Combining DNA Barcoding and HPLC Fingerprints to Trace Species of an Important Traditional Chinese Medicine Fritillariae Bulbus
Abstract
1. Introduction
2. Results
2.1. Efficiency of PCR Amplification and Sequencing
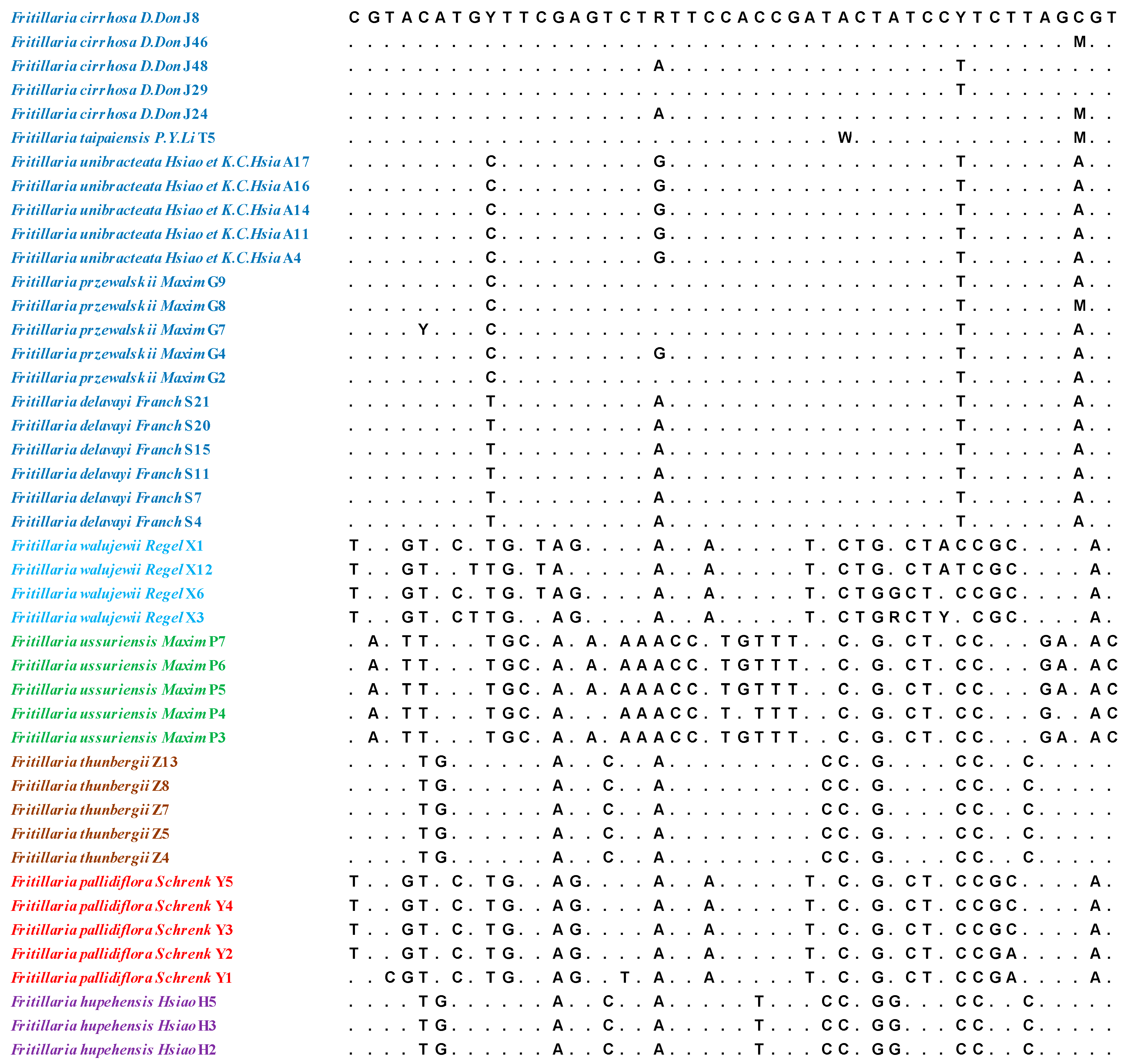
2.2. Genetic Divergence Determination
2.3. Neighbour-Joining (NJ) Tree Identification
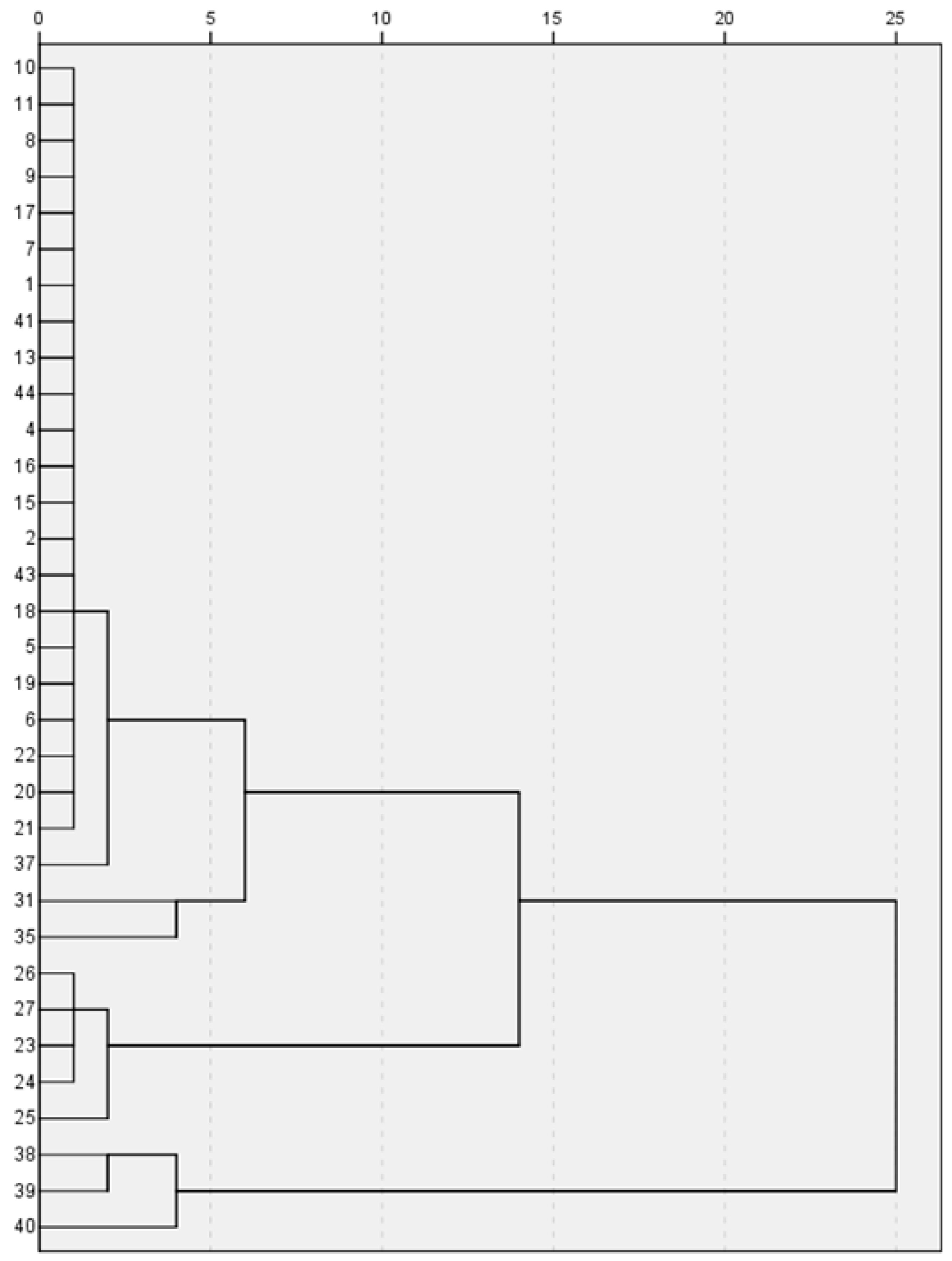
2.4. Cluster Analysis by High-Performance Liquid Chromatography
3. Discussion
3.1. Species Resources and DNA Barcodes of Fritillariae Bulbus
3.2. Identification of Fritillariae Bulbus
4. Materials and Methods
4.1. Sampling
4.2. DNA Extraction, Amplification, and Sequencing
4.3. Sequence Analysis
4.4. Chromatographic Conditions
4.4.1. Column and Instrument Conditions
4.4.2. Preparation of the Reference Solution
4.4.3. Preparation of Test Solution
4.4.4. Methodological Investigation
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, D.; Yang, J.; Du, Q.; Li, H.; Wang, S. The total alkaloid fraction of bulbs of Fritillaria cirrhosa displays anti-inflammatory activity and attenuates acute lung injury. J. Ethnopharmacol. 2016, 193, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; Chemical Industry Press: Beijing, China, 2015; Volume I. [Google Scholar]
- Mohammat, A.; Yili, A.; Aisa, H.A. Rapid Quantification and Quantitation of Alkaloids in Xinjiang Fritillaria by Ultra Performance Liquid Chromatography-Quadrupole Time-of-Flight Mass Spectrometry. Molecules 2017, 22, 719. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chen, X.; Atanasov, A.G.; Yi, X.; Wang, S. Plant Resource Availability of Medicinal Fritillaria Species in Traditional Producing Regions in Qinghai-Tibet Plateau. Front. Pharmacol. 2017, 8, 502. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, A.B.; Brinckmann, J.A.; Pei, S.J.; Luo, P.; Schippmann, U.; Long, X.; Bi, Y.F. High altitude species, high profits: Can the trade in wild harvested Fritillaria cirrhosa (Liliaceae) be sustained? J. Ethnopharmacol. 2018, 223, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Su, T.J.; Liu, Y.; Hou, K.; Chen, C.; Wu, W. Extraction, purification and antioxidation of a polysaccharide from Fritillaria unibracteata var. wabuensis. Int. J. Biol. Macromol. 