kinetic Inertness in Gd(III) Complexes? Effect of Coordinating and Noncoordinating Substituents on Inertness and Relaxivity of Gd(III) Chelates with DO3A-like Ligands. Inorg. Chem. 2013, 52, 4084–4096. [Google Scholar] [CrossRef]
- Caravan, P.; Esteban-Gómez, D.; Rodríguez-Rodríguez, A.; Platas-Iglesias, C. Water exchange in lanthanide complexes for MRI applications. Lessons learned over the last 25 years. Dalton Trans. 2019, 48, 11161–11180. [Google Scholar] [CrossRef]
- Drahoš, B.; Kubíček, V.; Bonnet, C.S.; Hermann, P.; Lukeš, I.; Tóth, É. Dissociation kinetics of Mn2+ complexes of NOTA and DOTA. Dalton Trans. 2011, 40, 1945–1951. [Google Scholar] [CrossRef]
- Wang, S.; Westmoreland, T.D. Correlation of Relaxivity with Coordination Number in Six-, Seven-, and Eight-Coordinate Mn(II) Complexes of Pendant-Arm Cyclen Derivatives. Inorg. Chem. 2009, 48, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Kálmán, F.K.; Baranyai, Z.; Tóth, I.; Bányai, I.; Király, R.; Brücher, E.; Aime, S.; Sun, X.; Sherry, A.D.; Kovács, Z. Synthesis, Potentiometric, Kinetic, and NMR Studies of 1,4,7,10-Tetraazacyclododecane-1,7-bis(acetic acid)-4,10-bis(methylenephosphonic acid) (DO2A2P) and its Complexes with Ca(II), Cu(II), Zn(II) and Lanthanide(III) Ions. Inorg. Chem. 2008, 47, 3851–3862. [Google Scholar] [CrossRef] [PubMed]
- Tóth, É.; Dhubhghaill, O.M.N.; Besson, G.; Helm, L.; Merbach, A.E. Coordination equilibrium—A clue for fast water exchange on potential magnetic resonance imaging contrast agents? Magn. Reson. Chem. 1999, 37, 701–708. [Google Scholar] [CrossRef]
- Kimpe, K.; D’Olieslager, W.; Görller-Walrand, C.; Figueirinha, A.; Kovács, Z.; Geraldes, C.F.G.C. Interaction of [Ln(DO2A)(H2O)2−3]+ and [Ln(DO2P)(H2O)2−3]− with Phosphate, Acetate and Fluoride Anions in Aqueous Solution. J. Alloys Compd. 2001, 323–324, 828–832. [Google Scholar] [CrossRef]
- Horrocks, W.D.; Sudnick, D.R. Lanthanide Ion Probes of Structure in Biology. Laser-Induced Luminescence Decay Constants Provide a Direct Measure of the Number of Metal-Coordinated Water Molecules. J. Am. Chem. Soc. 1979, 101, 334–340. [Google Scholar] [CrossRef]
- Clarkson, I.; Dickins, R.; de Sousa, A. Non-Radiative Deactivation of the Excited States of Europium, Terbium and Ytterbium Complexes by Proximate Energy-Matched OH, NH and CH Oscillators: An Improved Luminescence Method for Establishing Solution Hydration States. J. Chem. Soc. Perkin Trans. 2 1999, 493–504. [Google Scholar]
- Supkowski, R.M.; Horrocks, W.D., Jr. On the Determination of the Number of Water Molecules, q, Coordinated to Europium(III) Ions in Solution from Luminescence Decay Lifetimes. Inorg. Chim. Acta 2002, 340, 44–48. [Google Scholar] [CrossRef]
- Graeppi, N.; Hugh Powell, D.; Laurenczy, G.; Zékány, L.; Merbach, A. Coordination equilibria and water exchange kinetics of lanthanide(III) propylenediaminetetraacetates and other magnetic resonance imaging related complexes. Inorg. Chim. Acta 1995, 235, 311–326. [Google Scholar] [CrossRef]
- Perrin, D.D. Purification of Laboratory Chemicals; Pergamon Press: Oxford, UK, 1988. [Google Scholar]
- Kývala, M. OPIUM – Program for Solution Equilibria Analysis; Charles University: Prague, Czech Republic, 1995. [Google Scholar]
- Kývala, M.; Lukeš, I. Chemometrics ’95 (International conference); University of Pardubice: Pardubice, Czech Republic, 1995; p. 63. [Google Scholar]
- Martell, A.E.; Smith, R.M. Critical Stability Constants; Plenum Press: New York, NY, USA, 1974–1989; pp. 1–6. [Google Scholar]
- NIST Standard Reference Database 46 (Critically Selected Stability Constants of Metal Complexes), Version 7.0; National Institute for Standards and Technology: Gaithersburg, MD, USA, 2003.
