The Phenylethanol Glycoside Liposome Inhibits PDGF-Induced HSC Activation via Regulation of the FAK/PI3K/Akt Signaling Pathway
Abstract
1. Introduction
2. Results
2.1. Physical Properties of CPhG Liposome
2.2. In Vitro Release from the CPhG Liposomes and CPhGs
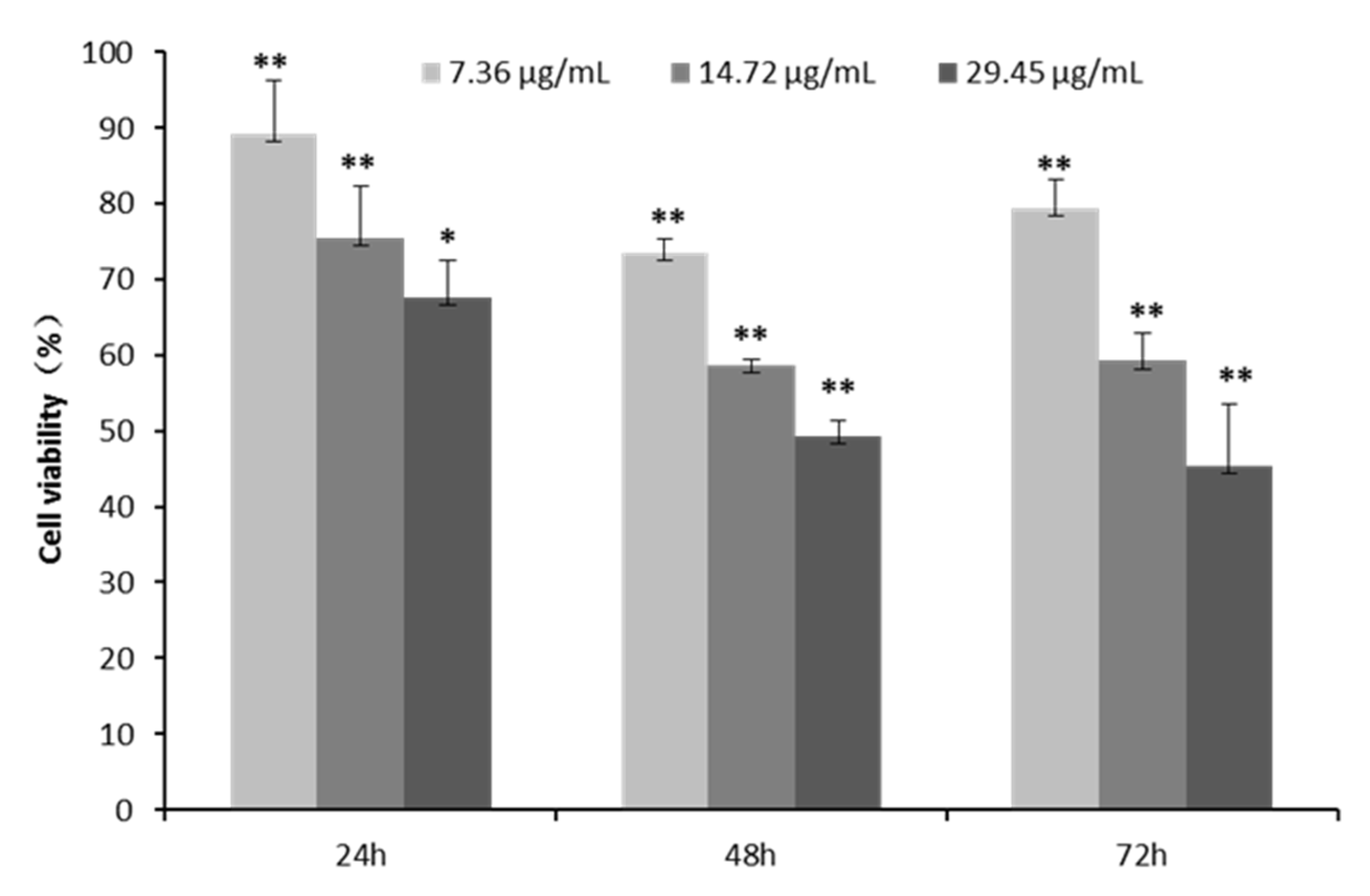
2.3. Cell Proliferation and Toxicity of CPhG Liposomes on HSCs
2.4. CPhG Liposomes Induce HSCs Apoptosis and Arrest the Cell Cycle
2.5. Study on the Mechanism(s) of Action of CPhG Liposomes In Vitro
2.5.1. CPhG Liposomes Inhibit HSCs Proliferation by rrPDGF-BB Stimulation
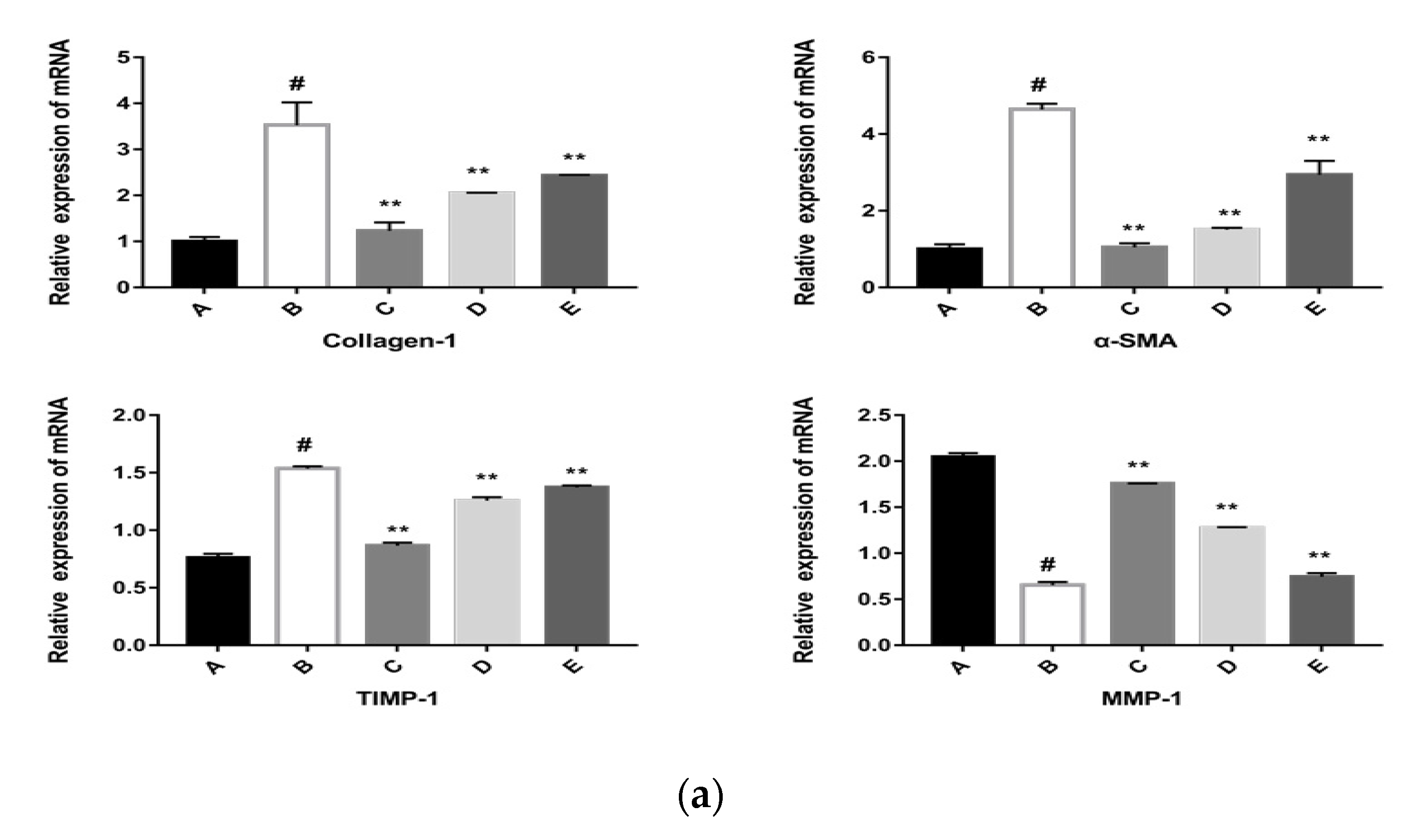
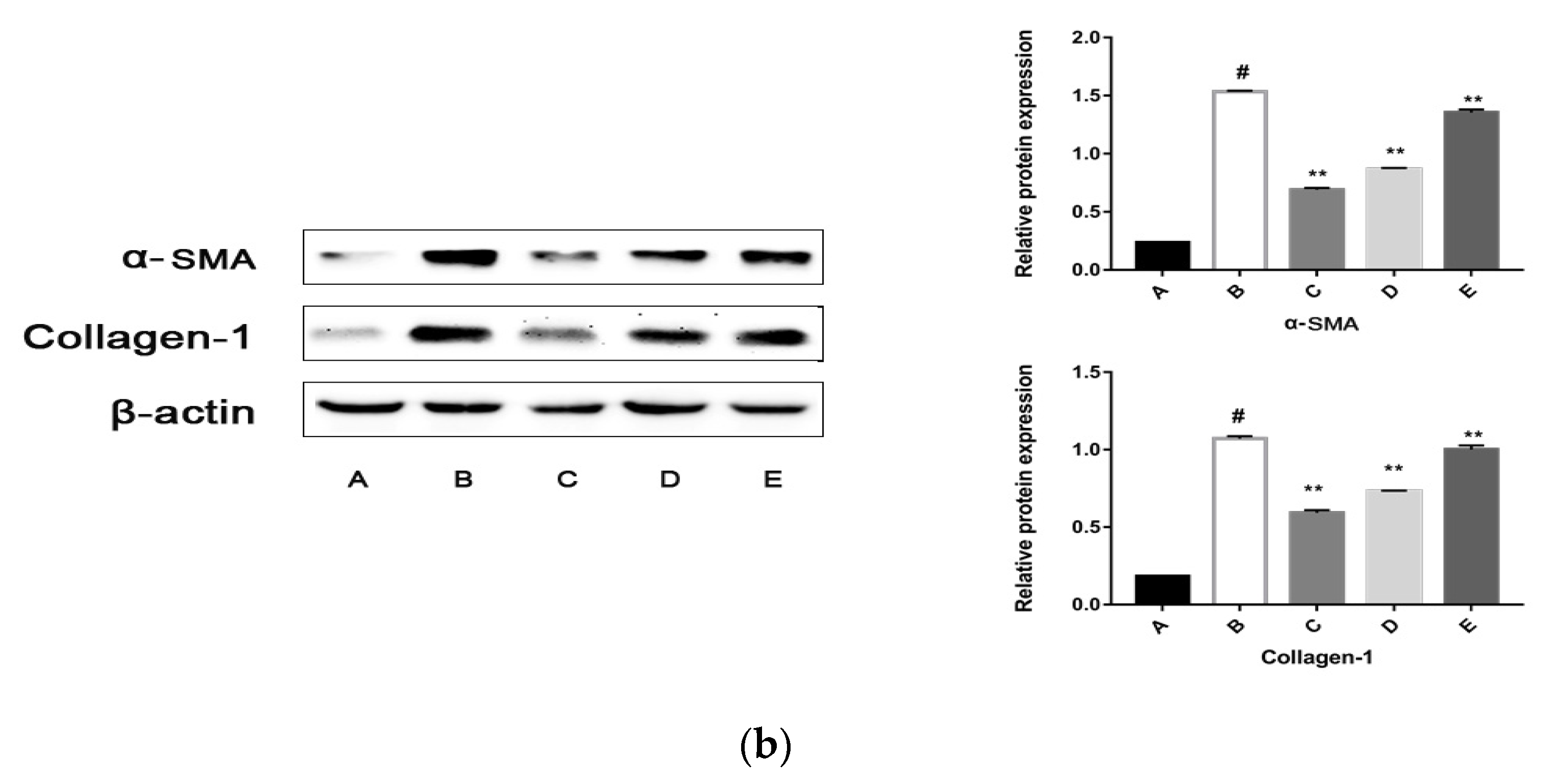
2.5.2. The CPhG Liposomes Inhibit HSC Activation In Vitro
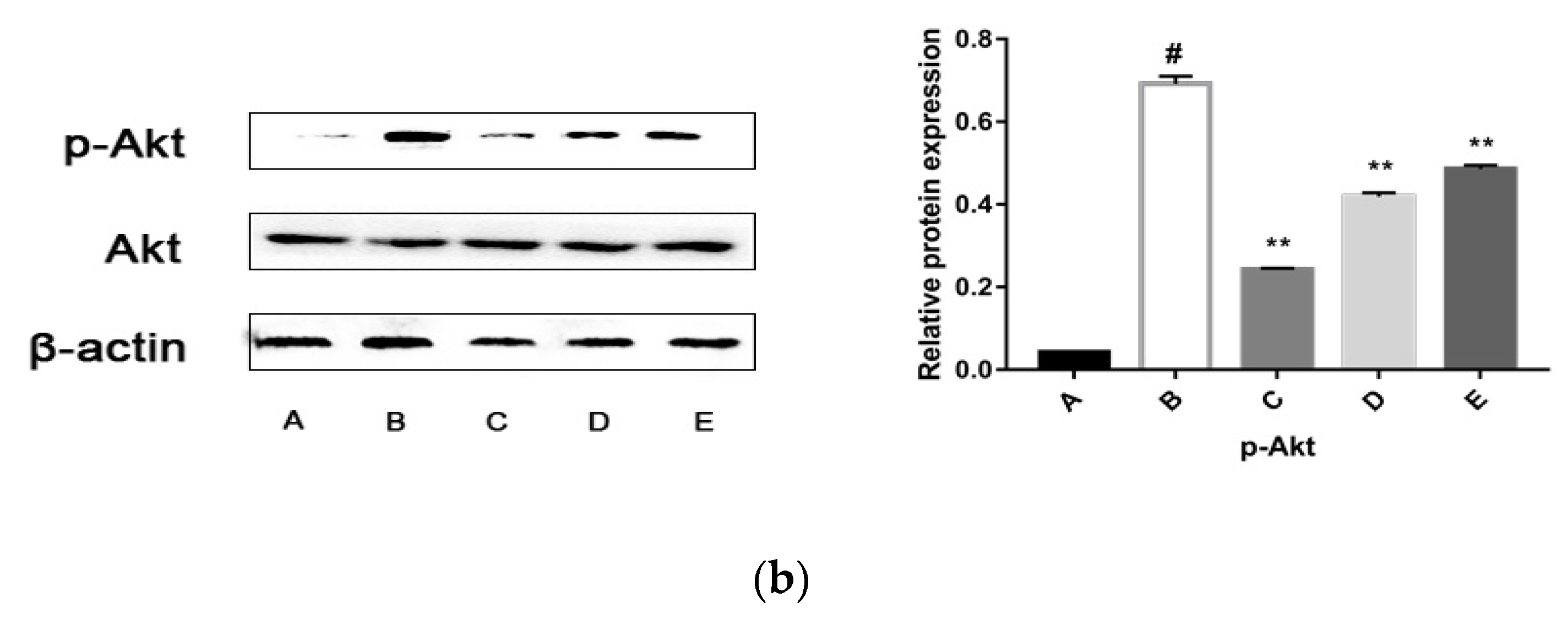
2.5.3. CPhG Liposomes Inhibit the PI3K/Akt Signaling Pathway
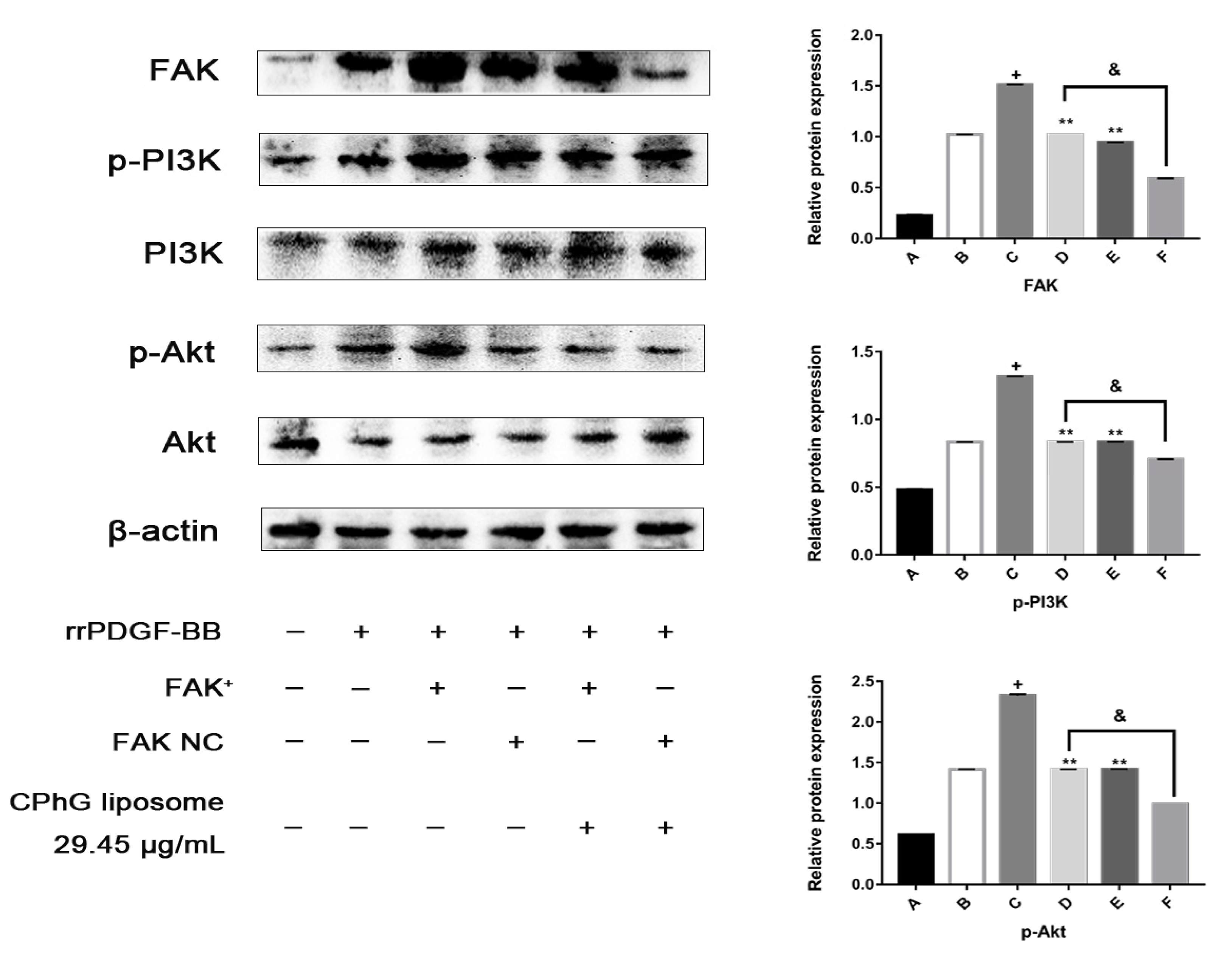
2.5.4. The CPhG Liposomes Can Down-Regulate the Expression of PI3K /Akt Pathway Proteins in HSCs of the rrPDGF-BB-Mediated FAK Overexpression Plasmid
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of the CPhG Liposomes
4.3. In Vitro Release from the CPhG Liposome and CPhGs
4.4. Cytotoxicity
4.5. Cell Toxicity of the CPhG Liposomes on HSCs
4.6. Apoptosis Analysis
4.7. Cell Cycle Arrest Analysis
4.8. Effect of the CPhG Liposome on the PDGF Mediated FAK and PI3K/Akt Signaling Pathway
4.8.1. Proliferation Assay
4.8.2. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.8.3. Western Blot Analysis
4.8.4. FAK Overexpression Experiments
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, H.; Sun, D.; Wang, G.; Cui, S.; Field, R.A.; Li, J.; Zang, Y. Alogliptin alleviates liver fibrosis via suppression of activated hepatic stellate cell. Biochem. Biophys. Res. Commun. 2019, 511, 387–393. [Google Scholar] [CrossRef]
