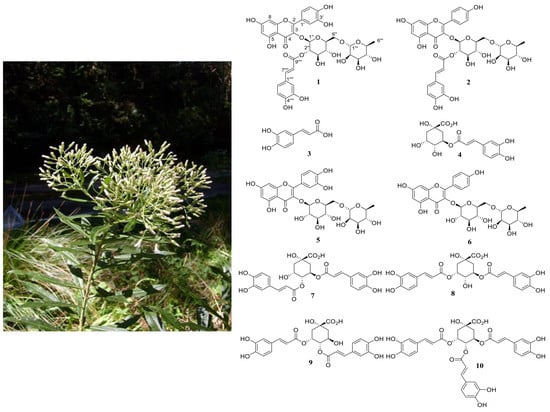
Two New Flavonoids from the Leaves of Baccharis oblongifolia (Ruiz and Pav.) Pers. (Asteraceae)
Abstract
:1. Introduction
2. Results
Structural Elucidation
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Antiradical Assay
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Müller, J. Systematics of Baccharis (Compositae–Astereae) in Bolivia, including an overview of the genus. Syst. Bot. Monographs 2006, 76, 1–341. [Google Scholar]
- Heiden, G.; Pirani, J.R. Novelties towards a phylogenetic infrageneric classification of Baccharis (Asteraceae, Astereae). Phytotaxa 2016, 289, 285–290. [Google Scholar] [CrossRef]
- Heiden, G.; Schneider, A. Baccharis in Lista de Espécies da Flora do Brasil. Jardim Botânico do Rio de Janeiro. 2015. Available online: http://floradobrasil.jbrj.gov.br/jabot/floradobrasil/FB5151 (accessed on 5 August 2019).
- Verdi, L.G.; Brighente, I.M.C.; Pizzolatti, M.G. Gênero Baccharis (Asteraceae): Aspectos químicos, econômicos e biológicos. Quim. Nova 2005, 28, 85–94. [Google Scholar] [CrossRef]
- Campos, F.R.; Bressan, J.; Jasinski, V.C.G.; Zuccolotto, T.; Silva, L.E.; Cerqueira, L.B. Baccharis (Asteraceae): Chemical constituents and biological activities. Chem. Biodivers. 2016, 13, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Abad, M.J.; Bermejo, P. Baccharis (Compositae): A review update. Arkivoc 2007, 7, 76–96. [Google Scholar] [CrossRef]
- Simões-Pires, C.A.; Queiroz, E.F.; Henriques, A.T.; Hostettmann, K. Isolation and on-line identification of antioxidant compounds from three Baccharis species by HPLC-UV-MS/MS with post-column derivatisation. Phytochem. Anal. 2005, 16, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Grecco, S.S.; Ferreira, M.J.P.; Romoff, P.; Fávero, O.A.; Lago, J.H.G. Phenolic derivatives from Baccharis retusa DC. (Asteraceae). Biochem. Syst. Ecol. 2012, 42, 21–24. [Google Scholar] [CrossRef]
- Grecco, S.S.; Félix, M.J.P.; Lago, J.H.G.; Pinto, E.G.; Tempone, A.G.; Ferreira, M.J.P.; Romoff, P.; Sartorelli, P. Anti-trypanosomal phenolic derivatives from Baccharis uncinella C. DC. (Asteraceae). Nat. Prod. Commun. 2014, 9, 171–173. [Google Scholar] [CrossRef]
- Toyama, D.O.; Ferreira, M.J.P.; Romoff, P.; Fávero, O.A.; Gaeta, H.H.; Toyama, M.H. Effect of chlorogenic acid (5-caffeoylquinic acid) isolated from Baccharis oxyodonta on the structure and pharmacological activities of secretory phospholipase A2 from Crotalus durissus terrificus. BioMed Res. Int. 2014, article ID 726585. [Google Scholar] [CrossRef]
- Sayuri, V.A.; Romoff, P.; Fávero, O.A.; Ferreira, M.J.P.; Lago, J.H.G.; Buturi, F.O.S. Chemical composition, seasonal variation and biosynthetic considerations of essential oils from Baccharis microdonta and B. elaeagnoides (Asteraceae). Chem. Biodivers. 2010, 7, 2771–2782. [Google Scholar] [CrossRef]
- Budel, J.M.; Wang, M.; Raman, V.; Zhao, J.; Khan, S.I.; Rehman, J.U.; Techen, N.; Tekwani, B.; Monteiro, L.M.; Heiden, G.; et al. Essential oils of five Baccharis species: investigations on the chemical composition and biological activities. Molecules 2018, 23, 2620. [Google Scholar] [CrossRef] [PubMed]