- Baes, C.F., Jr.; Mesmer, R.E. The Hydrolysis of Cations; Wiley: New York, NY, USA, 1976. [Google Scholar]
- Försterová, M.; Svobodová, I.; Lubal, P.; Táborský, P.; Kotek, J.; Hermann, P.; Lukeš, I. Thermodynamic Study of Lanthanide(III) Complexes with Bifunctional Monophosphinic Acid Analogues of H4dota and Comparative Kinetic Study of Yttrium(III) Complexes. Dalton Trans. 2007, 535–549. [Google Scholar] [CrossRef]
- Kubíček, V.; Kotek, J.; Hermann, P.; Lukeš, I. Aminoalkyl-bis(phosphonates): Their Complexation Properties in Solution and in the Solid State. Eur. J. Inorg. Chem. 2007, 333–344. [Google Scholar] [CrossRef]
- Otwinowski, Z.; Minor, W. HKL Denzo and Scalepack Program Package; Nonius BV: Delft, The Netherlands, 1997. [Google Scholar]
- Otwinowski, Z.; Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997, 276, 307–326. [Google Scholar]
- Sheldrick, G.M. SHELXS97. Program for Crystal Structure Solution from Diffraction Data; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. A Short History of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXL-2014/7. Program for Crystal Structure Refinement from Diffraction Data; University of Göttingen: Göttingen, Germany, 2014. [Google Scholar]
Sample Availability: Samples of the compounds H4L1, H2L2 and H2L3 are available from the authors. |
| Ligand | logKH1 | logKH2 | logKH3 | logKH4 | logKH5 |
|---|---|---|---|---|---|
| 1,4-H4do2p | 12.84 | 11.20 | 7.75 | 4.94 | 1.40 |
| 1,7-H4do2p [34] | 12.80 | 10.92 | 8.47 | 6.39 | – b |
| 1,4-H2do2pOEt | 11.38 | 8.57 | 1.29 | – c | – c |
| 1,7-H2do2pOEt [34] | 11.14 | 8.94 | – b | – b | – b |
| 1,4-H2Bn2do2pH | 11.22 | 6.78 | – c | – c | – c |
| H4dota [41] | 12.90 | 9.76 | 4.68 | 4.11 | 2.37 |
| 1,4-H2do2a [29] | 11.07 | 9.76 | 3.84 | 1.75 | – b |
| 1,7-H2do2a [28] | 10.91 | 9.45 | 4.09 | 3.18 | – b |
| Constant | Mg(II) | Ca(II) | Mn(II) | Cu(II) | Zn(II) | Gd(III) |
| 1,4-H4do2p | ||||||
| logKLM a | 7.36 | 9.29 | 15.41 | 26.45 | 21.11 | 19.15 |
| logKHLM b | 9.71 | 9.02 | 8.1 | 6.68 | 6.89 | 7.05 |
| logKH2LM b | 9.24 | – c | 5.24 | 4.67 | 4.11 | 6.02 |
| logKH3LM b | – c | – c | – c | – c | – c | 3.86 |
| logKLM(OH) d | – c | – c | – c | – c | 12.81 | – c |
| 1,4-H2do2pOEt | ||||||
| logKLM a | – c | 5.9 | 11.42 | 19.55 | 15.42 | – c |
| logKLM(OH) d | – c | – c | 12.2 | 12.71 | 9.51 | – c |
| 1,4-H2Bn2do2pH | ||||||
| logKLM a | – c | 3.96 | 9.39 | – c | 13.26 | – c |
| logKLM(OH) d | – c | 12.28 | 11.13 | – c | 9.12 | – c |
| logKLM(OH)2 d | – c | – c | – c | – c | 17.29 | – c |
| Ligand | logK(CuL) | logK(MnL) | logK(GdL) | pCu | pMn | pGd |
|---|---|---|---|---|---|---|
| 1,4-H4do2p | 26.45 | 15.41 | 19.15 | 9.9 | 4.7 | 6.3 |