- Parola, M.; Pinzani, M. Liver fibrosis: Pathophysiology, pathogenetic targets and clinical issues. Mol. Aspects Med. 2019, 65, 37–55. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.J.; Wu, Y.C.; Chen, I.F.; Wu, Y.L.; Chuang, C.W.; Huang, H.H.; Kuo, S.M. Reparative Effects of Astaxanthin-Hyaluronan Nanoaggregates against Retrorsine-CCl(4)-Induced Liver Fibrosis and Necrosis. Molecules 2018, 23, 726. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, Y.; Zuo, L.; Wang, Y.; Huang, Y. ASIC1a promotes high glucose and PDGF-induced hepatic stellate cell activation by inducing autophagy through CaMKKβ/ERK signaling pathway. Toxicol. Lett. 2019, 300, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Khadem, F.; Uzonna, J.E. Role of hepatic stellate cell (HSC)-derived cytokines in hepatic inflammation and immunity. Cytokine 2018. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Li, J.; Aipire, A.; Xia, L.; Yang, Y.; Chen, Q.; Lv, J.; Wang, X.; Li, J. Cistanche tubulosa phenylethanoid glycosides induce apoptosis in Eca-109 cells via the mitochondria-dependent pathway. Oncol. Lett. 2019, 17, 303–313. [Google Scholar] [CrossRef] [PubMed]
- You, S.P.; Ma, L.; Zhao, J.; Zhang, S.L.; Liu, T. Phenylethanol Glycosides from Cistanche tubulosa Suppress Hepatic Stellate Cell Activation and Block the Conduction of Signaling Pathways in TGF-beta1/smad as Potential Anti-Hepatic Fibrosis Agents. Molecules 2016, 21, 102. [Google Scholar] [CrossRef] [PubMed]
- You, S.P.; Zhao, J.; Ma, L.; Tudimat, M.; Zhang, S.L.; Liu, T. Preventive effects of phenylethanol glycosides from Cistanche tubulosa on bovine serum albumin-induced hepatic fibrosis in rats. DARU J. Pharm. Sci. 2015, 23, 52. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.L.; Wang, M.; Liu, L. The trap efficiency and the absorption of four kinds of proliposomes. J. Xin Jiang Med. Univ. 2007, 8, 787–790. [Google Scholar]
- Li, P.Y.; Du, S.Y.; Lu, Y.; Chen, X.N. Bio-adhesive drug delivery system and its application in traditional Chinese medicine. Chin. J. Mater. Medica 2017, 42, 4687–4693. [Google Scholar]