- Ascari, J.; Oliveira, M.S.; Nunes, D.S.; Granato, D.; Scharf, D.R.; Simionatto, E.; Otuki, M.; Soley, B.; Heiden, G. Chemical composition, antioxidant and anti-inflammatory activities of the essential oils from male and female specimens of Baccharis punctulata (Asteraceae). J. Ethnopharmacol. 2019, 234, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zuccolotto, T.; Bressan, J.; Lourenco, A.V.F.; Bruginski, E.; Veiga, A.; Marinho, J.V.N.; Raeski, P.A.; Heiden, G.; Salvador, M.J.; Murakami, F.S.; et al. Chemical, antioxidant, and antimicrobial evaluation of essential oils and an anatomical study of the aerial parts from Baccharis species (Asteraceae). Chem. Biodivers. 2019, 16, e1800547. [Google Scholar] [CrossRef]
- Sobrinho, A.C.N.; Souza, E.B.; Rocha, M.F.G.; Albuquerque, M.R.J.R.; Bandeira, P.N.; Santos, H.S.; Cavalcante, C.S.P.; Oliveira, S.S.; Aragão, P.R.; Morais, S.M.; et al. Chemical composition, antioxidant, antifungal and hemolytic activities of essential oil from Baccharis trinervis (Lam.) Pers. (Asteraceae). Ind. Crops Prod. 2016, 84, 108–115. [Google Scholar] [CrossRef]
- Grecco, S.S.; Gimenes, L.; Ferreira, M.J.P.; Romoff, P.; Fávero, O.A.; Zalewski, C.A.; Lago, J.H.G. Triterpenoids and phenolic derivatives from Baccharis uncinella C.DC. (Asteraceae). Biochem. Syst. Ecol. 2010, 38, 1234–1237. [Google Scholar] [CrossRef]
- Alvarenga, S.A.V.; Ferreira, M.J.P.; Rodrigues, G.V.; Emerenciano, V.P. A general survey and some taxonomic implications of diterpenes in the Asteraceae. Bot. J. Linn. Soc. 2005, 147, 291–308. [Google Scholar] [CrossRef]
- Seaman, F.; Bohlmann, F.; Zdero, C.; Mabry, T.J. Diterpenes of flowering plants – Compositae (Asteraceae); Springer: New York, NY, USA, 1990. [Google Scholar]
- Funes, M.; Garro, M.F.; Tosso, R.D.; Maria, A.O.; Saad, J.R.; Enriz, R.D. Antinociceptive effect of neo-clerodane diterpenes obtained from Baccharis flabellata. Fitoterapia 2018, 130, 94–99. [Google Scholar] [CrossRef]
- Moreira, C.P.S.; Oliveira, D.M.; Santos, C.N.; Zani, C.L.; Alves, T.M.A. Platypodiol a novel clerodane diterpene from Baccharis platypoda. Tetrah. Lett. 2014, 55, 4898–4900. [Google Scholar] [CrossRef]
- Ueno, A.K.; Barcellos, A.F.; Grecco, S.S.; Sartorelli, P.; Guadagnin, R.C.; Romoff, P.; Ferreira, M.J.P.; Tcacenco, C.M.; Lago, J.H.G. Sesquiterpenes, diterpenes, alkenyl p-coumarates and flavonoid from the aerial parts of Baccharis retusa (Asteraceae). Biochem. Syst. Ecol. 2018, 78, 39–42. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Quispe, C.; Bórquez, J.; Mocan, A.; Sepúlveda, B. High resolution metabolite fingerprinting of the resin of Baccharis tola Phil. from the Atacama Desert and its antioxidant capacities. Ind. Crops Prod. 2016, 94, 368–375. [Google Scholar] [CrossRef]
- Gomez, J.; Simirgiotis, M.J.; Lima, B.; Paredes, J.D.; Gabutti, C.M.V.; Gamarra-Luques, C.; Borquez, J.; Luna, L.; Wendel, G.H.; Maria, A.O.; et al. Antioxidant, gastroprotective, cytotoxic activities and UHPLC PDA-Q orbitrap mass spectrometry identification of metabolites in Baccharis grisebachii decoction. Molecules 2019, 24, 1085. [Google Scholar] [CrossRef] [PubMed]