| 1,4-H2do2pOEt | 19.55 | 11.42 | – a | 8.9 | 4.6 | – b |
| 1,4-H2Bn2do2pH | – a | 9.39 | – a | – b | 4.2 | – b |
| 1,7-H4do2p [34,36] | 28.7 | 18.1 | 18.2 | 10.8 | 5.5 | 6.0 |
| 1,7-H2do2pOEt [34] | – c | 11.03 | 9.81 | – b | 4.4 | 3.8 |
| 1,4-H2do2a [29,33] | 24.43 | 16.13 | – c | 10.6 | 6.6 | – b |
| 1,7-H2do2a [29,33] | 24.24 | 14.54 | 19.1–19.4 | 10.3 | 5.6 | 8.0–8.4 |
| H3do3a [29,33,46,47,48,49] | 25.75 | 19.40–19.43 | 21.0–22.02 | 11.0 | 7.8–8.3 | 9.0–9.1 |
| H4dota [28,29,33,48,49] | 24.83 | 19.33–19.89 | 24.67 | 10.7 | 7.9–8.1 | 10.5 |
| Complex | r1(1H) [9,10,30,31,54] mm–1 s–1 at 20 MHz and 37 °C | Denticity | q [3,52] |
|---|---|---|---|
| [Mn(1,4-do2p)]2− | 1.96 | 6 | 0 |
| [Mn(1,4-do2pOEt)] | 2.00 | 6 | 0 |
| [Mn(Bn2do2pH)] | 1.37 | 6 | 0 |
| [Mn(H2O)6]2+ | 6.76 | – a | 6 |
| [Mn(dota)]2− | 3.20 | 6 b | 0 |
| [Mn(edta)]2− | 3.30 | 6 | 1 |
| [Mn(do3a)]− | 1.3 | 7 | 0 |
| [Mn(1,7-do2a)] | 1.5 c | 6 | 0 |
| [Mn(1,4-do2a)] | 2.1 | 6 | 0–1 |
| [Gd(1,4-do2p)]− | 5.42 | 6 | 1–2 |
| [Gd(do3a)] | 4.80 | 7 | 2 |
| [Gd(dota)]− | 3.56 | 8 | 1 |
| [Gd(H2O)8]3+ | 10.5 | – a | 8 |
| Quantity | Eu(III)–1,4-H4do2p | Tb(III)–1,4-H4do2p |
|---|---|---|
| τem(H2O)/ms | 0.359 | 2.405 |
| τem(D2O)/ms | 1.679 | 4.142 |
| q [58] | 2.30 | 0.73 |
| q [59] | 2.33 | 0.57 |
| q [60] | 2.09 | not applicable |
| Species | Temperature (°C) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 5 | 10 | 25 | 30 | 40 | 50 | 60 | 70 | 80 | |
| [Eu(1,4-do2p)(H2O)2]− (%) | 97.7 | 97.0 | 93.0 | 90.5 | 85.0 | 77.5 | 70.9 | 61.9 | 57.5 |
| [Eu(1,4-do2p)(H2O)]− (%) | 2.3 | 3.0 | 7.0 | 9.5 | 15.0 | 22.5 | 29.1 | 38.1 | 42.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bárta, J.; Hermann, P.; Kotek, J. Coordination Behavior of 1,4-Disubstituted Cyclen Endowed with Phosphonate, Phosphonate Monoethylester, and H-Phosphinate Pendant Arms. Molecules 2019, 24, 3324. https://doi.org/10.3390/molecules24183324
Bárta J, Hermann P, Kotek J. Coordination Behavior of 1,4-Disubstituted Cyclen Endowed with Phosphonate, Phosphonate Monoethylester, and H-Phosphinate Pendant Arms. Molecules. 2019; 24(18):3324. https://doi.org/10.3390/molecules24183324
Chicago/Turabian StyleBárta, Jiří, Petr Hermann, and Jan Kotek. 2019. "Coordination Behavior of 1,4-Disubstituted Cyclen Endowed with Phosphonate, Phosphonate Monoethylester, and H-Phosphinate Pendant Arms" Molecules 24, no. 18: 3324. https://doi.org/10.3390/molecules24183324
APA StyleBárta, J., Hermann, P., & Kotek, J. (2019). Coordination Behavior of 1,4-Disubstituted Cyclen Endowed with Phosphonate, Phosphonate Monoethylester, and H-Phosphinate Pendant Arms. Molecules, 24(18), 3324. https://doi.org/10.3390/molecules24183324