- Li, Y.; Lu, A.; Long, M.; Cui, L.; Chen, Z.; Zhu, L. Nitroimidazole derivative incorporated liposomes for hypoxia-triggered drug delivery and enhanced therapeutic efficacy in patient-derived tumor xenografts. Acta Biomater. 2019, 83, 334–348. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.-X.; Zhao, T.-W.; Zheng, T.; Sheng, Y.-J.; Zheng, H.-S.; Zhang, Y.-S. Liver-targeting liposome drug delivery system and its research progress in liver diseases. World Chin. J. Digestol. 2016, 24, 4238. [Google Scholar] [CrossRef]
- Zhou, B.H.; Tan, P.P.; Jia, L.S.; Zhao, W.P.; Wang, J.C.; Wang, H.W. PI3K/AKT signaling pathway involvement in fluoride-induced apoptosis in C2C12cells. Chemosphere 2018, 199, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Guan, Q.; Guo, Y.; Du, C. Echinacoside Stimulates Cell Proliferation and Prevents Cell Apoptosis in Intestinal Epithelial MODE-K Cells by Up-Regulation of Transforming Growth Factor-β1 Expression. J. Pharm. Sci. 2012, 118, 99–108. [Google Scholar] [CrossRef]
- Lin, L.-W.; Hsieh, M.-T.; Tsai, F.-H.; Wang, W.-H.; Wu, C.-R. Anti-nociceptive and anti-inflammatory activity caused by Cistanche deserticola in rodents. J. Ethnopharmacol. 2002, 83, 177–182. [Google Scholar] [CrossRef]
- Li, T.-M.; Huang, H.-C.; Su, C.-M.; Ho, T.-Y.; Wu, C.-M.; Chen, W.-C.; Fong, Y.-C.; Tang, C.-H. Cistanche deserticola extract increases bone formation in osteoblasts. J. Pharm. Pharmacol. 2012, 64, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Yu, F.; Tong, Z.; Huang, Z. Effect of Cistanches Herba Aqueous Extract on Bone Loss in Ovariectomized Rat. Int. J. Mol. Sci. 2011, 12, 5060–5069. [Google Scholar] [CrossRef]
- Lu, M.-C. Studies on the sedative effect of Cistanche deserticola. J. Ethnopharmacol. 1998, 59, 161–165. [Google Scholar] [CrossRef]