2018, 112, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Zarei, O.; Yaghoobi, M.M. Cytotoxic effects of Fritillaria imperialis L. extracts on human liver cancer cells, breast cancer cells and fibroblast-like cells. Biomed. Pharmacother. Biomed. Pharmacother. 2017, 94, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chan, S.W.; Ma, J.; Li, P.; Shaw, P.C.; Lin, G. Investigation of association of chemical profiles with the tracheobronchial relaxant activity of Chinese medicinal herb Beimu derived from various Fritillaria species. J. Ethnopharmacol. 2017, 210, 39–46. [Google Scholar] [CrossRef]
- Zhao, B.; Shen, C.; Zheng, Z.; Wang, X.; Zhao, W.; Chen, X.; Peng, F.; Xue, L.; Shu, M.; Hou, X.; et al. Peiminine Inhibits Glioblastoma in Vitro and in Vivo Through Cell Cycle Arrest and Autophagic Flux Blocking. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 51, 1566–1583. [Google Scholar] [CrossRef]
- Gong, Q.; Li, Y.; Ma, H.; Guo, W.; Kan, X.; Xu, D.; Liu, J.; Fu, S. Peiminine Protects against Lipopolysaccharide-Induced Mastitis by Inhibiting the AKT/NF-kappaB, ERK1/2 and p38 Signaling Pathways. Int. J. Mol. Sci. 2018, 19, 2637. [Google Scholar] [CrossRef]
- Luo, D.; Liu, Y.; Wang, Y.; Zhang, X.; Huang, L.; Duan, B. Rapid identification of Fritillariae Cirrhosae Bulbus and its adulterants by UPLC-ELSD fingerprint combined with chemometrics methods. Biochem. Syst. Ecol. 2018, 76, 46–51. [Google Scholar] [CrossRef]
- Geng, Z.; Liu, Y.; Gou, Y.; Zhou, Q.; He, C.; Guo, L.; Zhou, J.; Xiong, L. Metabolomics study of cultivated bulbus fritillariae cirrhosae at different growth stages using UHPLC-QTOF-MS coupled with multivariate data analysis. Phytochem. Anal. 2018, 29, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Li, H.J.; Jiang, Y.; Li, P. Characterizing distribution of steroidal alkaloids in Fritillaria spp. and related compound formulas by liquid chromatography–mass spectrometry combined with hierarchial cluster analysis. J. Chromatogr. A 2009, 1216, 2142–2149. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.L.; Li, P.; Li, H.J.; Jiang, Y.; Ren, M.T.; Liu, Y. Development and validation of a liquid chromatography/electrospray ionization time-of-flight mass spectrometry method for relative and absolute quantification of steroidal alkaloids in Fritillaria species. J. Chromatogr. A 2008, 1177, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Pang, X.; Song, J.; Shi, L.; Yao, H.; Han, J.; Leon, C. A renaissance in herbal medicine identification: From morphology to DNA. Biotechnol. Adv. 2014, 32, 1237–1244. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, P.M. Refining the DNA barcode for land plants. Proc. Natl. Acad. Sci. USA 2011, 108, 19451–19452. [Google Scholar] [CrossRef] [PubMed]
- Chao, Z.; Zeng, W.; Liao, J.; Liu, L.; Liang, Z.; Li, X. DNA barcoding Chinese medicinal Bupleurum. Phytomed. Int. J. Phytother. Phytopharm. 2014, 21, 1767–1773. [Google Scholar] [CrossRef] [PubMed]
- Techen, N.; Parveen, I.; Pan, Z.; Khan, I.A. DNA barcoding of medicinal plant material for identification. Curr. Opin. Biotechnol. 2014, 25, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, Z.; Yang, J.; Lv, G. Complete chloroplast genome of seven Fritillaria species, variable DNA markers identification and phylogenetic relationships within the genus. PLoS ONE 2018, 13, e0194613. [Google Scholar] [CrossRef] [PubMed]
- Dawnay, N.; Ogden, R.; Mcewing, R.; Carvalho, G.R.; Thorpe, R.S. Validation of the barcoding gene COI for use in forensic genetic species identification. Forensic Sci. Int. 2007, 173, 1–6. [Google Scholar] [CrossRef]
- Eaton, M.J.; Meyers, G.L.; Kolokotronis, S.O.; Leslie, M.S.; Martin, A.P.; Amato, G. Barcoding bushmeat: Molecular identification of Central African and South American harvested vertebrates. Conserv. Genet. 2009, 11, 1389–1404. [Google Scholar] [CrossRef]
- Hebert, P.D.; Cywinska, A.; Ball, S.L.; deWaard, J.R. Biological identifications through DNA barcodes. Proc. Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.D.; Ratnasingham, S.; deWaard, J.R. Barcoding animal life: Cytochrome c oxidase subunit 1 divergences among closely related species. Proc. Biol. Sci. 2003, 270 (Suppl. 1), S96–S99. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Song, J.; Liu, C.; Luo, K.; Han, J.; Li, Y.; Pang, X.; Xu, H.; Zhu, Y.; Xiao, P.; et al. Use of ITS2 region as the universal DNA barcode for plants and animals. PLoS ONE 2010, 5, e13102. [Google Scholar] [CrossRef] [PubMed]