- Grecco, S.S.; Reimão, J.Q.; Tempone, A.G.; Sartorelli, P.; Romoff, P.; Ferreira, M.J.P.; Fávero, O.A.; Lago, J.H.G. Isolation of an antileishmanial and antitrypanosomal flavanone from the leaves of Baccharis retusa (Asteraceae). Parasitol. Res. 2010, 106, 1245–1248. [Google Scholar] [CrossRef] [PubMed]
- Grecco, S.S.; Reimão, J.Q.; Tempone, A.G.; Sartorelli, P.; Cunha, R.L.O.R.; Ferreira, M.J.P.; Romoff, P.; Fávero, O.A.; Lago, J.H.G. In vitro antileishmanial and antitrypanosomal activities of flavanones from Baccharis retusa DC. (Asteraceae). Exp. Parasitol. 2012, 130, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Bohm, B.A.; Stuessy, T.F. Flavonoids of the Sunflower Family; SpringerWien: New York, NY, USA, 2001. [Google Scholar]
- Sari, A.; Keçeci, Z. Phytochemical investigation on chemical constituents of Taraxacum bessarabicum (Hornem.) Hand.-Mazz. subsp. bessarabicum (Hornem.) Hand.-Mazz. Iranian J. Pharm. Res. 2019, 18, 400–405. [Google Scholar]
- Tamayose, C.I.; Torres, P.B.; Roque, N.; Ferreira, M.J.P. HIV-1 reverse transcriptase inhibitory activity of flavones and chlorogenic acid derivatives from Moquiniastrum floribundum (Asteraceae). South Afr. J. Bot. 2019, 123, 142–146. [Google Scholar] [CrossRef]
- Beck, M.-A.; Häberlein, H. Flavonol glycosides from Elchscholtzia californica. Phytochemistry 1999, 50, 329–332. [Google Scholar] [CrossRef]
- Sang, S.; Lapsley, K.; Jeong, W.-S.; Lachance, P.A.; Ho, C.-T.; Rosen, R.T. Antioxidative phenolic compounds isolated from almond skins (Prunus amygdalus Batch). J. Agric. Food Chem. 2002, 50, 2459–2463. [Google Scholar] [CrossRef] [PubMed]
- Tamayose, C.I.; Santos, E.A.; Roque, N.; Costa-Lotufo, L.V.; Ferreira, M.J.P. Caffeoylquinic acids: Separation method, antiradical properties and cytotoxicity. Chem. Biodivers. 2019, 16, e1900093. [Google Scholar] [CrossRef]
- Oliveira, S.; Souza, G.A.; Eckert, C.R.; Silva, T.A.; Sobral, E.S.; Fávero, O.A.; Ferreira, M.J.P.; Romoff, P.; Baader, W.J. Evaluation of antiradical assays used in determining the antioxidant capacity of pure compounds and plant extracts. Quim. Nova 2014, 37, 497–503. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1–10 are available from the authors. |

| Position | 1 | 2 | ||||
|---|---|---|---|---|---|---|
| δH | δC | HMBC | δH | δC | HMBC | |
| 2 | ----- | 157.4 | ----- | ----- | 157.5 | ----- |
| 3 | ----- | 133.4 | ----- | ----- | 133.3 | ----- |
| 4 | ----- | 177.6 | ----- | ----- | 177.7 | ----- |
| 5 | ----- | 161.7* | ----- | ----- | 161.7 | ----- |
| 6 | 6.13 d (2.0) | 99.5 | C5; C7; C8; C10 | 6.17 brs | 98.5 | C5; C7; C8; C10 |
| 7 | ----- | 165.2* | ----- | ----- | 164.5 | ----- |
| 8 | 6.29 d (2.0) | 93.8* | C6 | 6.35 brs | 93.5 | C6; C1⁗; C9⁗ |
| 9 | ----- | 157.0 | ----- | ----- | 157.0 | ----- |
| 10 | ----- | 103.4 | ----- | ----- | 104.4 | ----- |
| 1′ | ----- | 121.5 | ----- | ----- | 121.5 | ----- |
| 2′ | 7.57 d (2.1) | 122.1 | C2; C6′; C4′ | 7.99 d (8.4) | 130.8 | C2; C2′/6′; C3′/5′; C4′ |
| 3′ | ----- | 144.5 | ----- | 6.90 d (8.4) | 114.8 | C1′; C3′/5′; C4′ |