- Cai, R.-L.; Yang, M.-H.; Shi, Y.; Chen, J.; Li, Y.-C.; Qi, Y. Antifatigue activity of phenylethanoid-rich extract from Cistanche deserticola. Phytother. Res. 2010, 24, 313–315. [Google Scholar] [CrossRef]
- Geng, X.; Tian, X.; Tu, P.; Pu, X. Neuroprotective effects of echinacoside in the mouse MPTP model of Parkinson’s disease. Eur. J. Pharmacol. 2007, 564, 66–74. [Google Scholar] [CrossRef]
- Morikawa, T.; Pan, Y.; Ninomiya, K.; Imura, K.; Matsuda, H.; Yoshikawa, M.; Yuan, D.; Muraoka, O. Acylated phenylethanoid oligoglycosides with hepatoprotective activity from the desert plant Cistanche tubulosa1. Bioorg. Med. Chem. 2010, 18, 1882–1890. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.-R.; Wen, D.-S.; Fang, B.-W.; Lou, J.-S.; Meng, L. Prevention of Cistanche salsa Extract on Hepatic Fibrosis Induced by Carbon Tetrachloride in Rats. Chin. Herbal Med. 2013, 5, 199–204. [Google Scholar]
- Li, M.; Li, Y.; Liu, W.; Li, R.; Qin, C.; Liu, N.; Han, J. The preparation of Cistanche phenylethanoid glycosides liquid proliposomes: Optimized formulation, characterization and proliposome dripping pills in vitro and in vivo evaluation. Eur. J. Pharm. Sci. 2016, 93, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Ergen, C.; Niemietz, P.M.; Heymann, F.; Baues, M.; Gremse, F.; Pola, R.; van Bloois, L.; Storm, G.; Kiessling, F.; Trautwein, C.; et al. Liver fibrosis affects the targeting properties of drug delivery systems to macrophage subsets in vivo. Biomaterials 2019, 206, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Du, C.; Guo, N.; Teng, Y.; Meng, X.; Sun, H.; Li, S.; Yu, P.; Galons, H. Composition design and medical application of liposomes. Eur. J. Med. Chem. 2019, 164, 640–653. [Google Scholar] [CrossRef]