- Sheidai, M.; Tabaripour, R.; Talebi, S.M.; Noormohammadi, Z.; Koohdar, F. Adulteration in medicinally important plant species of Ziziphora in Iran market: DNA barcoding approach. Ind. Crops Products 2019, 130, 627–633. [Google Scholar] [CrossRef]
- Aziz, N.A.; Ahmad, M.I.; Naim, D.M. Molecular DNA identification of medicinal plants used by traditional healers in Malaysia. Genet. Mol. Res. 2015, 14, 15937–15947. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.M.; Tyagi, A.; Kumar, A.; Singh, A.; Singh, S.; Chaudhary, L.B.; Roy, S. The internal transcribed spacer (ITS) region and trnH-psbA [corrected] are suitable candidate loci for DNA barcoding of tropical tree species of India. PLoS ONE 2013, 8, e57934. [Google Scholar] [CrossRef] [PubMed]
- Vassou, S.L.; Kusuma, G.; Parani, M. DNA barcoding for species identification from dried and powdered plant parts: A case study with authentication of the raw drug market samples of Sida cordifolia. Gene 2015, 559, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Moon, B.C.; Park, I.; Kim, W.J.; Yang, S.; Kang, Y.M. The complete chloroplast genome sequence of Fritillaria thunbergii Miq., an important medicinal plant, and identification of DNA markers to authenticate Fritillariae Bulbus. Hortic. Environ. Biotechnol. 2018, 59, 1–10. [Google Scholar] [CrossRef]
- Wang, C.Z.; Li, P.; Ding, J.Y.; Peng, X.; Yuan, C.S. Simultaneous identification of Bulbus Fritillariae cirrhosae using PCR-RFLP analysis. Phytomed. Int. J. Phytother. Phytopharm. 2007, 14, 628–632. [Google Scholar] [CrossRef]
- Xin, G.Z.; Lam, Y.C.; Maiwulanjiang, M.; Chan, G.; Zhu, K.; Tang, W.L.; Dong, T.T.-X.; Shi, Z.Q.; Li, P.; Tsim, K.W.K. Authentication of Bulbus Fritillariae Cirrhosae by RAPD-derived DNA markers. Molecules 2014, 19, 3450–3459. [Google Scholar] [CrossRef]
- Zhang, D.; Mo, X.; Xiang, J.; Zhou, N. Molecular Identification of Original Plants of Fritillariae Cirrhosae Bulbus, A Tradtional Chinese Medicine (Tcm) Using Plant Dna Barcoding. Afr. J. Tradit. Complementary Altern. Med. Ajtcam 2016, 13, 74. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, C.; Sun, Y.Q.; Song, J.Y.; Li, C.J.; Li, X.W.; Zhang, X.W.; Li, Y.; Hu, S.N.; Luo, H.M.; Zhu, Y.J.; et al. Discovery of genes related to steroidal alkaloid biosynthesis in Fritillaria cirrhosa by generating and mining a dataset of expressed sequence tags (ESTs). J. Med. Plants Res. 2011, 5, 5307–5314. [Google Scholar]