| 4′ | ----- | 148.4 | ----- | ----- | 160.0 | ----- |
| 5′ | 6.86 d (8.2) | 114.8 | C1′; C3′; C4′ | 6.90 d (8.4) | 114.8 | C1′; C3′/5′; C4′ |
| 6′ | 7.56 dd (8.2, 2.1) | 115.9 | C4′; C5′ | 7.99 d (8.4) | 130.8 | C2; C2′/6′; C3′/5′; C4′ |
| 1″ | 5.48 d (8.0) | 99.5 | C3 | 5.55 d (8.0) | 99.4 | C3; C5″ |
| 2″ | 5.04 dd (9.6, 8.0) | 74.3 | C1″; C3″; C9⁗ | 5.01 dd (9.6, 8.0) | 74.3 | C1″; C3″; C9⁗ |
| 3″ | 3.60 d (9.6) | 74.9 | C2″; C4″ | 3.61 d (9.6) | 74.8 | C2″; C4″ |
| 4″ | 3.43 d (4.7) | 70.7 | C5″; C6″ | 3.35 d (2.4) | 70.4 | C5″; C6″ |
| 5″ | 3.38 d (8.5) | 75.8 | C4″; C6″ | 3.43 d (9.3) | 76.0 | C4″; C6″ |
| 6″ | 3.87 d (9.5) 3.55 d (9.5) | 66.9 | C5″; C1‴ | 3.87 d (9.3) 3.43 d (9.3) | 66.9 | C4″; C1‴ |
| 1‴ | 4.55 brs | 100.9 | C3‴; C5‴ | 4.54 d (1.4) | 100.9 | C3‴; C5‴ |
| 2‴ | 3.49 d (3.1) | 70.7 | 3.64 d (3.3) | 70.7 | ||
| 3‴ | 3.66 dd (3.3, 1.7) | 70.9 | C4‴ | 3.53 dd (9.5, 3.3) | 70.9 | C4‴ |
| 4‴ | 3.27 d (9.5) | 72.5 | C3‴; C5‴; C6‴ | 3.27 d (9.5) | 72.5 | C3‴; C5‴; C6‴ |
| 5‴ | 3.46 d (6.4) | 68.4 | C4‴ | 3.47 dd (9.5, 6.2) | 68.4 | C4‴ |
| 6‴ | 1.13 d (6.2) | 16.5 | C4‴; C5‴ | 1.13 d (6.2) | 16.5 | C4‴; C5‴ |
| 1⁗ | ----- | 126.5 | ----- | ----- | 126.5 | ----- |
| 2⁗ | 7.06 d (2.1) | 113.8 | C3⁗; C4⁗; C6⁗ | 7.06 d (2.0) | 113.8 | C3⁗; C4⁗; C6⁗ |
| 3⁗ | ----- | 145.4 | ----- | ----- | 145.5 | ----- |
| 4⁗ | ----- | 148.2 | ----- | ----- | 148.2 | ----- |
| 5⁗ | 6.78 d (8.2) | 115.1 | C1⁗; C3⁗; C4⁗; C6⁗ | 6.79 d (8.2) | 115.1 | C1⁗; C3⁗; C4⁗; C6⁗ |
| 6⁗ | 6.94 dd (8.2, 2.1) | 121.7 | C4⁗; C7⁗; C8⁗ | 6.95 dd (8.2, 2.0) | 121.7 | C4⁗; C7⁗; C8⁗ |
| 7⁗ | 7.62 d (15.8) | 146.0 | C1⁗; C2⁗; C6⁗; C9⁗ | 7.62 d (15.8) | 146.0 | C1⁗; C2⁗; C6⁗; C9⁗ |
| 8⁗ | 6.33 d (15.8) | 113.4 | C1⁗; C9⁗ | 6.32 d (15.8) | 113.8 | C1⁗; C9⁗ |
| 9⁗ | ----- | 167.2 | ----- | ----- | 167.1 | ----- |
| Compound | % Trolox (L·μmol−1) | IC50 (µmol·L−1) | n * |
|---|---|---|---|
| (1) Oblongifolioside A | 245.5 ± 0.1 | 13.3 ± 0.5 | 4.8 ± 0.1 |
| (2) Oblongifolioside B | 244.9 ± 0.2 | 12.4 ± 0.2 | 4.8 ± 0.2 |
| Trolox | -- | 45.4 ± 1.7 | 1.96 ± 0.06 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zampieri, P.R.F.; Tamayose, C.I.; Fávero, O.A.; Romoff, P.; Ferreira, M.J.P. Two New Flavonoids from the Leaves of Baccharis oblongifolia (Ruiz and Pav.) Pers. (Asteraceae). Molecules 2019, 24, 3198. https://doi.org/10.3390/molecules24173198
Zampieri PRF, Tamayose CI, Fávero OA, Romoff P, Ferreira MJP. Two New Flavonoids from the Leaves of Baccharis oblongifolia (Ruiz and Pav.) Pers. (Asteraceae). Molecules. 2019; 24(17):3198. https://doi.org/10.3390/molecules24173198
Chicago/Turabian StyleZampieri, Paulo R. F., Cinthia I. Tamayose, Oriana A. Fávero, Paulete Romoff, and Marcelo J. P. Ferreira. 2019. "Two New Flavonoids from the Leaves of Baccharis oblongifolia (Ruiz and Pav.) Pers. (Asteraceae)" Molecules 24, no. 17: 3198. https://doi.org/10.3390/molecules24173198
APA StyleZampieri, P. R. F., Tamayose, C. I., Fávero, O. A., Romoff, P., & Ferreira, M. J. P. (2019). Two New Flavonoids from the Leaves of Baccharis oblongifolia (Ruiz and Pav.) Pers. (Asteraceae). Molecules, 24(17), 3198. https://doi.org/10.3390/molecules24173198