- Paliwal, S.; Tilak, A.; Sharma, J.; Dave, V.; Sharma, S.; Verma, K.; Tak, K.; Reddy, K.R.; Sadhu, V. Flurbiprofen-loaded ethanolic liposome particles for biomedical applications. J. Microbiol. Methods 2019, 161, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Sakai-Kato, K.; Yoshida, K.; Izutsu, K.-I. Effect of surface charge on the size-dependent cellular internalization of liposomes. Chem. Phys. Lipids 2019. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, Q.; Li, H.; Zhang, H.; Zhu, Y.; Omari-Siaw, E.; Sun, C.; Wei, Q.; Deng, W.; Yu, J.; et al. Galangin-loaded, liver targeting liposomes: Optimization and hepatoprotective efficacy. J. Drug Deliv. Sci. Technol. 2018, 46, 339–347. [Google Scholar] [CrossRef]
- Ezhilarasan, D.; Sokal, E.; Najimi, M. Hepatic fibrosis: It is time to go with hepatic stellate cell-specific therapeutic targets. Hepatobiliary Pancreatic Dis. Int. 2018, 17, 192–197. [Google Scholar] [CrossRef]
- Moehrle, B.M.; Nattamai, K.; Brown, A.; Florian, M.C.; Ryan, M.; Vogel, M.; Bliederhaeuser, C.; Soller, K.; Prows, D.R.; Abdollahi, A. Stem Cell-Specific Mechanisms Ensure Genomic Fidelity within HSCs and upon Aging of HSCs. Cell Rep. 2015, 13, 2412–2424. [Google Scholar] [CrossRef]
- Brea, R.; Motiño, O.; Francés, D.; García-Monzón, C.; Vargas, J.; Fernández-Velasco, M.; Boscá, L.; Casado, M.; Martín-Sanz, P.; Agra, N. PGE2 induces apoptosis of hepatic stellate cells and attenuates liver fibrosis in mice by downregulating miR-23a-5p and miR-28a-5p. Biochim. Biophys. Acta Biophys. Incl. Photsynth. 2018, 1864, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Lauridsen, F.K.B.; Jensen, T.L.; Rapin, N.; Aslan, D.; Wilhelmson, A.S.; Pundhir, S.; Rehn, M.; Paul, F.; Giladi, A.; Hasemann, M.S.; et al. Differences in Cell Cycle Status Underlie Transcriptional Heterogeneity in the HSC Compartment. Cell Rep. 2018, 24, 766–780. [Google Scholar] [CrossRef] [PubMed]