- Li, X.; Su, Y.; Li, X.; Gang, X.; Qiang, W.; Shi, J.; Wang, L.; Chen, S. Identification of Fritillariae bulbus from adulterants using ITS2 regions. Plant Gene 2016, 7, S2352407316300051. [Google Scholar]
- Larkin, M.; Blackshields, G.; Brown, N.; Chenna, R.; Mcgettigan, P.; Mcwilliam, H.; Valentin, F.; Wallace, I.; Wilm, A.R. Clustal W and clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.; Paulay, G. DNA barcoding: Error rates based on comprehensive sampling. PLoS Biol. 2005, 3, e422. [Google Scholar] [CrossRef] [PubMed]
- Meier, R.; Shiyang, K.; Vaidya, G.; Ng, P.K.L. DNA Barcoding and Taxonomy in Diptera: A Tale of High Intraspecific Variability and Low Identification Success. Syst. Biol. 2006, 55, 715–728. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds peimisine, verticine, verticinone, and imperialine are available from the authors. |



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, Y.; Wang, H.; Wei, Q.; Cao, R.; Zhang, H.; He, Y.; Wang, L. Combining DNA Barcoding and HPLC Fingerprints to Trace Species of an Important Traditional Chinese Medicine Fritillariae Bulbus. Molecules 2019, 24, 3269. https://doi.org/10.3390/molecules24183269
Zhong Y, Wang H, Wei Q, Cao R, Zhang H, He Y, Wang L. Combining DNA Barcoding and HPLC Fingerprints to Trace Species of an Important Traditional Chinese Medicine Fritillariae Bulbus. Molecules. 2019; 24(18):3269. https://doi.org/10.3390/molecules24183269
Chicago/Turabian StyleZhong, Yingchun, Haiying Wang, Qianhe Wei, Rui Cao, Hailong Zhang, Yongzhi He, and Lizhi Wang. 2019. "Combining DNA Barcoding and HPLC Fingerprints to Trace Species of an Important Traditional Chinese Medicine Fritillariae Bulbus" Molecules 24, no. 18: 3269. https://doi.org/10.3390/molecules24183269
APA StyleZhong, Y., Wang, H., Wei, Q., Cao, R., Zhang, H., He, Y., & Wang, L. (2019). Combining DNA Barcoding and HPLC Fingerprints to Trace Species of an Important Traditional Chinese Medicine Fritillariae Bulbus. Molecules, 24(18), 3269. https://doi.org/10.3390/molecules24183269