- El-Lakkany, N.M.; El-Maadawy, W.H.; Seif El-Din, S.H.; Hammam, O.A.; Mohamed, S.H.; Ezzat, S.M.; Safar, M.M.; Saleh, S. Rosmarinic acid attenuates hepatic fibrogenesis via suppression of hepatic stellate cell activation/proliferation and induction of apoptosis. Asian Pac. J. Trop. Med. 2017, 10, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.X.; Zhang, X.Y.; Ding, Y.Z.; Li, X.; Guan, X.M.; Li, H.; Cheng, M.; Cui, X.D. Effects of endothelial progenitor cells on proliferation and biological function of hepatic stellate cells under shear stress. Chin. J. Appl. Physiol. 2018, 34, 404–407. [Google Scholar]
- Zhang, R.; Lin, X.H.; Liu, H.H.; Ma, M.; Chen, J.; Chen, J.; Gao, D.M.; Cui, J.F.; Chen, R.X. Activated hepatic stellate cells promote progression of post-heat residual hepatocellular carcinoma from autophagic survival to proliferation. Int. J. Hyperthermia 2019, 36, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Le, C.T.; Sung, K.Y.; Choi, D.H.; Cho, E.H. Succinate induces hepatic fibrogenesis by promoting activation, proliferation, and migration, and inhibiting apoptosis of hepatic stellate cells. Biochem. Biophys. Res. Commun. 2018, 496, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, S.; Parsons, C.J.; Rippe, R.A. Mechanisms of liver fibrosis. Clin. Chim. Acta 2006, 364, 33–60. [Google Scholar] [CrossRef]
- Copple, B.L.; Roth, K. 2.16—Mechanisms of Liver Fibrosis☆. In Comprehensive Toxicology, 3rd ed.; McQueen, C.A., Ed.; Elsevier: Oxford, UK, 2018; pp. 397–408. [Google Scholar]
- Breitkopf, K.; Roeyen, C.V.; Sawitza, I.; Wickert, L.; Floege, J.; Gressner, A.M. Expression patterns of PDGF-A, -B, -C and -D and the PDGF-receptors α and β in activated rat hepatic stellate cells (HSC). Cytokine 2005, 31, 349–357. [Google Scholar] [CrossRef]
- El-Lakkany, N.M.; El-Maadawy, W.H.; Seif El-Din, S.H.; Saleh, S.; Safar, M.M.; Ezzat, S.M.; Mohamed, S.H.; Botros, S.S.; Demerdash, Z.; Hammam, O.A. Antifibrotic effects of gallic acid on hepatic stellate cells: In vitro and in vivo mechanistic study. J. Tradit. Complement. Med. 2019, 9, 45–53. [Google Scholar] [CrossRef]
- Carloni, V.; Romanelli, R.G.; Pinzani, M.; Laffi, G.; Gentilini, P. Focal adhesion kinase and phospholipase C gamma involvement in adhesion and migration of human hepatic stellate cells. Gastroenterology 1997, 112, 522–531. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, J.; Chen, L.; Xie, X.L.; Jiang, H. Inhibition of Focal Adhesion Kinase on Hepatic Stellate-cell Adhesion and Migration. Am. J. Med. Sci. 2017, 353, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Gentilini, A.; Marra, F.; Gentilini, P.; Pinzani, M. Phosphatidylinositol-3 kinase and extracellular signal-regulated kinase mediate the chemotactic and mitogenic effects of insulin-like growth factor-I in human hepatic stellate cells. J. Hepatol. 2000, 32, 227–234. [Google Scholar] [CrossRef]
- Zhang, C.; An, R.; Bao, Y.W.; Meng, X.M.; Wang, T.Q.; Sun, H.N.; Pan, F.M.; Zhang, C. Inhibitory effects of octreotide on the progression of hepatic fibrosis via the regulation of Bcl-2/Bax and PI3K/AKT signaling pathways. Int. Immunopharmacol. 2019, 73, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Reif, S.; Lang, A.; Lindquist, J.N.; Yata, Y.; Gabele, E.; Scanga, A.; Brenner, D.A.; Rippe, R.A. The role of focal adhesion kinase-phosphatidylinositol 3-kinase-akt signaling in hepatic stellate cell proliferation and type I collagen expression. J. Biol. Chem. 2003, 278, 8083–8090. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |

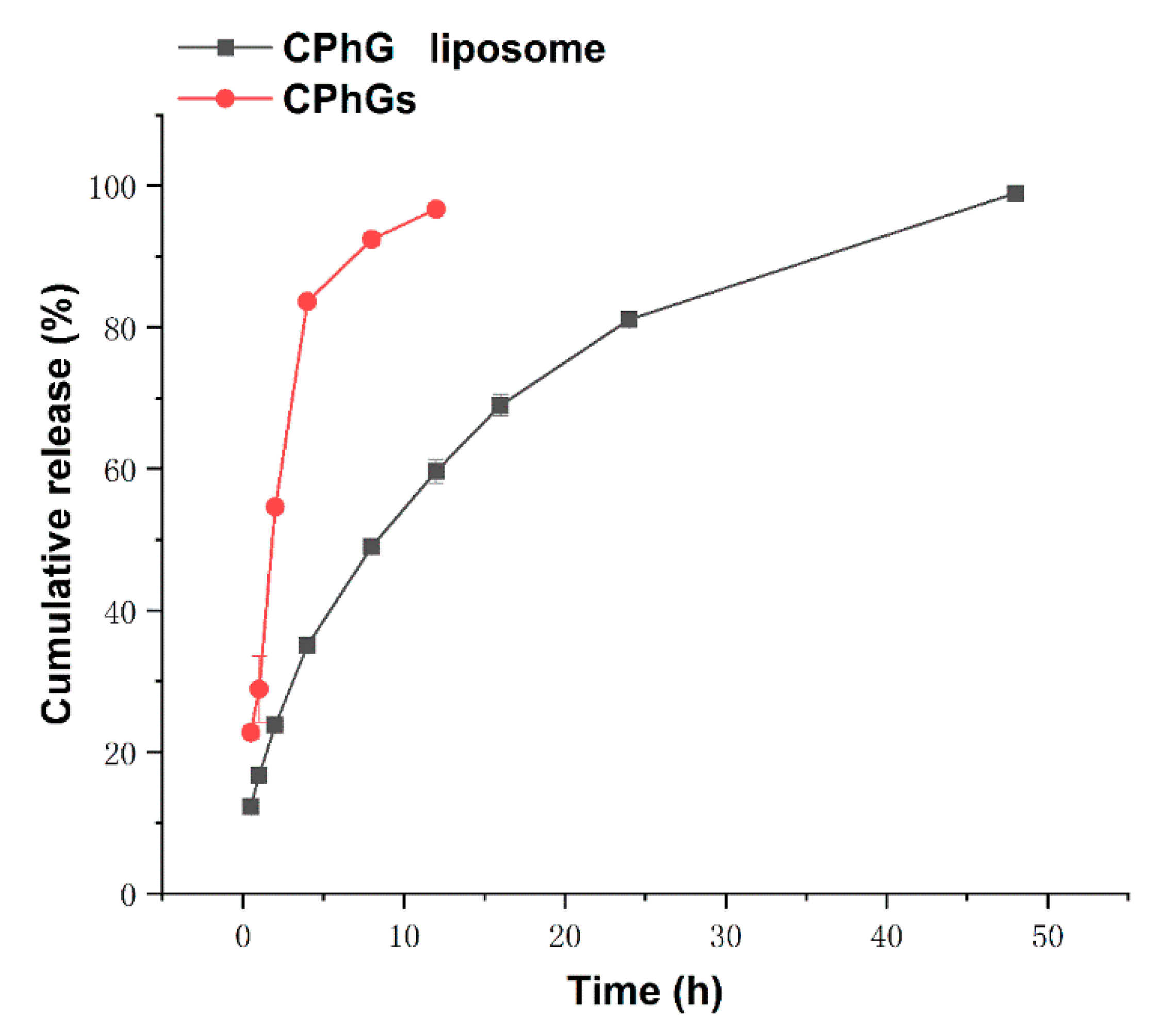



| Model | Equation | R | |
|---|---|---|---|
| CPhGs liposome | Zero-order kinetics | Q = 1.81t + 26.33 | 0.82442 |
| First-order kinetics | Q = 94.67(1 − e0.09t) | 0.95269 | |
| Higuchi | Q = 14.66t1/2 + 5.12 | 0.97661 | |
| CPhGs | Zero-order kinetics | Q = 6.26t + 34.45 | 0.70876 |
| First-order kinetics | Q = 97.37(1 − e0.43t) | 0.98557 | |
| Higuchi | Q = 28.48t1/2 + 8.98 | 0.86156 |
| Model | T50 (h) | |
|---|---|---|
| CPhGs liposome | Higuchi | 9.39139 |
| CPhGs | First-order kinetics | 1.6972 |
| Groups | n | LDH (U/104 cell) |
|---|---|---|
| Control | 9 | 0.62 ± 0.09 |
| CPhG liposomes 29.45 μg/mL | 9 | 0.67 ± 0.07 * |
| CPhG liposomes 14.72 μg/mL | 9 | 0.66 ±0.07 * |
| CPhG liposomes 7.36 μg/mL | 9 | 0.63 ± 0.05 * |
| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| collagen-1 | AACGCTATTGCCTGATGGACAGTC | CGCTGAGTCAAGGATGACGAAGAG |
| α-SMA | GCGTGGCTATTCCTTCGTGACTAC | CCATCAGGCAGTTCGTAGCTCTTC |
| MMP-1 | TGTTCGCCTTCTACAGAGGAGACC | TGTCGGTCCACGTCTCATCCAG |
| TIMP-1 | ATCTCTGGCCTCTGGCATCCTC | CGCTGGTATAAGGTGGTCTCGATG |
| β-actin | CAACCTTCTTGCAGCTCCTC | CGGTGTCCCTTCTGAGTGTT |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.-L.; Ma, L.; Zhao, J.; You, S.-P.; Ma, X.-T.; Ye, X.-Y.; Liu, T. The Phenylethanol Glycoside Liposome Inhibits PDGF-Induced HSC Activation via Regulation of the FAK/PI3K/Akt Signaling Pathway. Molecules 2019, 24, 3282. https://doi.org/10.3390/molecules24183282
Zhang S-L, Ma L, Zhao J, You S-P, Ma X-T, Ye X-Y, Liu T. The Phenylethanol Glycoside Liposome Inhibits PDGF-Induced HSC Activation via Regulation of the FAK/PI3K/Akt Signaling Pathway. Molecules. 2019; 24(18):3282. https://doi.org/10.3390/molecules24183282
Chicago/Turabian StyleZhang, Shi-Lei, Long Ma, Jun Zhao, Shu-Ping You, Xiao-Ting Ma, Xiao-Yan Ye, and Tao Liu. 2019. "The Phenylethanol Glycoside Liposome Inhibits PDGF-Induced HSC Activation via Regulation of the FAK/PI3K/Akt Signaling Pathway" Molecules 24, no. 18: 3282. https://doi.org/10.3390/molecules24183282
APA StyleZhang, S.-L., Ma, L., Zhao, J., You, S.-P., Ma, X.-T., Ye, X.-Y., & Liu, T. (2019). The Phenylethanol Glycoside Liposome Inhibits PDGF-Induced HSC Activation via Regulation of the FAK/PI3K/Akt Signaling Pathway. Molecules, 24(18), 3282. https://doi.org/10.3390/molecules24183282




